Abstract
OBJECTIVE
Kidney disease manifests clinically as elevated albumin excretion rate (AER), impaired glomerular filtration rate (GFR), or both, and is a cause of substantial morbidity and mortality in type 1 diabetes (T1D). The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) study tested whether intensive diabetes therapy (INT) aimed at lowering glucose concentrations as close as safely possible to the normal range reduces the risks of kidney disease and other diabetes complications.
RESEARCH DESIGN AND METHODS
In the DCCT, 1,441 participants with T1D were randomly assigned to INT or conventional diabetes therapy (CON) for a mean duration of 6.5 years. Subsequently, participants have been followed for 18 years in the ongoing observational EDIC. Standardized longitudinal measurements of AER, estimated GFR, and blood pressure were made throughout the DCCT/EDIC.
RESULTS
During the DCCT, INT reduced the risks of incident microalbuminuria (AER ≥40 mg/24 h) and macroalbuminuria (AER ≥300 mg/24 h) by 39% (95% CI 21–52%) and 54% (29–74%), respectively. During EDIC years 1–8, participants previously assigned to DCCT INT continued to experience lower rates of incident microalbuminuria and macroalbuminuria, with risk reductions of 59% (39–73%) and 84% (67–92%), respectively. Beneficial effects of INT on the development of impaired GFR (sustained estimated GFR <60 mL/min/1.73 m2) and hypertension became evident during combined DCCT/EDIC follow-up, with risk reductions of 50% (18–69%) and 20% (6–21%), respectively, compared with CON.
CONCLUSIONS
In the DCCT/EDIC, INT resulted in clinically important, durable reductions in the risks of microalbuminuria, macroalbuminuria, impaired GFR, and hypertension.
Introduction
Kidney disease manifests clinically as elevated albumin excretion rate (AER), impaired glomerular filtration rate (GFR), or both, and is a cause of substantial morbidity and mortality in type 1 diabetes (T1D). In early cohort studies, up to 40% of patients with T1D developed macroalbuminuria (AER ≥300 mg/24 h), and up to 75% of these patients progressed to end-stage renal disease (ESRD) within 10 years (1,2). Moreover, the presence and severity of kidney disease have been consistently and strongly associated with increased risks of cardiovascular disease (CVD) and death (3,4).
A large body of evidence demonstrates that hyperglycemia contributes to the damage of target organs, including the kidney (5). Moreover, poor glycemic control has been consistently associated with increased risk of developing kidney disease in T1D (2,6). The Diabetes Control and Complications Trial (DCCT) tested the hypothesis that intensive diabetes therapy (INT) aimed at lowering glucose concentrations as close as safely possible to the normal range would reduce the risk of developing microalbuminuria (AER ≥40 mg/24 h), macroalbuminuria, and other complications of T1D, compared with conventional therapy (CON) (7). The Epidemiology of Diabetes Interventions and Complications (EDIC) study—the observational study following the DCCT—has examined and continues to examine the extent to which the effects of INT observed during the DCCT are durable (8). In addition, the EDIC has examined the long-term effects of INT on related outcomes, such as impaired GFR and hypertension, as well as the evolving clinical course of kidney disease in T1D. This article summarizes the most important published DCCT/EDIC findings related to kidney disease.
Research Design and Methods
The DCCT enrolled 1,441 people with T1D from 1983–1989 to determine the effects of INT on the long-term complications of diabetes (7,9). The trial included two cohorts. The primary prevention cohort was characterized by diabetes duration 1–5 years, AER <40 mg/24 h, and no retinopathy by fundus photography. The secondary intervention cohort was characterized by diabetes duration 1–15 years, AER ≤200 mg/24 h, and at least one microaneurysm in either eye (but no more than moderate nonproliferative retinopathy). For both cohorts, serum creatinine <1.2 mg/dL or creatinine clearance >100 mL/min/1.73 m2 was required for eligibility. Hypertension, defined as systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or treatment with antihypertensive medications, was an exclusion criterion.
Participants were randomly assigned to receive INT or CON, as described in detail elsewhere (7,9). The DCCT ended in 1993 after a mean duration of 6.5 years. Subsequently, all surviving participants were invited to join the observational EDIC, which continues today. During EDIC, mean HbA1c levels, which were separated by ∼2% during DCCT, became similar for the two original DCCT treatment groups (8).
AER was measured yearly during DCCT and every other year during EDIC using supervised 4-h urine collections (10,11). Water intake was encouraged to maintain urine output throughout the collection period. Urine albumin was measured at the DCCT/EDIC Central Biochemistry Laboratory (CBL) at the University of Minnesota using a fluoroimmunoassay (interassay coefficient of variation 8.4%).
Serum creatinine was measured annually throughout DCCT/EDIC at the CBL (interassay coefficient of variation <3%). All results were calibrated to National Institute of Standards and Technology isotope dilution mass spectrometry assigned values (12). The Chronic Kidney Disease Epidemiology Collaboration equation was used to estimate GFR from calibrated serum creatinine, age, sex, and race (13).
Blood pressure was measured every 3 months during DCCT and yearly during EDIC. Hypertension was defined as two consecutive study visits with systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or the use of antihypertensive medications (14).
The effects of DCCT INT on AER, estimated GFR, and hypertension were tested according to intention to treat. Hazard ratios (HRs) were estimated using Cox proportional hazards models, which incorporated death as a competing risk when evaluating impaired GFR as the outcome (12). Risk reduction associated with INT was calculated as (1 − HR of INT vs. CON) × 100%. Proportion of effect explained by DCCT HbA1c was the proportion of INT effect explained by statistical adjustment for the treatment group difference in DCCT mean HbA1c. Cumulative incidence of microalbuminuria or macroalbuminuria by diabetes duration was quantified using a nonparametric estimator that allows for interval-censored observations (15,16).
Results
Baseline Characteristics
Of the 1,441 DCCT participants, 680 (47%) were female and 1,390 (96%) were Caucasian. At DCCT baseline, mean (SD) age was 26.8 (7.1) years and mean (SD) duration of diabetes was 5.9 (4.2) years. Measures of kidney disease were predominantly normal: median (interquartile range) AER was 11.5 (7.2–18.7) mg/24 h and mean (SD) serum creatinine concentration was 0.68 (0.14) mg/dL. AER was <40 mg/24 h for 1,365 participants (95%) and 40–200 mg/24 h for 76 participants (5%).
Microalbuminuria
During the DCCT, among the 1,365 participants with baseline AER <40 mg/24 h at baseline, INT reduced the risk of developing incident microalbuminuria (AER ≥40 mg/24 h) by 39% (95% CI 21–52%) (Table 1) (7). Significant effects were observed for participants in both the primary and secondary intervention cohorts, with risk reductions of 34% (2–56%) and 43% (21–58%), respectively (17). These effects were strongest beginning 4 years after randomization.
Table 1.
Effects of INT on the development of microalbuminuria and macroalbuminuria during the DCCT and during EDIC years 1–8
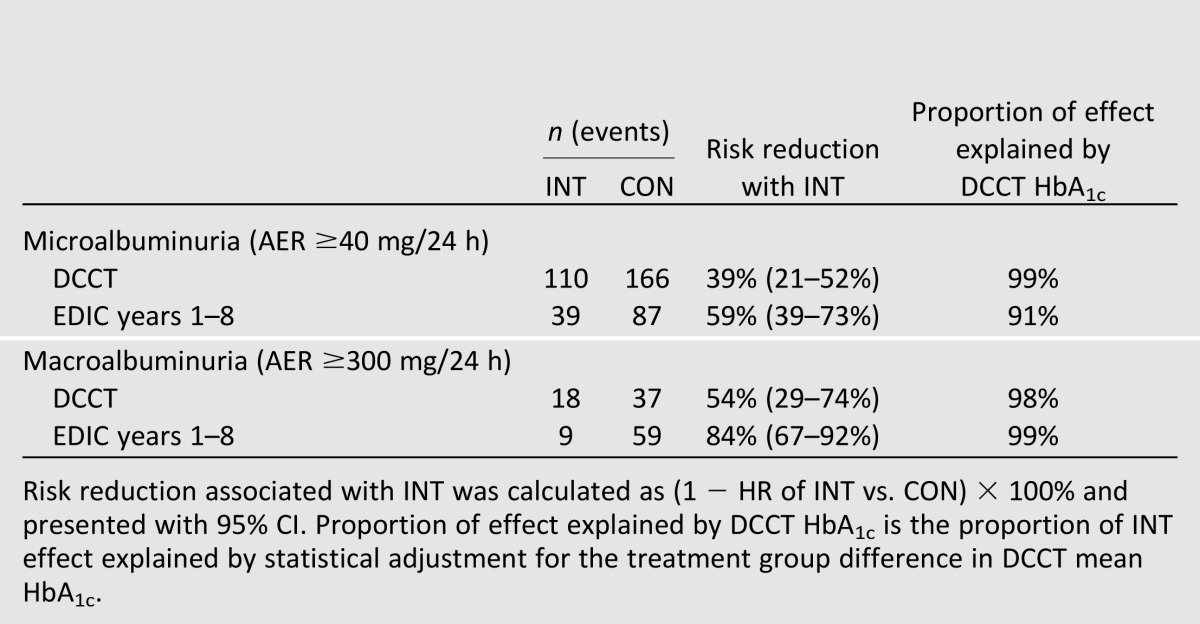
During EDIC years 1–8, among participants who did not develop microalbuminuria during the DCCT, participants previously assigned to DCCT INT continued to experience a lower risk of incident microalbuminuria (risk reduction 59% [95% CI 39–73%]) (8). The effects of INT on micro‐albuminuria during the DCCT and EDIC were largely explained by group differences in mean DCCT HbA1c (Table 1).
Macroalbuminuria
During the DCCT, INT reduced the risk of macroalbuminuria (AER ≥300 mg/24 h) by 54% (95% CI 29–74%) (Table 1) (7). This effect was significant among participants in the secondary prevention cohort (15 vs. 36 cases, risk reduction 56% [95% CI 18–76%]) but not in the primary prevention cohort, where few cases were observed (3 vs. 6 cases, risk reduction 44% [95% CI −124 to 86%]) (17).
During EDIC years 1–8, among participants who did not develop macroalbuminuria during the DCCT, participants previously assigned to DCCT INT had an 84% lower risk of developing incident macroalbuminuria (95% CI 67–92%) (8). As with microalbuminuria, the effects of INT on macroalbuminuria were largely explained by group differences in mean DCCT HbA1c (Table 1).
Impaired GFR
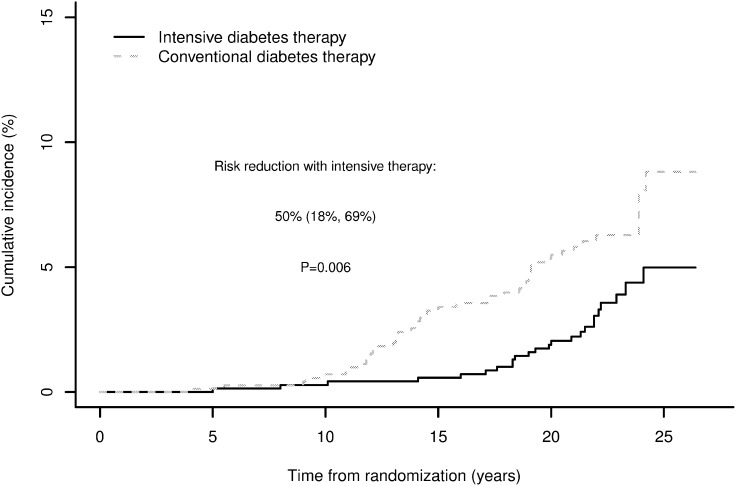
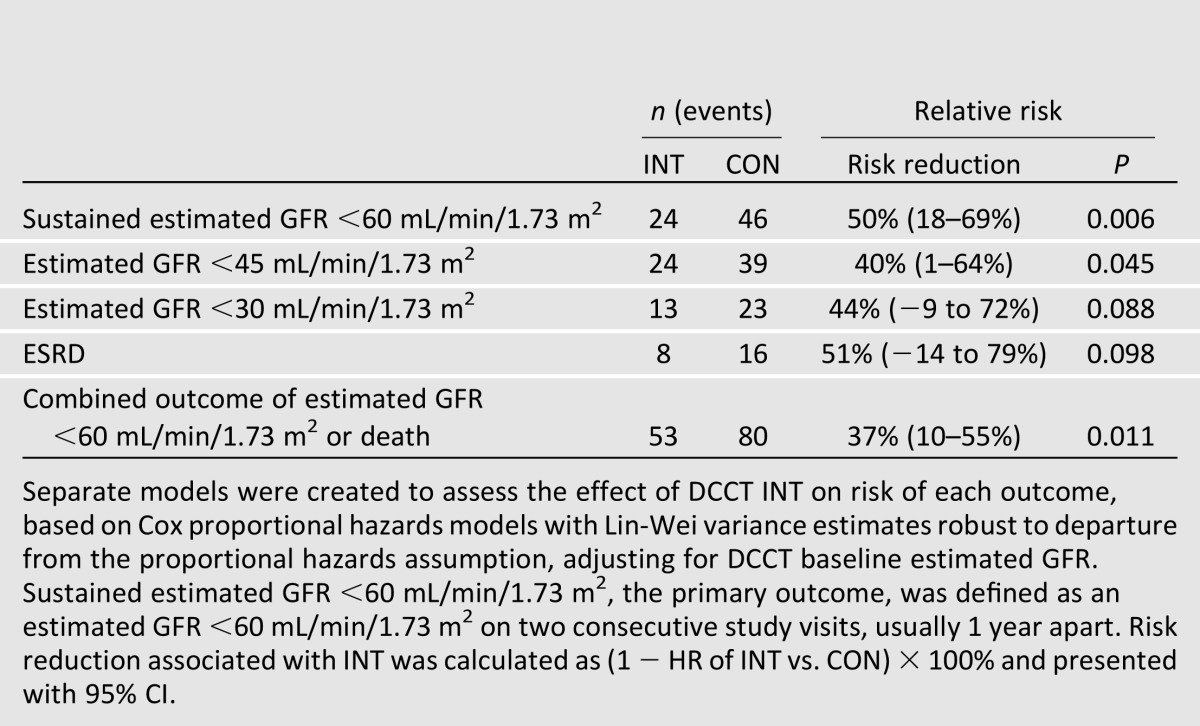
From DCCT baseline through EDIC year 16, 70 participants developed impaired GFR, defined as a sustained estimated GFR <60 mL/min/1.73 m2 on two consecutive study visits (12). Sixty-six of these (94%) occurred during EDIC. Cumulative incidence curves by treatment assignment began separating 10 years after DCCT randomization (Fig. 1). Over combined DCCT/EDIC follow-up, participants assigned to DCCT INT had a 50% lower risk of developing impaired GFR (95% CI 18–69%).
Figure 1.
Cumulative incidence of impaired GFR (sustained estimated GFR <60 mL/min/1.73 m2) by DCCT treatment group, accounting for death as a competing risk. Reproduced with permission from de Boer et al. (12).
Adjustment for DCCT mean HbA1c fully explained the effect of INT on impaired GFR. In parallel, adjustment for updated mean AER explained 90% of the effect of INT on impaired GFR (12). Relative risk reductions for more advanced stages of GFR loss were similar in magnitude to those for estimated GFR <60 mL/min/1.73 m2 (Table 2). However, these risk reductions were not all statistically significant, likely due to small numbers of events. INT reduced the risk of a composite outcome of impaired GFR or death, demonstrating that beneficial effects on impaired GFR were not attributable to a possible competing risk of death.
Table 2.
Effects of INT on the development of impaired GFR during the DCCT/EDIC
Hypertension
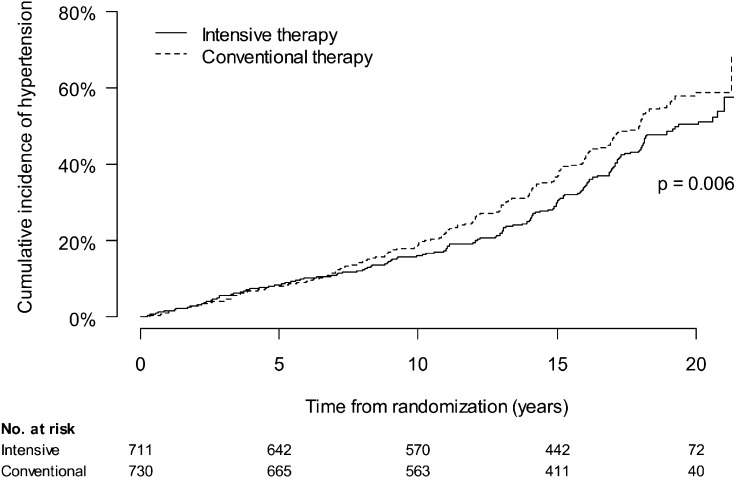
From DCCT baseline through EDIC year 12, 630 participants developed incident hypertension (Fig. 2) (14). During DCCT, the incidence of hypertension was similar in INT and CON treatment groups. However, INT during the DCCT reduced the risk of incident hypertension during EDIC follow-up. Over combined DCCT/EDIC follow-up, INT reduced the risk of hypertension by 20% (95% CI 6–21%). Adjustment for treatment group differences in DCCT mean HbA1c fully explained the effect of INT on incident hypertension. Parallel analyses suggested that AER explained a smaller portion of the effect of INT on hypertension and that weight gain with INT slightly blunted the effect on hypertension that would have otherwise been seen (14).
Figure 2.
Cumulative incidence of hypertension (two consecutive study visits with systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or the use of antihypertensive medications) by DCCT treatment assignment. Reproduced with permission from de Boer et al. (14).
Clinical Course
The detailed long-term data collected during DCCT/EDIC have enabled assessment of the clinical course of kidney disease in T1D. These analyses suggest that INT has modified the clinical course of kidney disease and have allowed further characterization of disease progression in this population.
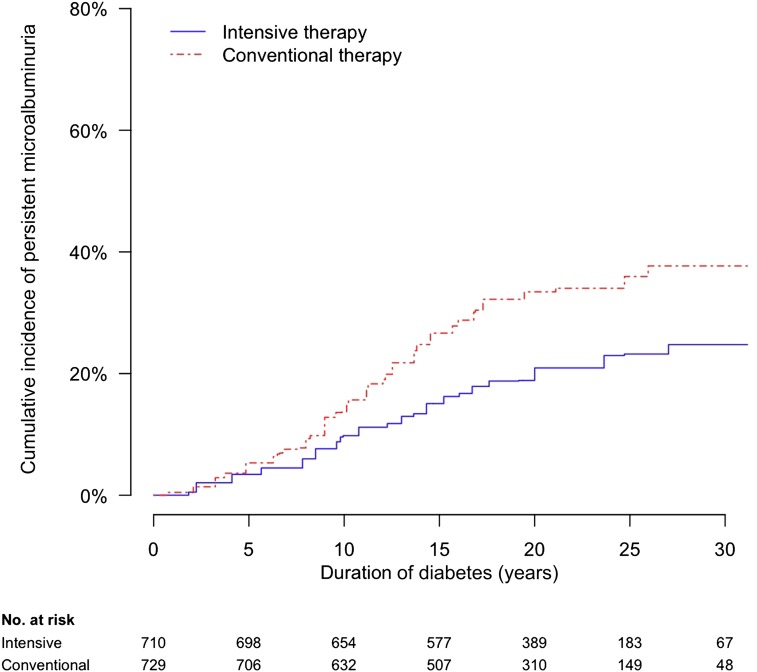
Thirty years after diabetes diagnosis, the cumulative incidences of microalbuminuriaand macroalbuminuria were lower among participants assigned to INT versus CON. The 30-year cumulative incidence of microalbuminuria, defined for the observational analyses as sustained AER ≥30 mg/24 h to be consistent with recent guidelines (18,19), was 25 or 38% among participants assigned to INT or CON, respectively (Fig. 3) (15). Similarly, the 30-year cumulative incidence of macroalbuminuria or worse, defined for this analysis as AER ≥300 mg/24 h or serum creatinine ≥2 mg/dL, was 9 or 25%, respectively (16).
Figure 3.
Cumulative incidence of persistent microalbuminuria (AER ≥30 mg/24 h on two consecutive study visits) in the DCCT/EDIC, by duration of T1D and DCCT treatment assignment. Reproduced with permission from de Boer et al. (15).
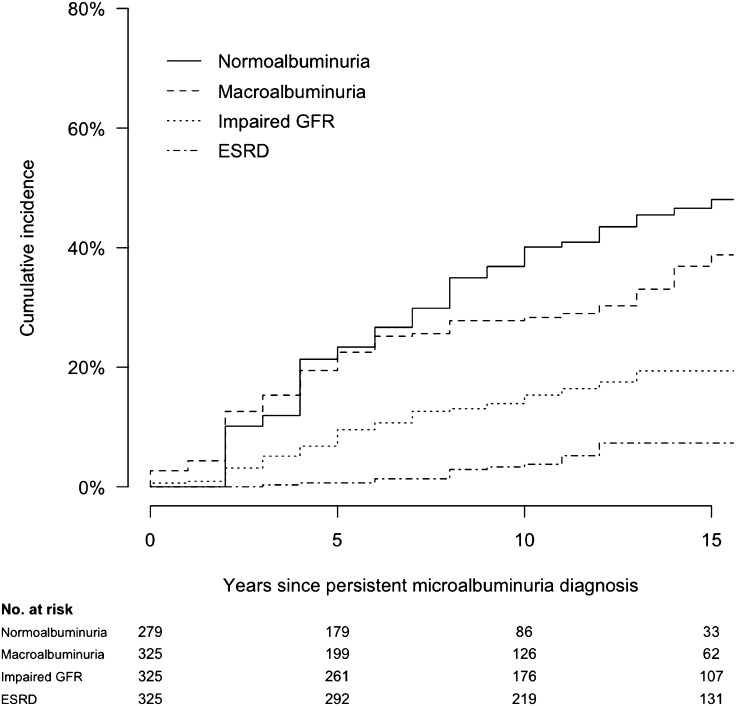
Renal outcomes after developing microalbuminuria were variable in the DCCT/EDIC. Ten years after developing persistent microalbuminuria, 28, 15, and 4% of participants went on to develop macroalbuminuria, impaired GFR, and ESRD, respectively (Fig. 4) (15). However, 40% of participants with incident microalbuminuria regressed to persistent AER <30 mg/24 h. Lower HbA1c was associated with more favorable renal outcomes after microalbuminuria diagnosis.
Figure 4.
Cumulative incidence of long-term renal outcomes after the development of persistent microalbuminuria (AER ≥30 mg/24 h on two consecutive study visits, defining time 0) among 325 participants in the DCCT/EDIC. Normoalbuminuria (AER <30 mg/24 h on two consecutive study visits), macroalbuminuria (AER ≥300 mg/24 h), impaired GFR (estimated GFR <60 mL/min/1.73 m2 on two consecutive study visits), and ESRD are evaluated as parallel outcomes and are not mutually exclusive. Reproduced with permission from de Boer et al. (15).
Macroalbuminuria was more strongly associated than microalbuminuria with GFR loss in the DCCT/EDIC. Mean rate of change in estimated GFR was 5.7% per year (95% CI 4.5–6.8%) in subjects who developed macroalbuminuria, compared with 1.2% per year (1.2–1.3%) with AER <30 mg/24 h and 1.8% per year (1.6–1.9%) in subjects with microalbuminuria (20). Moreover, macroalbuminuria was associated with a more than a 15-fold increase in risk of developing impaired GFR (HR 15.3 [95% CI 8.9–26.3]). Sixty-one percent of participants who developed impaired GFR first developed macroalbuminuria.
Additional work has identified genetic predisposition, male sex, obesity, blood pressure, inflammation, vitamin D deficiency, and dyslipidemia as risk factors for the development and progression of kidney disease in DCCT/EDIC (15,21–28).
Kidney and CVD
In EDIC, higher AER and higher blood pressure were associated with greater common and internal carotid intima-media thickness, a greater prevalence and severity of coronary artery calcium, reduced compliance of the aorta, and greater left ventricular mass (29–32). Moreover, during the combined DCCT/EDIC, microalbuminuria and macroalbuminuria were associated with nearly threefold increased risks of clinical CVD events (HR 2.93 [95% CI 1.85–4.65] and 2.57 [1.36–4.88], respectively) (33). Adjustment for treatment group differences in microalbuminuria and macroalbuminuria explained a minority of the beneficial effect of INT on risk of clinical CVD events.
Conclusions
In the DCCT/EDIC, INT resulted in substantial, durable reductions in the risks of microalbuminuria, macroalbuminuria, impaired GFR, and hypertension. For microalbuminuria and macroalbuminuria, beneficial effects were observed during the DCCT. Moreover, during EDIC follow-up, the incidences of microalbuminuria and macroalbuminuria continued to be lower among participants previously assigned to DCCT INT, with risk reductions during EDIC years 1–8 that were even greater than those observed during the DCCT itself. For impaired GFR and hypertension, beneficial effects of INT were not observed during the DCCT, but significant effects became apparent during long-term EDIC follow-up. The cumulative incidences of microalbuminuria and macroalbuminuria 30 years after diagnosis of diabetes were substantially lower among participants assigned to INT versus CON, providing further evidence that early INT alters the clinical course of kidney disease in T1D.
The term “metabolic memory” has been used to describe the differences in outcomes that persist or even expand after the original separation in chronic glycemia, based on treatment assignment, has disappeared. The persistent effects of INT on microalbuminuria and macroalbuminuria during EDIC years 1–8 are striking examples of this phenomenon. The subsequent beneficial effects of INT on impaired GFR and hypertension represent the long-term consequences of INT. The biological basis of metabolic memory remains a matter of speculation. Clinically, the important implication is that INT applied early in the course of T1D has strong and long-lasting effects to reduce the long-term risks of kidney disease.
Importantly, the DCCT/EDIC evaluated the effects of INT on each of the two most important clinical features of diabetic kidney disease—albuminuria and impaired GFR. To our knowledge, INT is the only intervention that has been demonstrated to reduce the risks of developing both albuminuria (microalbuminuria or macroalbuminuria) and impaired GFR in T1D. This emphasizes the importance of hyperglycemia in the pathophysiology of kidney disease in T1D and the primary role for glucose control in preventing both major manifestations of diabetic kidney disease in this population.
The beneficial effects of INT on the risks of microalbuminuria, macroalbuminuria, and impaired GFR were fully attenuated by statistical adjustment for HbA1c. Interestingly, in parallel, the beneficial effects of INT on the risk of impaired GFR were also fully attenuated by statistical adjustment for AER. This suggests that a common set of disease mechanisms leading from hyperglycemia to both albuminuria and impaired GFR is interrupted by INT. Additional observations from the DCCT/EDIC suggest that other mechanisms also contribute to the development of microalbuminuria, macroalbuminuria, and impaired GFR, and that these mechanisms may vary by disease manifestation. Most participants who developed microalbuminuria did not go on to develop impaired GFR, and most but not all participants who developed impaired GFR first developed macroalbuminuria. Blood pressure, obesity, and other metabolic factors appear to influence the development of kidney disease in T1D, in addition to hyperglycemia. These risk factors may offer additional treatment options to prevent and treat kidney disease in T1D, in addition to intensive glucose control.
Higher AER and blood pressure were associated with increased risks of CVDs, including carotid atherosclerosis, coronary atherosclerosis, stiffening of the aorta, and left ventricular hypertrophy. Taken together with similar observations in other cohorts (34,35), these results suggest a central role for kidney disease in the macrovascular complications of T1D. The beneficial effects of INT on kidney disease and blood pressure would therefore be expected to yield subsequent reductions in the risks of CVD. Statistical modeling suggested that reductions in AER explained a minority of the beneficial effects of INT on clinical CVD events, but such mediation analyses can underestimate the impact of covariates. Future studies will further evaluate the impact of kidney disease on CVD in the DCCT/EDIC, including the extent to which reductions in kidney disease with INT affect risk of CVD over long-term follow-up.
Strengths of the DCCT/EDIC include its randomized, controlled design with substantial separation in HbA1c achieved by treatment assignment; the detailed longitudinal characterization of diabetes complications; and the unusually long duration of follow-up, particularly for a clinical trial. These strengths were made possible by dedicated research staff, consistent sponsor support, and, most important, the invaluable contributions of the DCCT/EDIC participants. Low numbers of ESRD and CVD events have limited analyses of these outcomes to date. Continued follow-up of the DCCT/EDIC cohort will facilitate further examination of these events in the future.
In conclusion, INT applied early in the course of T1D resulted in clinically important, long-lasting reductions in the incidence of kidney disease. These benefits, combined with congruent reductions in the risks of retinopathy, neuropathy, CVD, and other diabetes complications, serve as a cornerstone of recommendations to control glycemia in T1D.
Article Information
Funding. The DCCT/EDIC has been supported by U01 Cooperative Agreement grants (1982–1993, 2011–2016) and contracts (1982–2011) with the Division of Diabetes Endocrinology and Metabolic Diseases of the National Institute of Diabetes and Digestive and Kidney Diseases (current grant numbers U01-DK-094176 and U01-DK-094157) and through support of the National Eye Institute, the National Institute of Neurological Disorders and Stroke, the Genetic Clinical Research Centers Program (1993–2007), and Clinical and Translational Science Center Program (2006–present), Bethesda, MD. I.H.d.B. was supported by grants R01-DK-087726, R01-DK-088762, and RC4-DK-090766 from the National Insititute of Diabetes and Digestive and Kidney Diseases.
Industry contributors have had no role in the DCCT/EDIC but have provided free or discounted supplies or equipment to support participants’ adherence to the study: Abbott Diabetes Care (Alameda, CA); Animas (West Chester, PA); Bayer Diabetes Care (Tarrytown, NY); Becton, Dickinson and Company (Franklin Lakes, NJ); CanAm (Atlanta, GA); Eli Lilly (Indianapolis, IN); LifeScan (Milpitas, CA); Medtronic Diabetes (Minneapolis, MI); Nova Diabetes Care (Bedford, MA); Omron (Shelton, CT); OmniPod Insulin Management System (Bedford, MA); Roche Diabetes Care (Indianapolis, IN); and Sanofi (Bridgewater, NJ).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. The DCCT/EDIC Research Group collected and analyzed the data. I.H.d.B. participated in data collection and analysis, and wrote the manuscript. I.H.d.B. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Clinical trial reg. nos. NCT00360893 and NCT00360815, clinicaltrials.gov.
A complete list of participants in the DCCT/EDIC Research Group can be found in N Engl J Med 2011;365:2366–2376.
References
- 1.Andersen AR, Christiansen JS, Andersen JK, Kreiner S, Deckert T. Diabetic nephropathy in type 1 (insulin-dependent) diabetes: an epidemiological study. Diabetologia 1983;25:496–501 [DOI] [PubMed] [Google Scholar]
- 2.Krolewski AS, Warram JH, Christlieb AR, Busick EJ, Kahn CR. The changing natural history of nephropathy in type I diabetes. Am J Med 1985;78:785–794 [DOI] [PubMed] [Google Scholar]
- 3.Groop PH, Thomas MC, Moran JL, et al. FinnDiane Study Group The presence and severity of chronic kidney disease predicts all-cause mortality in type 1 diabetes. Diabetes 2009;58:1651–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Orchard TJ, Secrest AM, Miller RG, Costacou T. In the absence of renal disease, 20 year mortality risk in type 1 diabetes is comparable to that of the general population: a report from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetologia 2010;53:2312–2319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes 2005;54:1615–1625 [DOI] [PubMed] [Google Scholar]
- 6.Chase HP, Jackson WE, Hoops SL, Cockerham RS, Archer PG, O’Brien D. Glucose control and the renal and retinal complications of insulin-dependent diabetes. JAMA 1989;261:1155–1160 [PubMed] [Google Scholar]
- 7.The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 8.Writing Team for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group Sustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy: the Epidemiology of Diabetes Interventions and Complications (EDIC) study. JAMA 2003;290:2159–2167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nathan DM, DCCT/EDIC Research Group The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study at 30 years: overview. Diabetes Care 2014;37:9–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Molitch ME, Steffes MW, Cleary PA, Nathan DM. Baseline analysis of renal function in the Diabetes Control and Complications Trial. The Diabetes Control and Complications Trial Research Group. [published correction appears in Kidney Int 1993;43:1196]. Kidney Int 1993;43:668–674 [DOI] [PubMed] [Google Scholar]
- 11.Younes N, Cleary PA, Steffes MW, et al. DCCT/EDIC Research Group Comparison of urinary albumin-creatinine ratio and albumin excretion rate in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study. Clin J Am Soc Nephrol 2010;5:1235–1242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Boer IH, Sun W, Cleary PA, et al. DCCT/EDIC Research Group Intensive diabetes therapy and glomerular filtration rate in type 1 diabetes. N Engl J Med 2011;365:2366–2376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inker LA, Schmid CH, Tighiouart H, et al. CKD-EPI Investigators Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 2012;367:20–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Boer IH, Kestenbaum B, Rue TC, et al. Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Study Research Group Insulin therapy, hyperglycemia, and hypertension in type 1 diabetes mellitus. Arch Intern Med 2008;168:1867–1873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Boer IH, Rue TC, Cleary PA, et al. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study Research Group Long-term renal outcomes of patients with type 1 diabetes mellitus and microalbuminuria: an analysis of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications cohort. Arch Intern Med 2011;171:412–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nathan DM, Zinman B, Cleary PA, et al. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Research Group Modern-day clinical course of type 1 diabetes mellitus after 30 years’ duration: the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications and Pittsburgh Epidemiology of Diabetes Complications experience (1983–2005). Arch Intern Med 2009;169:1307–1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The Diabetes Control and Complications (DCCT) Research Group Effect of intensive therapy on the development and progression of diabetic nephropathy in the Diabetes Control and Complications Trial. Kidney Int 1995;47:1703–1720 [DOI] [PubMed] [Google Scholar]
- 18.American Diabetes Association Standards of medical care in diabetes—2013. Diabetes Care 2013;36(Suppl. 1):S11–S66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Kidney FoundationKDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Diabetes and Chronic Kidney Disease Am J Kidney Dis 2007;49(Suppl. 2):S12–S154 [DOI] [PubMed] [Google Scholar]
- 20.Molitch ME, Steffes M, Sun W, et al. Epidemiology of Diabetes Interventions and Complications Study Group Development and progression of renal insufficiency with and without albuminuria in adults with type 1 diabetes in the Diabetes Control and Complications Trial and the Epidemiology of Diabetes Interventions and Complications Study. Diabetes Care 2010;33:1536–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Boer IH, Sachs MC, Cleary PA, et al. Diabetes Control and Complication Trial/Epidemiology of Diabetes Interventions and Complications Study Research Group Circulating vitamin D metabolites and kidney disease in type 1 diabetes. J Clin Endocrinol Metab 2012;97:4780–4788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Boer IH, Sibley SD, Kestenbaum B, et al. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study Research Group Central obesity, incident microalbuminuria, and change in creatinine clearance in the epidemiology of diabetes interventions and complications study. J Am Soc Nephrol 2007;18:235–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al-Kateb H, Boright AP, Mirea L, et al. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group Multiple superoxide dismutase 1/splicing factor serine alanine 15 variants are associated with the development and progression of diabetic nephropathy: the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Genetics study. Diabetes 2008;57:218–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Böger CA, Chen MH, Tin A, et al. CKDGen Consortium CUBN is a gene locus for albuminuria. J Am Soc Nephrol 2011;22:555–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boright AP, Paterson AD, Mirea L, et al. DCCT/EDIC Research Group Genetic variation at the ACE gene is associated with persistent microalbuminuria and severe nephropathy in type 1 diabetes: the DCCT/EDIC Genetics Study. Diabetes 2005;54:1238–1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Orchard TJ, Sun W, Cleary PA, et al. Haptoglobin genotype and the rate of renal function decline in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study. Diabetes 2013;62:3218–3223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lopes-Virella MF, Carter RE, Baker NL, Lachin J, Virella G. High levels of oxidized LDL in circulating immune complexes are associated with increased odds of developing abnormal albuminuria in type 1 diabetes. Nephrol Dial Transplant 2012;27:1416–1423 [DOI] [PMC free article] [PubMed]
- 28.Lopes-Virella MF, Baker NL, Hunt KJ, Cleary PA, Klein R, Virella G, DCCT/EDIC Research Group Baseline markers of inflammation are associated with progression to macroalbuminuria in type 1 diabetic subjects. Diabetes Care 2013;36:2317–2323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nathan DM, Lachin J, Cleary P, et al. Diabetes Control and Complications Trial. Epidemiology of Diabetes Interventions and Complications Research Group Intensive diabetes therapy and carotid intima-media thickness in type 1 diabetes mellitus. N Engl J Med 2003;348:2294–2303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cleary PA, Orchard TJ, Genuth S, et al. DCCT/EDIC Research Group The effect of intensive glycemic treatment on coronary artery calcification in type 1 diabetic participants of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study. Diabetes 2006;55:3556–3565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Turkbey EB, Backlund JY, Genuth S, et al. DCCT/EDIC Research Group Myocardial structure, function, and scar in patients with type 1 diabetes mellitus. Circulation 2011;124:1737–1746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Turkbey EB, Redheuil A, Backlund JY, et al. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group Aortic distensibility in type 1 diabetes. Diabetes Care 2013;36:2380–2387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nathan DM, Cleary PA, Backlund JY, et al. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kelbaek H, Jensen T, Feldt-Rasmussen B, et al. Impaired left-ventricular function in insulin-dependent diabetic patients with increased urinary albumin excretion. Scand J Clin Lab Invest 1991;51:467–473 [DOI] [PubMed] [Google Scholar]
- 35.Maahs DM, Jalal D, Chonchol M, Johnson RJ, Rewers M, Snell-Bergeon JK. Impaired renal function further increases odds of 6-year coronary artery calcification progression in adults with type 1 diabetes: the CACTI study. Diabetes Care 2013;36:2607–2614 [DOI] [PMC free article] [PubMed] [Google Scholar]