Abstract
OBJECTIVE
To describe the development and progression of neuropathy and related findings among patients with type 1 diabetes who participated in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) study.
RESEARCH DESIGN AND METHODS
The main diabetic peripheral neuropathy (DPN) outcome was assessed using clinical symptoms, signs, and nerve conduction study results during DCCT and repeated in EDIC year 13/14. Cardiovascular autonomic neuropathy (CAN) was assessed by R-R response to paced breathing, Valsalva ratio, and blood pressure response to standing during DCCT and in EDIC years 13/14 and 16/17. Additionally, symptoms reflecting neuropathic pain and autonomic function (including hypoglycemia awareness) were collected yearly in EDIC using standardized questionnaires; peripheral neuropathy was also assessed annually using the Michigan Neuropathy Screening Instrument. Assessments of genitourinary function were collected at EDIC year 10.
RESULTS
Intensive therapy during the DCCT significantly reduced the risk of DPN and CAN at DCCT closeout (64% and 45%, respectively, P < 0.01). The prevalence and incidence of DPN and CAN remained significantly lower in the DCCT intensive therapy group compared with the DCCT conventional therapy group through EDIC year 13/14.
CONCLUSIONS
The persistent effects of prior intensive therapy on neuropathy measures through 14 years of EDIC largely mirror those observed for other diabetes complications. DCCT/EDIC provides important information on the influence of glycemic control, and the clinical course of diabetic neuropathy, and, most important, on how to prevent neuropathy in type 1 diabetes.
Introduction
The Diabetes Control and Complications Trial (DCCT) enrolled 1,441 patients with type 1 diabetes between 1983 and 1989 (1). At study entry, participants were randomly assigned to intensive insulin therapy (INT), targeting near-normal glycemia, or conventional insulin therapy (CON) according to the standard of care at that time. Microvascular diabetes complications were assessed during the DCCT, including the development and progression of the peripheral and cardiovascular autonomic manifestations of diabetic neuropathy. In 1993, after an average of 6.5 years of follow-up, the DCCT investigators reported that INT significantly reduced the incidence of diabetic neuropathy, similar to findings for diabetic retinopathy and nephropathy (1–3). INT was subsequently widely accepted as the standard of care for type 1 diabetes. At DCCT end, DCCT CON participants were taught INT, and all participants were encouraged to adhere as closely as possible to an intensive diabetes treatment regimen and were returned to their prior health care providers for ongoing care. The observational Epidemiology of Diabetes Interventions and Complications (EDIC) follow-up was established to monitor the long-term effects of prior INT compared with prior CON treatment on the development and progression of more advanced microvascular complications and cardiovascular disease in the DCCT cohort.
Diabetic neuropathy represents a clinically diverse group of disorders having differing anatomic distribution, clinical course, and underlying pathophysiology, but ultimately thought to reflect metabolic and microvascular factors that result in axonal degeneration of large- and small-nerve fibers. The specific presentation of diabetic neuropathy reflects the distribution and size of nerve fibers involved, most commonly presenting as a distal symmetric sensory or sensorimotor neuropathy (diabetic peripheral neuropathy [DPN]). Manifestations of autonomic neuropathies, including cardiovascular autonomic neuropathy (CAN), may also develop. Although the specific clinical manifestations are heterogeneous, diabetic neuropathy is a major cause of disability, associated with reduced quality of life and high mortality. In this article, we detail and discuss the diabetic neuropathy outcomes, including DPN and CAN, during DCCT/EDIC.
Research Design and Methods
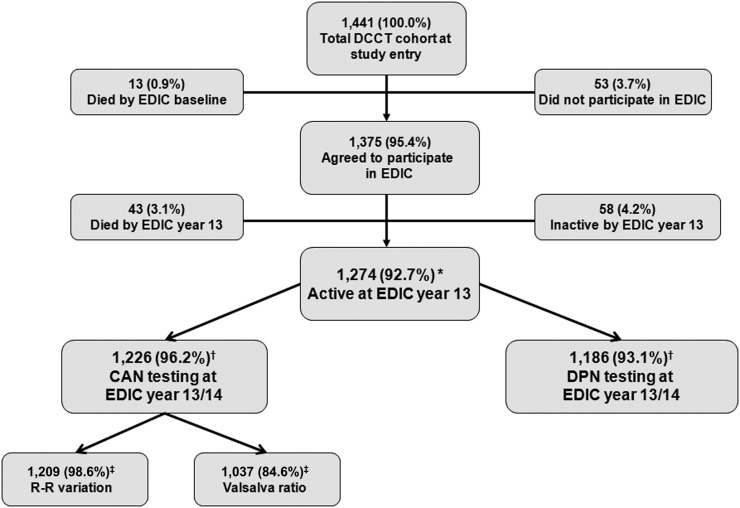
Detailed descriptions of DCCT and EDIC procedures and baseline characteristics have been described previously (1,4). The DCCT and EDIC protocols were approved by the institutional review boards of all participating centers, and all participants provided written informed consent. Participation in peripheral and cardiovascular autonomic neuropathy outcome assessments through EDIC years 13/14 are shown (Fig. 1).
Figure 1.
Flow diagram of DCCT/EDIC subject participation in CAN and DPN assessments at EDIC year 13/14. *Percentage based on the original number of EDIC participants (n = 1,375). †Percentage based on number of active EDIC participants at year 13/14 (n = 1,274). ‡Percentages shown for R-R variation and Valsalva ratio are based on number of EDIC participants with CAN test at EDIC year 13/14 (n = 1,226). DCCT/EDIC CAN testing included R-R variation during deep breathing, Valsalva maneuver, and postural testing. DPN testing included history and examination by a board-certified neurologist and nerve conduction studies. Adapted with permission from Pop-Busui et al. (16).
DPN
DPN was assessed by board-certified neurologists using a standardized evaluation to identify symptoms, signs, and nerve conduction abnormalities consistent with distal symmetrical peripheral neuropathy. These assessments were done at DCCT baseline, after 5 years of DCCT participation and/or at the end of the DCCT, and again during EDIC year 13/14. Vibration perception threshold testing was performed at EDIC year 13/14 using a forced-choice algorithm of decreasing vibration intensity at the dominant index finger and great toe (5). An annual neuropathy assessment was performed in EDIC using the Michigan Neuropathy Screening Instrument (MNSI), a 15-item symptom questionnaire and a structured examination for foot ulcers, deformities, infections, excessive dryness, and calluses, plus ankle reflexes and distal vibration perception (6–8).
DPN Definitions
DPN was defined using a three-level hierarchy of clinical findings and nerve conduction results:
-
1.
Clinically evident DPN, defined as at least two positive findings among sensory symptoms, signs, or reflex abnormalities consistent with a distal symmetrical polyneuropathy.
-
2.
Abnormal nerve conduction studies, defined by the presence of at least one abnormal nerve attribute (of amplitude, latency, F-wave, or nerve conduction velocity) in two or more nerves among the median, peroneal, and sural nerves.
-
3.
Confirmed DPN, defined as the presence of both clinically evident DPN and abnormal nerve conduction studies as defined above. Confirmed DPN was the primary outcome measure of peripheral neuropathy used during DCCT and EDIC.
Abnormal vibration perception threshold was defined as a threshold value more than 2.5 SDs above the age-adjusted mean value obtained from nondiabetic referents (5). The MNSI criterion for DPN was based on a symptom score of ≥7 or a physical examination score of >2 (6,8).
CAN
CAN was assessed at baseline and every 2 years in DCCT using a battery of tests that included R-R variation during deep breathing, the Valsalva maneuver, and postural testing. These tests were repeated in EDIC year 13/14 and again during year 16–17 (9). Subjects were uniformly prepared for CAN testing by instruction that included fasting; abstaining from caffeine, tobacco, and medications the morning of testing; avoidance of vigorous physical activity and alcohol consumption for 48 h prior to testing; and absence of hypoglycemia prior to and during testing (3,9).
CAN Definitions
The primary CAN outcome was defined as any of following criteria: R-R variation <15, R-R variation of <20 plus a Valsalva ratio ≤1.5, or a decrease of >10 mmHg in diastolic blood pressure at any point during 10 min of standing after a period of 30 min of supine rest (postural hypotension) (3,9). In EDIC, secondary CAN outcomes included the age-adjusted R-R variation and Valsalva ratio (9).
Other Neuropathy-Related Outcomes During EDIC
A structured medical history was obtained annually in EDIC including questions regarding the presence of painful or noxious symptoms of peripheral neuropathy, use of medications to treat painful neuropathic symptoms, symptoms of postural hypotension, gastroparesis, diarrhea, colonic atony, genitourinary dysfunction, and hypoglycemic unawareness.
The Uro-EDIC ancillary study was conducted at EDIC year 10 as a cross-sectional evaluation to describe the prevalence of diabetic genitourinary complications, including erectile dysfunction, lower urinary tract symptoms, and urinary incontinence, and to investigate relationships between these and other diabetes complications. Erectile dysfunction was defined as an erectile function domain score of <20 using the International Index of Erectile Function and by response to a single questionnaire item regarding “confidence to get and keep an erection” (10,11). Lower urinary tract symptoms were assessed using the American Urological Association Symptom Index with moderate to severe lower urinary tract symptoms defined by scores >7 (10). Urinary incontinence in women was measured using a standardized questionnaire (12,13).
Cardiac magnetic resonance imaging (MRI) was performed during EDIC year 15/16, affording an opportunity to relate cardiac structural and functional information to concurrent CAN findings (14).
Results
DPN
During DCCT, the prevalence of confirmed DPN increased slightly among INT group participants (from 7 to 9%), but substantially in CON subjects (from 5 to 17%, P < 0.001) (Table 1) (2,15,16). Significant treatment group differences were observed for all measures of incident DPN at the end of the DCCT. Adjusting for the presence of confirmed DPN at baseline, the INT risk reduction for incident neuropathy during the DCCT was 64% (95% CI 45–76) (1).
Table 1.
Prevalence of DPN and CAN outcomes at DCCT baseline, DCCT closeout, and EDIC year 13/14 (16)
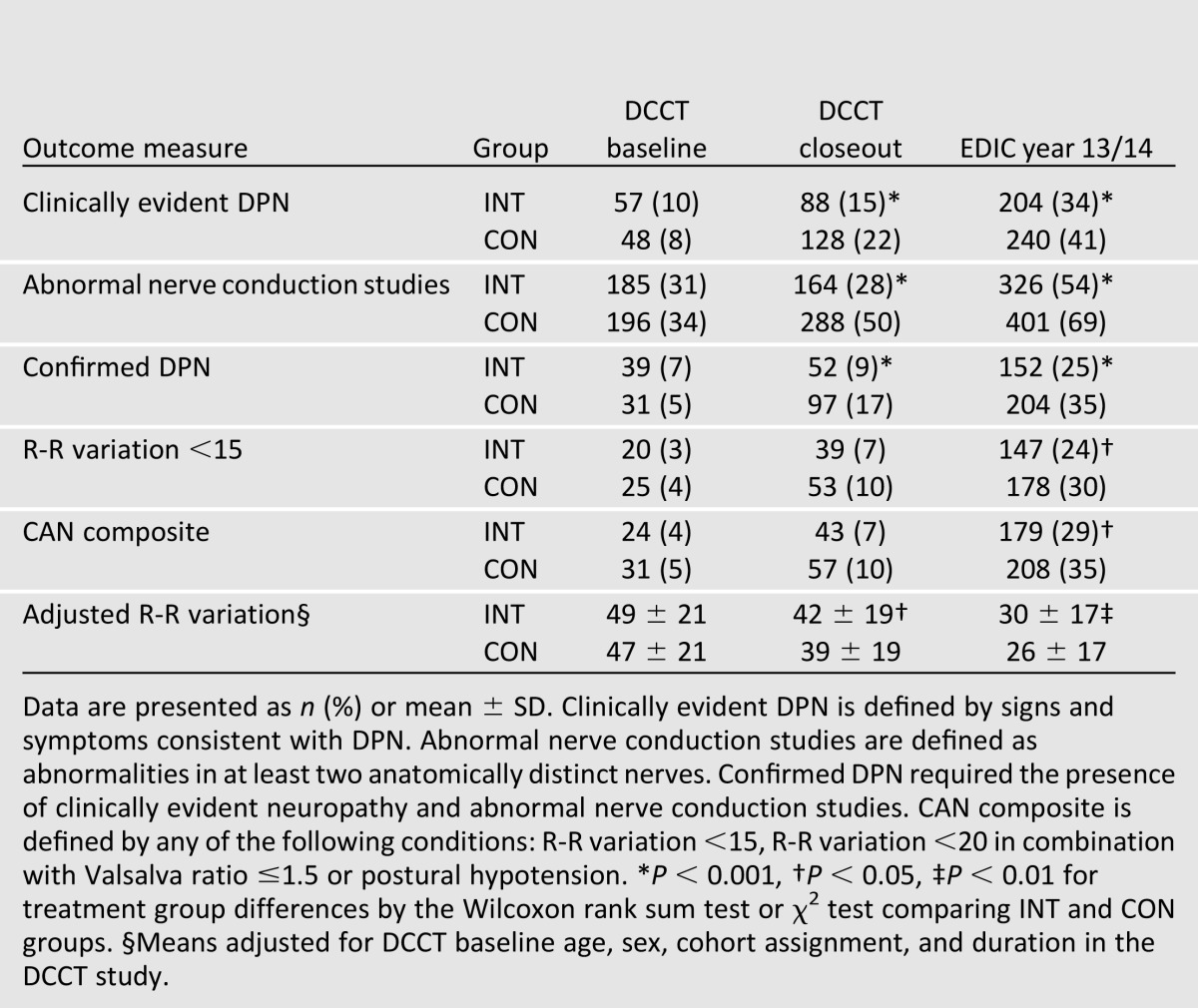
The prevalence of all measures of DPN increased during EDIC, and treatment group differences continued to be significant (Table 1). In EDIC, a 30% reduction in the risk of incident confirmed DPN was observed with prior INT (odds ratio [OR] 0.70 [95% CI 0.52– 0.93]) (Table 2). Similar magnitude reductions were documented for several nerve conduction study measures (15).
Table 2.
Incidence of clinically evident DPN, abnormal nerve conduction studies, and confirmed DPN at DCCT closeout and EDIC year 13/14* (15)
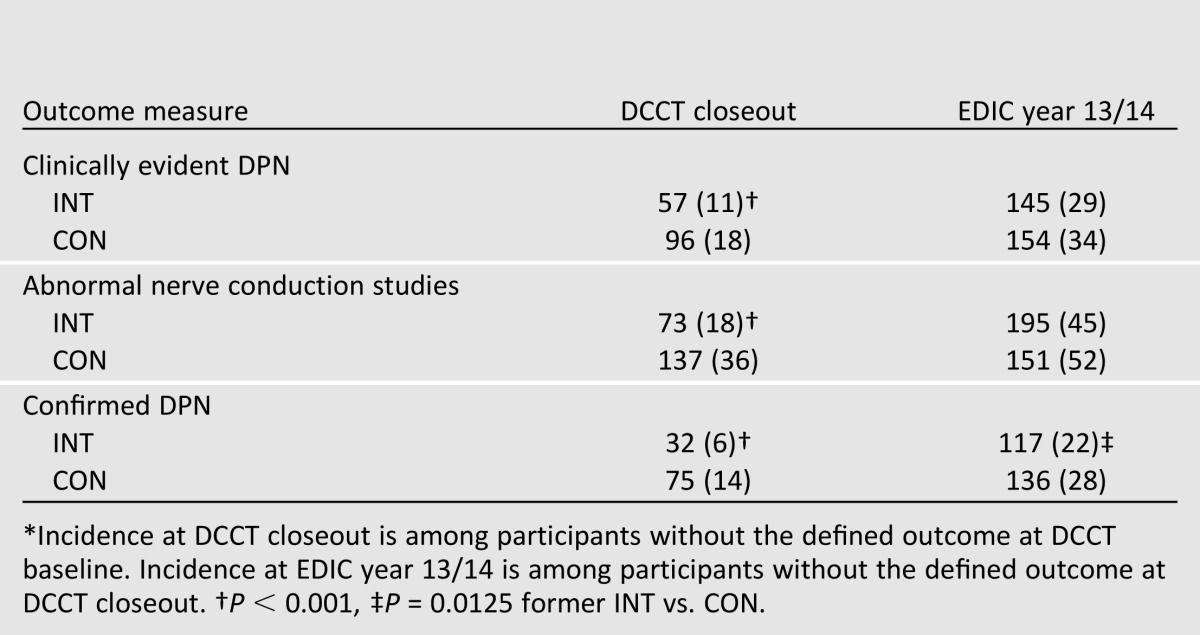
One explanation for the finding of a persistent benefit of INT for confirmed DPN at EDIC year 13/14 could be the presence of different levels of subclinical neuropathy in the INT and CON groups at DCCT closeout, as any significant group difference in the nerve conduction results could influence the subsequent development of confirmed DPN during EDIC (17). This possibility was addressed using analytic models of incident neuropathy that adjusted for nerve conduction study results at DCCT closeout. These additional models negated the treatment group differences in development of neuropathy observed during EDIC. Specifically, after correction for difference in nerve conduction study results at DCCT closeout, no significant risk reduction was associated with former INT at EDIC years 13/14 (OR 1.17 [95% CI 0.84 –1.63]) (15).
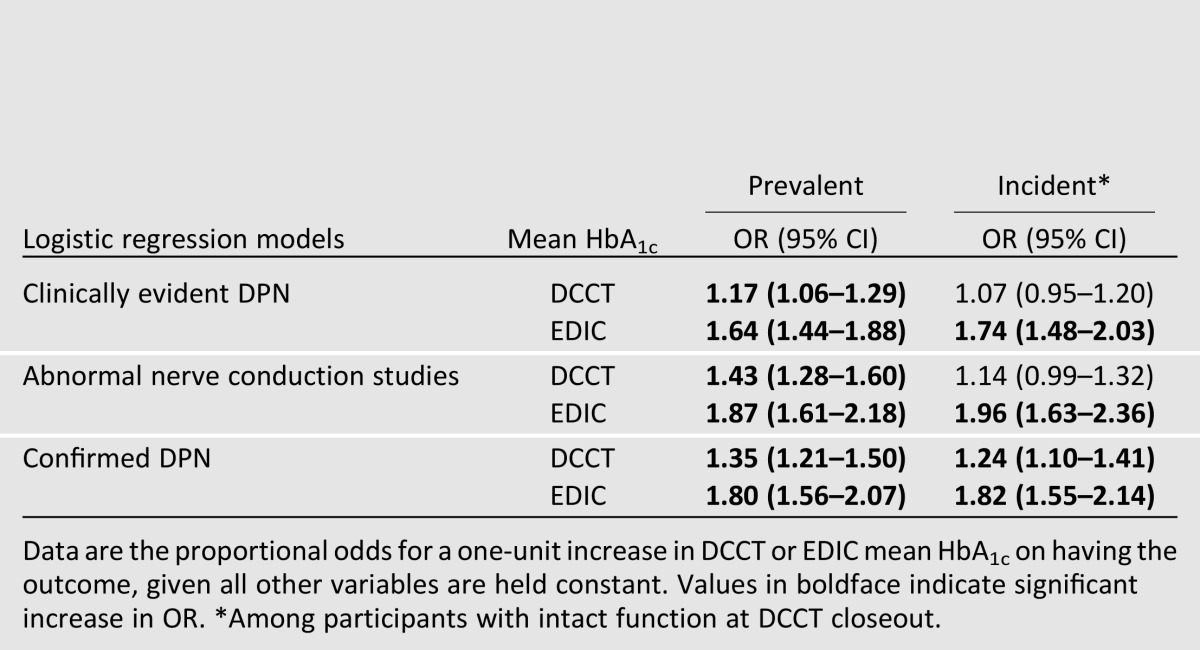
Logistic regression models were used to evaluate the effect of glycemic control on prevalent and incident DPN in EDIC. The odds of clinically evident DPN, abnormal nerve conduction studies, and confirmed DPN in EDIC (prevalence) each increased per unit (%) increase in DCCT mean HbA1c and EDIC mean HbA1c. The incidence of any of the three DPN outcomes during EDIC was increased with each unit increase in mean EDIC HbA1c. The DCCT mean HbA1c was associated only with increased odds for incident confirmed DPN in EDIC (Table 3) (15).
Table 3.
Effect of glycemic exposure in DCCT and EDIC (per unit increase in HbA1c) on the odds of prevalent (at EDIC year 13/14) or incident (during EDIC) clinically evident DPN, abnormal nerve conduction study, and confirmed DPN (15)
Vibration perception threshold was abnormally high at the great toe in 57% of former INT subjects versus 64% of former CON subjects (P < 0.05). The vibration perception threshold was lower (indicating better sensitivity) among former INT subjects (3.53 vibration units vs. 4.03 vibration units, P < 0.01) (5).
The prevalence of DPN as measured by MNSI at the first year of EDIC was 1.8% versus 4.7% in INT and CON, respectively (P < 0.0001) by MNSI questionnaire and 17.8% versus 28.0% (P < 0.0001) by MNSI examination. Significant treatment group effects using MNSI criteria for DPN persisted through at least EDIC year 8. A 1% lower cumulative mean HbA1c (e.g., 8% [64 mmol/mol] to 7% [53 mmol/mol]) reduced the odds of DPN by 38% and by 27% for the questionnaire and examination, respectively (8). At year 14 of EDIC, the overall prevalence of DPN using the MNSI examination was 33%, closely mirroring the overall prevalence of concurrently measured confirmed DPN (30%) (7).
Use of medication for neuropathic pain was reported by 7% of former INT participants and 6% of former CON participants at EDIC year 13/14. The difference was not statistically significant (15).
CAN
The prevalence of CAN was very low at the start of the DCCT (4% INT vs. 5% CON, P = NS). By DCCT end, the prevalence remained stable in INT, and had almost doubled in CON participants (5% vs. 9%, P = 0.0017) (Table 1) (3). The incidence of CAN was reduced by 45% with intensive treatment during the course of the DCCT.
The prevalence of CAN at EDIC year 13/14 was 29% INT vs. 35% CON, P = 0.018) (9). Group differences were primarily driven by differences in R-R variation. The continuous R-R variation remained significantly higher in the INT group compared with the CON group, even after adjusting for important covariates including age, sex, and duration in DCCT (adjusted means 29.9 vs. 25.6, P < 0.001) (9).
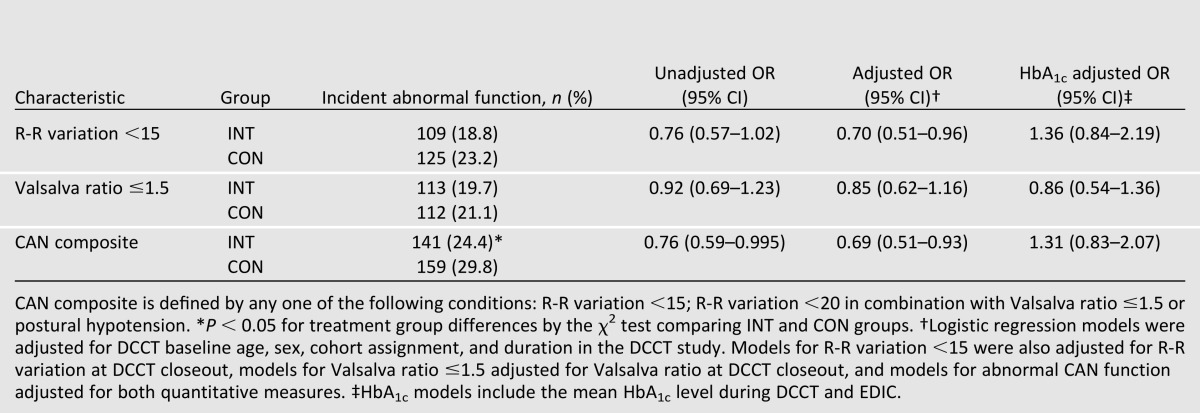
During EDIC, there was a 31% reduction in the risk for incident CAN in INT subjects who were free of CAN at DCCT closeout, with significant risk reductions remaining after adjustments that included age, R-R variation at DCCT closeout, medication use, and several other covariates (Table 4) (9,16). Virtually all of the treatment group difference in the incidence of CAN could be explained by the treatment group differences in mean HbA1c levels over time.
Table 4.
Incidence of abnormal CAN measurements at EDIC year 13/14 among subjects with intact function at DCCT closeout (9)
Comprehensive cardiovascular disease evaluations carried out during EDIC allowed for additional analyses to understand further the clinical implications of CAN. Associations between CAN and cardiac structure and function were analyzed in 966 EDIC participants with concomitant cardiac MRI and CAN measurements at EDIC year 16 (14). Although parameters of left ventricle systolic function, including the ejection fraction, did not differ among EDIC participants with and without CAN, those participants with CAN had higher left ventricular mass and mass-to-volume ratios compared with participants without CAN (P < 0.0001 for each), changes consistent with left ventricular concentric remodeling that were independent of age, sex, and other traditional cardiovascular risk factors.
Other Autonomic and Genitourinary Outcomes
A small number of participants from both former treatment groups reported autonomic symptoms. Decreased adrenergic awareness of hypoglycemia (20% INT vs. 25% CON, P = NS) excessive postprandial epigastric fullness (8% INT vs. 8% CON, P = NS), and male impotence were among the most commonly reported symptoms at EDIC year 13/14 (9).
Erectile dysfunction was ascertained in 591 men during EDIC year 10, as part of the Uro-EDIC (18). Erectile dysfunction was present in 23% of these men. No DCCT treatment group differences were observed for reported erectile dysfunction among men with diabetes duration of 1–5 years and who had no evidence of microvascular complications at DCCT entry (DCCT primary prevention cohort 17% INT vs. 20.3% CON, P = 0.49). Significant treatment group difference were observed among men with up to 15 years diabetes duration and retinopathy at DCCT entry (DCCT secondary intervention cohort 12.8% INT vs. 30.8% CON, P = 0.001). Age, DPN, and mean DCCT-EDIC HbA1c were significant risk factors associated with the presence of erectile dysfunction (18). Moderate to severe lower urinary tract symptoms were reported by 20% of men participating in Uro-EDIC and were associated with age and presence of peripheral neuropathy, but not with DCCT treatment group assignment (10). Of 550 women participating in Uro-EDIC, urinary incontinence was reported by 65%; 17% reported weekly or more frequent incontinence. There was no observed relationship with incontinence and DCCT treatment group assignment or presence of peripheral neuropathy (12,13).
Conclusions
The clinical burdens of diabetic neuropathic complications are well recognized and result in significant morbidity. Painful symptoms are frequently refractory to treatment, and loss of protective sensation heightens the risk for foot ulceration and lower-extremity amputations (19,20). While symptoms associated with painful DPN and advanced autonomic dysfunction are often particularly troublesome, the earliest manifestations of CAN are silent and easily overlooked in clinical practice. Yet, CAN is an independent predictor of mortality (21,22), possibly by promoting life-threatening arrhythmias and sudden death in response to a variety of insults including drug side effects, hypoglycemia, hypokalemia, hypotension, or ischemia (23).
DPN and CAN were uncommon at the start of the DCCT, partly due to the intentional exclusion of people with neuropathy sufficiently severe to require treatment, but were increasingly prevalent over the DCCT/EDIC follow-up. INT during the DCCT decreased the development and progression of confirmed DPN and CAN relative to CON. Remarkably, treatment group differences were still measurable through 14 years of EDIC follow-up despite similar levels of glycemic control during EDIC (9,15).
The durable impact of prior treatment, even after disappearance of prior glycemic separation, first observed for retinopathy and nephropathy, has been described as “metabolic memory” (24,25). Confirmed DPN increased in both INT and CON participants by EDIC year 13/14, but the treatment group differences observed for confirmed DPN at that time were eliminated by adjusting for nerve conduction variables at the end of the DCCT. Viewed differently, among subjects who did not have neuropathy at DCCT completion, those in the CON group were shown to have greater degrees of subclinical neuropathy than subjects in the INT group (17). This subclinical neuropathy represented an asymptomatic neuropathy that had not yet produced clinical signs or sufficiently abnormal electrophysiology and could partially explain the findings of continued difference in the development of DPN in the INT and CON groups during EDIC. Whether an additional influence of early intensive glucose control might have been apparent earlier during EDIC is unknown, but a persistent metabolic effect was not required to explain the durable beneficial effects on confirmed DPN in EDIC (17).
In contrast, a long-term beneficial influence of early intensive glucose control, first observed for retinopathy and nephropathy, was observed for CAN in EDIC. Modeling that adjusted for R-R variation at DCCT closeout did not negate the INT-associated risk reduction for development of CAN (9). The risk reduction for CAN is consistent with the “metabolic memory” effect observed for retinopathy and nephropathy (16). This apparent discordance in the impact of prior DCCT treatment group effect on longer-term outcomes for DPN and CAN may reflect differences in susceptibility of small- and large-nerve fibers to glycemic exposure.
In general, findings from studies of diabetic neuropathies have to be interpreted with caution, given the broad range of diagnostic methods employed and lack of consistency in the criteria used to define neuropathy. In the Wisconsin Epidemiologic Study of Diabetic Retinopathy (WESDR) (26), DPN was assessed by tactile or temperature sensitivity; and they reported abnormalities in 19–25% and 11–19% of participants, respectively, after 10 years of follow-up. In the Pittsburgh Epidemiology of Diabetes Complications Study (EDC) (27), the cumulative incidence of DPN over a period of 5.3 years was 29%. In the EURODIAB Prospective Complications Study (28), an observational study that included 1,172 subjects with type 1 diabetes from 31 centers across Europe, neuropathy was defined by the presence of neuropathic symptoms, absence of ankle or knee reflexes, and abnormal vibration perception threshold as assessed by biothesiometers. EURODIAB reported that after only 7.3 years of follow-up, neuropathy developed in 24% of subjects, considerably higher than the 9% overall incidence of confirmed DPN reported over 5 years of follow-up in DCCT, which arguably used more stringent criteria that included nerve conduction studies. Although the differences in study design do not allow for precise comparisons between the true incidence of neuropathy in the DCCT/EDIC and the EURODIAB cohorts, EURODIAB also reported that HbA1c was an important determinant of neuropathy incidence (28).
The DCCT/EDIC is the first large study to concurrently obtain high-quality, standardized cardiac MRI and CAN evaluations, allowing for additional analyses regarding clinical implications of CAN (14). Although these cross-sectional findings prevent analysis of any causal relationship between CAN and ventricular dysfunction, other studies have demonstrated a relationship between sympathetic activation and left ventricular hypertrophy (29,30).
Genitourinary problems associated with diabetes, with the likely exception of impotence, are frequently overlooked in clinical practice and are rarely considered in the context of diabetic neuropathies. The Uro-EDIC study affords an opportunity to define the extent of genitourinary complications of diabetes and to explore the relationships of these to well-defined micro- and macrovascular complications, including neuropathy. In cross-sectional analyses, both erectile dysfunction and lower urinary tract symptoms in men were associated with DPN, perhaps due to shared or overlapping mechanisms of neuronal damage (10,18). These cross-sectional findings of associations between male genitourinary complications and DPN must be interpreted cautiously. Uro-EDIC data are being collected longitudinally and this may, in the future, shed light on shared pathophysiologic mechanisms between genitourinary and neuropathic complications.
The EDIC study continues to evaluate risk factors for neuropathic complications in a large number of well-characterized patients with type 1 diabetes and continues to demonstrate the value of optimizing glucose control as early as possible in the course of the disease to ameliorate the long-term effects of hyperglycemia. The DCCT and EDIC confirm that glycemic control is a significant and robust predictor of neuropathy. However, they also show that for most patients with type 1 diabetes, current strategies for optimizing glucose control are insufficient to fully prevent or delay the development of neuropathic complications, as 25% of subjects in the former INT group and 35% of subjects in the former CON group had confirmed DPN by 14 years of EDIC follow-up.
The reproducible, standardized DPN and CAN testing protocols, the robust and consistent definitions of neuropathy outcomes, the large sample size, and, most important, the commitment of DCCT/EDIC participants have allowed the DCCT/EDIC to provide invaluable lessons on the clinical course and the means of ameliorating DPN and CAN in patients with type 1 diabetes (9,15,16).
Article Information
Acknowledgments. The manuscript was made possible because of the dedication and integrity of the DCCT/EDIC researchers and participants.
Funding. The DCCT/EDIC has been supported by U01 Cooperative Agreement grants (1982–1993, 2011–2016) and contracts (1982–2011) with the Division of Diabetes Endocrinology and Metabolic Diseases of the National Institute of Diabetes and Digestive and Kidney Diseases (current grant numbers U01-DK-094176 and U01-DK-094157) and through support of the National Eye Institute, the National Institute of Neurological Disorders and Stroke, the Genetic Clinical Research Centers Program (1993–2007), and Clinical and Translational Science Center Program (2006–present), Bethesda, MD.
Industry contributors have had no role in the DCCT/EDIC but have provided free or discounted supplies or equipment to support participants’ adherence to the study: Abbott Diabetes Care (Alameda, CA); Animas (West Chester, PA); Bayer Diabetes Care (Tarrytown, NY); Becton, Dickinson and Company (Franklin Lakes, NJ); CanAm (Atlanta, GA); Eli Lilly (Indianapolis, IN); LifeScan (Milpitas, CA); Medtronic Diabetes (Minneapolis, MI); Nova Diabetes Care (Bedford, MA); Omron (Shelton, CT); OmniPod Insulin Management System (Bedford, MA); Roche Diabetes Care (Indianapolis, IN); and Sanofi (Bridgewater, NJ).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. C.L.M., J.W.A., and R.P.-B. contributed equally to the development of the manuscript, including literature review, development of content, and development of the discussion. J.W.A. was the primary author for presentation and discussion of peripheral neuropathy findings. R.P.-B. was the primary author for presentation and discussion of autonomic neuropathy findings. C.L.M. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 73rd Scientific Sessions of the American Diabetes Association, Chicago, IL, 21–25 June 2013.
Footnotes
Clinical trial reg. nos. NCT00360815 and NCT00360893, clinicaltrials.gov.
C.L.M., J.W.A., and R.P.-B. contributed equally to this work.
*A complete list of participants in the DCCT/EDIC Research Group can be found in N Engl J Med 2011;365:2366–2376.
References
- 1.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 2.The Diabetes Control and Complications Trial Research Group. Effect of intensive diabetes treatment on nerve conduction in the Diabetes Control and Complications Trial. Ann Neurol 1995;38:869–880 [DOI] [PubMed] [Google Scholar]
- 3.The Diabetes Control and Complications Trial Research Group. The effect of intensive diabetes therapy on measures of autonomic nervous system function in the Diabetes Control and Complications Trial (DCCT). Diabetologia 1998;41:416–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Epidemiology of Diabetes Interventions and Complications (EDIC) Epidemiology of Diabetes Interventions and Complications (EDIC). Design, implementation, and preliminary results of a long-term follow-up of the Diabetes Control and Complications Trial cohort. Diabetes Care 1999;22:99–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin CL, Waberski BH, Pop-Busui R, et al. DCCT/EDIC Research Group Vibration perception threshold as a measure of distal symmetrical peripheral neuropathy in type 1 diabetes: results from the DCCT/EDIC study. Diabetes Care 2010;33:2635–2641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feldman EL, Stevens MJ, Thomas PK, Brown MB, Canal N, Greene DA. A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care 1994;17:1281–1289 [DOI] [PubMed] [Google Scholar]
- 7.Herman WH, Pop-Busui R, Braffett BH, et al. DCCT/EDIC Research Group Use of the Michigan Neuropathy Screening Instrument as a measure of distal symmetrical peripheral neuropathy in type 1 diabetes: results from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications. Diabet Med 2012;29:937–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin CL, Albers J, Herman WH, et al. DCCT/EDIC Research Group Neuropathy among the diabetes control and complications trial cohort 8 years after trial completion. Diabetes Care 2006;29:340–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pop-Busui R, Low PA, Waberski BH, et al. DCCT/EDIC Research Group Effects of prior intensive insulin therapy on cardiac autonomic nervous system function in type 1 diabetes mellitus: the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study (DCCT/EDIC). Circulation 2009;119:2886–2893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Den Eeden SK, Sarma AV, Rutledge BN, et al. Diabetes Control and Complications Trial/Epidemiology of Diabetes Research Group Effect of intensive glycemic control and diabetes complications on lower urinary tract symptoms in men with type 1 diabetes: Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) study. Diabetes Care 2009;32:664–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang CC, Porter MP, Penson DF. Comparison of the International Index of Erectile Function erectile domain scores and nocturnal penile tumescence and rigidity measurements: does one predict the other? BJU Int 2006;98:105–109; discussion 109 [DOI] [PubMed] [Google Scholar]
- 12.Sarma AV, Kanaya A, Nyberg LM, et al. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group Risk factors for urinary incontinence among women with type 1 diabetes: findings from the epidemiology of diabetes interventions and complications study. Urology 2009;73:1203–1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sarma AV, Kanaya AM, Nyberg LM, et al. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group Urinary incontinence among women with type 1 diabetes—how common is it? J Urol 2009;181:1224–1230; discussion 1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pop-Busui R, Cleary PA, Braffett BH, et al. DCCT/EDIC Research Group Association between cardiovascular autonomic neuropathy and left ventricular dysfunction: DCCT/EDIC study (Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications). J Am Coll Cardiol 2013;61:447–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Albers JW, Herman WH, Pop-Busui R, et al. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group Effect of prior intensive insulin treatment during the Diabetes Control and Complications Trial (DCCT) on peripheral neuropathy in type 1 diabetes during the Epidemiology of Diabetes Interventions and Complications (EDIC) study. Diabetes Care 2010;33:1090–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pop-Busui R, Herman WH, Feldman EL, et al. DCCT/EDIC Research Group DCCT and EDIC studies in type 1 diabetes: lessons for diabetic neuropathy regarding metabolic memory and natural history. Curr Diab Rep 2010;10:276–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Albers JW, Herman WH, Pop-Busui R, Martin CL, Cleary P, Waberski B, Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Intervention and Complications (EDIC) Research Group Subclinical neuropathy among Diabetes Control and Complications Trial participants without diagnosable neuropathy at trial completion: possible predictors of incident neuropathy? Diabetes Care 2007;30:2613–2618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wessells H, Penson DF, Cleary P, et al. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group Effect of intensive glycemic therapy on erectile function in men with type 1 diabetes. J Urol 2011;185:1828–1834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boulton AJ, Vileikyte L, Ragnarson-Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet 2005;366:1719–1724 [DOI] [PubMed] [Google Scholar]
- 20.Tesfaye S, Boulton AJ, Dyck PJ, et al. Toronto Diabetic Neuropathy Expert Group Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care 2010;33:2285–2293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maser RE, Mitchell BD, Vinik AI, Freeman R. The association between cardiovascular autonomic neuropathy and mortality in individuals with diabetes: a meta-analysis. Diabetes Care 2003;26:1895–1901 [DOI] [PubMed] [Google Scholar]
- 22.Pop-Busui R, Evans GW, Gerstein HC, et al. Action to Control Cardiovascular Risk in Diabetes Study Group Effects of cardiac autonomic dysfunction on mortality risk in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Diabetes Care 2010;33:1578–1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pop-Busui R. What do we know and we do not know about cardiovascular autonomic neuropathy in diabetes. J Cardiovasc Transl Res 2012;5:463–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Writing Team for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group Effect of intensive therapy on the microvascular complications of type 1 diabetes mellitus. JAMA 2002;287:2563–2569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Writing Team for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group Sustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy: the Epidemiology of Diabetes Interventions and Complications (EDIC) study. JAMA 2003;290:2159–2167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klein R, Klein BE, Moss SE. Relation of glycemic control to diabetic microvascular complications in diabetes mellitus. Ann Intern Med 1996;124:90–96 [DOI] [PubMed] [Google Scholar]
- 27.Maser RE, Steenkiste AR, Dorman JS, et al. Report from Pittsburgh Epidemiology of Diabetes Complications Study Epidemiological correlates of diabetic neuropathy. Diabetes 1989;38:1456–1461 [DOI] [PubMed] [Google Scholar]
- 28.Tesfaye S, Chaturvedi N, Eaton SE, et al. EURODIAB Prospective Complications Study Group Vascular risk factors and diabetic neuropathy. N Engl J Med 2005;352:341–350 [DOI] [PubMed] [Google Scholar]
- 29.Spallone V, Ziegler D, Freeman R, et al. Toronto Consensus Panel on Diabetic Neuropathy Cardiovascular autonomic neuropathy in diabetes: clinical impact, assessment, diagnosis, and management. Diabetes Metab Res Rev 2011;27:639–653 [DOI] [PubMed] [Google Scholar]
- 30.Sacre JW, Franjic B, Jellis CL, Jenkins C, Coombes JS, Marwick TH. Association of cardiac autonomic neuropathy with subclinical myocardial dysfunction in type 2 diabetes. JACC Cardiovasc Imaging 2010;3:1207–1215 [DOI] [PubMed] [Google Scholar]