Abstract
Old age is a major risk factor for cardiovascular diseases. Several lines of evidence in experimental animal models have indicated the central role of mitochondria both in lifespan determination and cardiovascular aging. In this article we review the evidence supporting the role of mitochondrial oxidative stress, mitochondrial damage and biogenesis as well as the crosstalk between mitochondria and cellular signaling in cardiac and vascular aging. Intrinsic cardiac aging in the murine model closely recapitulates age-related cardiac changes in humans (left ventricular hypertrophy, fibrosis and diastolic dysfunction), while the phenotype of vascular aging include endothelial dysfunction, reduced vascular elasticity and chronic vascular inflammation. Both cardiac and vascular aging involve neurohormonal signaling (e.g. renin-angiotensin, adrenergic, insulin-IGF1 signaling) and cell-autonomous mechanisms. The potential therapeutic strategies to improve mitochondrial function in aging and cardiovascular diseases are also discussed, with a focus on mitochondrial-targeted antioxidants, calorie restriction, calorie restriction mimetics and exercise training.
Introduction
Mitochondria play important roles in a myriad of cellular processes including ATP production via oxidative phosphorylation, biosynthetic pathways, cellular redox homeostasis, ion homeostasis, oxygen sensing, signaling and regulation of programmed cell death. Mitochondrial dysfunction is central to theories of aging, as age-related changes of mitochondria are likely to impair a host of cellular physiological functions in parallel and contribute to the development of all common age-related diseases.
Age-specific mortality rates from heart disease and stroke and the incidence of peripheral vascular disease and vascular cognitive impairment increase exponentially with age in people aged over 65. Previous studies established that mitochondria have a central role in age-related pathological alterations of the heart. In addition, there is growing evidence that mitochondria have also an important role in vascular pathophysiology. Development of novel therapeutic approaches for mitochondrial rejuvenation and attenuation of mitochondrial oxidative stress holds promise for reducing cardiovascular mortality in an aging population. In this review, the effects of aging on mitochondrial function and phenotype in the cardiovascular system and the signaling role of mitochondria in aging are considered. The possible benefits of therapeutic strategies that have the potential to improve mitochondrial function and delay the onset of age-related cardiovascular diseases are also discussed. The review is organized into four sections: 1) mitochondrial oxidative stress and aging; 2) mechanisms and signaling pathways mediating mitochondrial effects of cardiac aging; 3) therapeutic strategies to improve mitochondrial function in aging; 4) perspectives.
1. Mitochondrial oxidative stress theory and aging
1a. The free radical theory of aging
First proposed by Harman in 1956, the free radical theory of aging postulates that the production of intracellular reactive oxygen species (ROS) is the major determinant of lifespan1. Decline in cellular and organ functions as well as the associated degenerative diseases in old age could be attributed to deleterious effects of ROS on various cellular components. ROS are generated in multiple compartments and by multiple enzymes within the cell, such as NADPH oxidase at the plasma membrane, lipid oxidation within peroxisomes, oxidative phosphorylation within mitochondria, as well as various cyclooxygenases and xanthine oxidase in the cytoplasm. Although all of these sources contribute to the overall oxidative burden, the majority of ROS are produced during oxidative phosphorylation and ATP generation within the mitochondria in aging2. This has led to the extension of free radical theory in the 1970s to implicate mitochondrial production of ROS (including superoxide [O2.-] and hydrogen peroxide [H2O2]) as the main cause for age-related damage and degeneration3. Mitochondrial ROS might attack various mitochondrial constituents, causing mitochondrial DNA mutations and oxidative damage to respiratory enzymes. A defect in mitochondrial respiratory enzymes would increase mitochondrial production of ROS, causing further mitochondrial damage and dysfunction, leading to further decline in cellular and organ function that can eventually progress to death2. A large body of evidence has been published both in support of and against the free radical theory of aging. Key observations have been the lack of concordance between expected and observed results in knockout and transgenic mouse models4. Knockout mice for major cellular antioxidant enzymes show a relatively mild phenotype and rarely demonstrate a lifespan decrease despite significant increases in ROS. Conversely, overexpression of antioxidant enzymes has generally failed to extend mouse lifespan. In accord with this, oral antioxidant supplementation in humans with good nutritional status has generally not been shown to produce beneficial effects. However, the mitochondrial variant of the free radical theory of aging, which postulates that free radical generated by mitochondria is the main determinant of lifespan, has not been nearly as well tested. As described below, there is evidence that reducing mitochondrial ROS has lifespan and cardiac health benefits.
1b Mitochondrial oxidative stress in cardiac aging
Considerable evidence has been published that with advanced age mitochondrial production of ROS significantly increases both in the heart5 and the vasculature6. Age-dependent mitochondrial dysfunction is closely correlated with abnormal mitochondrial ROS production and detoxification (reviewed in 7-9). For example, an age-dependent reduction in mitochondrial oxidative phosphorylation is associated with the decline in mitochondrial state 3 respiration (maximal ADP-stimulated respiration in the presence of excess substrates) due to diminished activity of electron transport by complexes I and IV, which include critical components encoded by mitochondrial DNA. The complexes II, III and V are believed to be less affected in aging (see review 10). Such impairment of electron transport function might be directly related to elevated electron leakage and generation of mitochondrial ROS.
The heart is a vital organ with high metabolic demand and rich in mitochondria, and since ROS is produced in mitochondria as a byproduct of oxidative phosphorylation, the heart is especially prone to oxidative damage. Direct evidence of the critical role of mitochondrial ROS in aging was demonstrated in mice overexpressing catalase targeted to the mitochondria (mCAT), which had 18% prolongation of lifespan when compared with WT littermates, whereas mice overexpressing catalase targeted to peroxisomes (pCAT, the natural site of catalase) or the nucleus had little or no lifepan extension11. Work in the Rabinovitch laboratory further showed that mCAT mice were protected from the phenotypes of cardiac aging. Cardiac aging in mice closely recapitulates those found in human cardiac aging, as shown in Table 1, which include age-dependent increase in echocardiographic left ventricular mass index (LVMI, P<0.01 for age-dependent change), a modest decline in systolic function with age (FS%, P =0.03) and a significant decline in diastolic function measured by Ea/Aa (P<0.01) as well as impairment of myocardial performance with age, indicated by increase in myocardial performance index (indicated a greater fraction of systole was spent during isovolemic contraction and relaxation, P<0.01)12. Furthermore, the proportion of mice with diastolic dysfunction and left atrium dilatation also significantly increased with age12. All of the above phenotypes of cardiac aging were significantly attenuated in mCAT mice (Table 1, p<0.01 for old WT vs. old mCAT). Consistent with this, mitochondrial oxidative damage was increased in the aged hearts, as supported by an increase in mitochondrial protein carbonyls and a greater than 6 fold increase in mitochondrial DNA deletion (Table 1) and mutation12 frequencies compared with young wild type hearts. This mitochondrial damage stimulate signaling for mitochondrial biogenesis, seen in the aged heart by an increase in mtDNA copy number concomitant with significant upregulation of the master regulator PPAR-γ Coactivator-1-α (PGC-1α) and its downstream transcription factors 12. The mCAT mice showed significantly attenuated age-dependent increases in mitochondrial protein carbonyls and mtDNA deletions (Table 1), and consistent with this, the activation of PGC-1α and the increase in mtDNA copy number in aging was also attenuated12.
Table 1.
Physiologic and mitochondrial changes in cardiac aging
| Percent changes from YWT (4-6 months) |
||||
|---|---|---|---|---|
| Old (>24 months) | Middle age (13-14 months) | |||
| WT¶ | mCAT | Polg m/m† | Polg m/m/mCAT | |
| Physiologic parameters‡: | ||||
| Left Ventricular Mass Index (LVMI) | 76 | 37 | 175 | 120 |
| Fractional Shortening (FS) | -12 | -4.0 | -18 | -4.1 |
| Ea/Aa (Diastolic function) | -44 | -18 | -39 | -4.6 |
| Myocardial Performance Index (MPI) | 87 | 26 | 49 | 6.6 |
| Molecular parameters: | ||||
| Mitochondrial protein carbonyls | 76 | -28 | 119 | 59 |
| Mitochondrial DNA deletion frequency | 640 | 70 | 4470 | 2480 |
The success of targeted, specific ROS scavenging intervention by mCAT suggests that a key to successful intervention lies in specificity. Given the complexity of the systems involved it seems possible that mitochondrial dysfunction and aberrant ROS production may contribute to aging through interference with normal signaling and energetics as much, or more than by the direct damaging effect to cellular macromolecules. Age-dependent decline in the rate of mitochondrial electron transfer also favors mitochondrial superoxide production, leading to a positive feedback between complex I inhibition and mitochondrial ROS production, as well as the more classical vicious cycle of mitochondrial DNA mutation and protein damage amplifying ROS. When viewed in the light of alterations in both signaling and energetics, this may be a critical factor in cardiac (and other organ system) aging (Figure 1).
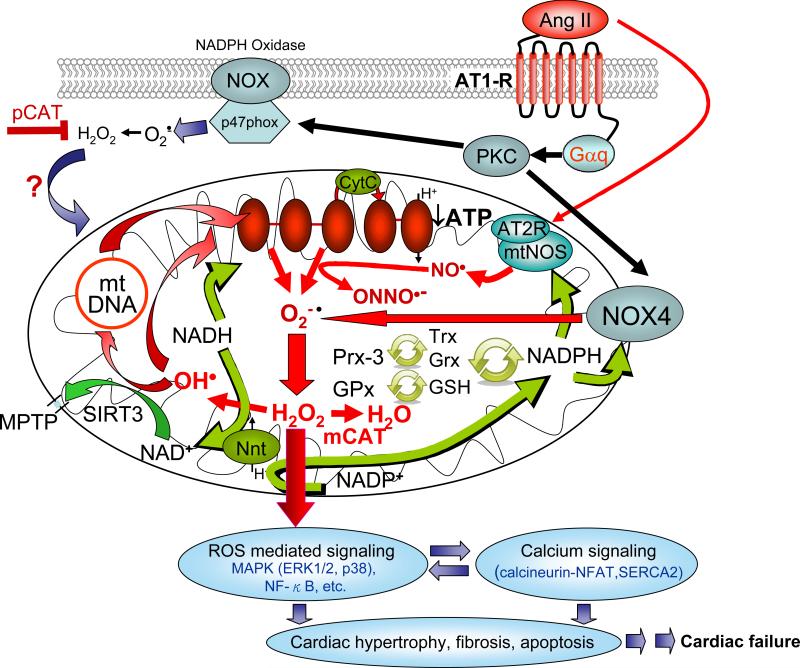
Figure 1.
Proposed signaling mechanism of Angiotensin/Gαq and mitochondrial ROS amplification in aging and cardiovascular diseases. AT1-R = angiotensin receptor-1; Nnt = nicotinamide nucleotide transferase
Further evidence supporting the role of mitochondria in aging was demonstrated using mice with homozygous mutation of mitochondrial polymerase gamma (PolgaD257A/D257A , abbreviated Polgm/m), which impair the proofreading capacity of polymerase gamma and hence induced substantial increase in mtDNA point mutations and deletions with age13, 14. These mice have shortened lifespan (maximal lifespan ~15 months) and several progeroid phenotypes, including kyphosis, graying and loss of hair, anemia, osteoporosis, sarcopenia (loss of muscle mass) and presbycusis (age-related hearing loss)13. It has been shown that accumulation of mtDNA deletions is better correlated with the “premature” aging-like phenotype in these mice than are mtDNA point mutations15. The accumulation of mitochondrial DNA damage has been shown to increase apoptotic rate14 as well as mitochondrial oxidative damage in the mouse heart (Table 1). Cardiomyopathy is evident in middle age (13-14 months) Polgm/m mice, to a degree that is much more severe than the usual cardiac aging in WT mice 13, 16. Cardiac hypertrophy, as indicated by LVMI, is much greater in Polgm/m mice in middle age than in WT mice of 24-30 months old (Table 1). Likewise, systolic and diastolic function in Polgm/m mice is also worse than the usual cardiac aging (Table 1). The observations that mitochondrial damage and cardiomyopathy in these mice can be partially rescued by mCAT (Table 1) suggests that mitochondrial ROS and mitochondrial DNA damage are part of a vicious cycle of ROS-induced ROS release (Figure 1)16. Interestingly, a recent paper reports the striking observation that endurance exercise in Polgm/m mice can prevent both their skeletal muscle and cardiac progeroid phenotypes17. The authors suggest that the augmented level of mitochondrial biogenesis seen with exercise in these mice is a critical factor in maintaining mitochondrial and organ function.
Additional implication of mitochondria in cardiac aging comes from observations of mice with a targeted mutation of the p66Shc gene. These mice display prolonged lifespan, reduced production of ROS and increased resistance to ROS-mediated apoptosis18. The p66Shc localizes to the mitochondrial intermembranous space and has been shown to be a mitochondrial redox enzyme, forming ROS by using electrons from the respiratory chain to produce H2O219. A recent study from the same group showed that p66Shc was phosphorylated by PKC-beta together with prolyl isomerase Pin-1, then the phosphorylated p66Shc accumulated within mitochondria to activate mitochondrial Ca2+ response, and subsequently induce apoptosis20. Disruption of p66Shc prevents Angiotensin-II induced LV hypertrophy and cardiomyocytes apoptosis as well as reducing oxidative damage in cardiac progenitor cells, cardiomyocytes and endothelial cells in a diabetic mouse model21-23.
Consistent with all of the above, a deficiency of mitochondrial energetics has been documented in human and experimental animals with heart failure 24. Mechanisms may include mitochondrial biogenesis that does not keep up with the increasing demand (see review 25), mitochondrial uncoupling and decreased substrate availability 26, and increased mitochondrial DNA deletions27.
1c. Mitochondrial oxidative stress in vascular aging
There is growing evidence that increased mitochondrial production of ROS also has an important role in age-related vascular impairment. Previous studies suggest that increased ROS in aging promotes mitochondrial protein oxidation and increased mitochondrial DNA mutations in the heart and other organs, but it is yet to be determined whether similar aging-induced mitochondrial DNA and protein damage plays an important role in vascular endothelial and smooth muscle cells. Importantly, mitochondria-derived ROS are likely to contribute to the development of chronic low-grade vascular inflammation in aging6 by activating redox signaling pathways (see below). Furthermore, recent studies suggest that mitochondria-derived ROS contribute to accelerated development of the senescent phenotype in endothelial cells (i.e. by activating Akt28). Endothelial cell senescence may impair the regenerative and angiogenic capacity of the endothelium, its reactivity and promote the progression of atherosclerosis by altering the secretion of cytokines, growth factors and proteases in the vascular wall. Another potentially important link between mitochondrial oxidative stress and vascular aging is the induction of apoptosis29. Oxidative stress in aging is associated with an increased rate of endothelial apoptosis30, 31, which may contribute to microvascular rarefaction impairing the blood supply of the heart32 and the brain33. Cerebrovascular endothelial cells are rich in mitochondria and normal mitochondrial function is essential to maintain the integrity of the blood-brain barrier. On the basis of the available data with mitochondrial inhibitors34 we posit that age-related mitochondrial dysfunction may contribute to breakdown of the blood-brain barrier promoting neuroinflammation in aging35. Mitochondrial-derived ROS may also impact endothelium-dependent vasodilation36, 37. Further studies are warranted to determine whether novel therapies that reduce mitochondrial oxidative stress are able to prevent the development of endothelial senescence and apoptosis, improve vasodilator function in the aged vasculature and/or maintain the integrity of the blood brain barrier.
2. Mechanisms and signaling pathways mediating mitochondrial effects of cardiovascular aging
2a. Molecular mechanisms contributing to mitochondrial oxidative stress in the aging cardiovascular system
The molecular mechanisms underlying age-related increases in mitochondrial oxidative stress in the cardiovascular system are multifaceted and likely involve cell-autonomous effects, including a significant decline in reduced glutathione content38, dysregulation of antioxidant defense mechanisms (e.g. peroxynitrite-mediated nitration and inhibition of MnSOD39) and a dysfunctional electron transport chain40. Furthermore, there is also evidence that a cross-talk exists between mitochondrial and cytosolic sources of ROS production (see below). There is a significant age-related increase in NADPH oxidase activity both in heart and vasculature41, which is likely to exacerbate mitochondrial oxidative stress in aging. In addition to the aforementioned age-related mechanisms, several cardiovascular risk factors, including oxidatively modified lipoproteins, cigarette smoke constituents42, high methionine diet and hyperhomocysteinemia, and diabetes43, either directly or indirectly, may increase ROS production in mitochondria of vascular cells and/or cardiac myocytes. Recent studies suggest that age-related changes in endocrine/paracrine regulatory mechanisms, including activation of the renin-angiotensin-aldosterone system, adrenergic signaling and an age-related dysfunction of growth hormone/IGF-1 signaling, alos have an important role in promoting mitochondrial oxidative stress in the aged cardiovascular system.
Renin-angiotensin-aldosterone system
The renin-angiotensin-aldosterone system is the key player implicated in a broad spectrum of cardiovascular diseases, including hypertension, coronary heart disease and congestive heart failure, as well as atrial fibrillation. The prevalence of these diseases has been shown to increase with age in the American Heart Association Heart Disease and Stroke Statistics update44. Indeed, both renin and angiotensin II (Ang II) have been shown to induce cardiac hypertrophy and cardiomyocytes apoptosis, increases cardiac fibrosis and impairs cardiomyocyte relaxation45. All of the above changes are compatible with the phenotypes associated with cardiac aging. The concentration of Ang II in cardiac tissue has been shown to increase significantly in aged rodent hearts46, which is presumably related to increase in local angiotensin II converting enzyme (ACE) level in cardiac and vascular tissues47. Although the mechanism of increased ACE in aging is not well understood, long-term inhibition with angiotensin receptor blockers or disruption of angiotensin receptor type I has been shown to reduce age-dependent cardiac pathology and prolong rat 48 and mouse49 survival. Thus, the activation of renin-angiotensin system might play a central role in cardiac aging and age-associated cardiovascular diseases. At the molecular level, Angiotensin has been shown to induce an increase in total cellular and mitochondrial ROS, and it is likely that Angiotensin II induced mitochondrial ROS is the proximal mediator of cardiac aging changes. The mechanistic relationship of Angiotensin II and mitochondria in cardiac hypertrophy and failure is discussed below.
Adrenergic signaling
It is well accepted that chronic stimulation of adrenergic signaling by catecholamine is deleterious to the heart, as this activation increased heart rate, contractility, afterload (blood pressure) as well as cardiac wall stress, which subsequently increased cardiac metabolic demand. Adenylate cyclase is a key enzyme producing c-AMP as a secondary messenger downstream to β-adrenergic signaling and adenylate cyclase type 5 (AC5) is the major form in the heart. Disruption of AC5 has been shown to protect against chronic pressure overload-induced cardiac hypertrophy, apoptosis and failure by chronic catecholamine stimulation or aortic banding50, 51. These animals were shown to have prolonged lifespan which might be mediated through upregulation of Raf-1/pMEK/pERK pathway, which confers protection against oxidative stress52. Furthermore, AC5 knock-out mice were also shown to attenuate aging changes in the heart, including cardiac hypertrophy, systolic dysfunction, apoptosis and fibrosis52. Although several clinical trials have shown the efficacy of specific beta-blockers to provide survival benefit in patients with heart failure, the effects of beta-blockers on human cardiac aging are yet to be determined.
Emerging evidence have shown that activation of adrenergic signaling could induce mitochondrial ROS. Chronic β-adrenergic stimulation induces mitochondrial membrane depolarization and apoptosis in adult rat cardiomyocytes that is inhibited by Mn-SOD/catalase mimetics and by overexpression of catalase53. Furthermore, in vitro stimulation of β-adrenergic receptor also induces mitochondrial ROS in mouse neonatal ventricular cardiomyocytes via cAMP-PKA pathways. A recent study demonstrated that β-adrenergic stimulation induced increase in cardiomyocytes Ca2+ transient amplitude was diminished by the antioxidant N-acetyl cysteine as well as the mitochondrial-targeted antioxidant SS31 54. The above findings support the significant role in mitochondrial ROS in β-adrenergic signaling.
Growth hormone and IGF1 signaling
Previous studies suggest that a causal relationship exists among declining levels of insulin-like growth factor-1 and growth hormone (GH, which regulates the synthesis of IGF-1) and age-related cardiovascular morbidity and mortality in humans and in laboratory animals (for a review see55). There is increasing evidence that the beneficial cardiovascular effects of IGF-1, at least in part, can be related to mitochondrial protection mechanisms. Importantly, treatment of cultured endothelial cells and cardiomyocytes with IGF-1 decreases mitochondrial superoxide production56. Further, low plasma levels of GH and IGF-1 in Ames dwarf mice (which exhibit defective development of the pituitary gland due to a mutation in Prop-1) are associated with increased mitochondrial oxidative stress both in the vasculature and the heart56, mimicking the aging phenotype. Interestingly, mitochondrial oxidative stress in the heart of Ames dwarf mice appears to be associated with impaired contractile function57. Recent studies show that treatment of aged rodents with IGF-1 confers mitochondrial protection, including an attenuation of mitochondrial ROS generation in the liver58. The available data suggest that treatments that increase circulating IGF-1 levels exert cardiovascular protective effects in aging46, 59, 60. Thus, further studies are warranted to determine the role of mitochondrial mechanisms in the beneficial effects of GH replacement and/or IGF-1 treatment in the aged heart and vasculature, including the effects of IGF-1 on autophagy of dysfunctional mitochondria and apoptosis.
Age-related Nrf2 dysfunction
In the heart and the vasculature of young animals in response to increased production of mitochondria-derived ROS an adaptive NF-E2-related factor 2 (Nrf2)-driven antioxidant defense mechanism manifests, which up-regulates antioxidant response element (ARE)-driven expression of detoxifying and antioxidant enzymes and the cystine/glutamate transporter involved in glutathione biosynthesis61, 62. This homeostatic response serves to attenuate oxidative stress in the mitochondria and the cytoplasm and limit the cellular dysfunction caused by the increased production of ROS. Recent findings demonstrate that in aging vessels increased production of ROS by mitochondria and other sources fails to activate Nrf2 resulting in increased cellular sensitivity to the deleterious effects of oxidative stressors61, 63, 64. In that regard it is significant that in arteries of successfully aging species the same stressors elicit a lower level of mitochondrial oxidative stress than in blood vessels of shorter-lived species43, suggesting a possible link between Nrf2-dependent mechanisms regulating cellular oxidative stress resistance and slower rate of aging in longer-living species.
Dysregulation of mitochondrial turnover
Because macromolecules in mitochondria (including the mitochondrial DNA) are particularly susceptible to oxidative damage, effective control of mitochondrial turnover is critical for the maintenance of a healthy mitochondrial phenotype, normal energy production, and the promotion of healthy aging65. Mitochondria are highly dynamic organelles and dysregulation of mitochondrial turnover is likely one of the intrinsic causes of mitochondrial dysfunction, which contributes to dysregulation of cell metabolism, oxidative stress, and altered signal transduction during the aging process.
Autophagy is a catabolic process that contributes to the maintenance of cellular homeostasis in the cardiovascular system through the degradation of damaged mitochondria in lysosomes. In the heart of young mice angiotensin-II induced mitochondrial oxidative stress mediates induction of autophagy and mitochondrial biogenesis, likely to replenish the damaged mitochondria and restore energy production66. The available evidence suggest that there is an age-dependent decline in autophagic function, which likely contributes to the accumulation of damaged non-functional mitochondria in the heart and the vasculature67. In addition, dysfunction of the proteasomes68 may also contribute to the accumulation of damaged mitochondrial proteins in the aged cardiovascular system.
Previous studies demonstrate that mitochondrial biogenesis increases in the aged heart, as indicated by the increase in mtDNA copy number concomitant with significant upregulation of the peroxisome proliferator-activated receptor gamma coactivator-1α (PGC-1α) and its downstream effectors, mitochondrial transcription factor A (TFAM) and nuclear respiratory factors69. It is thought that increased mitochondrial biogenesis in the aged heart represents a compensatory maladaptive response in response to energy (ATP) deficiency, which is also stimulated by age-related oxidative damage to mitochondria69. In contrast, aging is associated with impaired mitochondrial biogenesis and reduced mitochondrial mass in the vascular endothelial and smooth muscle cells40, 70, 71. The available evidence suggest that in the aged vasculature, due to an increased production of ROS and down-regulation and uncoupling of eNOS, the bioavailability of nitric oxide is significantly decreased41, which results in a down-regulation of PGC-1α and consequential dysregulation of constituents of the electron transport chain and other mitochondrial proteins40. It is likely that decreased NO bioavailability is causally linked to dysfunction of mitochondrial biogenesis in other organs as well during aging65, 72.
2b. Mitochondria as signaling organelles in aging
The original formulation of the mitochondrial theory of aging3 postulates that increased production of mitochondria-derived ROS results in a variety of macromolecular oxidative modifications, which are a primary causal factor in the aging process as well as in development of age-related diseases. In the past decade cellular signaling events originating in mitochondria have moved into the spotlight in aging research. Mitochondrial retrograde signaling is a pathway of communication from mitochondria to the nucleus, which involves multiple factors that sense and transmit mitochondrial signals to alter nuclear gene expression. This crosstalk between mitochondria and the nucleus influences many cellular functions and is believed to play an important role in the aging process. There are multiple signaling cascades that involve the mitochondria, including release of ROS from the mitochondria, Ca2+ signaling, which activate downstream effectors pathways and transcription factors and the nutrient sensing mTOR pathway that regulates growth and cellular metabolism. Recent studies suggest that longevity is regulated by both cell-autonomous and non-autonomous mitochondrial stress pathways triggered by mild mitochondrial impairment73. According to this model adaptive mitochondrial retrograde pathways relay mitochondrial stress signals to the nucleus, activating genes involved in maintenance of mitochondrial integrity and cellular function. The cross-talk between the aforementioned pathways and cellular redox signaling mechanisms in the aging cardiovascular system is an important area of ongoing research74. Here we review some of the recent advances on the signaling role of mitochondria-derived ROS in age-related pathophysiological alterations of the heart and the vasculature.
Role of mitochondrial signaling in cardiac pathophysiology in aging
As discussed above, both RAAS activation and mitochondrial ROS play a central role in the pathophysiology of cardiac aging. Chronic administration of Angiotensin II via subcutaneous osmotic pump in mice results in left ventricular hypertrophy, diastolic dysfunction as well as cardiac fibrosis, all of which closely recapitulate the phenotypes of cardiac aging. Studies from Rabinovitch`s laboratory demonstrated that Angiotensin II for 4 weeks increased cardiac mitochondrial DNA deletion frequency as well as mitochondrial protein carbonyls27, both of which are related to oxidative damage to mitochondria. Angiotensin II-induced mitochondrial damage also activated mitochondrial autophagy. As a homeostic mechanism, ROS-induced mitochondrial damage and turnover could induce signaling for mitochondrial biogenesis through activation of PGC-1α and its target genes27. This is consistent with previous report that PGC-1α is transcriptionally upregulated by ROS 75.
The critical role of mitochondrial ROS in Angiotensin II-induced cardiomyopathy is reinforced by the observation that mice overexpressing catalase targeted to mitochondria (mCAT), but not mice overexpressing peroxisomal catalase (pCAT, natural site of catalase), are resistant to cardiac hypertrophy, fibrosis and diastolic dysfunction induced by Angiotensin II as well as systolic heart failure induced by cardiac-specific overexpression of Gαq (the αq subunit of guanine nucleotide-binding protein) 27. As shown in Figure 1, Angiotensin II binds to ATR1, a Gαq protein coupled-receptor (GPCR), then activates NADPH oxidase (NOX2) via a protein kinase C-dependent mechanism76. ROS produced by NADPH oxidase (NOX2 and / or NOX4) might increase mitochondrial ROS production in endothelial, vascular smooth muscle cells as well as neonatal cardiomyocytes77, 78. The fact that mCAT but not pCAT attenuates Angiotensin II- and Gαq-induced cardiac hypertrophy and failure emphasize the central role of ROS amplification within mitochondria 27. Mechanisms of ROS amplification might include ROS induced ROS release as well as a ROS-mtDNA damage vicious cycle (Figure 1). ROS production from NOX2/ p47 phox at the cell membrane, and more specifically, NOX4 at the mitochondrial membrane (see below) could lead to electron leakage from the electron transports chain, which might further stimulate ROS production. The involvement of mitochondrial DNA mutations/ deletions in this vicious cycle is supported by the observation that primary damage to mtDNA in Polgm/m mice is sufficient to increase mitochondrial ROS, induce cardiac hypertrophy and systolic dysfunction 16, 27. Thus, breaking the ROS vicious cycle within mitochondria by mCAT or mitochondrial targeted antioxidants (see below) is effective to attenuate both cardiac hypertrophy and failure.
Emerging evidence have shown that NOX4 is localized to the mitochondrial membrane79. Angiotensin II induces upregulation of NOX4 and contributes ROS to the mitochondria, which might simultaneously consume NADPH 80, 81. Detoxification of hydrogen peroxide generated by dismutation of superoxide is normally performed in mitochondria by Peroxiredoxin-3 (Prx-3) and Glutathione peroxidase (GPx). After their oxidation by hydrogen peroxide these enzymes are regenerated using the ultimate reductive power of NADPH. However, the consumption of NADPH by NOX4 establishes another potential mitochondrial vicious cycle (Figure 1). Note that mitochondrial-targeted catalase or other mitochondrial antioxidants (see below) can break the vicious cycle by removing hydrogen peroxide or superoxide without consuming glutathione or NADPH. NADPH can itself be regenerated from NADP+ by electron exchange with NADH, catalyzed by nicotinamide nucleotide transferase (Nnt). Thus, cardiomyocyte mitochondrial redox status is intimately bound with nicotinamide adenine dinucleotide metabolism. This further implicates sirtuins (sensors of the ratio of NAD+/NADH), particularly SIRT3, in the cardiac response to stress (see below).
Recently, it has been reported that mitochondrial nitric oxide synthtase (mtNOS) is also activated by angiotensin II subsequent to binding of angiotensin II to a AT2R receptor located in the mitochondrial inner membrane (mtAT2R) 82. The mtNOS uses NADPH and arginine as substrates to generate the nitric oxide radical (NO.). NO. has been shown to regulate oxygen consumption by inhibition of cytochrome oxidase, Complex IV of the electron transport chain83,84. Thus, while consuming NADPH, production of NO could compromise respiratory function (Figure 1). In addition, formation of peroxynitrite by reaction of NO. with O2.- leads to increased nitrosative stress in the mitochondrial compartment85 (Figure 1), including damage to respiratory complexes and opening of the MPTP86. However, Abadir et al. note that mtAT2R is decreased and mtAT1R density is increased in density with aging and they speculate that activation of mtNOS by mtAT2R could be a cardioprotective mechanism 87. Thus, additional study is needed to determine in which circumstances this novel mechanisms is protective and in which it is pathologic.
Role of mitochondrial signaling in vascular inflammation in aging
Inflammatory processes, particularly those mediating chronic inflammation, are known to contribute to the development of age-related cardiovascular disease, heart failure, stroke, peripheral artery disease and vascular cognitive impairment. However, the underlying biology of aging-induced inflammation in the cardiovascular system is not completely understood. Age-related pro-inflammatory alterations in endothelial phenotype, known as “endothelial activation,” involve up-regulation of cellular adhesion molecules, an increase in endothelial–leukocyte interactions, as well as alterations in the secretion of autocrine/paracrine mediators, which are pivotal to inflammatory responses. Previous studies both in humans and animal models of aging have demonstrated that advanced age per se alters cytokine expression profiles and promote the expression of pro-inflammatory genes in the wall of the large arteries, in the perivascular adipose tissue, in the microcirculation and in the cardiac muscle as well41. There is increasing evidence that activation of the redox-sensitive transcription factor NF-κB plays a key role in endothelial activation and vascular inflammatory changes in aging in humans88, non-human primates61 and laboratory rodents6.
Studies continue to support a role for mitochondria-derived ROS in chronic low-grade inflammation, including NF-κB activation, which is associated with aging in the cardiovascular system6, 41. The current view is that O2.-, overproduced in the aged mitochondria, is dismutated to H2O2 by Mn-SOD and it is the increased release of H2O2 that activates NF-κB in the cytoplasm (O2.- is membrane-impermeable, whereas H2O2 easily penetrates the mitochondrial membranes)6. Recent studies suggest that aging is associated with impairment of endogenous Nrf2-driven antioxidant defense mechanisms that protect the cardiovascular system against sustained oxidative stress61, 62 and that diminished Nrf2/ARE activity in aged vessels contributes to increased mitochondrial oxidative stress exacerbating NF-κB activation in the vasculature61. Further support for the link between oxidative stress and vascular inflammation in aging comes from the findings that pharmacological scavenging of mitochondria-derived H2O2 inhibits NF-κB activation in aged arteries6. Overexpression of human catalase in the mitochondria of aging mice delays cardiac pathology and attenuates age-related oxidative stress and inflammation69. Future studies should elucidate whether in mCAT mice attenuation of mitochondrial H2O2 production in the endothelial and smooth muscle also prevents low-grade vascular inflammation associated with aging.
Recent studies also point to a new and potentially important function of mitochondria in vascular aging: production of cytoprotective factors89-92. Among them, the best characterized is humanin, a mitochondria-derived peptide expressed from an open reading frame within the mitochondrial 16S ribosomal RNA. It was first described as a rescue factor against neuronal cell death associated with Alzheimer's disease but subsequent studies demonstrated its expression in vascular endothelial and smooth muscle cells as well90-92. Importantly, humanin was shown to confer endothelial protection inhibiting apoptosis and preventing progression of atherosclerotic plaque development in mouse models of accelerated vascular aging91. Expression of humanin declines with age both in humans and mice89. Thus, future studies are warranted to determine whether overexpression of humanin or treatment with humanin analogs confer vasoprotection in aging inhibiting endothelial apoptosis and/or attenuating vascular inflammation. There are other small humanin-like mitochondria-derived peptides, which are known to confer pro-survival cellular effects. Future studies should characterize age-related changes in the expression profile of these peptides in the cardiovascular system and determine their role in vasoprotection. Recent studies in C. elegans show that perturbing mitochondrial function in a subset of cells sends systemic signals, termed “mitokines”, governing stress resistance and longevity of the entire organism73. Future studies should test the possibility that modulation of the mitochondrial electron transport chain in mammalian cardiovascular cells by circulating dietary factors or pharmacological agents may also result in the secretion of diffusible mediators, which would regulate stress response pathways and inflammatory processes.
3. Therapeutic strategies to improve mitochondrial function in aging
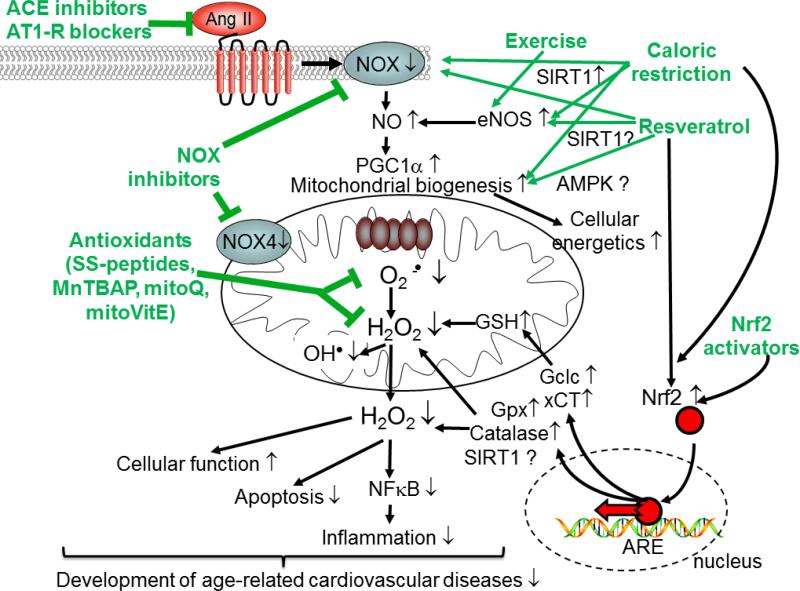
Pathways that improve mitochondrial function, attenuate mitochondrial oxidative stress and/or regulate mitochondrial biogenesis have recently emerged as potential therapeutic targets for prevention of the development of age-related cardiovascular diseases (Figure 2).
Figure 2.
Summary of mitochondrial-targeted interventions and their therapeutic potential in aging. xCT= cysteine transporter
3a. Mitochondrial-targeted antioxidants
As meta-analyses of clinical studies applying non-targeted antioxidants have shown disappointing results93, several specific mitochondrial-targeted antioxidants have been developed. Although the experiments in rodents have been quite promising, their efficacy in attenuating mitochondrial oxidative stress in cardiovascular aging and inhibiting vascular inflammation remains as yet to be established.
TPP+ conjugated antioxidants
Triphenylphosphonium ion (TPP+) has been successfully used to deliver several lipophylic antioxidants to the mitochondrial matrix, including Mito-Q (coenzyme Q), mitovitamin E and mitophenyltertbutyline as well as SkQ1 (plastoquinone). These mitochondrial-targeted drugs can achieve concentrations in the mitochondrial matrix 100 to 1000-fold higher than those in the cytosol because of their strong positive charge, as mitochondria have a highly negative membrane potential (approximately -150mV). Mito-Q pretreatment has been shown effective in reducing ischemia-reperfusion injury in the isolated perfused hearts. Likewise, SkQ1 has also been shown to reduce infarct size in rodent model of coronary artery ligation. A recent study in spontaneous hypertensive rats demonstrated that MitoQ treatment for 8 weeks significantly reduced systolic blood pressure and reduced cardiac mass94. The blood pressure lowering effect might be explained by the improved bioavailability of endothelial nitric oxide. The efficacy of MitoQ to reduce blood pressure provides proof of concept that mitochondria-targeted antioxidants may confer vasoprotection in aging as well. Furthermore, oral supplementation with the mitochondrial antioxidants alpha-lipoic acid and coenzyme Q10 reduced apoptosis in the cochlea aged mice95 and a similar treatment paradigm was shown to improve endothelial function in the aged aorta96.
SS-Peptides
The Szeto-Schiller (SS) compounds are tetrapeptides with an alternating aromatic-cationic amino acids motif, and demonstrated to concentrate in the inner mitochondrial membrane more than 1000 fold compared with the cytosolic concentration 97-99. Although the positive charge might explain the mitochondrial-targeting effect, the mitochondrial uptake of these SS peptides appears to be independent on mitochondrial potential, as they are concentrated even in depolarized mitochondria 97, 98. SS-31 (H-D-Arg-Dmt-Lys-Phe-NH2) contains dimethyl tyrosine moieties which is similar to (but even more effectively than) tyrosine to have intrinsic oxygen free radical scavenging activity 100. SS-31 is able to scavenge H2O2 hydroxyl radical, and peroxynitrite in vitro in a dose-dependent manner 97, 101. SS-31 has been shown to reduce ischemia reperfusion injury and reperfusion arrhythmia and better preserve myocardial function in various infarct models 101, 102. Furthermore, it has also been shown to attenuate several age-related diseases, including Parkinson's Disease 103, Alzheimer's Disease 104, muscle weakness 105, heart failure106 and insulin resistance 107. Studies from our laboratory demonstrated that SS-31 ameliorates Angiotensin-II induced cardiac hypertrophy and Gαq-overexpression induced heart failure, despite the absence of a blood pressure lowering effect108.
Thus, preclinical studies have shown promise for both TPP+conjugated antioxidants and SS-Peptides in prevention of cardiovascular diseases. Early phase clinical studies are ongoing and although these are generally designed to address the efficacy of these agents in treatment of acute cardiovascular disease, it will eventually be important to also establish whether they can delay aging in the cardiovascular system.
3b. Calorie restriction
An increasing amount of data suggest that the calorie restriction confers cardiovascular protection in aging and in pathological conditions associated with accelerated cardiac and vascular aging (recently reviewed elsewhere64). The mechanisms underlying the beneficial cardiovascular effects of calorie restriction are multifaceted, and include normalization of mitochondrial biogenesis109, attenuation of mitochondrial ROS production64, 110, 111 and consequential inhibition of signaling pathways regulated by mitochondria-derived ROS (e.g. NF-κB)112. The cellular pathways involved in mitochondrial protection induced by calorie restriction appear to depend on an increased expression/activity of the NAD+-dependent histone deacetylase SIRT1113 and activation of its downstream effectors, including PGC-1α65, 109. Expression of SIRT1 in mice confer vasoprotection, reducing endothelial ROS production, inhibiting NF-κB signaling and attenuating vascular inflammation, mimicking the effects of calorie restriction114. These effects mediated by SIRT1 are likely potentiated by an increased bioavailability of nitric oxide115 and increased levels of adiponectin116. Calorie restriction can also activate the transcription factor Nrf2, which controls the expression of numerous ROS detoxifying and antioxidant genes involved in regulation of mitochondrial redox homeostasis117. Interestingly, in the heart of Nrf2 knockout mice calorie restriction-mediated changes in SIRT1 expression are attenuated (Ungvari, Csiszar and de Cabo, unpublished observation), suggesting that a cross-talk exists between Nrf2 and SIRT1 signaling. Recent studies suggest that in addition to SIRT1 (a nuclear enzyme), activation of mitochondrial SIRT3 may also contribute to the mitochondrial protective effects of calorie restriction118. In this context it is significant that SIRT3 knockout mice show accelerated signs of cardiac aging119, including reduced ATP levels120.
3c. SIRT1 activators and other calorie restriction mimetics
Recent studies focus on the development of calorie restriction mimetics, to identify compounds that mimic the effects of calorie restriction by targeting cellular metabolic and stress response pathways without actually restricting calorie intake. The polyphenol resveratrol is one the first compounds, which was shown to mimic the cardiovascular protective effects of calorie restriction31, 111, 121, 122, including induction of mitochondrial biogenesis123 and attenuation of mitochondrial oxidative stress124, 125 in vascular endothelial cells and/or in cardiomyocytes. The effects of resveratrol, in part, are attributed to its ability to up-regulate and/or activate SIRT1, which deacetylates and activates PGC-1α and other regulators of mitochondrial function126. In addition, resveratrol can also activate Nrf2 in the endothelial cells125, which may also contribute to its mitochondrial protective effects.
Synthetic activators of SIRT1, such as SRT1720, were also reported to exert mitochondrial protective effects and to induce mitochondrial biogenesis in vitro127. In vivo treatment with SRT1720 was recently shown to affect mitochondrial respiration in a SIRT1- and PGC-1α-dependent manner and to extend lifespan of mice fed a high-fat diet128. Importantly, SRT1720 treatment in high fat diet-fed mice significantly reduced the number of ischemic foci in the heart and attenuated inflammatory gene expression both in the heart and the liver128. Further studies are definitely warranted to test whether mitochondrial protective effects of SRT1720 and related compounds are associated with functional improvement in the cardiovascular system of aged animals fed a standard diet.
The AMP-activated protein kinase (AMPK) has emerged as a key nutrient sensor, which acts as a master regulator of mitochondrial biogenesis, turnover, mitochondrial metabolism and mitochondrial antioxidant defenses129. There is increasing evidence suggesting that AMPK up-regulates SIRT1 activity130 and that AMPK activation may contribute to the cardioprotective effects of calorie restriction131. Because polyphenols can activate AMPK132, this effect may contribute to the robust increases in cellular SIRT1 activity and other calorie restriction-like effects in vascular cells observed upon resveratrol treatment. The AMPK activator metformin has been shown to ameliorate cardiac ischemia133, myocardial infarction134, diabetic cardiomyopathy135 and various animal models of heart failure136, 137. Furthermore, it has also been shown to improve aging-related cardiomyocyte dysfunction138, despite the absence of any beneficial effect on lifespan139, and improve endothelial vasodilation in rodent models of accelerated vascular aging140. Further studies are warranted to determine whether metformin treatment also confer mitochondrial protective effects promoting cardiovascular health in aged humans.
3d. Exercise
Population-based studies clearly show that regular physical activity can reduce the risk of cardiovascular diseases and it is assumed that some of the beneficial effects of exercise are due to its effect on mitochondrial function. The available evidence in experimental animals suggests that long-term voluntary exercise reduces mitochondrial ROS production in the heart of old rats141. As mentioned above, a recent study on mitochondrial mutator (Polgm/m) mice also shows that 5 months of endurance exercise induces mitochondrial biogenesis, prevents mtDNA depletion and mutations, increases mitochondrial oxidative capacity and respiratory chain assembly, restores mitochondrial morphology, and blunts pathological levels of apoptosis in multiple tissues, including the heart, of this model of premature aging17. The potential mechanisms underlying the mitochondrial effects of exercise are likely multifaceted and may include an increased shear stress-induced NO production, altered metabolism and neurohormonal effects. A recent study demonstrated that in 9 sedentary individuals older than 65 years of age prescribed a year of progressive and vigorous exercise training failed to improved age-dependent cardiac stiffening and diastolic dysfunction. However, it reduced arterial elastance and improved maximal aerobic exercise capacity142. Further clinical studies are needed to determine whether mitochondrial rejuvenation through exercise is an effective therapeutic approach to mitigate cardiac and vascular mitochondrial dysfunction associated with ‘healthy’ aging in humans as well.
3e. Other potential therapeutic strategies
As discussed above, genetic disruption of AC5 is protective against cardiac hypertrophy, apoptosis and cardiac failure by chronic catecholamine stimulation50, 51. Earlier experiment from the same group reported that direct inhibition of AC5 by 1R,4R-3-(6-aminopurin-9-yl)-cyclopentanecarboxylic acid hydroxyamide prevent isoproterenol induced apoptosis in isolated neonatal cardiomyocytes143. Further in vivo experiments using various inhibitors of AC5 are needed to elucidate the clinical potential of AC5 inhibition.
4. Perspectives
The important role of mitochondrial oxidative stress and mitochondrial dysfunction in age-related cardiovascular pathologies is evident and we are at the beginning of an exciting phase of research on understanding the genetic and epigenetic mechanisms underlying the mitochondrial alterations that occur with age. Importantly, a consensus should be reached whether mitochondrial contributions to cardiovascular aging occur primarily via increased macromolecular damage induced by mitochondria-derived ROS or other mechanisms by which mitochondrial ROS and signaling affect the cellular aging process play an equally important role. The role of neurohormonal changes in age-related mitochondrial alterations needs to be elucidated further. Further research is also needed to investigate mitochondrial fusion/fission, the homeostasis of mitochondrial damage and turnover as well as the role of cellular energetic changes, and to elucidate the precise connections between mitochondrial ROS production and mitochondrial retrograde signaling in the context of cardiovascular aging and deciphering the relationships between these processes. Future studies also should continue elucidating the cross-talk between mitochondria-derived ROS and increased activity of cell membrane-associated and cytoplasmic oxidases, including NADPH oxidases.
Mitochondria-targeted antioxidants show efficacy in various animal models and the existing preliminary clinical data show promising results in humans. Therefore, it is likely that novel classes of mitochondria-targeted antioxidants will be developed to improve mitochondrial function promoting cardiovascular health in the elderly in various human diseases. It will be particularly interesting to test the efficacy of these novel compounds for cardiovascular indications in animal models of aging. Because age-related changes in mitochondria are likely organ-specific, it is evident that much future work is required to improve the specificity of the biodistribution and penetration of antioxidant molecules delivered to mitochondria in vivo. In addition to targeting antioxidant compounds to the mitochondria, interventions that up-regulate intrinsic antioxidant systems in the mitochondria can be exploited for therapeutic advantage. In particular, pharmacological or nutritional modulation of evolutionarily conserved, Nrf2/ARE-driven and/or sirtuin-dependent pro-survival pathways may be effective in restoring a youthful mitochondrial function in the aged cardiovascular system, delaying the onset of age-related cardiovascular diseases. Interventions that modulate processes involved in regulation of mitochondrial turnover are also of particular interest. Finally, a deeper understanding of the mitochondria-derived signals that activate inflammatory processes may also yield new therapies for the prevention of cardiovascular diseases.
Table 2.
Influence of genetic and pharmacological manipulations related to mitochondria on aging and cardiovascular phenotypes in rodent models
| Animal models | Description | Aging phenotypes | Cardiovascular phenotypes | Reference | |
|---|---|---|---|---|---|
| Genotypes | mCAT | Overexpression of catalase targeted to mitochondria | 18% extension of lifespan. Attenuated cardiac aging, aging-related sarcopenia, presbyacusis and cancer incidence. | Protect against cardiac hypertophy and heart failure | 11, 12, 27, 95, 144 |
| Polg m/m | Homozygous mutation of mitochondrial polymerase gamma D257A | “Accelerated aging”: sarcopenia, graying and alopecia, kyphosis, presbyacusis, anemia, age-dependent cardiomyopathy | Aggravate heart failure in response to Angiotensin II | 13, 14, 16, 18, 27 | |
| p66shc | Targeted mutation of the p66Shc gene | Extension of lifespan. Reduction of ROS and apoptosis | Attenuate Angiotensin II induced LV hypertrophy and cardiomyocytes apoptosis; reduce oxidative damage in cardiac progenitor cells, cardiomyocytes and endothelial cells in diabetes | 18, 20-22 | |
| Tg-Sirt1 | α-myosin heavy chain (α-MHC) promoter-driven, cardiac-specific overexpression of SIRT1 | Delayed cardiac aging | Attenuates age-dependent increases in cardiac hypertrophy, apoptosis/fibrosis, cardiac dysfunction, and expression of senescence markers; reduces myocardial infarction/area at risk after ischemia/reperfusion | 145,146 | |
| Sirt1flox/flox,αMHC-Cre | cardiac-specific Sirt1–/– mice | increases size of myocardial infarction after ischemia/reperfusion | 146 | ||
| SIRT3-/- | SIRT3-deficient mice | accelerated cardiac aging, age-dependent increase in mitochondrial swelling due to increased mPTP opening | early-age onset of hypertrophy associated with fibrosis, increased mortality after transaortic constriction | 119 | |
| Nrf2-/- | Nrf2 deficient mice | pathological cardiac hypertrophy, myocardial fibrosis and apoptosis, overt heart failure, and increased mortality after transaortic constriction; exacerbation of high fat diet-induced endothelial dysfunction and vascular oxidative stress and inflammation | 125, 147, 148 | ||
| Tg-IGF-1 | Cardiac-specific overexpression of IGF-1 | attenuates aging-associated cardiac diastolic contractile dysfunction | 149 | ||
| Prop1df/Prop1df | GH- and IGF-1 deficient Ames dwarf mice | Extension of lifespan due to reduced cancer incidence | Mitochondrial oxidative stress in vasculature and the heart; cardiac dysfunction | 56, 57 | |
| dw-4/dw-4 | GH- and IGF-1 deficient Lewis dwarf rats | Increased incidence of stroke, no change in lifespan | Vascular oxidative stress, increased high fat diet-induced vascular inflammation | 150, 151 | |
| Igf1f/f+MUP-iCre-AAV8 | AAV-mediated hepatic knockdown of IGF-1 | Impaired vascular oxidative stress resistance, Nrf2 dysfunction, increased apoptosis | 152 | ||
| Pharmacological treatments | Resveratrol | Pharmacological activator of SIRT1 and Nrf2 | Ameliorate shortened lifespan and metabolic derangement in aged mice fed with high fat diet | Promotes mitochondrial biogenesis, attenuates mitochondrial oxidative stress and improves cardiac and vascular function in aged rodents and/or in rodent models of type 2 diabetes; protect against cardiac ischemia-reperfusion injury; attenuate atherosclerosis | 31, 42, 122, 124, 125, 153-156 |
| SRT1720 | Pharmacological activator of SIRT1 | Ameliorate shortened lifespan and metabolic derangement in mice fed with high fat diet; | Reduce ischemic damage in heart of high fat diet-fed mice; attenuates atherosclerosis in LDLR-/- mice | 128, (Price N, Ungvari Z and Sinclair D, unpublished data, 2010) | |
| Metformin | AMPK activator | improve aging-related cardiomyocyte dysfunction, no effect on lifespan | ameliorate cardiac ischemia, myocardial infarction, diabetic cardiomyopathy and various animal models of heart failure | 133-139 | |
| MitoQ | Ubiquinone (antioxidant) conjugated with TPP+ | Reduction of blood pressure and cardiac hypertrophy in spontaneous hypertensive rats | 100 | ||
| SS-31 | Tetrapeptide antioxidant targeted to mitochondria | Attenuation of Angiotensin II induced cardiac hypertrophy and Gaq overexpression induced heart failure | 108 |
Acknowledgement
This work was supported by grants from the American Diabetes Association, the Oklahoma Center for the Advancement of Science and Technology, The Ellison Medical Foundation and the NIH (AT006526, AG038747, AG11370, HL101186, AG013280, AG000751).
Footnotes
Disclosure: The authors have nothing to disclose
References
- 1.Harman D. Aging: A theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 2.Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Harman D. The biologic clock: The mitochondria? J Am Geriatr Soc. 1972;20:145–147. doi: 10.1111/j.1532-5415.1972.tb00787.x. [DOI] [PubMed] [Google Scholar]
- 4.Perez VI, Bokov A, Van Remmen H, Mele J, Ran Q, Ikeno Y, Richardson A. Is the oxidative stress theory of aging dead? Biochim Biophys Acta. 2009;1790:1005–1014. doi: 10.1016/j.bbagen.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Judge S, Jang YM, Smith A, Hagen T, Leeuwenburgh C. Age-associated increases in oxidative stress and antioxidant enzyme activities in cardiac interfibrillar mitochondria: Implications for the mitochondrial theory of aging. Faseb J. 2005;19:419–421. doi: 10.1096/fj.04-2622fje. [DOI] [PubMed] [Google Scholar]
- 6.Ungvari ZI, Orosz Z, Labinskyy N, Rivera A, Xiangmin Z, Smith KE, Csiszar A. Increased mitochondrial h2o2 production promotes endothelial nf-kb activation in aged rat arteries. Am J Physiol Heart Circ Physiol. 2007;293:H37–47. doi: 10.1152/ajpheart.01346.2006. [DOI] [PubMed] [Google Scholar]
- 7.Mammucari C, Rizzuto R. Signaling pathways in mitochondrial dysfunction and aging. Mech Ageing Dev. 2010;131:536–543. doi: 10.1016/j.mad.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trifunovic A, Larsson NG. Mitochondrial dysfunction as a cause of ageing. J Intern Med. 2008;263:167–178. doi: 10.1111/j.1365-2796.2007.01905.x. [DOI] [PubMed] [Google Scholar]
- 9.Terzioglu M, Larsson NG. Mitochondrial dysfunction in mammalian ageing. Novartis Found Symp. 2007;287:197–208. doi: 10.1002/9780470725207.ch14. discussion 208-113. [DOI] [PubMed] [Google Scholar]
- 10.Navarro A, Boveris A. The mitochondrial energy transduction system and the aging process. Am J Physiol Cell Physiol. 2007;292:C670–686. doi: 10.1152/ajpcell.00213.2006. [DOI] [PubMed] [Google Scholar]
- 11.Schriner SE, Linford NJ, Martin GM, Treuting P, Ogburn CE, Emond M, Coskun PE, Ladiges W, Wolf N, Van Remmen H, Wallace DC, Rabinovitch PS. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science. 2005;308:1909–1911. doi: 10.1126/science.1106653. [DOI] [PubMed] [Google Scholar]
- 12.Dai DF, Santana LF, Vermulst M, Tomazela DM, Emond MJ, MacCoss MJ, Gollahon K, Martin GM, Loeb LA, Ladiges WC, Rabinovitch PS. Overexpression of catalase targeted to mitochondria attenuates murine cardiac aging. Circulation. 2009;119:2789–2797. doi: 10.1161/CIRCULATIONAHA.108.822403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trifunovic A, Wredenberg A, Falkenberg M, Spelbrink JN, Rovio AT, Bruder CE, Bohlooly YM, Gidlof S, Oldfors A, Wibom R, Tornell J, Jacobs HT, Larsson NG. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature. 2004;429:417–423. doi: 10.1038/nature02517. [DOI] [PubMed] [Google Scholar]
- 14.Kujoth GC, Hiona A, Pugh TD, Someya S, Panzer K, Wohlgemuth SE, Hofer T, Seo AY, Sullivan R, Jobling WA, Morrow JD, Van Remmen H, Sedivy JM, Yamasoba T, Tanokura M, Weindruch R, Leeuwenburgh C, Prolla TA. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science. 2005;309:481–484. doi: 10.1126/science.1112125. [DOI] [PubMed] [Google Scholar]
- 15.Vermulst M, Wanagat J, Kujoth GC, Bielas JH, Rabinovitch PS, Prolla TA, Loeb LA. DNA deletions and clonal mutations drive premature aging in mitochondrial mutator mice. Nat Genet. 2008;40:392–394. doi: 10.1038/ng.95. [DOI] [PubMed] [Google Scholar]
- 16.Dai DF, Chen T, Wanagat J, Laflamme M, Marcinek DJ, Emond MJ, Ngo CP, Prolla TA, Rabinovitch PS. Age-dependent cardiomyopathy in mitochondrial mutator mice is attenuated by overexpression of catalase targeted to mitochondria. Aging Cell. 2010;9:536–544. doi: 10.1111/j.1474-9726.2010.00581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Safdar A, Bourgeois JM, Ogborn DI, Little JP, Hettinga BP, Akhtar M, Thompson JE, Melov S, Mocellin NJ, Kujoth GC, Prolla TA, Tarnopolsky MA. Endurance exercise rescues progeroid aging and induces systemic mitochondrial rejuvenation in mtdna mutator mice. Proc Natl Acad Sci U S A. 2011;108:4135–4140. doi: 10.1073/pnas.1019581108. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Migliaccio E, Giorgio M, Mele S, Pelicci G, Reboldi P, Pandolfi PP, Lanfrancone L, Pelicci PG. The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature. 1999;402:309–313. doi: 10.1038/46311. [DOI] [PubMed] [Google Scholar]
- 19.Giorgio M, Migliaccio E, Orsini F, Paolucci D, Moroni M, Contursi C, Pelliccia G, Luzi L, Minucci S, Marcaccio M, Pinton P, Rizzuto R, Bernardi P, Paolucci F, Pelicci PG. Electron transfer between cytochrome c and p66shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell. 2005;122:221–233. doi: 10.1016/j.cell.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 20.Pinton P, Rimessi A, Marchi S, Orsini F, Migliaccio E, Giorgio M, Contursi C, Minucci S, Mantovani F, Wieckowski MR, Del Sal G, Pelicci PG, Rizzuto R. Protein kinase c beta and prolyl isomerase 1 regulate mitochondrial effects of the life-span determinant p66shc. Science. 2007;315:659–663. doi: 10.1126/science.1135380. [DOI] [PubMed] [Google Scholar]
- 21.Rota M, LeCapitaine N, Hosoda T, Boni A, De Angelis A, Padin-Iruegas ME, Esposito G, Vitale S, Urbanek K, Casarsa C, Giorgio M, Luscher TF, Pelicci PG, Anversa P, Leri A, Kajstura J. Diabetes promotes cardiac stem cell aging and heart failure, which are prevented by deletion of the p66shc gene. Circ Res. 2006;99:42–52. doi: 10.1161/01.RES.0000231289.63468.08. [DOI] [PubMed] [Google Scholar]
- 22.Camici GG, Schiavoni M, Francia P, Bachschmid M, Martin-Padura I, Hersberger M, Tanner FC, Pelicci P, Volpe M, Anversa P, Luscher TF, Cosentino F. Genetic deletion of p66(shc) adaptor protein prevents hyperglycemia-induced endothelial dysfunction and oxidative stress. Proc Natl Acad Sci U S A. 2007;104:5217–5222. doi: 10.1073/pnas.0609656104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Francia P, Cosentino F, Schiavoni M, Huang Y, Perna E, Camici GG, Luscher TF, Volpe M. P66(shc) protein, oxidative stress, and cardiovascular complications of diabetes: The missing link. J Mol Med (Berl) 2009;87:885–891. doi: 10.1007/s00109-009-0499-3. [DOI] [PubMed] [Google Scholar]
- 24.Ventura-Clapier R, Garnier A, Veksler V. Transcriptional control of mitochondrial biogenesis: The central role of pgc-1alpha. Cardiovasc Res. 2008;79:208–217. doi: 10.1093/cvr/cvn098. [DOI] [PubMed] [Google Scholar]
- 25.Goffart S, von Kleist-Retzow J-C, Wiesner RJ. Regulation of mitochondrial proliferation in the heart: Power-plant failure contributes to cardiac failure in hypertrophy. Cardiovascular Research. 2004;64:198–207. doi: 10.1016/j.cardiores.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 26.Murray AJ, Anderson RE, Watson GC, Radda GK, Clarke K. Uncoupling proteins in human heart. Lancet. 2004;364:1786–1788. doi: 10.1016/S0140-6736(04)17402-3. [DOI] [PubMed] [Google Scholar]
- 27.Dai DF, Johnson SC, Villarin JJ, Chin MT, Nieves-Cintron M, Chen T, Marcinek DJ, Dorn GW, 2nd, Kang YJ, Prolla TA, Santana LF, Rabinovitch PS. Mitochondrial oxidative stress mediates angiotensin ii-induced cardiac hypertrophy and g{alpha}q overexpression-induced heart failure. Circ Res. 2011;108:837–846. doi: 10.1161/CIRCRESAHA.110.232306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Erusalimsky JD. Vascular endothelial senescence: From mechanisms to pathophysiology. J Appl Physiol. 2009;106:326–332. doi: 10.1152/japplphysiol.91353.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Phaneuf S, Leeuwenburgh C. Cytochrome c release from mitochondria in the aging heart: A possible mechanism for apoptosis with age. Am J Physiol Regul Integr Comp Physiol. 2002;282:R423–430. doi: 10.1152/ajpregu.00296.2001. [DOI] [PubMed] [Google Scholar]
- 30.Csiszar A, Ungvari Z, Koller A, Edwards JG, Kaley G. Proinflammatory phenotype of coronary arteries promotes endothelial apoptosis in aging. Physiol Genomics. 2004;17:21–30. doi: 10.1152/physiolgenomics.00136.2003. [DOI] [PubMed] [Google Scholar]
- 31.Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E, Jamieson HA, Zhang Y, Dunn SR, Sharma K, Pleshko N, Woollett LA, Csiszar A, Ikeno Y, Le Couteur D, Elliott PJ, Becker KG, Navas P, Ingram DK, Wolf NS, Ungvari Z, Sinclair DA, de Cabo R. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8:157–168. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anversa P, Li P, Sonnenblick EH, Olivetti G. Effects of aging on quantitative structural properties of coronary vasculature and microvasculature in rats. Am J Physiol. 1994;267:H1062–1073. doi: 10.1152/ajpheart.1994.267.3.H1062. [DOI] [PubMed] [Google Scholar]
- 33.Sonntag WE, Lynch CD, Cooney PT, Hutchins PM. Decreases in cerebral microvasculature with age are associated with the decline in growth hormone and insulin-like growth factor 1. Endocrinology. 1997;138:3515–3520. doi: 10.1210/endo.138.8.5330. [DOI] [PubMed] [Google Scholar]
- 34.Kim GW, Gasche Y, Grzeschik S, Copin JC, Maier CM, Chan PH. Neurodegeneration in striatum induced by the mitochondrial toxin 3-nitropropionic acid: Role of matrix metalloproteinase-9 in early blood-brain barrier disruption? J Neurosci. 2003;23:8733–8742. doi: 10.1523/JNEUROSCI.23-25-08733.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Farrall AJ, Wardlaw JM. Blood-brain barrier: Ageing and microvascular disease--systematic review and meta-analysis. Neurobiol Aging. 2009;30:337–352. doi: 10.1016/j.neurobiolaging.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 36.Gao Q, Zhao X, Ahmad M, Wolin MS. Mitochondrial-derived hydrogen peroxide inhibits relaxation of bovine coronary arterial smooth muscle to hypoxia through stimulation of erk map kinase. Am J Physiol Heart Circ Physiol. 2009;297:H2262–2269. doi: 10.1152/ajpheart.00817.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wenzel P, Schuhmacher S, Kienhofer J, Muller J, Hortmann M, Oelze M, Schulz E, Treiber N, Kawamoto T, Scharffetter-Kochanek K, Munzel T, Burkle A, Bachschmid MM, Daiber A. Manganese superoxide dismutase and aldehyde dehydrogenase deficiency increase mitochondrial oxidative stress and aggravate age-dependent vascular dysfunction. Cardiovasc Res. 2008;80:280–289. doi: 10.1093/cvr/cvn182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Csiszar A, Labinskyy N, Jimenez R, Pinto JT, Ballabh P, Losonczy G, Pearson KJ, de Cabo R, Ungvari Z. Anti-oxidative and anti-inflammatory vasoprotective effects of caloric restriction in aging: Role of circulating factors and sirt1. Mech Ageing Dev. 2009 doi: 10.1016/j.mad.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van der Loo B, Labugger R, Skepper JN, Bachschmid M, Kilo J, Powell JM, Palacios-Callender M, Erusalimsky JD, Quaschning T, Malinski T, Gygi D, Ullrich V, Lüscher TF. Enhanced peroxynitrite formation is associated with vascular aging. J Exp Med. 2000;192:1731–1744. doi: 10.1084/jem.192.12.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ungvari ZI, Labinskyy N, Gupte SA, Chander PN, Edwards JG, Csiszar A. Dysregulation of mitochondrial biogenesis in vascular endothelial and smooth muscle cells of aged rats. Am J Physiol Heart Circ Physiol. 2008;294:H2121–2128. doi: 10.1152/ajpheart.00012.2008. [DOI] [PubMed] [Google Scholar]
- 41.Ungvari Z, Kaley G, de Cabo R, Sonntag WE, Csiszar A. Mechanisms of vascular aging: New perspectives. J Gerontol A Biol Sci Med Sci. 2010;65:1028–1041. doi: 10.1093/gerona/glq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Csiszar A, Labinskyy N, Podlutsky A, Kaminski PM, Wolin MS, Zhang C, Mukhopadhyay P, Pacher P, Hu F, de Cabo R, Ballabh P, Ungvari Z. Vasoprotective effects of resveratrol and sirt1: Attenuation of cigarette smoke-induced oxidative stress and proinflammatory phenotypic alterations. Am J Physiol Heart Circ Physiol. 2008;294:H2721–2735. doi: 10.1152/ajpheart.00235.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Labinskyy N, Mukhopadhyay P, Toth J, Szalai G, Veres M, Losonczy G, Pinto JT, Pacher P, Ballabh P, Podlutsky A, Austad SN, Csiszar A, Ungvari Z. Longevity is associated with increased vascular resistance to high glucose-induced oxidative stress and inflammatory gene expression in peromyscus leucopus. Am J Physiol Heart Circ Physiol. 2009;296:H946–956. doi: 10.1152/ajpheart.00693.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics--2011 update: A report from the american heart association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Domenighetti AA, Wang Q, Egger M, Richards SM, Pedrazzini T, Delbridge LM. Angiotensin ii-mediated phenotypic cardiomyocyte remodeling leads to age-dependent cardiac dysfunction and failure. Hypertension. 2005;46:426–432. doi: 10.1161/01.HYP.0000173069.53699.d9. [DOI] [PubMed] [Google Scholar]
- 46.Groban L, Pailes NA, Bennett CD, Carter CS, Chappell MC, Kitzman DW, Sonntag WE. Growth hormone replacement attenuates diastolic dysfunction and cardiac angiotensin ii expression in senescent rats. J Gerontol A Biol Sci Med Sci. 2006;61:28–35. doi: 10.1093/gerona/61.1.28. [DOI] [PubMed] [Google Scholar]
- 47.Wang M, Takagi G, Asai K, Resuello RG, Natividad FF, Vatner DE, Vatner SF, Lakatta EG. Aging increases aortic mmp-2 activity and angiotensin ii in nonhuman primates. Hypertension. 2003;41:1308–1316. doi: 10.1161/01.HYP.0000073843.56046.45. [DOI] [PubMed] [Google Scholar]
- 48.Basso N, Cini R, Pietrelli A, Ferder L, Terragno NA, Inserra F. Protective effect of long-term angiotensin ii inhibition. Am J Physiol Heart Circ Physiol. 2007;293:H1351–1358. doi: 10.1152/ajpheart.00393.2007. [DOI] [PubMed] [Google Scholar]
- 49.Benigni A, Corna D, Zoja C, Sonzogni A, Latini R, Salio M, Conti S, Rottoli D, Longaretti L, Cassis P, Morigi M, Coffman TM, Remuzzi G. Disruption of the ang ii type 1 receptor promotes longevity in mice. J Clin Invest. 2009;119:524–530. doi: 10.1172/JCI36703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Okumura S, Vatner DE, Kurotani R, Bai Y, Gao S, Yuan Z, Iwatsubo K, Ulucan C, Kawabe J, Ghosh K, Vatner SF, Ishikawa Y. Disruption of type 5 adenylyl cyclase enhances desensitization of cyclic adenosine monophosphate signal and increases akt signal with chronic catecholamine stress. Circulation. 2007;116:1776–1783. doi: 10.1161/CIRCULATIONAHA.107.698662. [DOI] [PubMed] [Google Scholar]
- 51.Okumura S, Takagi G, Kawabe J, Yang G, Lee MC, Hong C, Liu J, Vatner DE, Sadoshima J, Vatner SF, Ishikawa Y. Disruption of type 5 adenylyl cyclase gene preserves cardiac function against pressure overload. Proc Natl Acad Sci U S A. 2003;100:9986–9990. doi: 10.1073/pnas.1733772100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yan L, Vatner DE, O'Connor JP, Ivessa A, Ge H, Chen W, Hirotani S, Ishikawa Y, Sadoshima J, Vatner SF. Type 5 adenylyl cyclase disruption increases longevity and protects against stress. Cell. 2007;130:247–258. doi: 10.1016/j.cell.2007.05.038. [DOI] [PubMed] [Google Scholar]
- 53.Remondino A, Kwon SH, Communal C, Pimentel DR, Sawyer DB, Singh K, Colucci WS. Beta-adrenergic receptor-stimulated apoptosis in cardiac myocytes is mediated by reactive oxygen species/c-jun nh2-terminal kinase-dependent activation of the mitochondrial pathway. Circ Res. 2003;92:136–138. doi: 10.1161/01.res.0000054624.03539.b4. [DOI] [PubMed] [Google Scholar]
- 54.Andersson DC, Fauconnier J, Yamada T, Lacampagne A, Zhang SJ, Katz A, Westerblad H. Mitochondrial production of reactive oxygen species contributes to the beta-adrenergic stimulation of mouse cardiomycytes. J Physiol. 2011;589:1791–1801. doi: 10.1113/jphysiol.2010.202838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Khan AS, Sane DC, Wannenburg T, Sonntag WE. Growth hormone, insulin-like growth factor-1 and the aging cardiovascular system. Cardiovasc Res. 2002;54:25–35. doi: 10.1016/s0008-6363(01)00533-8. [DOI] [PubMed] [Google Scholar]
- 56.Csiszar A, Labinskyy N, Perez V, Recchia FA, Podlutsky A, Mukhopadhyay P, Losonczy G, Pacher P, Austad SN, Bartke A, Ungvari Z. Endothelial function and vascular oxidative stress in long-lived gh/igf-deficient ames dwarf mice. Am J Physiol Heart Circ Physiol. 2008;295:H1882–1894. doi: 10.1152/ajpheart.412.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ren J, Brown-Borg HM. Impaired cardiac excitation-contraction coupling in ventricular myocytes from ames dwarf mice with igf-i deficiency. Growth Horm IGF Res. 2002;12:99–105. doi: 10.1054/ghir.2002.0267. [DOI] [PubMed] [Google Scholar]
- 58.Puche JE, Garcia-Fernandez M, Muntane J, Rioja J, Gonzalez-Baron S, Castilla Cortazar I. Low doses of insulin-like growth factor-i induce mitochondrial protection in aging rats. Endocrinology. 2008;149:2620–2627. doi: 10.1210/en.2007-1563. [DOI] [PubMed] [Google Scholar]
- 59.Rivera EJ, Goldin A, Fulmer N, Tavares R, Wands JR, de la Monte SM. Insulin and insulin-like growth factor expression and function deteriorate with progression of alzheimer's disease: Link to brain reductions in acetylcholine. J Alzheimers Dis. 2005;8:247–268. doi: 10.3233/jad-2005-8304. [DOI] [PubMed] [Google Scholar]
- 60.Lopez-Lopez C, Dietrich MO, Metzger F, Loetscher H, Torres-Aleman I. Disturbed cross talk between insulin-like growth factor i and amp-activated protein kinase as a possible cause of vascular dysfunction in the amyloid precursor protein/presenilin 2 mouse model of alzheimer's disease. The Journal of neuroscience. 2007;27:824–831. doi: 10.1523/JNEUROSCI.4345-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ungvari Z, Bailey-Downs L, Gautam T, Sosnowska D, Wang M, Monticone RE, Telljohann R, Pinto JT, de Cabo R, Sonntag WE, Lakatta E, Csiszar A. Age-associated vascular oxidative stress, nrf2 dysfunction and nf-kb activation in the non-human primate macaca mulatta. J Gerontol Biol Med Sci. 2011;66:866–875. doi: 10.1093/gerona/glr092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ungvari Z, Bailey-Downs L, Sosnowska D, Gautam T, Koncz P, Losonczy G, Ballabh P, de Cabo R, Sonntag WE, Csiszar A. Vascular oxidative stress in aging: A homeostatic failure due to dysregulation of nrf2-mediated antioxidant response. Am J Physiol. 2011;301:H363–372. doi: 10.1152/ajpheart.01134.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ungvari Z, Bailey-Downs L, Gautam T, Koncz P, Losonczy G, Ballabh P, de Cabo R, Sonntag WE, Csiszar A. Vascular oxidative stress in aging: A homeostatic failure due to dysregulation of nrf2-mediated antioxidant response. Am J Physiol. 2011 doi: 10.1152/ajpheart.01134.2010. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ungvari Z, Parrado-Fernandez C, Csiszar A, de Cabo R. Mechanisms underlying caloric restriction and lifespan regulation: Implications for vascular aging. Circ Res. 2008;102:519–528. doi: 10.1161/CIRCRESAHA.107.168369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lopez-Lluch G, Irusta PM, Navas P, de Cabo R. Mitochondrial biogenesis and healthy aging. Exp Gerontol. 2008;43:813–819. doi: 10.1016/j.exger.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dai DF, Rabinovitch P. Mitochondrial oxidative stress mediates induction of autophagy and hypertrophy in angiotensin-ii treated mouse hearts. Autophagy. 2011;7:917–918. doi: 10.4161/auto.7.8.15813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sazonova M, Budnikov E, Khasanova Z, Sobenin I, Postnov A, Orekhov A. Studies of the human aortic intima by a direct quantitative assay of mutant alleles in the mitochondrial genome. Atherosclerosis. 2009;204:184–190. doi: 10.1016/j.atherosclerosis.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 68.Li F, Zhang L, Craddock J, Bruce-Keller AJ, Dasuri K, Nguyen A, Keller JN. Aging and dietary restriction effects on ubiquitination, sumoylation, and the proteasome in the heart. Mech Ageing Dev. 2008;129:515–521. doi: 10.1016/j.mad.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dai DF, Rabinovitch PS. Cardiac aging in mice and humans: The role of mitochondrial oxidative stress. Trends Cardiovasc Med. 2009;19:213–220. doi: 10.1016/j.tcm.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Burns EM, Kruckeberg TW, Comerford LE, Buschmann MT. Thinning of capillary walls and declining numbers of endothelial mitochondria in the cerebral cortex of the aging primate, macaca nemestrina. J Gerontol. 1979;34:642–650. doi: 10.1093/geronj/34.5.642. [DOI] [PubMed] [Google Scholar]
- 71.Burns EM, Kruckeberg TW, Gaetano PK. Changes with age in cerebral capillary morphology. Neurobiol Aging. 1981;2:283–291. doi: 10.1016/0197-4580(81)90037-3. [DOI] [PubMed] [Google Scholar]
- 72.Nisoli E, Clementi E, Paolucci C, Cozzi V, Tonello C, Sciorati C, Bracale R, Valerio A, Francolini M, Moncada S, Carruba MO. Mitochondrial biogenesis in mammals: The role of endogenous nitric oxide. Science. 2003;299:896–899. doi: 10.1126/science.1079368. [DOI] [PubMed] [Google Scholar]
- 73.Durieux J, Wolff S, Dillin A. The cell-non-autonomous nature of electron transport chain-mediated longevity. Cell. 2011;144:79–91. doi: 10.1016/j.cell.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Linford NJ, Beyer RP, Gollahon K, Krajcik RA, Malloy VL, Demas V, Burmer GC, Rabinovitch PS. Transcriptional response to aging and caloric restriction in heart and adipose tissue. Aging Cell. 2007;6:673–688. doi: 10.1111/j.1474-9726.2007.00319.x. [DOI] [PubMed] [Google Scholar]
- 75.St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jager S, Handschin C, Zheng K, Lin J, Yang W, Simon DK, Bachoo R, Spiegelman BM. Suppression of reactive oxygen species and neurodegeneration by the pgc-1 transcriptional coactivators. Cell. 2006;127:397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 76.Mollnau H, Wendt M, Szocs K, Lassegue B, Schulz E, Oelze M, Li H, Bodenschatz M, August M, Kleschyov AL, Tsilimingas N, Walter U, Forstermann U, Meinertz T, Griendling K, Munzel T. Effects of angiotensin ii infusion on the expression and function of nad(p)h oxidase and components of nitric oxide/cgmp signaling. Circ Res. 2002;90:E58–65. doi: 10.1161/01.res.0000012569.55432.02. [DOI] [PubMed] [Google Scholar]
- 77.Doughan AK, Harrison DG, Dikalov SI. Molecular mechanisms of angiotensin ii-mediated mitochondrial dysfunction: Linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ Res. 2008;102:488–496. doi: 10.1161/CIRCRESAHA.107.162800. [DOI] [PubMed] [Google Scholar]
- 78.Kimura S, Zhang GX, Nishiyama A, Shokoji T, Yao L, Fan YY, Rahman M, Abe Y. Mitochondria-derived reactive oxygen species and vascular map kinases: Comparison of angiotensin ii and diazoxide. Hypertension. 2005;45:438–444. doi: 10.1161/01.HYP.0000157169.27818.ae. [DOI] [PubMed] [Google Scholar]
- 79.Ago T, Matsushima S, Kuroda J, Zablocki D, Kitazono T, Sadoshima J. The nadph oxidase nox4 and aging in the heart. Aging (Albany NY) 2010;2:1012–1016. doi: 10.18632/aging.100261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ago T, Kuroda J, Pain J, Fu C, Li H, Sadoshima J. Upregulation of nox4 by hypertrophic stimuli promotes apoptosis and mitochondrial dysfunction in cardiac myocytes. Circ Res. 2010;106:1253–1264. doi: 10.1161/CIRCRESAHA.109.213116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kuroda J, Ago T, Matsushima S, Zhai P, Schneider MD, Sadoshima J. Nadph oxidase 4 (nox4) is a major source of oxidative stress in the failing heart. Proc Natl Acad Sci U S A. 2010;107:15565–15570. doi: 10.1073/pnas.1002178107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Abadir PM, Foster DB, Crow M, Cooke CA, Rucker JJ, Jain A, Smith BJ, Burks TN, Cohn RD, Fedarko NS, Carey RM, O'Rourke B, Walston JD. Identification and characterization of a functional mitochondrial angiotensin system. Proc Natl Acad Sci U S A. 2011;108:14849–14854. doi: 10.1073/pnas.1101507108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Finocchietto PV, Franco MC, Holod S, Gonzalez AS, Converso DP, Arciuch VG, Serra MP, Poderoso JJ, Carreras MC. Mitochondrial nitric oxide synthase: A masterpiece of metabolic adaptation, cell growth, transformation, and death. Exp Biol Med (Maywood) 2009;234:1020–1028. doi: 10.3181/0902-MR-81. [DOI] [PubMed] [Google Scholar]
- 84.Poderoso JJ, Carreras MC, Lisdero C, Riobo N, Schopfer F, Boveris A. Nitric oxide inhibits electron transfer and increases superoxide radical production in rat heart mitochondria and submitochondrial particles. Arch Biochem Biophys. 1996;328:85–92. doi: 10.1006/abbi.1996.0146. [DOI] [PubMed] [Google Scholar]
- 85.Kanski J, Behring A, Pelling J, Schoneich C. Proteomic identification of 3-nitrotyrosine-containing rat cardiac proteins: Effect of biological aging. Am J Physiol Heart Circ Physiol. 2004 doi: 10.1152/ajpheart.01030.2003. [DOI] [PubMed] [Google Scholar]
- 86.Radi R, Cassina A, Hodara R, Quijano C, Castro L. Peroxynitrite reactions and formation in mitochondria. Free Radic Biol Med. 2002;33:1451–1464. doi: 10.1016/s0891-5849(02)01111-5. [DOI] [PubMed] [Google Scholar]
- 87.Jones SP, Bolli R. The ubiquitous role of nitric oxide in cardioprotection. J Mol Cell Cardiol. 2006;40:16–23. doi: 10.1016/j.yjmcc.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 88.Donato AJ, Black AD, Jablonski KL, Gano LB, Seals DR. Aging is associated with greater nuclear nf kappa b, reduced i kappa b alpha, and increased expression of proinflammatory cytokines in vascular endothelial cells of healthy humans. Aging Cell. 2008;7:805–812. doi: 10.1111/j.1474-9726.2008.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Muzumdar RH, Huffman DM, Atzmon G, Buettner C, Cobb LJ, Fishman S, Budagov T, Cui L, Einstein FH, Poduval A, Hwang D, Barzilai N, Cohen P. Humanin: A novel central regulator of peripheral insulin action. PLoS ONE. 2009;4:e6334. doi: 10.1371/journal.pone.0006334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jung SS, Van Nostrand WE. Humanin rescues human cerebrovascular smooth muscle cells from abeta-induced toxicity. J Neurochem. 2003;84:266–272. doi: 10.1046/j.1471-4159.2003.01524.x. [DOI] [PubMed] [Google Scholar]
- 91.Oh YK, Bachar AR, Zacharias DG, Kim SG, Wan J, Cobb LJ, Lerman LO, Cohen P, Lerman A. Humanin preserves endothelial function and prevents atherosclerotic plaque progression in hypercholesterolemic apoe deficient mice. Atherosclerosis. 2011 doi: 10.1016/j.atherosclerosis.2011.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bachar AR, Scheffer L, Schroeder AS, Nakamura HK, Cobb LJ, Oh YK, Lerman LO, Pagano RE, Cohen P, Lerman A. Humanin is expressed in human vascular walls and has a cytoprotective effect against oxidized ldl-induced oxidative stress. Cardiovasc Res. 2010;88:360–366. doi: 10.1093/cvr/cvq191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: Systematic review and meta-analysis. JAMA. 2007;297:842–857. doi: 10.1001/jama.297.8.842. [DOI] [PubMed] [Google Scholar]
- 94.Dikalova AE, Bikineyeva AT, Budzyn K, Nazarewicz RR, McCann L, Lewis W, Harrison DG, Dikalov SI. Therapeutic targeting of mitochondrial superoxide in hypertension. Circ Res. 2010 doi: 10.1161/CIRCRESAHA.109.214601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Someya S, Xu J, Kondo K, Ding D, Salvi RJ, Yamasoba T, Rabinovitch PS, Weindruch R, Leeuwenburgh C, Tanokura M, Prolla TA. Age-related hearing loss in c57bl/6j mice is mediated by bak-dependent mitochondrial apoptosis. Proc Natl Acad Sci U S A. 2009;106:19432–19437. doi: 10.1073/pnas.0908786106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Smith AR, Visioli F, Frei B, Hagen TM. Lipoic acid significantly restores, in rats, the age-related decline in vasomotion. Br J Pharmacol. 2008;153:1615–1622. doi: 10.1038/bjp.2008.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhao K, Zhao GM, Wu D, Soong Y, Birk AV, Schiller PW, Szeto HH. Cell-permeable peptide antioxidants targeted to inner mitochondrial membrane inhibit mitochondrial swelling, oxidative cell death, and reperfusion injury. J Biol Chem. 2004;279:34682–34690. doi: 10.1074/jbc.M402999200. [DOI] [PubMed] [Google Scholar]
- 98.Doughan AK, Dikalov SI. Mitochondrial redox cycling of mitoquinone leads to superoxide production and cellular apoptosis. Antioxid Redox Signal. 2007;9:1825–1836. doi: 10.1089/ars.2007.1693. [DOI] [PubMed] [Google Scholar]
- 99.Bakeeva LE, Barskov IV, Egorov MV, Isaev NK, Kapelko VI, Kazachenko AV, Kirpatovsky VI, Kozlovsky SV, Lakomkin VL, Levina SB, Pisarenko OI, Plotnikov EY, Saprunova VB, Serebryakova LI, Skulachev MV, Stelmashook EV, Studneva IM, Tskitishvili OV, Vasilyeva AK, Victorov IV, Zorov DB, Skulachev VP. Mitochondria-targeted plastoquinone derivatives as tools to interrupt execution of the aging program. 2. Treatment of some ros- and age-related diseases (heart arrhythmia, heart infarctions, kidney ischemia, and stroke). Biochemistry (Mosc) 2008;73:1288–1299. doi: 10.1134/s000629790812002x. [DOI] [PubMed] [Google Scholar]
- 100.Graham D, Huynh NN, Hamilton CA, Beattie E, Smith RA, Cocheme HM, Murphy MP, Dominiczak AF. Mitochondria-targeted antioxidant mitoq10 improves endothelial function and attenuates cardiac hypertrophy. Hypertension. 2009;54:322–328. doi: 10.1161/HYPERTENSIONAHA.109.130351. [DOI] [PubMed] [Google Scholar]
- 101.Szeto HH. Mitochondria-targeted cytoprotective peptides for ischemia-reperfusion injury. Antioxid Redox Signal. 2008;10:601–619. doi: 10.1089/ars.2007.1892. [DOI] [PubMed] [Google Scholar]
- 102.Petri S, Kiaei M, Damiano M, Hiller A, Wille E, Manfredi G, Calingasan NY, Szeto HH, Beal MF. Cell-permeable peptide antioxidants as a novel therapeutic approach in a mouse model of amyotrophic lateral sclerosis. J Neurochem. 2006;98:1141–1148. doi: 10.1111/j.1471-4159.2006.04018.x. [DOI] [PubMed] [Google Scholar]
- 103.Plecita-Hlavata L, Jezek J, Jezek P. Pro-oxidant mitochondrial matrix-targeted ubiquinone mitoq10 acts as anti-oxidant at retarded electron transport or proton pumping within complex i. Int J Biochem Cell Biol. 2009;41:1697–1707. doi: 10.1016/j.biocel.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 104.Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Small molecule activators of sirtuins extend saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 105.Cho S, Szeto HH, Kim E, Kim H, Tolhurst AT, Pinto JT. A novel cell-permeable antioxidant peptide, ss31, attenuates ischemic brain injury by down-regulating cd36. J Biol Chem. 2007;282:4634–4642. doi: 10.1074/jbc.M609388200. [DOI] [PubMed] [Google Scholar]
- 106.Dai DF, Johnson SC, Villarin JJ, Chin MT, Nieves-Cintron M, Chen T, Marcinek DJ, Dorn GW, 2nd, Kang YJ, Prolla TA, Santana LF, Rabinovitch PS. Mitochondrial oxidative stress mediates angiotensin ii-induced cardiac hypertrophy and g{alpha}q overexpression-induced heart failure. Circ Res. 2011 doi: 10.1161/CIRCRESAHA.110.232306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Anderson EJ, Lustig ME, Boyle KE, Woodlief TL, Kane DA, Lin CT, Price JW, 3rd, Kang L, Rabinovitch PS, Szeto HH, Houmard JA, Cortright RN, Wasserman DH, Neufer PD. Mitochondrial h2o2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest. 2009 doi: 10.1172/JCI37048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dai DF, Chen T, Szeto H, Nieves-Cintron M, Kutyavin V, Santana LF, Rabinovitch PS. Mitochondrial targeted antioxidant peptide ameliorates hypertensive cardiomyopathy. J Am Coll Cardiol. 2011;58:73–82. doi: 10.1016/j.jacc.2010.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lopez-Lluch G, Hunt N, Jones B, Zhu M, Jamieson H, Hilmer S, Cascajo MV, Allard J, Ingram DK, Navas P, de Cabo R. Calorie restriction induces mitochondrial biogenesis and bioenergetic efficiency. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:1768–1773. doi: 10.1073/pnas.0510452103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nisoli E, Tonello C, Cardile A, Cozzi V, Bracale R, Tedesco L, Falcone S, Valerio A, Cantoni O, Clementi E, Moncada S, Carruba MO. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of enos. Science. 2005;310:314–317. doi: 10.1126/science.1117728. [DOI] [PubMed] [Google Scholar]
- 111.Shinmura K, Tamaki K, Sano M, Nakashima-Kamimura N, Wolf AM, Amo T, Ohta S, Katsumata Y, Fukuda K, Ishiwata K, Suematsu M, Takeshi A. Caloric restriction primes mitochondria for ischemic stress by deacetylating specific mitochondrial proteins of the electron transport chain. Circ Res. 2011 doi: 10.1161/CIRCRESAHA.111.243097. [DOI] [PubMed] [Google Scholar]
- 112.Csiszar A, Labinskyy N, Jimenez R, Pinto JT, Ballabh P, Losonczy G, Pearson KJ, de Cabo R, Ungvari Z. Anti-oxidative and anti-inflammatory vasoprotective effects of caloric restriction in aging: Role of circulating factors and sirt1. Mech Ageing Dev. 2009;130:518–527. doi: 10.1016/j.mad.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cohen HY, Miller C, Bitterman KJ, Wall NR, Hekking B, Kessler B, Howitz KT, Gorospe M, de Cabo R, Sinclair DA. Calorie restriction promotes mammalian cell survival by inducing the sirt1 deacetylase. Science. 2004;305:390–392. doi: 10.1126/science.1099196. [DOI] [PubMed] [Google Scholar]
- 114.Stein S, Schafer N, Breitenstein A, Besler C, Winnik S, Lohmann C, Heinrich K, Brokopp CE, Handschin C, Landmesser U, Tanner FC, Luscher TF, Matter CM. Sirt1 reduces endothelial activation without affecting vascular function in apoe-/- mice. Aging (Albany NY) 2010;2:353–360. doi: 10.18632/aging.100162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mattagajasingh I, Kim CS, Naqvi A, Yamamori T, Hoffman TA, Jung SB, DeRicco J, Kasuno K, Irani K. Sirt1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:14855–14860. doi: 10.1073/pnas.0704329104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shinmura K, Tamaki K, Saito K, Nakano Y, Tobe T, Bolli R. Cardioprotective effects of short-term caloric restriction are mediated by adiponectin via activation of amp-activated protein kinase. Circulation. 2007;116:2809–2817. doi: 10.1161/CIRCULATIONAHA.107.725697. [DOI] [PubMed] [Google Scholar]
- 117.Pearson KJ, Lewis KN, Price NL, Chang JW, Perez E, Cascajo MV, Tamashiro KL, Poosala S, Csiszar A, Ungvari Z, Kensler TW, Yamamoto M, Egan JM, Longo DL, Ingram DK, Navas P, de Cabo R. Nrf2 mediates cancer protection but not prolongevity induced by caloric restriction. Proc Natl Acad Sci U S A. 2008;105:2325–2330. doi: 10.1073/pnas.0712162105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Someya S, Yu W, Hallows WC, Xu J, Vann JM, Leeuwenburgh C, Tanokura M, Denu JM, Prolla TA. Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell. 2010;143:802–812. doi: 10.1016/j.cell.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hafner AV, Dai J, Gomes AP, Xiao CY, Palmeira CM, Rosenzweig A, Sinclair DA. Regulation of the mptp by sirt3-mediated deacetylation of cypd at lysine 166 suppresses age-related cardiac hypertrophy. Aging (Albany NY) 2010;2:914–923. doi: 10.18632/aging.100252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ahn BH, Kim HS, Song S, Lee IH, Liu J, Vassilopoulos A, Deng CX, Finkel T. A role for the mitochondrial deacetylase sirt3 in regulating energy homeostasis. Proc Natl Acad Sci U S A. 2008;105:14447–14452. doi: 10.1073/pnas.0803790105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Barger JL, Kayo T, Vann JM, Arias EB, Wang J, Hacker TA, Wang Y, Raederstorff D, Morrow JD, Leeuwenburgh C, Allison DB, Saupe KW, Cartee GD, Weindruch R, Prolla TA. A low dose of dietary resveratrol partially mimics caloric restriction and retards aging parameters in mice. PLoS ONE. 2008;3:e2264. doi: 10.1371/journal.pone.0002264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Csiszar A, Labinskyy N, Pinto JT, Ballabh P, Zhang H, Losonczy G, Pearson K, de Cabo R, Pacher P, Zhang C, Ungvari Z. Resveratrol induces mitochondrial biogenesis in endothelial cells. Am J Physiol Heart Circ Physiol. 2009;297:H13–20. doi: 10.1152/ajpheart.00368.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ungvari Z, Labinskyy N, Mukhopadhyay P, Pinto JT, Bagi Z, Ballabh P, Zhang C, Pacher P, Csiszar A. Resveratrol attenuates mitochondrial oxidative stress in coronary arterial endothelial cells. Am J Physiol Heart Circ Physiol. 2009;297:H1876–1881. doi: 10.1152/ajpheart.00375.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ungvari Z, Bagi Z, Feher A, Recchia FA, Sonntag WE, Pearson K, de Cabo R, Csiszar A. Resveratrol confers endothelial protection via activation of the antioxidant transcription factor nrf2. Am J Physiol Heart Circ Physiol. 2010;299:H18–24. doi: 10.1152/ajpheart.00260.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating sirt1 and pgc-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 127.Funk JA, Odejinmi S, Schnellmann RG. Srt1720 induces mitochondrial biogenesis and rescues mitochondrial function after oxidant injury in renal proximal tubule cells. J Pharmacol Exp Ther. 2010;333:593–601. doi: 10.1124/jpet.109.161992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Minor RK, Baur JA, Gomes AP, Ward TM, Csiszar A, Mercken EM, Abdelmohsen K, Shin Y-K, Canto C, Scheibye-Knudsen M, Krawczyk M, Irusta PM, Martin-Montalvo A, Hubbard BP, Zhang Y, Lehrmann E, White AA, Price NL, Swindell WR, Pearson KJ, Becker KG, Bohr VA, Gorospe M, Egan JM, Talan MI, Auwerx J, Westphal CH, Ellis JL, Ungvari Z, Vlasuk GP, Elliott PJ, Sinclair DA, de Cabo R. Srt1720 improves survival and healthspan of obese mice. Sci. Rep. 2011;1 doi: 10.1038/srep00070. doi:10.1038/srep00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Quintero M, Colombo SL, Godfrey A, Moncada S. Mitochondria as signaling organelles in the vascular endothelium. Proc Natl Acad Sci U S A. 2006;103:5379–5384. doi: 10.1073/pnas.0601026103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J. Ampk regulates energy expenditure by modulating nad+ metabolism and sirt1 activity. Nature. 2009;458:1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Edwards AG, Donato AJ, Lesniewski LA, Gioscia RA, Seals DR, Moore RL. Life-long caloric restriction elicits pronounced protection of the aged myocardium: A role for ampk. Mech Ageing Dev. 2010;131:739–742. doi: 10.1016/j.mad.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zang M, Xu S, Maitland-Toolan KA, Zuccollo A, Hou X, Jiang B, Wierzbicki M, Verbeuren TJ, Cohen RA. Polyphenols stimulate amp-activated protein kinase, lower lipids, and inhibit accelerated atherosclerosis in diabetic ldl receptor-deficient mice. Diabetes. 2006;55:2180–2191. doi: 10.2337/db05-1188. [DOI] [PubMed] [Google Scholar]
- 133.Bhamra GS, Hausenloy DJ, Davidson SM, Carr RD, Paiva M, Wynne AM, Mocanu MM, Yellon DM. Metformin protects the ischemic heart by the akt-mediated inhibition of mitochondrial permeability transition pore opening. Basic Res Cardiol. 2008;103:274–284. doi: 10.1007/s00395-007-0691-y. [DOI] [PubMed] [Google Scholar]
- 134.Calvert JW, Gundewar S, Jha S, Greer JJ, Bestermann WH, Tian R, Lefer DJ. Acute metformin therapy confers cardioprotection against myocardial infarction via ampk-enos-mediated signaling. Diabetes. 2008;57:696–705. doi: 10.2337/db07-1098. [DOI] [PubMed] [Google Scholar]
- 135.Xie Z, Lau K, Eby B, Lozano P, He C, Pennington B, Li H, Rathi S, Dong Y, Tian R, Kem D, Zou MH. Improvement of cardiac functions by chronic metformin treatment is associated with enhanced cardiac autophagy in diabetic ove26 mice. Diabetes. 2011;60:1770–1778. doi: 10.2337/db10-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sasaki H, Asanuma H, Fujita M, Takahama H, Wakeno M, Ito S, Ogai A, Asakura M, Kim J, Minamino T, Takashima S, Sanada S, Sugimachi M, Komamura K, Mochizuki N, Kitakaze M. Metformin prevents progression of heart failure in dogs: Role of amp-activated protein kinase. Circulation. 2009;119:2568–2577. doi: 10.1161/CIRCULATIONAHA.108.798561. [DOI] [PubMed] [Google Scholar]
- 137.Gundewar S, Calvert JW, Jha S, Toedt-Pingel I, Ji SY, Nunez D, Ramachandran A, Anaya-Cisneros M, Tian R, Lefer DJ. Activation of amp-activated protein kinase by metformin improves left ventricular function and survival in heart failure. Circ Res. 2009;104:403–411. doi: 10.1161/CIRCRESAHA.108.190918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Turdi S, Fan X, Li J, Zhao J, Huff AF, Du M, Ren J. Amp-activated protein kinase deficiency exacerbates aging-induced myocardial contractile dysfunction. Aging Cell. 2010;9:592–606. doi: 10.1111/j.1474-9726.2010.00586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Smith DL, Jr., Elam CF, Jr., Mattison JA, Lane MA, Roth GS, Ingram DK, Allison DB. Metformin supplementation and life span in fischer-344 rats. J Gerontol A Biol Sci Med Sci. 2010;65:468–474. doi: 10.1093/gerona/glq033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Iida KT, Kawakami Y, Suzuki M, Shimano H, Toyoshima H, Sone H, Shimada K, Iwama Y, Watanabe Y, Mokuno H, Kamata K, Yamada N. Effect of thiazolidinediones and metformin on ldl oxidation and aortic endothelium relaxation in diabetic gk rats. Am J Physiol Endocrinol Metab. 2003;284:E1125–1130. doi: 10.1152/ajpendo.00430.2002. [DOI] [PubMed] [Google Scholar]
- 141.Judge S, Jang YM, Smith A, Selman C, Phillips T, Speakman JR, Hagen T, Leeuwenburgh C. Exercise by lifelong voluntary wheel running reduces subsarcolemmal and interfibrillar mitochondrial hydrogen peroxide production in the heart. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1564–1572. doi: 10.1152/ajpregu.00396.2005. [DOI] [PubMed] [Google Scholar]
- 142.Fujimoto N, Prasad A, Hastings JL, Arbab-Zadeh A, Bhella PS, Shibata S, Palmer D, Levine BD. Cardiovascular effects of 1 year of progressive and vigorous exercise training in previously sedentary individuals older than 65 years of age. Circulation. 2010;122:1797–1805. doi: 10.1161/CIRCULATIONAHA.110.973784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Iwatsubo K, Minamisawa S, Tsunematsu T, Nakagome M, Toya Y, Tomlinson JE, Umemura S, Scarborough RM, Levy DE, Ishikawa Y. Direct inhibition of type 5 adenylyl cyclase prevents myocardial apoptosis without functional deterioration. J Biol Chem. 2004;279:40938–40945. doi: 10.1074/jbc.M314238200. [DOI] [PubMed] [Google Scholar]
- 144.Treuting PM, Linford NJ, Knoblaugh SE, Emond MJ, Morton JF, Martin GM, Rabinovitch PS, Ladiges WC. Reduction of age-associated pathology in old mice by overexpression of catalase in mitochondria. J Gerontol A Biol Sci Med Sci. 2008;63:813–822. doi: 10.1093/gerona/63.8.813. [DOI] [PubMed] [Google Scholar]
- 145.Alcendor RR, Gao S, Zhai P, Zablocki D, Holle E, Yu X, Tian B, Wagner T, Vatner SF, Sadoshima J. Sirt1 regulates aging and resistance to oxidative stress in the heart. Circulation research. 2007;100:1512–1521. doi: 10.1161/01.RES.0000267723.65696.4a. [DOI] [PubMed] [Google Scholar]
- 146.Hsu CP, Zhai P, Yamamoto T, Maejima Y, Matsushima S, Hariharan N, Shao D, Takagi H, Oka S, Sadoshima J. Silent information regulator 1 protects the heart from ischemia/reperfusion. Circulation. 2010;122:2170–2182. doi: 10.1161/CIRCULATIONAHA.110.958033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Li J, Ichikawa T, Villacorta L, Janicki JS, Brower GL, Yamamoto M, Cui T. Nrf2 protects against maladaptive cardiac responses to hemodynamic stress. Arterioscler Thromb Vasc Biol. 2009;29:1843–1850. doi: 10.1161/ATVBAHA.109.189480. [DOI] [PubMed] [Google Scholar]
- 148.Ungvari ZI, Bailey-Downs L, Gautam T, Jimenez R, Losonczy G, Zhang C, Ballabh P, Recchia FA, Wilkerson DC, Sonntag WE, Pearson KJ, de Cabo R, Csiszar A. Adaptive induction of nf-e2-related factor-2-driven antioxidant genes in endothelial cells in response to hyperglycemia. Am J Physiol Heart Circ Physiol. 2011;300:H1133–1140. doi: 10.1152/ajpheart.00402.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Li Q, Wu S, Li SY, Lopez FL, Du M, Kajstura J, Anversa P, Ren J. Cardiac-specific overexpression of insulin-like growth factor 1 attenuates aging-associated cardiac diastolic contractile dysfunction and protein damage. American journal of physiology. 2007;292:H1398–1403. doi: 10.1152/ajpheart.01036.2006. [DOI] [PubMed] [Google Scholar]
- 150.Ungvari Z, Gautam T, Koncz P, Henthorn JC, Pinto JT, Ballabh P, Yan H, Mitschelen M, Farley J, Sonntag WE, Csiszar A. Vasoprotective effects of life span-extending peripubertal gh replacement in lewis dwarf rats. J Gerontol A Biol Sci Med Sci. 2010;65:1145–1156. doi: 10.1093/gerona/glq147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sonntag WE, Carter CS, Ikeno Y, Ekenstedt K, Carlson CS, Loeser RF, Chakrabarty S, Lee S, Bennett C, Ingram R, Moore T, Ramsey M. Adult-onset growth hormone and insulin-like growth factor i deficiency reduces neoplastic disease, modifies age-related pathology, and increases life span. Endocrinology. 2005;146:2920–2932. doi: 10.1210/en.2005-0058. [DOI] [PubMed] [Google Scholar]
- 152.Bailey-Downs LC, Mitschelen M, Sosnowska D, Toth P, Pinto JT, Ballabh P, Valcarcel-Ares MN, Farley J, Koller A, Henthorn JC, Bass C, Sonntag WE, Ungvari Z, Csiszar A. Liver-specific knockdown of igf-1 decreases vascular oxidative stress resistance by impairing the nrf2-dependent antioxidant response: A novel model of vascular aging. J Gerontol Biol Med Sci. 2011 doi: 10.1093/gerona/glr164. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Csiszar A, Labinskyy N, Pinto JT, Ballabh P, Zhang H, Losonczy G, Pearson KJ, de Cabo R, Pacher P, Zhang C, Ungvari ZI. Resveratrol induces mitochondrial biogenesis in endothelial cells. Am J Physiol Heart Circ Physiol. 2009 doi: 10.1152/ajpheart.00368.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Csiszar A, Smith K, Labinskyy N, Orosz Z, Rivera A, Ungvari Z. Resveratrol attenuates tnf-{alpha}-induced activation of coronary arterial endothelial cells: Role of nf-{kappa}b inhibition. Am J Physiol. 2006;291:H1694–1699. doi: 10.1152/ajpheart.00340.2006. [DOI] [PubMed] [Google Scholar]
- 155.Zhang H, Morgan B, Potter BJ, Ma L, Dellsperger KC, Ungvari ZI, Zhang C. Resveratrol improves left ventricular diastolic relaxation in type 2 diabetes by inhibiting oxidative/nitrative stress. Am J Physiol Heart Circ Physiol. 2010;299:H985–994. doi: 10.1152/ajpheart.00489.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zhang H, Zhang J, Ungvari Z, Zhang C. Resveratrol improves endothelial function: Role of tnf{alpha} and vascular oxidative stress. Arterioscler Thromb Vasc Biol. 2009;29:1164–1171. doi: 10.1161/ATVBAHA.109.187146. [DOI] [PMC free article] [PubMed] [Google Scholar]