Abstract
OBJECTIVE
To evaluate the cost-effectiveness of a genetic testing policy for HNF1A-, HNF4A-, and GCK-MODY in a hypothetical cohort of type 2 diabetic patients 25–40 years old with a MODY prevalence of 2%.
RESEARCH DESIGN AND METHODS
We used a simulation model of type 2 diabetes complications based on UK Prospective Diabetes Study data, modified to account for the natural history of disease by genetic subtype to compare a policy of genetic testing at diabetes diagnosis versus a policy of no testing. Under the screening policy, successful sulfonylurea treatment of HNF1A-MODY and HNF4A-MODY was modeled to produce a glycosylated hemoglobin reduction of −1.5% compared with usual care. GCK-MODY received no therapy. Main outcome measures were costs and quality-adjusted life years (QALYs) based on lifetime risk of complications and treatments, expressed as the incremental cost-effectiveness ratio (ICER) (USD/QALY).
RESULTS
The testing policy yielded an average gain of 0.012 QALYs and resulted in an ICER of 205,000 USD. Sensitivity analysis showed that if the MODY prevalence was 6%, the ICER would be ∼50,000 USD. If MODY prevalence was >30%, the testing policy was cost saving. Reducing genetic testing costs to 700 USD also resulted in an ICER of ∼50,000 USD.
CONCLUSIONS
Our simulated model suggests that a policy of testing for MODY in selected populations is cost-effective for the U.S. based on contemporary ICER thresholds. Higher prevalence of MODY in the tested population or decreased testing costs would enhance cost-effectiveness. Our results make a compelling argument for routine coverage of genetic testing in patients with high clinical suspicion of MODY.
Introduction
Completion of the human genome sequence and advances in sequencing are bringing the promises of personalized genetic medicine to fruition. Its applications will not only unravel the mysteries of complex diseases but will also advance the practice of individualized care for disorders whose pathogenic basis is known. It will be important to consider the scenarios in which genetically personalized health care can be applied at costs reflective of society’s willingness to pay for health benefits. Currently, there are few rigorous cost-effectiveness analyses of genetic testing policies that lead to changes in the selection of treatments.
Maturity-onset diabetes of the young (MODY) is the most prevalent form of monogenic diabetes, all types of which account for 1–2% of diabetes cases (1). MODY is classically defined as autosomal dominant, non–insulin dependent diabetes with diagnosis typically before 25 years of age (2). Additional features of MODY depend upon its molecular genetic basis.
Heterozygous mutations in three genes, HNF1A, HNF4A, and GCK, together account for >90% of all MODY with a known genetic cause (3). Diagnosing these subtypes of MODY has important implications for treatment. The first-line therapy for HNF1A- and HNF4A-MODY is sulfonylurea pills, which result in stable or improved glycemic control and improved quality of life related to diabetes care compared with insulin or metformin therapy (4–7). GCK-MODY has a unique phenotype of mild, nonprogressive hyperglycemia, with HbA1c typically <7% (53 mmol/mol). It is not associated with increased risk of microvascular and macrovascular complications seen in other forms of diabetes (8). Generally, treatment does not change HbA1c. Molecular diagnosis of GCK-MODY allows pharmacologic therapy to be discontinued and decreases the needed frequency of medical surveillance (9,10). Genetic diagnosis of MODY additionally allows for identification of at-risk first-degree family members, who have a 50% chance of inheriting a gene mutation.
Screening for MODY is not a routine practice for diabetes classification despite the fact that there is significant clinical overlap between MODY and type 1 and 2 diabetes. Genetic testing is generally pursued only in those with classic features of MODY. However, only ∼50% of subjects with genetically diagnosed MODY meet classic criteria (3). Apart from clinical features, there are established biomarkers to aid in patient selection for genetic testing, such as high-sensitivity CRP or urinary C-peptide–to–creatinine ratio. However, these tests are not yet in routine clinical practice in the U.S., and not all biomarkers are useful at diabetes onset (11,12). Irrespective of these clinical considerations, insurance coverage of MODY genetic testing may be denied even in those meeting classic MODY criteria (University of Chicago Monogenic Diabetes Registry, unpublished data,) (13). Thus, many cases of MODY are being missed. In the case of GCK-MODY, HNF1A-MODY, and HNF4A-MODY, misclassification is likely to lead to overtreatment or unnecessary treatment with increased health care expenditures for individuals (9). Assessing the economic value of genetic testing for these MODY subtypes in diabetes may clarify practices for selection of patients for testing and inform policy decisions regarding medical insurance coverage for genetic testing costs.
Research Design and Methods
Framework of the Study
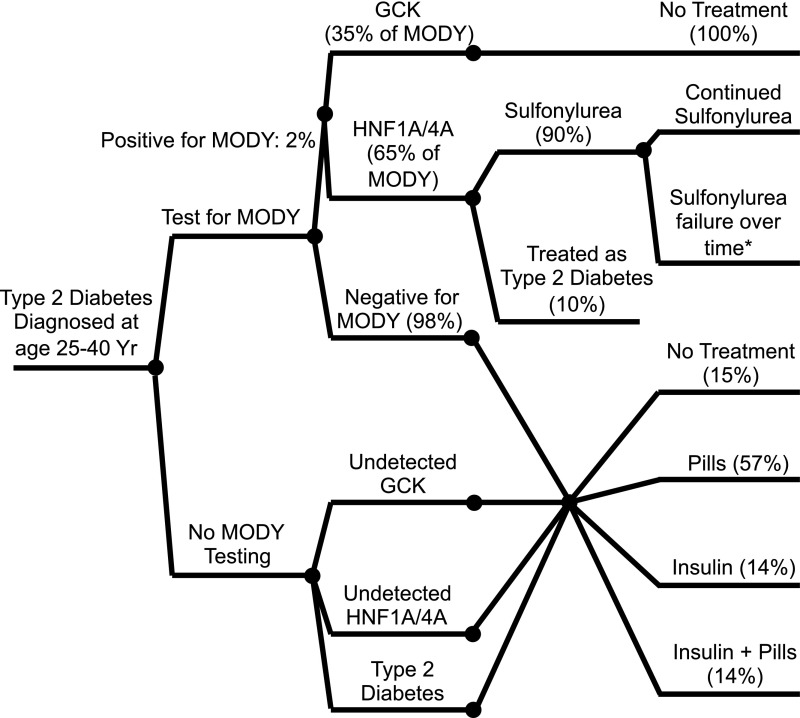
We used a simulation model of diabetes complications to compare a policy of routine genetic testing for GCK-, HNF1A-, and HNF4A-MODY in newly diagnosed patients 25–40 years of age with presumed type 2 diabetes with a policy of no genetic testing. Cost of diabetes care and health outcomes, including rates of diabetes complications, was analyzed for all hypothetical patients. Analysis was conducted from a health care system perspective over the lifetime of the study population. Costs were expressed in 2011 USD. Future costs and quality-adjusted life years (QALYs) were discounted at an annual rate of 3%. The conceptual framework for the cost analysis is depicted in Fig. 1.
Figure 1.
Policy decision for genetic testing for MODY due to mutations in HNF1A, HNF4A, and GCK. Yr, years.
Population Characteristics
Our hypothetical cohort consisted of patients between the ages of 25–40 years newly diagnosed with type 2 diabetes. We assumed that the background prevalence of MODY due to mutations in HNF1A, HNF4A, and GCK for this population was 2% and that 98% had true type 2 diabetes (1). Of those with MODY, we assumed that 65% had HNF1A- or HNF4A-MODY and 35% had GCK-MODY (3). We used data from the combined National Health and Nutrition Examination Survey (NHANES) 1999–2006 data releases as well as case series of HNF1A-, HNF4A-, and GCK-MODY to assign clinical parameters—including initial HbA1c; total cholesterol, HDL, LDL, and triglyceride levels; blood pressure; and BMI—to the cohort (8,14–16).
Genetic Testing Impact on Treatment Decisions
In our model, hypothetical patients underwent one-time genetic testing for mutations in GCK, HNF1A, and HNF4A within the first year of diabetes diagnosis. We assumed a perfectly sensitive and specific genetic test that would uncover all pathologic mutations. Our model did not allow for more than one type of MODY in a single individual or cooccurrence of MODY and type 2 diabetes in a single patient, though there are rare examples of such in the literature (17,18).
In both the testing and no testing policies, patients with true type 2 diabetes were treated based on the observed distributions of medications in national studies: 15% no pharmacologic treatment, 57% pills/noninsulin injectables, 14% insulin, and 14% insulin and pills/noninsulin injectables (19,20). Under the genetic testing policy, those with a diagnosis of MODY were treated according to their genetic subtype: sulfonylureas in the case of HNF1A- and HNF4A-MODY and no medical treatment in GCK-MODY. We assumed that 90% of patients with HNF1A- and HNF4A-MODY would be successfully treated with sulfonylureas associated with a 1.5% decrease in HbA1c at the time of therapy initiation (4,21). For those with initial successful sulfonylurea treatment, we modeled increasing rates of sulfonylurea failure with duration of diabetes in hypothetical patients, such that the rate of sulfonylurea failure and insulin initiation was 50% at 20 years postdiagnosis (14). Hypothetical patients who initially failed sulfonylurea treatment were assigned the same therapy as for those with true type 2 diabetes. For GCK-MODY, we assumed that all patients would be able to successfully discontinue therapy without a change in HbA1c. We did not account for changes or escalation of therapy over time in the model for those with GCK-MODY or true type 2 diabetes. Under the no testing policy, undetected MODY was treated the same as true type 2 diabetes.
Simulation Model for Diabetes Complications
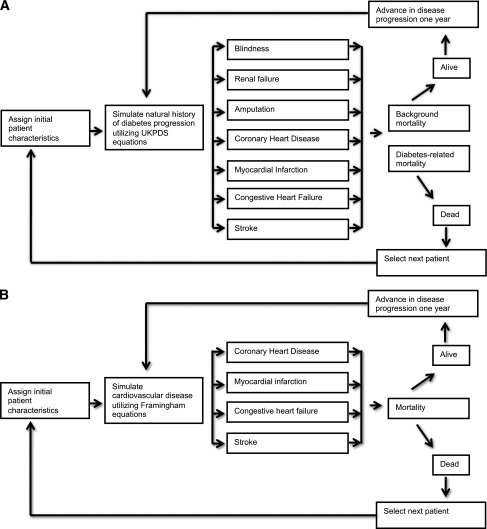
To account for future complications and costs of diabetes associated with different policies, we constructed two distinctive diabetes complications models (Fig. 2). For type 2 diabetes and HNF1A- and HNF4A-MODY, we tracked microvascular-related complications of blindness, renal failure, and amputation and macrovascular complications of angina, myocardial infarction, congestive heart failure, and stroke. Rates of these complications were modeled based upon well-established UK Prospective Diabetes Study (UKPDS) risk equations (22).
Figure 2.
A: Simulation model for complications for type 2 and HNF1A- and HNF4A-MODY. B: Simulation model for complications for GCK-MODY.
For patients with GCK-MODY, we only tracked cardiovascular complications because the mild hyperglycemia in GCK-MODY is not associated with increased frequency of microvascular complications. In these patients, Framingham cardiovascular risk equations were used to simulate cardiovascular disease and background mortality rates (23). Hypoglycemia as an adverse reaction to insulin and sulfonylurea therapy was also assessed in all patients (24).
When we were running the analysis, each hypothetical patient progressed through major categories of complications, as well as a mortality module, over a 1-year cycle. Calculations were done using Excel (Microsoft, Redmond, WA) and @Risk 5.5 software (Palisades, Newfield, NY). Cycles were repeated over the lifetime of the subject. For each specific scenario, the model was run for 10,000 iterations using Monte Carlo recalculation.
Quality-of-Life Effects
To calculate QALYs, we assumed that there were quality-of-life effects of diabetes treatments and complications. Treatment of diabetes with an oral medication was assigned a health utility of 0.77, while treatment with insulin was assigned a utility of 0.64 (25). Microvascular and macrovascular complications resulted in a decremental change in health state based on published health state utilities (Supplementary Table 1). If a patient had multiple treatment and complications health states in a given year, we assigned the utility based on the minimum utility method.
Costs
The cost of genetic testing was set at 2,580 USD per individual tested, reflecting the cost of simultaneously sequencing GCK, HNF1A, and HNF4A (Commercial Reference Laboratory pricing). Individuals were assigned an annual treatment cost based upon their diabetes treatment group. The annual per-individual treatment cost of insulin-only therapy was 2,641 USD, which includes the cost of insulin, injection supplies, and self–blood glucose monitoring (26). The annual treatment cost of pills/noninsulin injectables was 767 USD (27). These costs were combined for those treated with insulin and pills/noninsulin injectables. The annual treatment cost of sulfonylureas in HNF1A- and HNF4A-MODY was 96 USD (28). Those on no therapy were assigned a cost of 0 USD. We did not account for self–blood glucose monitoring in non–insulin-treated patients or in patients on no therapy, as the cost-effectiveness and glycemic efficacy of self–blood glucose monitoring has not been established in these populations (29,30).
Study Outcomes
The main outcome measures were costs and QALYs, based on lifetime risk of complications and treatments, expressed as the incremental cost-effectiveness ratio (ICER) (USD/QALY). Future costs and quality-of-life effects were discounted at an annual rate of 3% with one-way sensitivity analyses performed around the discount rate (31).
Sensitivity Analysis
We conducted one-way and two-way sensitivity analyses to evaluate the impact of changes in several key assumptions including MODY prevalence and testing costs on cost utility of a genetic testing policy. We also performed a threshold analysis to understand at which prevalence of MODY a genetic testing policy would become cost saving.
Scenario Analysis
We also considered that treatment reported in case series of MODY differs from the typical treatment selection reported for type 2 diabetes. MODY case series are likely subject to selection bias; those suspected of having MODY and referred for testing would be more likely to be treated appropriately for their suspected MODY diagnosis than someone misclassified as having type 2 diabetes. Moreover, many case series do not define whether treatment followed MODY diagnosis. Nevertheless, we assessed the impact of modeling treatment of undetected MODY based on data extrapolated from case series as follows: 75% no treatment, 20% pills/noninsulin injectables (modeled to reflect the 2011 annual rates of use of noninsulin antidiabetes treatment), and 5% insulin for GCK-MODY and 10% no treatment, 45% pills/noninsulin injectables, and 45% insulin for HNF1A- and HNF4A-MODY (14,15,32–34).
Results
Health Effects
In the base case analysis, the genetic testing policy did not significantly change the rate of diabetes complications or overall life expectancy. The genetic testing policy resulted in small quality-of-life benefits of 0.012 QALYs that were due to increased use of pills rather than insulin therapy in the case of HNF1A- and HNF4A-MODY and a larger percentage of individuals on no therapy in the case of GCK-MODY (Table 1).
Table 1.
Base case cost-effectiveness analysis results

Cost
In the base case analysis, the total cost difference between the testing and no testing scenarios was 2,400 USD, accounted for almost entirely by the costs of screening and treatment.
ICER
Considering the health and cost effects together, the genetic testing policy resulted in an overall ICER of 205,000 USD/QALY for detecting GCK-, HNF1A-, and HNF4A-MODY in incident cases of presumed type 2 diabetes among 25- to 40-year-old patients.
Sensitivity and Scenario Analyses
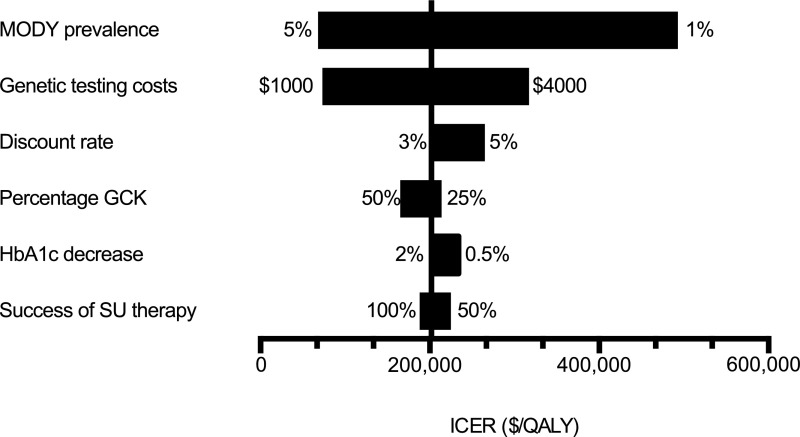
We performed one- and two-way sensitivity analyses around important parameters varied over plausible ranges to determine how changes would impact the results of the model (Fig. 3). Total costs were most sensitive to the prevalence of MODY and the cost of genetic testing. An increase in MODY prevalence from 2 to 6% resulted in an ICER just over 50,000 USD/QALY, which is often used as a benchmark for assessing cost-effectiveness of a health intervention (35). Similarly, decreasing the cost of genetic testing from 2,580 to 700 USD led to an ICER of 50,000 USD/QALY.
Figure 3.
Sensitivity analyses for lifetime ICER. SU, sulfonylurea.
As the pick-up rate of MODY had a large impact on the overall cost-effectiveness of the model, we performed a threshold analysis to determine how prevalent MODY would need to be in the screened population to make a policy of genetic testing cost saving. At an expected pick-up rate of ∼30%, the testing scenario dominated the no testing policy.
Finally, we evaluated the scenario of modeling undetected MODY in the no testing arm with treatment extrapolated from MODY case series. There was no change in QALY, but the ICER increased from 205,000 USD/QALY in the base case to 228,000 USD/QALY. Under this scenario, a MODY prevalence of 8% led to an ICER of ∼50,000 USD/QALY.
Conclusions
Currently, there is no uniform policy on insurance coverage decisions for MODY genetic testing. Valuation of MODY genetic testing may help inform and shape coverage policies, and our modeled study is a first step in this process. Conventional benchmarks of cost-effectiveness are typically set at 50,000 USD/QALY (35), although this threshold has been questioned and benchmarks of 109,000–297,000 USD/QALY may be more reflective of actual U.S. resource-allocation decisions (36). In our simulation model of type 2 diabetes, screening all hypothetical patients with a clinical diagnosis of type 2 diabetes resulted in an ICER just over 200,000 USD. With a conservative interpretation of cost-effectiveness, small increases in MODY prevalence in our hypothetical cohort to 6% made the genetic screening policy cost-effective with an ICER of ∼50,000 USD. This suggests that testing for subtypes of MODY in an appropriately selected population may be cost-effective. As our conclusions are based on modeled data rather than patient cases, our results may over- or undervalue MODY genetic testing among those with a clinical diagnosis of type 2 diabetes and must be interpreted cautiously. However, they serve as an important indication that MODY genetic testing may improve health outcomes at acceptable societal costs, and thus improved access to testing should be sought.
Our sensitivity analyses showed that the prevalence of MODY in the screened population and the cost of genetic testing had large effects on the cost-effectiveness of a genetic screening policy. An increase in MODY prevalence from 2% in the base case analysis to 6% or a decrease in testing costs to 700 USD resulted in an ICER of ∼50,000 USD/QALY. At a prevalence of just over 30%, a genetic testing policy for MODY was cost saving. Literature has also shown that the prevalence of HNF1A-, HNF4A-, or GCK-MODY in individuals clinically diagnosed with type 2 diabetes but without metabolic features approaches 15% (21), a prevalence where genetic testing would be predicted to be cost-effective by our model. The likelihood of individuals with classic MODY criteria having a mutation in HNF1A, HNF4A, or GCK is nearly 50% (3), a prevalence at which genetic testing for MODY would be predicted to be cost saving in our model. Currently available tools, such as a “MODY calculator” could aid in identifying individuals for genetic testing (14,37). A formal health care policy of routine medical coverage for genetic testing in defined groups of patients for these subtypes of MODY could serve as a model for developing cost-effective health care policies around the application of personalized genetic medicine. Follow-up of the success of such a policy could guide future decisions on expansion of MODY testing coverage as well as form a framework for general decision making in medical insurance coverage policies for genetic testing.
Some important limitations of our model must be considered. The UKPDS outcome model and the natural history of HbA1c are not validated for HNF1A- or HNF4A-MODY. Thus, our model may have inflated complication rates for HNF1A- and HNF4A-MODY, accounting for the lack of difference in the testing versus no testing scenarios. These assumptions likely biased against a genetic testing program for MODY.
We modeled GCK-MODY without microvascular complications and used data from the Framingham study to model cardiovascular disease. Additionally, we modeled no treatment over the lifetime of hypothetical patients. Many case series support these assumptions. However, Martin et al. (38) report long-term deterioration in glycemia due to insulin resistance in GCK-MODY with 15% of subjects requiring medication at follow-up. This insulin resistance correlated with increases in BMI and age. Insulin resistance is seen under these same circumstances in the nondiabetic population; thus, it is not clear that deterioration in glycemia was a specific feature of GCK-MODY.
We extrapolated from the literature to model failure of sulfonylurea therapy over time in those with HNF1A- and HNF4A-MODY. However, there are little data on the durability of successful use of sulfonylurea therapy in MODY. Studies and case reports have demonstrated deterioration of glycemic control in individuals over time, but there are some reports of successful use of sulfonylurea therapy for decades (6). Thus, modeled failure rates and therapy changes were based on limited data in our study.
Our model did not account for the effects of identifying family members with MODY, where testing would occur at a substantially lower cost nearly 10-fold less than the cost of propositus testing (Commercial Reference Laboratory pricing). Thus, additional cost savings attributable to this “spillover effect” were not assessed or accounted for, potentially undervaluing the impact of MODY genetic testing.
A final important limitation of our study is the extrapolation of data from MODY case series largely derived from Caucasian European populations for important clinical inputs for the model. As we are interpreting our results in the U.S. context, it is unclear how representative the data are for type 2 diabetes within the U.S. Specifically, the larger non-Caucasian population and higher prevalence of obesity and metabolic syndrome within the U.S. may result in a different background MODY prevalence compared with European countries, and the true U.S. prevalence of MODY is unknown (39). As practices to identify and genetically diagnose MODY increase in the U.S., we will be able to more accurately assess the incidence and prevalence of MODY and modify health care and economic policy appropriately.
With these limitations taken into account, the results of our simulated model suggest that MODY genetic testing for mutations in GCK, HNF1A, and HNF4A in incident cases of type 2 diabetes is cost-effective if the prevalence of MODY is 6% in the screened cohort. Moreover, as genetic testing costs decrease over time with advancements in sequencing technology, we can expect generalized screening for subtypes of MODY in type 2 diabetes to be a cost-effective application of personalized genetic medicine.
Acknowledgments
Funding. This study was supported by the Chicago Center for Diabetes Translational Research grant P30 DK-092949-01 from the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (NIH), the University of Chicago Diabetes Research and Training Center grant P60 DK-020595 from the NIH, the National Center for Monogenic Diabetes award 1-11-CT-41 from the American Diabetes Association, and the Kovler Family Foundation. R.N.N. was supported by Minority Post-Doctoral Fellowship award 7-10-MI-08 from the American Diabetes Association. S.A.W.G. is funded by award K23 DK-094866 from the NIH.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. R.N.N. developed the study concept and design, acquired data, analyzed and interpreted data, drafted the manuscript, and critically revised the manuscript for important intellectual content. P.M.J. developed the study concept and design, acquired data, analyzed and interpreted data, and critically revised the manuscript for important intellectual content. A.N.W. analyzed and interpreted data, critically revised the manuscript for important intellectual content, and provided administrative, technical, or material support. D.C. acquired data, analyzed and interpreted data, and critically revised the manuscript for important intellectual content. S.A.W.G. developed the study concept and design and critically revised the manuscript for important intellectual content. L.H.P. developed the study concept and design, critically revised the manuscript for important intellectual content, and obtained funding. G.I.B. and E.S.H. developed the study concept and design; analyzed and interpreted data; drafted the manuscript; critically revised the manuscript for important intellectual content; obtained funding; provided administrative, technical, or material support; and supervised the study. R.N.N. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 72nd Scientific Sessions of the American Diabetes Association, Philadelphia, Pennsylvania, 8–12 June 2012.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc13-0410/-/DC1.
References
- 1.Frayling TM, Evans JC, Bulman MP, et al. beta-cell genes and diabetes: molecular and clinical characterization of mutations in transcription factors. Diabetes 2001;50(Suppl. 1):S94–S100 [DOI] [PubMed] [Google Scholar]
- 2.Tattersall RB, Fajans SS. A difference between the inheritance of classical juvenile-onset and maturity-onset type diabetes of young people. Diabetes 1975;24:44–53 [DOI] [PubMed] [Google Scholar]
- 3.Shields BM, Hicks S, Shepherd MH, Colclough K, Hattersley AT, Ellard S. Maturity-onset diabetes of the young (MODY): how many cases are we missing? Diabetologia 2010;53:2504–2508 [DOI] [PubMed] [Google Scholar]
- 4.Shepherd M, Pearson ER, Houghton J, Salt G, Ellard S, Hattersley AT. No deterioration in glycemic control in HNF-1alpha maturity-onset diabetes of the young following transfer from long-term insulin to sulphonylureas. Diabetes Care 2003;26:3191–3192 [DOI] [PubMed] [Google Scholar]
- 5.Pearson ER, Starkey BJ, Powell RJ, Gribble FM, Clark PM, Hattersley AT. Genetic cause of hyperglycaemia and response to treatment in diabetes. Lancet 2003;362:1275–1281 [DOI] [PubMed] [Google Scholar]
- 6.Fajans SS, Brown MB. Administration of sulfonylureas can increase glucose-induced insulin secretion for decades in patients with maturity-onset diabetes of the young. Diabetes Care 1993;16:1254–1261 [DOI] [PubMed] [Google Scholar]
- 7.Shepherd M, Shields B, Ellard S, Rubio-Cabezas O, Hattersley AT. A genetic diagnosis of HNF1A diabetes alters treatment and improves glycaemic control in the majority of insulin-treated patients. Diabet Med 2009;26:437–441 [DOI] [PubMed] [Google Scholar]
- 8.Velho G, Blanché H, Vaxillaire M, et al. Identification of 14 new glucokinase mutations and description of the clinical profile of 42 MODY-2 families. Diabetologia 1997;40:217–224 [DOI] [PubMed] [Google Scholar]
- 9.Schnyder S, Mullis PE, Ellard S, Hattersley AT, Flück CE. Genetic testing for glucokinase mutations in clinically selected patients with MODY: a worthwhile investment. Swiss Med Wkly 2005;135:352–356 [DOI] [PubMed] [Google Scholar]
- 10.Murphy R, Ellard S, Hattersley AT. Clinical implications of a molecular genetic classification of monogenic beta-cell diabetes. Nat Clin Pract Endocrinol Metab 2008;4:200–213 [DOI] [PubMed] [Google Scholar]
- 11.Thanabalasingham G, Shah N, Vaxillaire M, et al. A large multi-centre European study validates high-sensitivity C-reactive protein (hsCRP) as a clinical biomarker for the diagnosis of diabetes subtypes. Diabetologia 2011;54:2801–2810 [DOI] [PubMed] [Google Scholar]
- 12.Besser REJ, Shepherd MH, McDonald TJ, et al. Urinary C-peptide creatinine ratio is a practical outpatient tool for identifying hepatocyte nuclear factor 1-alpha/hepatocyte nuclear factor 4-alpha maturity-onset diabetes of the young from long-duration type 1 diabetes. Diabetes Care 2011;34:286–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.University of Chicago Kovler Diabetes Center. University of Chicago Monogenic Diabetes Registry. Available from http://monogenicdiabetes.uchicago.edu/registry/ Accessed 22 January 2013
- 14.Bellanné-Chantelot C, Lévy DJ, Carette C, et al. French Monogenic Diabetes Study Group Clinical characteristics and diagnostic criteria of maturity-onset diabetes of the young (MODY) due to molecular anomalies of the HNF1A gene. J Clin Endocrinol Metab 2011;96:E1346–E1351 [DOI] [PubMed] [Google Scholar]
- 15.Pearson ER, Pruhova S, Tack CJ, et al. Molecular genetics and phenotypic characteristics of MODY caused by hepatocyte nuclear factor 4alpha mutations in a large European collection. Diabetologia 2005;48:878–885 [DOI] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey [internet]. Available from http://www.cdc.gov/nchs/nhanes.htm Accessed 10 January 2013
- 17.Forlani G, Zucchini S, Di Rocco A, et al. Double heterozygous mutations involving both HNF1A/MODY3 and HNF4A/MODY1 genes: a case report. Diabetes Care 2010;33:2336–2338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beijers HJBH, Losekoot M, Odink RJ, Bravenboer B. Hepatocyte nuclear factor (HNF)1A and HNF4A substitution occurring simultaneously in a family with maturity-onset diabetes of the young. Diabet Med 2009;26:1172–1174 [DOI] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention. National Health Interview Survey [internet]. Available from http://www.cdc.gov/nchs/nhis.htm Accessed 22 January 2013
- 20.Alexander GC, Sehgal NL, Moloney RM, Stafford RS. National trends in treatment of type 2 diabetes mellitus, 1994-2007. Arch Intern Med 2008;168:2088–2094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thanabalasingham G, Pal A, Selwood MP, et al. Systematic assessment of etiology in adults with a clinical diagnosis of young-onset type 2 diabetes is a successful strategy for identifying maturity-onset diabetes of the young. Diabetes Care 2012;35:1206–1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clarke PM, Gray AM, Briggs A, et al. UK Prospective Diabetes Study (UKDPS) Group A model to estimate the lifetime health outcomes of patients with type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS) Outcomes Model (UKPDS no. 68). Diabetologia 2004;47:1747–1759 [DOI] [PubMed] [Google Scholar]
- 23.Risk score profiles [internet], 2013 Available from http://www.framinghamheartstudy.org/risk/index.html Accessed 22 January 2013
- 24.Leese GP, Wang J, Broomhall J, et al. DARTS/MEMO Collaboration Frequency of severe hypoglycemia requiring emergency treatment in type 1 and type 2 diabetes: a population-based study of health service resource use. Diabetes Care 2003;26:1176–1180 [DOI] [PubMed] [Google Scholar]
- 25.Huang ES, Shook M, Jin L, Chin MH, Meltzer DO. The impact of patient preferences on the cost-effectiveness of intensive glucose control in older patients with new-onset diabetes. Diabetes Care 2006;29:259–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reinke T. New diabetes medications drive up costs and stymie formulary design. Manag Care 2012;21:16–18 [PubMed] [Google Scholar]
- 27.Yeaw J, Lee WC, Aagren M, Christensen T. Cost of self-monitoring of blood glucose in the United States among patients on an insulin regimen for diabetes. J Manag Care Pharm 2012;18:21–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Desai NR, Shrank WH, Fischer MA, et al. Patterns of medication initiation in newly diagnosed diabetes mellitus: quality and cost implications. Am J Med 2012;125:302.e1–302.e7 [DOI] [PMC free article] [PubMed]
- 29.Farmer AJ, Wade AN, French DP, et al. Blood glucose self-monitoring in type 2 diabetes: a randomised controlled trial. Health Technol Assess 2009;13:iii–iv, ix–xi, 1–50 [DOI] [PubMed]
- 30.Malanda UL, Welschen LMC, Riphagen II, Dekker JM, Nijpels G, Bot SDM. Self-monitoring of blood glucose in patients with type 2 diabetes mellitus who are not using insulin. Cochrane Database Syst Rev 2012;1:CD005060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB. Recommendations of the Panel on Cost-Effectiveness in Health and Medicine. JAMA 1996;276:1253–1258 [PubMed] [Google Scholar]
- 32.Froguel P, Zouali H, Vionnet N, et al. Familial hyperglycemia due to mutations in glucokinase. Definition of a subtype of diabetes mellitus. N Engl J Med 1993;328:697–702 [DOI] [PubMed] [Google Scholar]
- 33.Codner E, Rocha A, Deng L, et al. Mild fasting hyperglycemia in children: high rate of glucokinase mutations and some risk of developing type 1 diabetes mellitus. Pediatr Diabetes 2009;10:382–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pruhova S, Dusatkova P, Sumnik Z, et al. Glucokinase diabetes in 103 families from a country-based study in the Czech Republic: geographically restricted distribution of two prevalent GCK mutations. Pediatr Diabetes 2010;11:529–535 [DOI] [PubMed] [Google Scholar]
- 35.Evans C, Tavakoli M, Crawford B. Use of quality adjusted life years and life years gained as benchmarks in economic evaluations: a critical appraisal. Health Care Manage Sci 2004;7:43–49 [DOI] [PubMed] [Google Scholar]
- 36.Braithwaite RS, Meltzer DO, King JT, Jr, Leslie D, Roberts MS. What does the value of modern medicine say about the $50,000 per quality-adjusted life-year decision rule? Med Care 2008;46:349–356 [DOI] [PubMed] [Google Scholar]
- 37.Shields BM, McDonald TJ, Ellard S, Campbell MJ, Hyde C, Hattersley AT. The development and validation of a clinical prediction model to determine the probability of MODY in patients with young-onset diabetes. Diabetologia 2012;55:1265–1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martin D, Bellanné-Chantelot C, Deschamps I, Froguel P, Robert J-J, Velho G. Long-term follow-up of oral glucose tolerance test-derived glucose tolerance and insulin secretion and insulin sensitivity indexes in subjects with glucokinase mutations (MODY2). Diabetes Care 2008;31:1321–1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kopelman PG. Obesity as a medical problem. Nature 2000;404:635–643 [DOI] [PubMed] [Google Scholar]