Abstract
Fetal life is a critical time for female fertility, when germ cells complete proliferation, initiate meiosis and ultimately form the lifetime stock of primordial follicles. Female fertility may be reduced by in utero exposure to cigarette smoke, which contains ligands for the aryl hydrocarbon receptor (AhR). The AhR is a critical regulator of ovarian germ cell survival in mice; thus activation of this receptor in the ovaries of fetuses exposed to maternal cigarette smoke in utero may provide a mechanism by which female fertility is reduced in later life. We have therefore investigated AhR expression in the human fetal ovary, and examined the effects of an AhR ligand present in cigarette smoke, on germ cells in human fetal ovaries cultured in vitro. The results showed that AHR mRNA expression increased 2-fold between first and late second trimester (P = 0.008). AhR protein was confined to germ cells at all gestations, but varied from expression in most germ cells during the first trimester, to only patchy expression by clusters of germ cells at later gestations. Culture of human fetal ovaries with the AhR ligand 9,10-dimethyl-1,2-benzanthracene-3,4-dihydrodiol (DMBA-DHD; a component of cigarette smoke) did not affect germ cell number in vitro, but significantly reduced the proportion of proliferating germ cells by 29% (as assessed by phospho-histone H3 staining (P = 0.04)). Germ cell apoptosis was not significantly affected. These results reveal that germ cells in the human fetal ovary express AhR from the proliferative stage of development through entry into meiosis and beyond, and demonstrate that AhR ligands found in cigarette smoke have the capacity to impair human fetal ovarian germ cell proliferation.
Keywords: germ cell, smoking, fertility, oogenesis, ovary
Introduction
Germ cell development in the human fetal ovary results in the formation of the finite primordial follicle pool that is the ultimate determinant of female fertility and reproductive lifespan (Maheshwari and Fowler, 2008; Tingen et al., 2009). Following migration of primordial germ cells to the gonadal ridge, the key stages are germ cell proliferation, entry into meiosis with subsequent meiotic arrest and association with somatic cells to form primordial follicles (Byskov, 1986; Pepling and Spradling, 2001). The first germ cells enter meiosis in the third month of fetal development with primordial follicles present from ∼18 weeks of gestation (equal to 16 weeks post conception) (Baker, 1963; Kurilo, 1981; Gondos et al., 1986; Sforza et al., 2003; Bendsen et al., 2006). In the human fetal ovary, germ cell proliferation continues long after some cells have entered meiosis, such that during the second trimester of pregnancy a developmental gradient is established across the ovary with less mature and mitotic germ cells present around the periphery of the ovary, with those at increasing stages of maturity towards the centre where the first primordial follicles are formed (Fulton et al., 2005; Stoop et al., 2005; Anderson et al., 2007; Childs et al., 2012).
As entry to meiosis precludes further expansion of the germ cell pool by mitosis, generating an adequate germ cell number prior to meiosis is a key step in establishing female fertility. In addition to intrinsic genetic variability, this process is potentially vulnerable to external influence, and there are increasing data regarding the adverse effects of a range of chemicals on ovarian development in humans as well as other species (Susiarjo et al., 2007; Fowler et al., 2008; Allard and Colaiacovo, 2010; Brieno-Enriquez et al., 2011; Hunt et al., 2012). Cigarette smoking is well recognized to have a deleterious effect on the fertility of both men and women (Vine et al., 1994; Ramlau-Hansen et al., 2007; Dechanet et al., 2011) and may also affect fetal androgen exposure (Fowler et al., 2011). Smoking advances the age of the menopause (Gold et al., 2013), and in utero exposure of human female fetuses to cigarette smoke has been associated with decreased numbers of germ cells and somatic cells in the developing ovary (Lutterodt et al., 2009; Mamsen et al., 2010), and reduced adult female fertility (Jensen et al., 1998, 2006; Ye et al., 2010).
The chemicals in cigarette smoke include polycyclic aromatic hydrocarbons (PAHs), which are ligands for the aryl hydrocarbon receptor (AhR), a transcription factor that mediates the cellular response to a broad range of xenobiotic molecules with adverse effects on female reproduction (Pocar et al., 2005; Hernandez-Ochoa et al., 2009). We have previously demonstrated that human germ cells in the male express the AhR, and that its activation in vitro induces germ cell apoptosis (Coutts et al., 2007). In the fetal mouse ovary, AhR activation results in germ cell apoptosis (Matikainen et al., 2002) and also results in the loss of more mature oocytes in both mouse and human (Matikainen et al., 2001). Consistent with this, Ahr−/− mice have increased numbers of ovarian follicles in the early post-natal period (Benedict et al., 2000; Robles et al., 2000). In the present study we have explored the expression of the AhR in the human fetal ovary and investigated the effect of an AhR ligand on germ cell proliferation and apoptosis, to explore a mechanism whereby cigarette smoke PAHs might impact on female reproductive potential.
Methods
Tissue
Human fetal ovaries were obtained following medical termination of pregnancy during both the first and second trimesters (7–20 weeks of gestational age). Women gave consent according to national guidelines and the study was approved by the Lothian Research Ethics Committee (REC 08/S1101/1). Termination of pregnancy was induced by treatment with mifepristone (200 mg orally) followed 48 h later by misoprostol (800 μg) three hourly per vaginum. None of the terminations were for reasons of fetal abnormality, and all fetuses appeared morphologically normal. Gestational age was determined by ultrasound examination before termination and confirmed by subsequent direct measurement of foot length. Sex of first trimester specimens was determined by PCR genotyping for the SRY gene (primers: Fwd: 5′-ACAGTAAAGGCAACGTCCAG-3′, Rev: 5′-ATCTGCGGGAAGCAAACTGC-3′ (Friel et al., 2002)). Ovaries were dissected and either snap frozen and stored at −70°C, fixed in Bouin's for 2 h, followed by processing for immunohistochemistry or immunofluorescence, or cultured in vitro as detailed below. Extra-ovarian tissue was dissected from ovaries to be fixed or frozen, but the mesonephros was left attached to samples used in culture experiments.
Quantitative PCR
For quantification of AHR and aryl hydrocarbon receptor nuclear translocator (ARNT) transcript levels, total RNA was extracted from frozen human fetal ovaries using the RNeasy Mini/Micro Kit (Qiagen, Crawley, UK) with on-column DNaseI digestion, and cDNA synthesized using the Superscript VILO cDNA synthesis kit (Applied Biosystems, Paisley, UK), with duplicate cDNA reactions in which the reverse transcriptase enzyme was omitted prepared as no-template controls for qPCR. qPCR was performed using an ABI HT7900 real-time PCR instrument (Applied Biosystems) and Power SYBR Green PCR Master Mix (Applied Biosystems). Calculations of mRNA concentrations were made relative to the housekeeping gene RPL32, to allow comparisons between cDNAs. Sequences of the oligonucleotide primers used in qPCR are as follows: AHR Fwd: 5′-ATACTGAAACAGAGCTGTGC-3′, Rev: 5′- AAAGCAGGCGTGCATTAGAC-3′ (Ikuta and Kawajiri, 2006); ARNT Fwd: 5′-GCTGCTGCCTACCCTAGTCTCA-3′, Rev: 5′-GCTGCTCGTGTCTGGAATTGT-3′ (Ginis et al., 2004); RPL32 Fwd: 5′-CATCTCCTTCTCGGCATCA-3′, Rev: 5′-AACCCTGTTGTCAATGCCTC-3′.
Immunofluorescence
Paraffin-embedded ovaries were cut into 5 µm sections and mounted onto electrostatically charged microscope slides (VWR, Poole, UK), dried overnight, and then dewaxed and rehydrated using conventional methods. Endogenous peroxidases were quenched in 3% hydrogen peroxide in methanol for 30 min (min) at room temperature. After a wash in water, slides were transferred into phospho-buffered saline (PBS) (Sigma-Aldrich, Poole, UK) for 5 min and blocked for 30 min in normal serum (Diagnostics Scotland, Carluke, UK) diluted 1:4 in PBS containing 5% bovine serum albumin (BSA). Sections were blocked with avidin (0.01M; 15 min) and then biotin (0.001M; 15 min; both from Vector Laboratories, Peterborough, UK) with washes in PBS in between. AHR antibody (Affinity BioReagents/Thermo Fisher Scientific, Cramlington, UK) was diluted 1:150 and applied to sections at 4°C overnight in a humidified chamber. AHR was visualized by tyramide-enhanced fluorescein via an HRP conjugated goat anti-mouse secondary antibody diluted 1:200. Sections were counterstained with propidium iodide 1:1000. Fluorescent images were captured using a LSM510 confocal microscope. Negative controls incubated with mouse IgG, omitting primary antisera, were included in all runs and showed no positive immunostaining.
Culture of fetal ovaries
Human fetal ovary-mesonephros complexes (8–9 weeks of gestational age) were cultured as previously described (Childs et al., 2010) on cell culture inserts (Greiner Bio-One, Stonehouse, UK) in serum free medium (αMEM + GlutaMAX with 1X nonessential amino acids (Applied Biosystems); 2 mM sodium pyruvate and 3 mg/ml BSA Fraction V (both from Sigma-Aldrich); and penicillin/streptomycin/amphotericin B (Cambrex Biosciences, MD, USA)) in the presence of a final concentration of 0.01% dimethyl sulfoxide (DMSO; Sigma-Aldrich) or the AHR ligand 9,10-dimethyl-1,2-benzanthracene-3,4-dihydrodiol (DMBA-DHD (an active metabolite of DMBA); 1μM in DMSO; NCI Chemical Carcinogen Reference Standards Repository, MO, USA) for 7 days in a humidified incubator (37°C, 5% CO2) to determine effects on PGC number, proliferation and apoptosis. Paired ovaries were used for control and treatment. A complete medium change was performed every 48 h. After culture, tissues were fixed in Bouin's solution and processed into paraffin for histological assessment.
Immunohistochemical determination of germ cell number, proliferation and apoptosis
Immunohistochemistry was performed to estimate total germ cell number (Activator Protein-2gamma; AP-2γ), germ cell proliferation (phosphorylated histone-H3 (phospho-H3)) and apoptosis (cleaved caspase 3) on adjacent serial sections every fifth section as previously described (Martins da Silva et al., 2004; Childs et al., 2010). Slides were incubated in primary antibody (rabbit polyclonal antibodies to AP-2γ; Santa Cruz Biotechnology, CA, USA; #sc-8977), and cleaved caspase 3 (New England Biolabs, Hitchin, UK; #9601), both diluted 1:100 in Tris Buffered Saline (TBS) supplemented with 20% normal goat serum (NGS) and 5% BSA, at 4°C overnight. Primary antibodies were detected using a biotinylated goat anti-rabbit secondary antibody (Dako, Cambridge, UK), diluted 1:500 in TBS/NGS/BSA and incubated for 1 h at room temperature. Staining was visualized using streptavidin-horseradish peroxidase (diluted 1:1000 in TBS) followed by 3,3′-diaminobenzidine tetrahydrochloride (DAB; Dako). Immunohistochemical detection of phospho-H3 was performed on an automated Bond Immunostaining Robot using a rabbit polyclonal to phospho-H3 (Upstate Biotechnology, Milton Keynes, UK, #06-570) as the primary antibody, with secondary antibody and detection as above. Images were captured using an Olympus Provis microscope (Olympus, London, UK). PGC counts and determination of areas were determined using a Zeiss Axio Imager A1 microscope (Carl Zeiss) fitted with a camera and automatic stage (Prior Scientific Instruments Ltd, Cambridge, UK) with Image Pro Plus software 4.5.1 with Stereologer Pro 5 software (Media Cybernetics, Workingham, UK). Germ cell numbers were counted using the point-counting tool, and ovarian areas calculated using the freehand draw tool to outline the edge of the tissue section.
Statistical analysis
Data are presented as mean ± SEM. Gene expression across gestation was analysed by ANOVA. Tissue culture experiments were analysed by paired t-test or Wilcoxon tests for data expressed as percentages, as the experimental design involved comparison of treatment effects on paired gonads from each fetal specimen.
Results
AHR gene expression is up-regulated during human fetal ovarian development
Expression of AHR mRNA was detected in human fetal ovaries at all gestations by qPCR. AHR transcript levels increased with gestation, rising 2-fold between the first trimester (8–9 weeks of gestation) and late second trimester (17–20 weeks of gestation; P = 0.008, n = 5–6 per group; Fig. 1A). Expression of ARNT, which encodes the Aryl Hydrocarbon Nuclear Translocator required for AhR transcriptional activity, was unchanged across this period (Fig. 1B).
Figure 1.
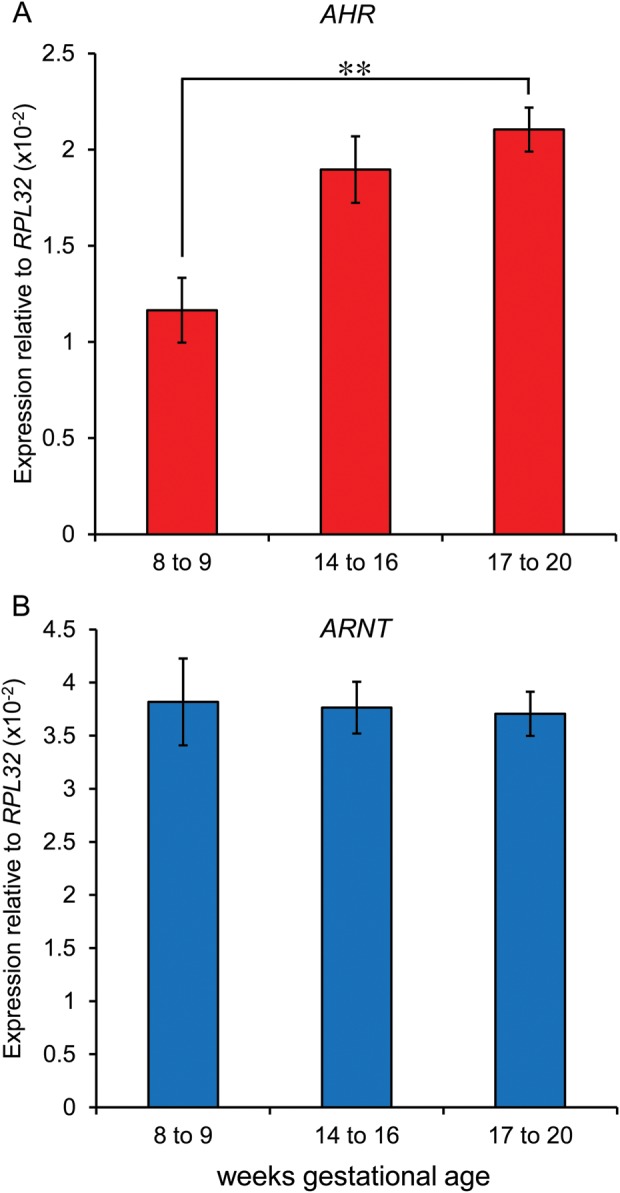
Expression of the aryl hydrocarbon receptor AHR (A) increases with gestation (P = 0.008), but ARNT (aryl hydrocarbon translocator, an AhR co-factor) (B) was unchanged (n = 5–6 ovaries per group).
AhR protein is expressed exclusively by germ cells in the human fetal ovary
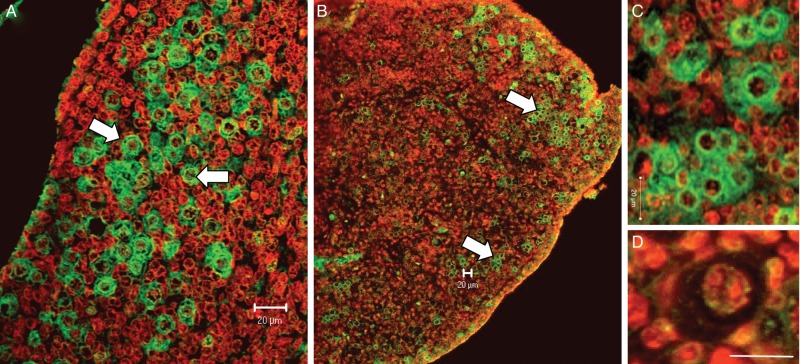
AhR was detected in human fetal ovaries in all specimens across the gestational range examined. At all stages of development, AhR expression was exclusively confined to germ cells. In the first trimester, AhR was expressed by all germ cells (Fig. 2A), whereas in the second trimester AhR was expressed by clusters of germ cells with others not showing expression (Fig. 2B and C). AhR-expressing germ cells were predominantly around the periphery of the ovary (i.e. in less mature germ cells) but scattered clusters of immunopositive germ cells were detected throughout the ovary (Fig. 2B). Oocytes within primordial follicles (Fig. 2D) showed weak/no immunostaining.
Figure 2.
In the first trimester (A, 7 weeks of gestation), AhR was expressed by all germ cells (arrows) with no expression in somatic cells. At later gestations (B, 19 weeks and C, 18 weeks), AhR expression remained confined to germ cells in clusters, predominantly but not exclusively localized to the more peripheral regions of the ovary (arrows). AhR expression was low/absent in primordial follicles (D, 19 weeks). All scale bars, 20 μm.
The AhR ligand DMBA-DHD reduces germ cell proliferation in the human fetal ovary in vitro
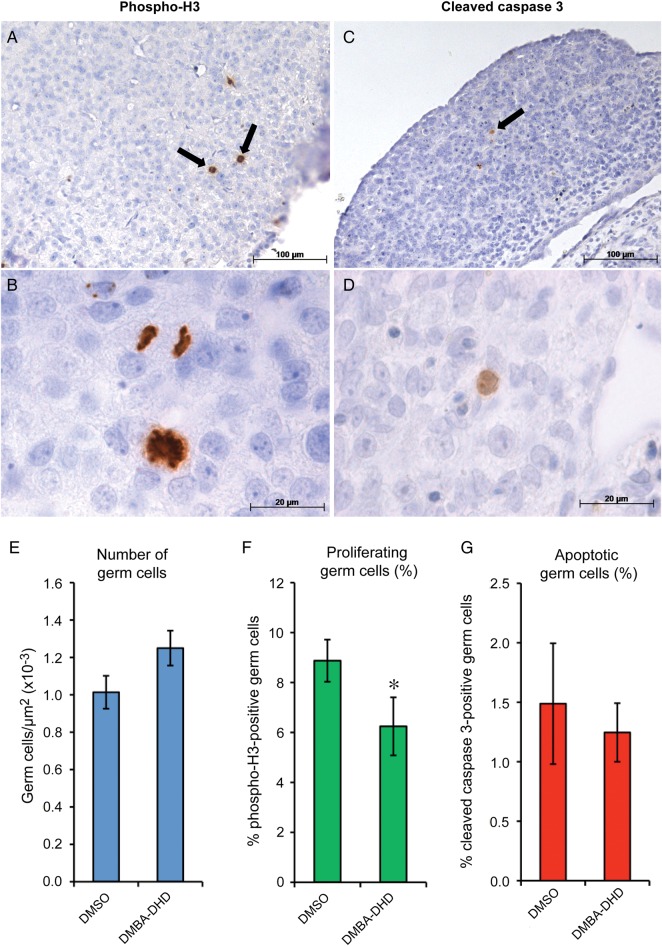
To establish the effect of AhR activation on human fetal germ cell behaviour, first trimester human fetal ovaries were maintained in vitro for 7 days in the presence of vehicle (0.01% DMSO) or the AhR agonist DMBA-DHD (1 µM), before histological assessment of germ cell number, proliferation and apoptosis. First trimester samples were used for this part of the study since (i) all germ cells expressed AhR at this stage, (ii) germ cells at this stage are less heterogeneous than at later stages of development and (iii) first trimester human fetal ovaries can be maintained in culture for at least 7 days, which we have previously demonstrated to be a sufficient period to analyse changes in germ cell number, proliferation or apoptosis in response to external stimuli (Childs et al., 2010). Ovarian tissue showed well-preserved morphology after 7 days in culture, with ongoing germ cell mitosis detected (as determined by phospho-H3 immunostaining; Fig. 3A). Germ cell number (determined by quantifying the number of AP-2γ-positive cells in sections (Childs et al., 2010)) was not affected by treatment with DMBA-DHD (1.01 ± 0.08 in vehicle controls versus 1.25 ± 0.09 × 10−4/µm2 in DMBA-DHD treated; Fig. 3C); however, exposure to DMBA-DHD did reduce the proportion of proliferating (phospho-H3-positive) germ cells by ∼30% (8.9 ± 0.8% in controls versus 6.3 ± 1.2% in DMBA-DHD treated; P = 0.04, n = 4; Fig. 3F). Apoptotic (cleaved caspase 3-positive) germ cells were rare (Fig. 3C), and the proportion of apoptotic germ cells was not affected by exposure to DMBA-DHD (1.49 ± 5.1% control versus 1.25 ± 2.5% treated, ns; Fig. 3G).
Figure 3.
In vitro exposure of human fetal ovaries (8–9 weeks of gestation) to an AhR ligand reduces germ cell proliferation. Representative images of human fetal ovaries cultured for 7 days and immunostained for phosphorylated histone H3 (A and B) and cleaved caspase 3 (C and D) indicating mitotic proliferation and apoptosis, respectively (arrows indicate immunostained cells in (A) and (C)). Exposure of first trimester fetal ovaries to the AhR ligand DMBA-DHD (1 µM) did not affect germ cell number (E), but significantly reduced human fetal ovarian germ cell proliferation relative to vehicle (DMSO) controls (F; quantified by detection of phospho-H3). Germ cell apoptosis (assessed by caspase 3 immunostaining) was not affected by DMBA-DHD treatment (G). Data are mean ± SEM of 4 independent experiments.
Discussion
These data demonstrate that germ cells in the human fetal ovary are a site of expression of the AhR, and that expression of the AhR is developmentally regulated at the gene, protein and cellular level. In the first trimester the great majority of germ cells express the AhR, whereas in the second trimester, after the onset of meiosis, AhR expression was more restricted, with AhR detected in clusters of germ cells, while others showed no expression. There was a modest increase in AHR mRNA expression with increasing gestation, interpretation of which is complicated by the changing cellular constituents of the ovary. There was no change in the expression of ARNT, which encodes the aryl hydrocarbon translocator, an AhR co-factor. Importantly, we have shown for the first time that functional activation of the AhR by a PAH found in cigarette smoke reduced germ cell proliferation in the first trimester human fetal ovary, but did not affect germ cell apoptosis. Collectively, these data provide a mechanism whereby in utero exposure to AhR ligands, as found for example in cigarette smoke and other products of combustion, may influence germ cell proliferation in the ovary and potentially impact on later female reproductive function. This may therefore at least in part contribute to the observed reduced fertility in women exposed to cigarettes prenatally (Jensen et al., 1998; Ye et al., 2010).
The AhR was expressed in germ cells, but not in other cell types in the fetal ovary across the gestational range examined. This pattern of expression is similar to that we previously reported in the fetal testes (Coutts et al., 2007), as is the finding that AhR expression becomes restricted to specific populations of germ cells in the fetal ovary with increasing developmental age. The progressive restriction of AhR expression to a subset of germ cells in the second trimester human fetal ovary implies only a certain stage or stages of germ cell development are associated with AhR expression, following the initiation of meiosis from 11 weeks of gestation onwards. The functional significance of this is unclear, but it may be of relevance that the AhR has been associated with regulation of the cell cycle (Denison and Heath-Pagliuso, 1998). In keeping with this, our functional data indicate that treatment of first trimester fetal ovary with a specific AhR ligand significantly reduced germ cell proliferation, as detected by expression of phosphorylated histone H3. The importance of germ cell proliferation prior to meiotic entry is indicated by the phenotype of mice deficient for Pin1, a regulator of the rate of mitosis, the absence of which results in markedly reduced primordial follicle numbers (Atchison et al., 2003). We were unable to detect a significant reduction in the number of germ cells in the fetal ovary in response to DMBA-DHD. The doubling time of the human fetal ovarian germ cell population has been estimated at ∼6 days (Bendsen et al., 2006); thus it is likely that the reduction in germ cell proliferation observed here is too small to effect a change in germ cell number of sufficient magnitude to be detected within the short (7-day) period of culture, although this method was able to detect changes in germ cell number associated with increased apoptosis (Childs et al., 2010). Fetuses in utero are likely to be exposed chronically to cigarette smoke over a period of weeks or months, which may be long enough for an effect on germ cell proliferation to become manifest as a reduction in germ cell number, and thus reduced adult fertility.
There was no change in the proportion of germ cells undergoing apoptosis, as indicated by detection of cleaved caspase 3. This result therefore differs from our findings in the fetal testes where AhR activation resulted in an increase in germ cell apoptosis (Coutts et al., 2007). Human embryonic stem cells induced to differentiate towards the germ cell lineage also express the AhR, and are sensitive to PAHs (Kee et al., 2010). In that model, DMBA-DHD resulted in reduced expression of primordial germ cell genes, and increased apoptosis, although the suitability of the ES cell system as a model for human ovarian germ cell development in vivo remains to be determined. Female mice exposed in utero to the AhR ligand benzo(a)pyrene have reduced fertility (MacKenzie and Angevine, 1981), and exposure to dioxin (2,3,7,8-tetrachlorodibenzo-p-dioxin, TCDD), also an AhR ligand, has diverse adverse effects on the developing reproductive tract (Wolf et al., 1999; Bruner-Tran and Osteen, 2011). In vitro studies suggest that PAH exposure resulted in increased germ cell apoptosis in the mouse fetal ovary (Matikainen et al., 2002). This effect on apoptosis therefore differs from the results presented here, possibly reflecting different stages of development of the germ cells exposed to the PAH. Germ cell apoptosis is infrequent in the first trimester human ovary, but is thought to be an important part of germ cell selection at later stages before primordial follicle formation, a hypothesis consistent with the marked increase in the number of apoptotic germ cells observed in the late second trimester human fetal ovary (Fulton et al., 2005). The fetal mouse germ cells exposed to PAH by Matikainen et al were at embryonic day 13.5, coincident with the onset of meiosis in the fetal mouse ovary. Exposure of meiotic germ cells in second trimester human fetal ovary to an AhR ligand might induce germ cell apoptosis, in contrast to the phenotype of reduced proliferation we see in response to DMBA-DHD treatment of first trimester human fetal ovaries. Interestingly, female mice with targeted disruptions of the Ahr gene display increased numbers of primordial follicles in the early post-natal period (Benedict et al., 2000; Robles et al., 2000). This suggests that activation of the AhR by as-yet-unidentified endogenous ligands in the fetal ovary may contribute to the process of widespread germ cell death that occurs during fetal oogenesis under normal physiological conditions.
The results presented here are consistent with a previous report of reduced numbers of germ cells in the ovaries of fetuses of women who smoked (Mamsen et al., 2010), and indicate that this effect may be mediated by direct effects of PAHs in cigarette smoke on the fetal ovary. Smoking was also associated with a reduced number of somatic cells in the ovary (Mamsen et al., 2010), and while we found no evidence in the present study that somatic cells expressed the AHR, the close inter-dependency of the two cell types, and extensive bidirectional signalling between them (Robinson et al., 2001; Martins da Silva et al., 2004; Coutts et al., 2008; Childs and Anderson, 2009), makes a secondary, germ cell-mediated effect on the development of ovarian somatic cells very plausible.
In summary, these data provide a functional basis for an adverse effect of in utero exposure to AhR ligands including many that are found in cigarette smoke, providing a mechanism for observational studies that have examined the gonads of smoke exposed fetuses (Lutterodt et al., 2009; Mamsen et al., 2010), and epidemiological studies on the subsequent fertility of such individuals (Jensen et al., 1998, 2006; Ye et al., 2010). Together with substantial experimental and epidemiological evidence for an adverse effect of smoking exposure in utero on male reproductive function (Jensen et al., 2004; Coutts et al., 2007; Ramlau-Hansen et al., 2007) these data highlight the vulnerability of fetal germ cells of both females and males to adverse environmental influences in utero.
Authors' roles
R.A.A.: study conception and design, analysis, preparation of the manuscript, revision and final approval; L.M.: acquisition of data, analysis, revision and approval of the manuscript; S.C.: acquisition of data, analysis, revision and approval of the manuscript; H.L.K.: acquisition of data, analysis, revision and approval of the manuscript; P.A.F.: interpretation of data, revision and approval of the manuscript; A.J.C.: study conception and design, analysis, revision and approval of the manuscript.
Funding
This work was funded by the Medical Research Council (G1100357). We are grateful to Anne Saunderson, Joan Creiger and the staff of the Bruntsfield Suite, Royal Infirmary of Edinburgh, for their considerable assistance in patient recruitment. Funding to pay the Open Access publication charges for this article was provided by MRC grant G1100357.
Conflict of interest
None declared.
References
- Allard P, Colaiacovo MP. Bisphenol A impairs the double-strand break repair machinery in the germline and causes chromosome abnormalities. Proc Natl Acad Sci USA. 2010;107:20405–20410. doi: 10.1073/pnas.1010386107. doi:10.1073/pnas.1010386107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RA, Fulton N, Cowan G, Coutts S, Saunders PTK. Conserved and divergent patterns of gene expression in female and male germ cells during development of the human fetal gonad. BMC Dev Biol. 2007;7:136–145. doi: 10.1186/1471-213X-7-136. doi:10.1186/1471-213X-7-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atchison FW, Capel B, Means AR. Pin1 regulates the timing of mammalian primordial germ cell proliferation. Development. 2003;130:3579–3586. doi: 10.1242/dev.00584. doi:10.1242/dev.00584. [DOI] [PubMed] [Google Scholar]
- Baker TG. A quantitative and cytological study of germ cells in human ovaries. Proc R Soc Lond B Biol Sci. 1963;158:417–433. doi: 10.1098/rspb.1963.0055. doi:10.1098/rspb.1963.0055. [DOI] [PubMed] [Google Scholar]
- Bendsen E, Byskov AG, Andersen CY, Westergaard LG. Number of germ cells and somatic cells in human fetal ovaries during the first weeks after sex differentiation. Hum Reprod. 2006;21:30–35. doi: 10.1093/humrep/dei280. doi:10.1093/humrep/dei280. [DOI] [PubMed] [Google Scholar]
- Benedict JC, Lin TM, Loeffler IK, Peterson RE, Flaws JA. Physiological role of the aryl hydrocarbon receptor in mouse ovary development. Toxicol Sci. 2000;56:382–388. doi: 10.1093/toxsci/56.2.382. doi:10.1093/toxsci/56.2.382. [DOI] [PubMed] [Google Scholar]
- Brieno-Enriquez MA, Reig R, Cabero L, Toran N, Martinez F, Roig I, Garcia Caldes M. Gene expression is altered after Bisphenol A exposure in human fetal oocytes in vitro. Mol Hum Reprod. 2011;18:171–183. doi: 10.1093/molehr/gar074. doi:10.1093/molehr/gar074. [DOI] [PubMed] [Google Scholar]
- Bruner-Tran KL, Osteen KG. Developmental exposure to TCDD reduces fertility and negatively affects pregnancy outcomes across multiple generations. Reprod Toxicol. 2011;31:344–350. doi: 10.1016/j.reprotox.2010.10.003. doi:10.1016/j.reprotox.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byskov AG. Differentiation of mammalian embryonic gonad. Physiol Rev. 1986;66:71–117. doi: 10.1152/physrev.1986.66.1.71. [DOI] [PubMed] [Google Scholar]
- Childs AJ, Anderson RA. Activin A selectively represses expression of the membrane-bound isoform of Kit ligand in human fetal ovary. Fertil Steril. 2009;92:1416–1419. doi: 10.1016/j.fertnstert.2009.03.095. doi:10.1016/j.fertnstert.2009.03.095. [DOI] [PubMed] [Google Scholar]
- Childs AJ, Kinnell HL, Collins CS, Hogg K, Bayne RA, Green SJ, McNeilly AS, Anderson RA. BMP Signaling in the human fetal ovary is developmentally regulated and promotes primordial germ cell apoptosis. Stem Cells. 2010;28:1368–1378. doi: 10.1002/stem.440. doi:10.1002/stem.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs AJ, Kinnell HL, He J, Anderson RA. LIN28 is selectively expressed by primordial and pre-meiotic germ cells in the human fetal ovary. Stem Cells Dev. 2012;21:2343–2349. doi: 10.1089/scd.2011.0730. doi:10.1089/scd.2011.0730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutts SM, Fulton N, Anderson RA. Environmental toxicant-induced germ cell apoptosis in the human fetal testis. Hum Reprod. 2007;22:2912–2918. doi: 10.1093/humrep/dem300. doi:10.1093/humrep/dem300. [DOI] [PubMed] [Google Scholar]
- Coutts SM, Childs AJ, Fulton N, Collins C, Bayne RA, McNeilly AS, Anderson RA. Activin signals via SMAD2/3 between germ and somatic cells in the human fetal ovary and regulates kit ligand expression. Dev Biol. 2008;314:189–199. doi: 10.1016/j.ydbio.2007.11.026. doi:10.1016/j.ydbio.2007.11.026. [DOI] [PubMed] [Google Scholar]
- Dechanet C, Anahory T, Mathieu Daude JC, Quantin X, Reyftmann L, Hamamah S, Hedon B, Dechaud H. Effects of cigarette smoking on reproduction. Hum Reprod Update. 2011;17:76–95. doi: 10.1093/humupd/dmq033. doi:10.1093/humupd/dmq033. [DOI] [PubMed] [Google Scholar]
- Denison MS, Heath-Pagliuso S. The Ah receptor: a regulator of the biochemical and toxicological actions of structurally diverse chemicals. Bull Environ Contam Toxicol. 1998;61:557–568. doi: 10.1007/pl00002973. doi:10.1007/PL00002973. [DOI] [PubMed] [Google Scholar]
- Fowler PA, Dora NJ, McFerran H, Amezaga MR, Miller DW, Lea RG, Cash P, McNeilly AS, Evans NP, Cotinot C, et al. In utero exposure to low doses of environmental pollutants disrupts fetal ovarian development in sheep. Mol Hum Reprod. 2008;14:269–280. doi: 10.1093/molehr/gan020. doi:10.1093/molehr/gan020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler PA, Bhattacharya S, Flannigan S, Drake AJ, O'Shaughnessy PJ. Maternal cigarette smoking and effects on androgen action in male offspring: unexpected effects on second-trimester anogenital distance. J Clin Endocrinol Metab. 2011;96:E1502–E1506. doi: 10.1210/jc.2011-1100. doi:10.1210/jc.2011-1100. [DOI] [PubMed] [Google Scholar]
- Friel A, Houghton JA, Glennon M, Lavery R, Smith T, Nolan A, Maher M. A preliminary report on the implication of RT-PCR detection of DAZ, RBMY1, USP9Y and Protamine-2 mRNA in testicular biopsy samples from azoospermic men. Int J Androl. 2002;25:59–64. doi: 10.1046/j.1365-2605.2002.00326.x. doi:10.1046/j.1365-2605.2002.00326.x. [DOI] [PubMed] [Google Scholar]
- Fulton N, Martins da Silva SJ, Bayne RAL, Anderson RA. Germ cell proliferation and apoptosis in the developing human ovary. J Clin Endocrinol Metab. 2005;90:4664–4670. doi: 10.1210/jc.2005-0219. doi:10.1210/jc.2005-0219. [DOI] [PubMed] [Google Scholar]
- Ginis I, Luo Y, Miura T, Thies S, Brandenberger R, Gerecht-Nir S, Amit M, Hoke A, Carpenter MK, Itskovitz-Eldor J, et al. Differences between human and mouse embryonic stem cells. Dev Biol. 2004;269:360–380. doi: 10.1016/j.ydbio.2003.12.034. doi:10.1016/j.ydbio.2003.12.034. [DOI] [PubMed] [Google Scholar]
- Gold EB, Crawford SL, Avis NE, Crandall CJ, Matthews KA, Waetjen LE, Lee JS, Thurston R, Vuga M, Harlow SD. Factors related to age at natural menopause: longitudinal analyses from SWAN. Am J Epidemiol. 2013;178:70–83. doi: 10.1093/aje/kws421. doi:10.1093/aje/kws421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gondos B, Westergaard L, Byskov AG. Initiation of oogenesis in the human fetal ovary: ultrastructural and squash preparation study. Am J Obstet Gynaecol. 1986;155:189–195. doi: 10.1016/0002-9378(86)90109-2. [DOI] [PubMed] [Google Scholar]
- Hernandez-Ochoa I, Karman BN, Flaws JA. The role of the aryl hydrocarbon receptor in the female reproductive system. Biochem Pharmacol. 2009;77:547–559. doi: 10.1016/j.bcp.2008.09.037. doi:10.1016/j.bcp.2008.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt PA, Lawson C, Gieske M, Murdoch B, Smith H, Marre A, Hassold T, VandeVoort CA. Bisphenol A alters early oogenesis and follicle formation in the fetal ovary of the rhesus monkey. Proc Natl Acad Sci USA. 2012;109:17525–17530. doi: 10.1073/pnas.1207854109. doi:10.1073/pnas.1207854109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikuta T, Kawajiri K. Zinc finger transcription factor Slug is a novel target gene of aryl hydrocarbon receptor. Exp Cell Res. 2006;312:3585–3594. doi: 10.1016/j.yexcr.2006.08.002. doi:10.1016/j.yexcr.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Jensen TK, Henriksen TB, Hjollund NH, Scheike T, Kolstad H, Giwercman A, Ernst E, Bonde JP, Skakkebaek NE, Olsen J. Adult and prenatal exposures to tobacco smoke as risk indicators of fertility among 430 Danish couples. Am J Epidemiol. 1998;148:992–997. doi: 10.1093/oxfordjournals.aje.a009576. doi:10.1093/oxfordjournals.aje.a009576. [DOI] [PubMed] [Google Scholar]
- Jensen TK, Jorgensen N, Punab M, Haugen TB, Suominen J, Zilaitiene B, Horte A, Andersen AG, Carlsen E, Magnus O, et al. Association of in utero exposure to maternal smoking with reduced semen quality and testis size in adulthood: a cross-sectional study of 1,770 young men from the general population in five European countries. Am J Epidemiol. 2004;159:49–58. doi: 10.1093/aje/kwh002. doi:10.1093/aje/kwh002. [DOI] [PubMed] [Google Scholar]
- Jensen TK, Joffe M, Scheike T, Skytthe A, Gaist D, Petersen I, Christensen K. Early exposure to smoking and future fecundity among Danish twins. Int J Androl. 2006;29:603–613. doi: 10.1111/j.1365-2605.2006.00701.x. doi:10.1111/j.1365-2605.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- Kee K, Flores M, Cedars MI, Reijo Pera RA. Human primordial germ cell formation is diminished by exposure to environmental toxicants acting through the AHR signaling pathway. Toxicol Sci. 2010;117:218–224. doi: 10.1093/toxsci/kfq179. doi:10.1093/toxsci/kfq179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurilo LF. Oogenesis in antenatal development in man. Hum Genet. 1981;57:86–92. doi: 10.1007/BF00271175. doi:10.1007/BF00271175. [DOI] [PubMed] [Google Scholar]
- Lutterodt MC, Sorensen KP, Larsen KB, Skouby SO, Andersen CY, Byskov AG. The number of oogonia and somatic cells in the human female embryo and fetus in relation to whether or not exposed to maternal cigarette smoking. Hum Reprod. 2009;24:2558–2566. doi: 10.1093/humrep/dep226. doi:10.1093/humrep/dep226. [DOI] [PubMed] [Google Scholar]
- MacKenzie KM, Angevine DM. Infertility in mice exposed in utero to benzo(a)pyrene. Biol Reprod. 1981;24:183–191. doi: 10.1095/biolreprod24.1.183. doi:10.1095/biolreprod24.1.183. [DOI] [PubMed] [Google Scholar]
- Maheshwari A, Fowler PA. Primordial follicular assembly in humans—revisited. Zygote. 2008;16:285–296. doi: 10.1017/S0967199408004802. doi:10.1017/S0967199408004802. [DOI] [PubMed] [Google Scholar]
- Mamsen LS, Lutterodt MC, Andersen EW, Skouby SO, Sorensen KP, Andersen CY, Byskov AG. Cigarette smoking during early pregnancy reduces the number of embryonic germ and somatic cells. Hum Reprod. 2010;25:2755–2761. doi: 10.1093/humrep/deq215. doi:10.1093/humrep/deq215. [DOI] [PubMed] [Google Scholar]
- Martins da Silva SJ, Bayne RAL, Cambray N, Hartley PS, McNeilly AS, Anderson RA. Expression of activin subunits and receptors in the developing human ovary: activin A promotes germ cell survival and proliferation prior to primordial follicle formation. Dev Biol. 2004;266:334–345. doi: 10.1016/j.ydbio.2003.10.030. doi:10.1016/j.ydbio.2003.10.030. [DOI] [PubMed] [Google Scholar]
- Matikainen T, Perez GI, Jurisicova A, Pru JK, Schlezinger JJ, Ryu HY, Laine J, Sakai T, Korsmeyer SJ, Casper RF, et al. Aromatic hydrocarbon receptor-driven Bax gene expression is required for premature ovarian failure caused by biohazardous environmental chemicals. Nat Genet. 2001;28:355–360. doi: 10.1038/ng575. doi:10.1038/ng575. [DOI] [PubMed] [Google Scholar]
- Matikainen TM, Moriyama T, Morita Y, Perez GI, Korsmeyer SJ, Sherr DH, Tilly JL. Ligand activation of the aromatic hydrocarbon receptor transcription factor drives Bax-dependent apoptosis in developing fetal ovarian germ cells. Endocrinology. 2002;143:615–620. doi: 10.1210/endo.143.2.8624. doi:10.1210/en.143.2.615. [DOI] [PubMed] [Google Scholar]
- Pepling ME, Spradling AC. Mouse ovarian germ cell cysts undergo programmed breakdown to form primordial follicles. Dev Biol. 2001;234:339–351. doi: 10.1006/dbio.2001.0269. doi:10.1006/dbio.2001.0269. [DOI] [PubMed] [Google Scholar]
- Pocar P, Fischer B, Klonisch T, Hombach-Klonisch S. Molecular interactions of the aryl hydrocarbon receptor and its biological and toxicological relevance for reproduction. Reproduction. 2005;129:379–389. doi: 10.1530/rep.1.00294. doi:10.1530/rep.1.00294. [DOI] [PubMed] [Google Scholar]
- Ramlau-Hansen CH, Thulstrup AM, Storgaard L, Toft G, Olsen J, Bonde JP. Is prenatal exposure to tobacco smoking a cause of poor semen quality? A follow-up study. Am J Epidemiol. 2007;165:1372–1379. doi: 10.1093/aje/kwm032. doi:10.1093/aje/kwm032. [DOI] [PubMed] [Google Scholar]
- Robinson LLL, Gaskell TL, Saunders PTK, Anderson RA. Germ cell specific expression of c-kit in the human fetal gonad. Mol Hum Reprod. 2001;7:845–852. doi: 10.1093/molehr/7.9.845. doi:10.1093/molehr/7.9.845. [DOI] [PubMed] [Google Scholar]
- Robles R, Morita Y, Mann KK, Perez GI, Yang S, Matikainen T, Sherr DH, Tilly JL. The aryl hydrocarbon receptor, a basic helix-loop-helix transcription factor of the PAS gene family, is required for normal ovarian germ cell dynamics in the mouse. Endocrinology. 2000;141:450–453. doi: 10.1210/endo.141.1.7374. doi:10.1210/en.141.1.450. [DOI] [PubMed] [Google Scholar]
- Sforza C, Vizzotto L, Ferrario VF, Forabosco A. Position of follicles in normal human ovary during definitive histogenesis. Early Hum Dev. 2003;74:27–35. doi: 10.1016/s0378-3782(03)00081-1. doi:10.1016/S0378-3782(03)00081-1. [DOI] [PubMed] [Google Scholar]
- Stoop H, Honecker F, Cools M, de Krijger R, Bokemeyer C, Looijenga LH. Differentiation and development of human female germ cells during prenatal gonadogenesis: an immunohistochemical study. Hum Reprod. 2005;20:1466–1476. doi: 10.1093/humrep/deh800. doi:10.1093/humrep/deh800. [DOI] [PubMed] [Google Scholar]
- Susiarjo M, Hassold TJ, Freeman E, Hunt PA. Bisphenol A exposure in utero disrupts early oogenesis in the mouse. PLoS Genet. 2007;3:e5. doi: 10.1371/journal.pgen.0030005. doi:10.1371/journal.pgen.0030005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tingen C, Kim A, Woodruff TK. The primordial pool of follicles and nest breakdown in mammalian ovaries. Mol Hum Reprod. 2009;15:795–803. doi: 10.1093/molehr/gap073. doi:10.1093/molehr/gap073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vine MF, Margolin BH, Morrison HI, Hulka BS. Cigarette smoking and sperm density: a meta-analysis. Fertil Steril. 1994;61:35–43. [PubMed] [Google Scholar]
- Wolf CJ, Ostby JS, Gray LE., Jr Gestational exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) severely alters reproductive function of female hamster offspring. Toxicol Sci. 1999;51:259–264. doi: 10.1093/toxsci/51.2.259. doi:10.1093/toxsci/51.2.259. [DOI] [PubMed] [Google Scholar]
- Ye X, Skjaerven R, Basso O, Baird DD, Eggesbo M, Uicab LA, Haug K, Longnecker MP. In utero exposure to tobacco smoke and subsequent reduced fertility in females. Hum Reprod. 2010;25:2901–2906. doi: 10.1093/humrep/deq235. doi:10.1093/humrep/deq235. [DOI] [PMC free article] [PubMed] [Google Scholar]