INTRODUCTION
Chronic neuropathic pain affects 8.2% of adults, extrapolated to roughly 18 million people every year in the United States.1,2 Patients who have pain that cannot be controlled with pharmacologic management or less invasive techniques can be considered for deep brain stimulation (DBS) or motor cortex stimulation (MCS).3 These techniques are not currently approved by the American Food and Drug Administration for chronic pain and are, thus, considered off-label use of medical devices for this patient population. Conclusive effectiveness studies are still needed to demonstrate the best targets as well as the reliability of the results with these approaches.
MCS
In the early 1990s, stimulation of feline and rodent cortex via epidural leads was found to modulate thalamic hyperactivity in a model of deafferentation.4,5 This concept, when applied to patients with chronic central or peripheral deafferentation pain, demonstrated initial success.6 However, subsequent studies have exhibited mixed clinical outcomes of MCS.7,8 MCS has been explored as an option to treat trigeminal neuralgia, poststroke central pain, spinal cord injury pain, and other pain disorders.7,9,10 In a review of 22 chronic pain studies, Lima and Fregni11 found that epidural MCS showed a significant effect in chronic pain and recommended further clinical trials to elucidate the role of MCS. It is important to note that, as for all meta-analysis, the conclusions are limited by the level of evidence of the literature included. Because there is no large, randomized placebo-controlled trial to date, the meta-analysis included mostly uncontrolled case series with various technical approaches. A recent randomized, double-blind, placebo-controlled, crossover trial examined the efficacy of MCS in a small number of patients with a variety of peripheral neuropathies.12 Although MCS efficacy was considered good or satisfactory in 60% of the patients during the open phase, no significant differences in pain ratings were detected between the ON and OFF stimulation groups when adjusting for multiple comparisons.12 The mixed clinical outcomes of MCS indicate that the therapy would benefit from a multicenter, prospective, randomized controlled trial with systematic patient selection, surgical technique, programing methodology, and follow-up. In the meantime, it is likely that MCS will continue to be used sporadically for selected patients in need of alternatives for refractory pain. MCS has risks that are typical of most craniotomies, including infection, hemorrhage, and neurologic deficits but is considered to be, overall, safe. MCS has been associated with seizures during stimulator programing and active stimulation; however, seizures and epilepsy do not seem to be a long-term complication.13
PAIN PATHWAYS
Pain transmission and its pathways are complex. It is thought that activity in two pathways, the lateral pain system and the medial pain system, can be modulated with DBS. The lateral pain system consists of spinothalamic tracts, which connect to the ventral posterior lateral (VPL), ventral posterior medial (VPM), and ventral posterior inferior nuclei of the thalamus, which then project to the primary and secondary somatosensory cortices. This pathway, thought to be involved in central pain, is seen in the Dejerine-Roussy syndrome (or thalamic pain syndrome) whereby damage to the thalamus or afferent and efferent fiber bundles can cause chronic pain with or without allodynia and hyperalgesia. The medial pain system consists of spinothalamic projections to the medial thalamic nuclei, limbic cortices, anterior cingulate cortex, and reticular formation and has been found to modulate emotional and affective perception with painful stimuli.14,15 The periaqueductal gray (PAG) is part of a pain inhibitory pathway in which dopamine and serotonin signaling are linked with pain suppression and analgesia whereas norepinephrine facilitates pain transmission.16,17
DBS TARGETS
DBS for modulation of refractory pain goes back to studies beginning in the 1950s, with targets including the septal region, central medial, and parafascicular thalamic nuclei.18–20 The most frequently reported targets are the sensory nucleus of the thalamus (ventral caudal or VPL/ VPM) and the PAG and periventricular gray matter (PVG) (Fig. 1A).17,21,22 New targets under exploration include the mesial thalamic nuclei and the area of the ventral anterior limb of the internal capsule (VC) and ventral striatum (VS) (see Fig. 1B).23,24
Fig. 1.
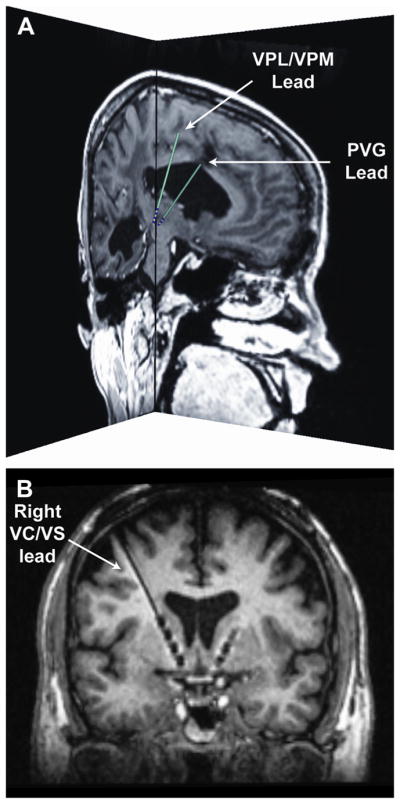
DBS targets for pain management. (A) More traditional DBS targets aimed at treating the sensory-discriminative component of pain. The image shows the preoperative magnetic resonance imaging (MRI) and corresponding lead (model 3387, Medtronic, Minneapolis, MN) locations for a patient with one DBS lead in the VPL/VPM and a second DBS lead in the PVG. Both sagittal and coronal slices are shown near the distal ends of the leads. The patient-specific lead locations and trajectories were determined using the software Cicerone v1.3.25 (B) A recently proposed DBS target aimed at treating the affective-motivational component of pain.24 The image shows an oblique coronal view of the postoperative MRI for a patient with bilateral DBS leads (Medtronic model 3391) implanted in the ventral capsule and ventral striatal (VC/VS) area. It is possible to see the 4 electrodes in the right hemisphere as well as the distal end of the DBS lead implanted in the left hemisphere.
Sensory Nucleus of the Thalamus (VPL, VPM)
In the early 1970s, Mazars and colleagues26 chronically implanted electrodes to stimulate the sensory thalamic relay nuclei based on prior work in the 1960s in which they stimulated the ventral posterolateral thalamic relay nucleus. Hosobuchi and colleagues21 chronically implanted electrodes in the sensory thalamus and were able to alleviate refractory facial pain in 4 of 5 patients for up to 2 years of follow-up. Turnbull and colleagues27 studied 18 cases of sensory thalamic implantation, and they found 72% of successful pain relief over a 10-month average follow-up. In a retrospective evaluation of 76 patients implanted with chronic stimulators in the thalamic somatosensory area for deafferentation pain, 44 patients reported substantial pain relief for longer than 2 years.28 Levy and colleagues29 reviewed a series of 84 patients with deafferentation pain implanted in either the VPL (extremity deafferentation) or the VPM (facial deafferentation); they found that 61% had initial success (patients using stimulator regularly with pain relief), but only 30% had long-term success after at least 2 years. Patients with peripheral neuropathy had greater long-term success (50%) compared with central anesthesia dolorosa (18%) or pain associated with spinal cord injury (0%), demonstrating great variability of outcomes depending on the location of the precipitating injury. Ten years later, Kumar and colleagues30 published a study in which they prospectively evaluated 20 patients who had a negative response to a morphine-naloxone test and were diagnosed with deafferentation pain and implanted with externalized DBS leads targeting the sensory thalamus, 3 of which had dual-implanted PVG and sensory thalamus electrodes. Twelve patients underwent chronic implantation, and 6 patients reported long-term relief at an average of 3.8 years. In a prospective, double-blind, placebo-controlled trial, Marchand and colleagues31 evaluated the effect of sensory thalamic stimulation in 6 patients who had been implanted for at least 2 years. These patients reported a significant reduction in daily pain with DBS, but a strong placebo component was noted. Hamani and colleagues32 demonstrated much less favorable results after implantation of 21 patients whereby only 5 patients (4 patients with DBS in the sensory thalamus and one patient with DBS in both the sensory thalamus and the PAG/PVG) experienced at least 1 year of pain relief.
PAG and PVG Matter
Reynolds described an application of focal stimulation to the PAG in rats that induced electroanesthesia via chronic monopolar stainless steel electrodes. The anesthetic effect was so profound that investigators were able to perform laparotomies under the analgesia provided by DBS.33 This study helped serve as the groundwork to translate the concept of central gray matter stimulation to the human as seen in work by Richardson and Akil.22 These studies initiated with a group of 5 patients who received a short period of acute stimulation before thalamic ablation. Monopolar stainless steel electrodes were stereotactically implanted into the PAG/PVG, transversing through other locations, which were tested. The 5 patients had diverse conditions, including phantom limb leg pain, abdominal cancer pain, brachial plexus pain, thalamic stroke pain, and intention tremor without pain. During stimulation, numerous side effects were seen, including nystagmus and dizziness. The best relief was seen around the nucleus parafascicularis in the medial thalamus with the least amount of side effects.34 Based on these findings, the group then decided to test the effects of DBS at a site medial to the parafascicularis nucleus in the PVG, with externalized leads for 1 to 2 weeks. Eight patients with various pain syndromes, including cervical and lumbar back pain, brachial plexus pain, and cancer pain, were included. Six of the eight patients obtained significant pain relief with their implant.34 The investigators then expanded their case series to include 30 patients, 27 of whom were chronically implanted. Of those implanted, 66% reported significant long-term relief with stimulation.35 In agreement with these results, Hosobuchi28 reported successful pain relief in 50 out of 65 patients (77%) with PAG stimulation for pain of peripheral origin. However, the investigator also noted a buildup of tolerance to DBS that was often accompanied by a tolerance to opiate analgesics. DBS analgesic efficacy was usually restored by intermittent stimulation breaks as well as concurrent use of L-tryp-tophan.28 Young and colleagues20 reported a 57% success rate in the stimulation of 26 patients over a 20-month mean follow-up period with implantation of PVG or PAG region alone with excellent pain relief.10,29 Levy and colleagues29 reported more modest results, with only a 32% rate of long-term relief in 57 patients at 7 years of follow-up, indicating that the long-term outcome was much less successful than initially anticipated in patients with nociceptive pain with PVG and VPL leads. Kumar and colleagues30 implanted 49 patients with PVG electrodes who had positive responses to the morphine-naloxone test and were diagnosed with nociceptive pain. The study found that 71% of those who received a permanent implant after an externalized trial reported adequate pain relief at a mean follow-up of 7.1 years. Additional studies have suggested a benefit from targeting the PAG/PVG regions, but similar limitations of previous studies apply.36 Recently, the group at Oxford led by Tipu Aziz37 has reported on the combined targeting of sensory thalamic nucleus and PVG in series of patients with central or peripheral pain syndromes. Unlike prior studies, this group has tried PVG DBS in patients with pain of neuropathic characteristics, showing that the potential analgesic effects of this target are not limited to those with pain of nociceptive characteristics.29,36
A NEED FOR NEW TARGETS AND RIGOROUS STUDY DESIGN
Coffey38 analyzed the results of 2 multicenter clinical trials investigating the effects of DBS for managing chronic pain of various causes. The study found that, although 46% of patients had greater than a 50% reduction of pain at 1 year, only 18% maintained pain relief at 2 years when an older lead model was used. The success rate was even lower for the second clinical trial with a newer electrode lead model. This low efficacy contributed to the manufacturer’s choice to not pursue approval by the Food and Drug Administration for the use of DBS to treat chronic pain.38 Coffey and Lozano39 described the need for specific diagnosis and randomized, controlled, and double-blinded studies to more systematically evaluate potential stimulation targets.
NEW STIMULATION TARGETS
The Neuromatrix and the Link Between Neuropsychiatric Illness and Pain
In 1965, Melzack and Wall40 proposed the gate-control theory of pain, which described the regulation or gating of pain signals at the level of the spinal dorsal horn. The gate-control theory led to advances in pain research and therapy and was a major driving force behind the development of spinal cord stimulation for pain management. However, in response to limitations of the gate-control theory, Melzack41 proposed a modified theory, called the neuromatrix theory. Melzack described the neuromatrix as a widespread neural network that consisted of loops between the thalamus and cortex and the cortex and the limbic system.41 The neuromatrix emphasized the multidimensional (ie, sensory-discriminative, affective-motivational, and evaluative-cognitive) nature of the pain with all components contributing equally to the overall pain experience.
The limbic circuits mentioned by Melzack were delineated by several anatomic studies, including seminal work by Nauta.42 Initial surgeries to affect chronic pain and psychiatric illness involved radio-frequency ablation of these networks at the cingulate cortex.43 Other targets, including the anterior limb of the internal capsule, were mainly targeted for treating obsessive-compulsive and anxiety disorders.44,45 Broadmann areas 24/25 and the VC/VS have also been considered as possible targets for treating mood disorders.46,47 Stimulation of these areas seems to be safe and is not associated with cognitive decline, as previously shown with ablative procedures.47
Ventral Striatum and Anterior Limb of the Internal Capsule
The orbitofrontal cortico-striato-pallido-thalamo-cortical system and the circuit of Papez are involved in the control of emotion and behavior. In addition to projections from frontal and prefrontal cortical areas into the dorsal striatum, there are also direct projections from the anterior frontal cortical areas and orbitofrontal cortical areas to the ventral striatum, as well as direct projections to the thalamus via the anterior limb of the internal capsule. The authors have previously shown that acute stimulation of the ventral striatum and ventral part of the anterior limb of the capsule is associated with changes in mood and anxiety and, in some patients, sensation of warmth, flushing, and, less commonly, dizziness.46 Some patients were noted to smile and laugh. Stimulation in more dorsal areas of the internal capsule rarely produces the same effects. Chronic stimulation of these same areas is associated with improvements in patients with refractory major depressive disorder and obsessive-compulsive disorder.47 These areas, although affecting mood, may in turn modulate the affective part of chronic pain.
A SYSTEMATIC APPROACH TO EXPLORE A NOVEL TARGET AND EVALUATE THERAPEUTIC EFFECTS
Intracranial neurostimulation for pain management has a strong history of case reports and clinical trials that were often uncontrolled, included multiple pain causes, and considered multiple brain targets. The mixed results of these studies illustrate the need for systematic evaluation of these therapies and the need for novel research and exploration of novel targets. As discussed earlier, future research will be more informative if performed with a sham-controlled phase and allowed investigators to quantify the placebo effect, which has been shown to be significant in intracranial neuro-stimulation for pain.31,48 In the authors’ ongoing work for evaluating the effects of VC/VS stimulation, they chose a multiphase design, including a prolonged titration phase and a 6-month sham-controlled phase.
STUDY DESIGN
In a prospective, double-blind, controlled trial under Institutional Review Board approval and a physician-sponsored Food and Drug Administration Investigational Device Exemption, 10 patients with refractory intractable hemibody pain secondary to a lesion of the contralateral thalamic area (or related afferent and efferent pathways) are enrolling. The main phase of the pilot study is randomized and sham-controlled, with a 2-group crossover. The exclusion criteria include patients with severe, unmanaged psychiatric or cognitive comorbidities and usual contraindications for DBS or intracranial procedures. The details can be seen at the study’s page in clinicaltrials.gov.49 In a 6-phase study, the patients are initially enrolled (phase I) after meeting the selection criteria and then undergo baseline evaluation, which includes functional neuroimaging and neurologic, cognitive, and behavioral assessments (phase II). Phase III involves the implantation of the DBS electrodes bilaterally in the VC/VS area and implantation of the implantable pulse generator. Phase IV involves initial programing, which will occur at least one 1 month after implantation to allow for healing after surgery and to minimize the influence of the microlesional effects.32,50 A 1-month break occurs before proceeding to the (main) phase V portion of the study to washout possible carryover effects of titration. The patients are then randomized to either the sham DBS control group or the active stimulation group and, after 3 months, will cross over to the other group. The final phase (VI) will allow all patients to receive active, unblinded stimulation and follow-up through 24 months. Functional neuroimaging is acquired during each part of the blinded phase and is then repeated at 12 and 24 months. This imaging is an explorative part of the research, aimed at identifying possible mechanisms underlying the observed effects. The primary outcome measure for the study is the pain disability index, matching the authors’ primary hypothesis that stimulation of this novel target area will reduce pain-related disability via modulation of the affective sphere of neuropathic pain.
SUMMARY
DBS and MCS can alleviate chronic pain in select patients, particularly those with peripheral neuropathies. Common targets are the sensory thalamic nuclei and PVG/PAG areas. Given the vast amount of retrospective reports and uncontrolled case series, there is a paucity of reliable data in the literature, and the magnitude of the placebo effect is not well known. The future of this application depends on an organized, systematic approach with randomized studies in which the effects of stimulation can be differentiated from placebo and other confounding features. Novel approaches, including those targeting the networks related to the affective sphere of chronic pain, may show improvements in quality of life and reduce disability.
KEY POINTS.
Motor cortex stimulation (MCS) and deep brain stimulation (DBS) have been used for the treatment of refractory pain with good early results.
MCS and DBS are used off label for pain applications because conclusive effectiveness studies are still needed to prove therapeutic value.
New targets are being evaluated with new clinical trials that will explore pain with regard to the afferent component.
Acknowledgments
Fig. 1 was generated with assistance from Angela M. Noecker, Case Western Reserve University.
References
- 1.Torrance N, Smith BH, Bennett MI, et al. The epidemiology of chronic pain of predominantly neuropathic origin. Results from a general population survey. J Pain. 2006;7(4):281–9. doi: 10.1016/j.jpain.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 2.United States Census Bureau. The 2012 statistical abstract. [Accessed on May 16, 2013];The national data book, “Table 7: resident population by sex and age: 1980 to 2010”. 2013 Available at: http://www.census.gov/compendia/statab/2012/tables/12s0007.pdf.
- 3.Machado A, Kopell BH, Rezai AR. Chronic electrical stimulation for refractory chronic pain. In: Star PA, Barbaro NM, Larson PS, editors. Neurosurgical operative atlas. Functional neurosurgery. 2. New York: Thieme Medical Publishers; 2009. p. 134. [Google Scholar]
- 4.Hirayama T, Tsubokawa T, Katayama Y, et al. Chronic changes in activity of thalamic lemniscal relay neurons following spino-thalamic tractotomy in cats: effects of motor cortex stimulation. Pain. 1990;41:S273. [Google Scholar]
- 5.Yamasiro K, Mukawa J, Terada Y, et al. Neurons with high-frequency discharge in the central nervous system in chronic pain. Stereotact Funct Neurosurg. 1994;62:290–4. doi: 10.1159/000098634. [DOI] [PubMed] [Google Scholar]
- 6.Tsubokawa T, Katayama Y, Yamamoto T, et al. Chronic motor cortex stimulation in patients with thalamic pain. J Neurosurg. 1993;78(3):393–401. doi: 10.3171/jns.1993.78.3.0393. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen JP, Nizard J, Keravel Y, et al. Invasive brain stimulation for the treatment of neuropathic pain. Nat Rev Neurol. 2011;7(12):699–709. doi: 10.1038/nrneurol.2011.138. [DOI] [PubMed] [Google Scholar]
- 8.Meyerson BA, Lindblom U, Linderoth B, et al. Motor cortex stimulation as treatment of trigeminal neuropathic pain. Acta Neurochir Suppl (Wien) 1993;58:150–3. doi: 10.1007/978-3-7091-9297-9_34. [DOI] [PubMed] [Google Scholar]
- 9.Fontaine D, Hamani C, Lozano A. Efficacy and safety of motor cortex stimulation for chronic neuropathic pain: critical review of the literature. J Neurosurg. 2009;110(2):251–6. doi: 10.3171/2008.6.17602. [DOI] [PubMed] [Google Scholar]
- 10.Levy R, Deer TR, Henderson J. Intracranial neuro-stimulation for pain control: a review. Pain Physician. 2010;13(2):157–65. [PubMed] [Google Scholar]
- 11.Lima MC, Fregni F. Motor cortex stimulation for chronic pain: systematic review and meta-analysis of the literature. Neurology. 2008;70(24):2329–37. doi: 10.1212/01.wnl.0000314649.38527.93. [DOI] [PubMed] [Google Scholar]
- 12.Lefaucheur JP, Drouot X, Cunin P, et al. Motor cortex stimulation for the treatment of refractory peripheral neuropathic pain. Brain. 2009;132(Pt 6):1463–71. doi: 10.1093/brain/awp035. [DOI] [PubMed] [Google Scholar]
- 13.Henderson JM, Heit G, Fisher RS. Recurrent seizures related to motor cortex stimulator programming. Neuromodulation. 2010;13(1):37–43. doi: 10.1111/j.1525-1403.2009.00256.x. [DOI] [PubMed] [Google Scholar]
- 14.Kandell ER, Schwartz JH, Jessell TM, editors. Principles of neural science. 4. New York: McGraw-Hill; 2000. pp. 472–91. [Google Scholar]
- 15.Ossipov MH, Dussor GO, Porreca F. Central modulation of pain. J Clin Invest. 2010;120(11):3779–87. doi: 10.1172/JCI43766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akil H, Mayer DJ. Antagonism of stimulation-produced analgesia by p-CPA, a serotonin synthesis inhibitor. Brain Res. 1972;44(2):692–7. doi: 10.1016/0006-8993(72)90338-1. [DOI] [PubMed] [Google Scholar]
- 17.Akil H, Liebeskind JC. Monoaminergic mechanisms of stimulation-produced analgesia. Brain Res. 1975;94(2):279–96. doi: 10.1016/0006-8993(75)90062-1. [DOI] [PubMed] [Google Scholar]
- 18.Heath RG. Electrical self-stimulation of the brain in man. Am J Psychiatry. 1963;120:571–7. doi: 10.1176/ajp.120.6.571. [DOI] [PubMed] [Google Scholar]
- 19.Ervin FR, Brown CE, Mark VH. Striatal influence on facial pain. Confin Neurol. 1966;27(1):75–90. doi: 10.1159/000103936. [DOI] [PubMed] [Google Scholar]
- 20.Young RF, Kroening R, Fulton W, et al. Electrical stimulation of the brain in treatment of chronic pain. Experience over 5 years. J Neurosurg. 1985;62(3):389–96. doi: 10.3171/jns.1985.62.3.0389. [DOI] [PubMed] [Google Scholar]
- 21.Hosobuchi Y, Adams JE, Rutkin B. Chronic thalamic stimulation for the control of facial anesthesia dolorosa. Arch Neurol. 1973;29(3):158–61. doi: 10.1001/archneur.1973.00490270040005. [DOI] [PubMed] [Google Scholar]
- 22.Richardson DE, Akil H. Pain reduction by electrical brain stimulation in man. Part 1: acute administration in periaqueductal and periventricular sites. J Neurosurg. 1977;47(2):178–83. doi: 10.3171/jns.1977.47.2.0178. [DOI] [PubMed] [Google Scholar]
- 23.Kraus T, Hosl K, Kiess O, et al. BOLD fMRI deactivation of limbic and temporal brain structures and mood enhancing effect by transcutaneous vagus nerve stimulation. J Neural Transm. 2007;114(11):1485–93. doi: 10.1007/s00702-007-0755-z. [DOI] [PubMed] [Google Scholar]
- 24.Machado AG, Baker KB, Plow E, et al. Cerebral stimulation for the affective component of neuropathic pain. Neuromodulation. 2012 doi: 10.1111/j.1525-1403.2012.00517.x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miocinovic S, Noecker AM, Maks CB, et al. Cicerone: stereotactic neurophysiological recording and deep brain stimulation electrode placement software system. Acta Neurochir Suppl. 2007;97(Pt 2):561–7. doi: 10.1007/978-3-211-33081-4_65. [DOI] [PubMed] [Google Scholar]
- 26.Mazars G, Merienne L, Ciolocca C. Intermittent analgesic thalamic stimulation. Preliminary note Rev Neurol (Paris) 1973;128(4):273–9. [PubMed] [Google Scholar]
- 27.Turnbull IM, Shulman R, Woodhurst WB. Thalamic stimulation for neuropathic pain. J Neurosurg. 1980;52(4):486–93. doi: 10.3171/jns.1980.52.4.0486. [DOI] [PubMed] [Google Scholar]
- 28.Hosobuchi Y. Subcortical electrical stimulation for control of intractable pain in humans. Report of 122 cases (1970–1984) J Neurosurg. 1986;64(4):543–53. doi: 10.3171/jns.1986.64.4.0543. [DOI] [PubMed] [Google Scholar]
- 29.Levy RM, Lamb S, Adams JE. Treatment of chronic pain by deep brain stimulation: long term follow-up and review of the literature. Neurosurgery. 1987;21(6):885–93. doi: 10.1227/00006123-198712000-00017. [DOI] [PubMed] [Google Scholar]
- 30.Kumar K, Toth C, Nath RK. Deep brain stimulation for intractable pain: a 15-year experience. Neurosurgery. 1997;40(4):736–46. doi: 10.1097/00006123-199704000-00015. discussion: 746–7. [DOI] [PubMed] [Google Scholar]
- 31.Marchand S, Kupers RC, Bushnell MC, et al. Analgesic and placebo effects of thalamic stimulation. Pain. 2003;105:481–8. doi: 10.1016/S0304-3959(03)00265-3. [DOI] [PubMed] [Google Scholar]
- 32.Hamani C, Schwalb JM, Rezai AR, et al. Deep brain stimulation for chronic neuropathic pain: long-term outcome and the incidence of insertional effect. Pain. 2006;125(1–2):188–96. doi: 10.1016/j.pain.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 33.Reynolds DV. Surgery in the rat during electrical analgesia induced by focal brain stimulation. Science. 1969;164(3878):444–5. doi: 10.1126/science.164.3878.444. [DOI] [PubMed] [Google Scholar]
- 34.Richardson DE, Akil H. Pain reduction by electrical brain stimulation in man. Part 2: Chronic self-administration in the periventricular gray matter. J Neurosurg. 1977;47:184–94. doi: 10.3171/jns.1977.47.2.0184. [DOI] [PubMed] [Google Scholar]
- 35.Richardson DE, Akil H. Long term results of peri-ventricular gray self-stimulation. Neurosurgery. 1977;1(2):199–202. doi: 10.1097/00006123-197709000-00018. [DOI] [PubMed] [Google Scholar]
- 36.Boccard SG, Pereira EA, Moir L, et al. Long-term outcomes of deep brain stimulation for neuropathic pain. Neurosurgery. 2013;72(2):221–30. doi: 10.1227/NEU.0b013e31827b97d6. discussion: 231. [DOI] [PubMed] [Google Scholar]
- 37.Owen SLF, Green AL, Stein JF, et al. Deep brain stimulation for the alleviation of post-stroke neuropathic pain. Pain. 2006;120(1–2):202–6. doi: 10.1016/j.pain.2005.09.035. [DOI] [PubMed] [Google Scholar]
- 38.Coffey RJ. Deep brain stimulation for chronic pain: results of two multicenter trials and a structured review. Pain Med. 2001;2(3):183–92. doi: 10.1046/j.1526-4637.2001.01029.x. [DOI] [PubMed] [Google Scholar]
- 39.Coffey RJ, Lozano AM. Neurostimulation for chronic noncancer pain: an evaluation of the clinical evidence and recommendations for future trial designs. J Neurosurg. 2006;105(2):175–89. doi: 10.3171/jns.2006.105.2.175. [DOI] [PubMed] [Google Scholar]
- 40.Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150(3699):971–9. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- 41.Melzack R. From the gate to the neuromatrix. Pain. 1999;(Suppl 6):S121–6. doi: 10.1016/S0304-3959(99)00145-1. [DOI] [PubMed] [Google Scholar]
- 42.Nauta WJ. Hippocampal projections and related neural pathways to the midbrain in the cat. Brain. 1958;81(3):319–40. doi: 10.1093/brain/81.3.319. [DOI] [PubMed] [Google Scholar]
- 43.Ballantine HT, Jr, Cassidy WL, Flanagan NB, et al. Stereotaxic anterior cingulotomy for neuropsychiatric illness and intractable pain. J Neurosurg. 1967;26(5):488–95. doi: 10.3171/jns.1967.26.5.0488. [DOI] [PubMed] [Google Scholar]
- 44.Nyman H, Andreewitch S, Lundback E, et al. Executive and cognitive functions in patients with extreme obsessive-compulsive disorder treated by capsulotomy. Appl Neuropsychol. 2001;8(2):91–8. doi: 10.1207/S15324826AN0802_3. [DOI] [PubMed] [Google Scholar]
- 45.Lippitz B, Mindus P, Meyerson BA, et al. Obsessive compulsive disorder and the right hemisphere: topographic analysis of lesions after anterior capsulotomy performed with thermocoagulation. Acta Neurochir Suppl. 1997;68:61–3. doi: 10.1007/978-3-7091-6513-3_11. [DOI] [PubMed] [Google Scholar]
- 46.Machado A, Haber S, Sears N, et al. Functional topography of the ventral striatum and anterior limb of the internal capsule determined by electrical stimulation of awake patients. Clin Neurophysiol. 2009;120(11):1941–8. doi: 10.1016/j.clinph.2009.05.030. [DOI] [PubMed] [Google Scholar]
- 47.Malone DA, Jr, Dougherty DD, Rezai AR, et al. Deep brain stimulation of the ventral capsule/ventral striatum for treatment-resistant depression. Biol Psychiatry. 2009;65(4):267–75. doi: 10.1016/j.biopsych.2008.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rasche D, Ruppolt M, Stippich C, et al. Motor cortex stimulation for long-term relief of chronic neuropathic pain: a 10 year experience. Pain. 2006;121(1–2):43–52. doi: 10.1016/j.pain.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 49.Machado A. [Accessed June 13, 2013];2013 Available at: http://clinical-trials.gov/ct2/show/NCT01072656?term=macha-do&rank=10.
- 50.Deuschl G, Herzog J, Kleiner-Fisman G, et al. Deep brain stimulation: postoperative issues. Mov Disord. 2006;21(Suppl 14):S219–37. doi: 10.1002/mds.20957. [DOI] [PubMed] [Google Scholar]


