Abstract
Protecting the brain in vulnerable infants undergoing surgery is a central aspect of perioperative care. Understanding the link between blood flow, oxygen delivery and oxygen consumption leads to a more informed approach to bedside care. In some cases, we need to consider how high can we let the partial pressure of carbon dioxide go before we have concerns about risk of increased cerebral blood volume and change in intracranial hydrodynamics? Alternatively, in almost all such cases, we have to address the question of how low can we let the blood pressure drop before we should be concerned about brain perfusion? This review, provides a basic understanding of brain bioenergetics, hemodynamics, hydrodynamics, autoregulation and vascular homeostasis to changes in blood gases that is fundamental to our thinking about bedside care and monitoring.
Keywords: Cerebral blood flow, cerebral blood volume, intracranial pressure, autoregulation, infant, perioperative
Protecting the brain in vulnerable infants undergoing surgery is a central aspect of perioperative care. Understanding the link between blood flow, oxygen (O2) delivery and O2 consumption will lead to a more informed approach to bedside care. For example: what are the limits to normality?, how do we know when a significant perturbation has occurred?, and what preventive measures can we take to protect the brain? This brief review is not going to be able to answer these questions, as we are still in the dark ages in regard to differentiating between cause and effect. Rather, it will provide a basic understanding of brain bioenergetics, hemodynamics, hydrodynamics, autoregulation and vascular homeostasis to changes in blood gases that is fundamental to our thinking about bedside care and monitoring. Each section will start from what we know in man, and identify relevant information in neonates.
Brain bioenergetics
The brain is one of the most metabolically active organs of the body and it requires a constant supply of O2 and nutrient, mainly glucose (1). In the adult, the brain consumes about one-fifth of total body O2 utilization. Cerebral metabolic rate for oxygen (CMRO2) in normal, conscious young men is approximately 3.5 mL O2/100g brain/min. The CMRO2 by an entire adult brain of average weight (i.e., 1.4 kg) is therefore about 50 mL O2/min. A 70 kg adult consumes about 250 ml O2/min in the basal resting state. Therefore the brain, which represents only about 2% of total body weight, accounts for 20% of the resting total body O2 consumption.
The functions of nervous tissues are mainly excitation and conduction of nerve impulses, and these are reflected in the increasing activity of the brain. Electrical energy, ultimately, is derived from chemical processes, and it is likely that most of the energy consumption of the brain is used for active transport of ions. Oxygen is used in the brain almost entirely for the oxidation of carbohydrate. The energy equivalent of the total cerebral metabolic rate is, therefore, approximately 20 W, or 0.25 kcal/min. Let us assume that this energy is used mainly for the synthesis of high-energy phosphate bonds, that the efficiency of the energy conservation is approximately 20%, and that the free energy hydrolysis of the terminal phosphate of adenosine triphosphate (ATP) is 7 kcal/Mol. This energy expenditure supports the steady turnover of close to 7 mMol, or approximately 4 × 1021 molecules of ATP per minute in the entire brain.
In the normal, in vivo state, glucose is the only significant substrate for energy metabolism in the brain. The stoichiometry of glucose utilization and CMRO2 is as follows. The normal, conscious human brain produces carbon dioxide (CO2) at about the same rate of CMRO2 of 156 µMol/100g tissue/min – leading to a respiratory exchange ratio of 1. CMRO2 and CO2 production are equivalent to a rate of glucose utilization of 26 µMol/100g tissue/min, assuming 6 µMol of O2 consumed and CO2 produced for each µMol of glucose completely oxidized to CO2 and water. (The actual glucose utilization is, however, 31 µMol/100g/min. For complete oxidation of glucose, the theoretical ratio of O2:glucose utilization is 6; the excess glucose utilization is responsible for a measured ratio of only 5.5 µMol O2/µMol glucose. The fate of the excess glucose is unknown but, probably, is distributed in lactate, pyruvate and other intermediary metabolites.)
Development and cerebral glucose metabolism
Cerebral glucose utilization in 5-week-old infants is three-quarters that of the adult brain. The developing brain also metabolizes lactate, ketone bodies, amino acids and free fatty acids. Adult rates of glucose utilization are first achieved by the age of 2 years; after this age there is a further increase through to the age of 8 years, followed by a decline in metabolic rate through to the age of 20 years. This crescendo-decrescendo pattern of change likely represents the consequence of brain development and subsequent ‘pruning’ of neurons, synapses and pathways that occurs with maturation (2).
Cerebral oxygen kinetics
Human brain maturation is incomplete at birth and continues to progress during the first years of life. As already discussed, brain development is associated with regional changes in glucose metabolism. Given that O2 needs to be delivered to the tissue at a rate that is biochemically-proportionate to metabolic needs, it should come as no surprise that regional cerebral blood flow (CBF) also changes with development.
CMRO2 must be equal to the total amount of O2 delivered to cerebral tissue per unit time minus the amount leaving in the venous circulation per unit time and the amount that accumulates in the cerebral tissue per unit time (Equation 1, Table 1). The extraction of O2 from cerebral tissue is so closely matched to the brain’s metabolic needs that O2 content of brain tissue is small. The vast majority of the arteriovenous O2 difference (AVDO2) is made up of O2 off-loaded from hemoglobin, and the amount of O2 off-loaded from hemoglobin in the cerebral circulation is tightly regulated by many physiologic factors including brain pH, brain temperature, concentration of cerebral metabolites, and amount of adult hemoglobin.
Table 1.
Equations describing the pathway for blood and oxygen in the brain
| Equations |
| [1] CMRO2 = (CBF • CaO2) – (CBF • CvO2) – CiO2 |
| [2] Q = ΔP ÷ R |
| [3] Q = (π • r4 • ΔP) ÷ (8 η • L) |
| [4] Q = CBV ÷ t` |
| [5] CBV = 1.09 • CBF0.29 |
| [6] OEF = (SaO2 – SjvO2) ÷ SaO2 |
| [7] OEF = CMRO2 ÷ (CBF × 1.34 × [Hb] × SaO2) |
Key:
CaO2 is the oxygen content of arterial blood
CBF is the cerebral blood flow
CBV is the cerebral blood volume
CiO2 is the oxygen content of brain tissue
CMRO2 is the cerebral metabolic rate for oxygen
CvO2 is the oxygen content of venous blood
[Hb] is the hemoglobin concentration
η is blood viscosity
L is vessel length
OEF is oxygen extraction fraction of brain
ΔP is the pressure gradient between inflow and outflow
Q is flow
R is resistance to flow
r is vessel radius
SaO2 is oxyhemoglobin saturation of arterial blood
SjvO2 is oxyhemoglobin saturation of jugular venous blood
t` is mean transit time of blood
Cerebrovascular hemodynamics
CBF at birth is, on average, 50 mL/100 g/min, increasing after birth to a maximum of 70 at 5 years, and then decreasing to reach adult levels after 19 years. The global CBF represents more than 50% of cardiac output at the peak value around 1 to 3 years of age, indicating why this age group is at risk for cerebrovascular catastrophes consequent to perioperative systemic disorders.
Under physiologic conditions CBF is closely coupled to the O2 requirements of the tissue. CMRO2, in turn, depend on the density of neurons, and on their state of functional activation. CBF, therefore, differs in different species and with different types of anesthesia: it is higher as the brain is smaller (because neurons are more closely packed) and as the level of anesthesia is shallower. Typical flow rate of CBF of the cerebral cortex in adults in the awake-state is 80 mL/100g/min. Under anesthesia, CBF decreases by about 20%, and under deep general anesthesia by up to 50%. In white matter, blood flow is about 25% of that of the cortex in the awake-state, but it is not markedly influenced by anesthesia; differences between white and grey matter diminish when subjects are deeply anesthetized.
The physical laws that describe steady laminar flow of uniform fluids through non-distensible tubes are helpful in understanding in vivo cerebrovascular hemodynamics. Ohm’s law predicts that flow is proportional to the pressure gradient between inflow and outflow divided by the resistance to flow (Equation 2, Table 1). In brain, cerebral perfusion pressure (CPP) is taken as the driving pressure for CBF. CPP is the difference between intra-arterial pressure and the pressure in the thin walled veins, collapsible at the point of entry into the venous sinuses. Venous pressure changes in parallel with intracranial pressure (ICP) and is normally 2–5 mm Hg higher than ICP. Therefore, the driving pressure is calculated as the difference between mean arterial pressure (MAP) and the cerebral venous pressure or ICP, whichever is higher. Resistance is determined principally by vessel radius and can be calculated from Equation 2 (Table 1) to estimate total cerebrovascular resistance (CVR) or resistance of any vascular segment of interest in which flow and upstream and downstream pressure gradients are known. Poiseuille’s law shows that the major determinants of CBF are perfusion pressure, blood viscosity, and vessel radius (Equation 3, Table 1). Vessel length is an unchanging parameter.
Cerebral blood volume
Cerebral blood volume (CBV) is determined by two factors, CBF and capacitance vessel diameter (i.e., small veins and venules). CBV increases with vasodilation and decreases with vasoconstriction. Although CBF frequently changes in the same direction as CBV, these variables are inversely related under normal situations (e.g., autoregulation) or in pathological situations. Further, blood volume is not equally distributed throughout brain; blood volume per unit weight is greater in gray than white matter with further variation among the various nuclei. Average CBV in humans is 3–4 mL/100 g tissue. Pathology, which effects either CBF or cerebral venous capacitance, may modulate CBV with subsequent effects on ICP. More quantitatively, the central volume principle relates the volume that intravascular blood occupies within brain (CBV in ml) and the volume of blood that moves through the brain per unit time (CBF in ml/min) (Equation 4, Table 1). For example, although CBV is increased during vasodilation, CBF may not change if blood flow velocity is correspondingly reduced. Surplus blood volume accumulates primarily within cerebral veins, known to receive sympathetic innervation and to respond to sympathetic stimulation, and within capillaries to a smaller degree. Normally, increases in CBV can be physiologically controlled by two maneuvres: increased blood outflow to the extracranial venous circulation and restricted inflow via constriction of the major feeding arteries.
Cerebral vascular supply and drainage
The arterial blood supply to the brain comes from two circulations, anterior and posterior. The anterior circulation of the brain arises from the internal carotid artery, to middle cerebral artery (MCA), to anterior cerebral artery (ACA). The posterior circulation of the brain arises from the subclavian artery, to vertebral, basilar and posterior cerebral arteries (PCA). The anterior circulation supplies the eyes, basal ganglia, part of the hypothalamus, the frontal and parietal lobes, and a large part of the temporal lobes. The posterior circulation supplies the brain stem, cerebellum, inner ear, occipital lobes, the thalamus, part of the hypothalamus, and a smaller portion of the temporal lobes. Venous drainage of the brain via the superficial and deep cerebral veins drains through the dural venous sinuses into the internal jugular veins and then into the superior vena cava. Superficial cortical veins carry blood from the outer 1–2 cm of the brain surface to the superior and inferior sagittal sinuses, the vein of Galen, the straight sinus and the tentorial veins. The superficial cerebellar veins drain to the superior vermian vein, great cerebral vein, straight sinus and the transverse sinuses. The deep cerebral veins drain from the inner region of the brain.
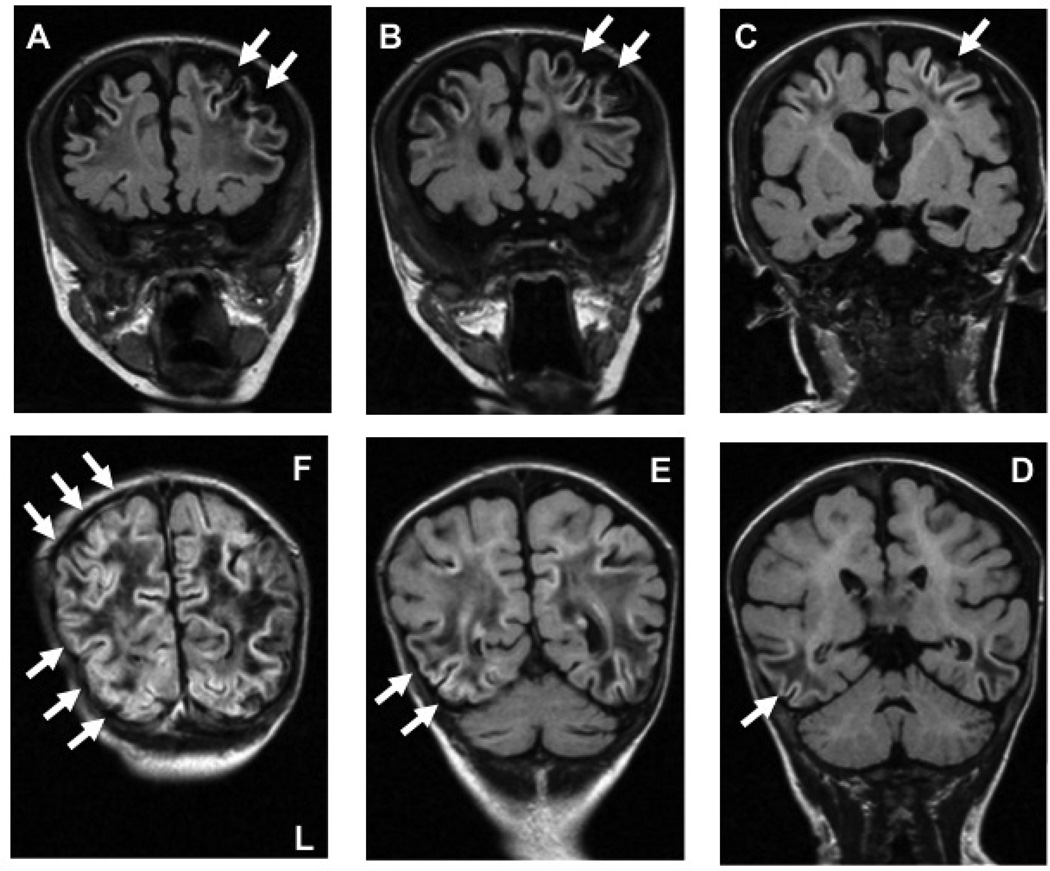
Arterial watersheds or border zones are areas that lie at the junction of two different supply and drainage areas. The vascular supply of the cerebral parenchyma can be thought of in a similar manner, with defined boundaries between different major cerebral arteries. These territories can be further classified in two broad categories as either external (cortical), or internal (subcortical) zones. The internal border zone watershed areas are located between the ACA, MCA and PCA, and the areas supplied by the Heubner, lenticulostriate, and anterior choroidal arteries. This pattern of injury is seen after acute catastrophic loss in cardiac output. The external or cortical border zones are located at the junctions of the ACA, MCA, and PCA territories. Infarcts in the anterior external border zones and paramedian white matter are found at the junction of the territories supplied by the ACA and MCA, and those in the parieto-occipital areas (posterior external border zones) are found at the junction of the territories supplied by the MCA and PCA. Clinically, pure frontal cortical watershed infarcts are very rare, and in most cases they are associated with internal border zone infarcts. However, cortical watershed infarcts, often bilateral, and involving the posterior convexity – much more so than the anterior lobes – is the usual pattern of watershed injury (Figure 1). Isolated external arterial border zone or watershed injury is thought to be due to an acute decrease in CBF as a result of a brief episode of cerebral hypoperfusion, not severe enough to cause frank infarction or widespread necrosis. The affected areas will therefore be those that are most susceptible to hypotension and a fall in perfusion, such as the regions in the posterior lobes that represent the end fields of all three main cerebral arteries. This pattern of injury is well described in adults, children, and acutely ill young infants with hypotension (3).
Figure 1. Perioperative external watershed injury.
Coronal inversion recovery with long echo time (TE) T1-weighted magnetic resonance images MRI, clockwise A to F, showing slices from frontal through to the occipital lobes. The arrows indicate evidence of external cortical watershed ischemia as evidenced by T1 hyperintensity in the cortex and subcortical areas, with centrum semiovale and occipital lobe white matter hypointensity: plates A–C, the anterior cerebral artery – middle cerebral artery watershed; plates D–F, the middle cerebral artery – posterior cerebral artery watershed.
Cerebral hydrodynamics
Eighty percent of cerebrospinal fluid (CSF) is produced in the choroid plexus of the lateral and fourth ventricles. The remainder is produced in the interstitial space and ependymal lining. In the adult, normal CSF volume is 150 mL (50% intracranial and the rest intraspinal). In the neonate CSF volume is 50 mL. The rate of CSF production across all ages is 0.15 to 0.30 mL/min.
There are two pathways for CSF circulation: a major, adult, pathway with CSF absorption through arachnoid villi (arachnoid granulation) into the venous sinuses; and a minor, infantile, pathway with CSF drainage through the ventricular ependyma, the interstitial and perivascular space, and perineural lymphatics. The need for CSF circulation begins early during intrauterine development because the choroid plexus is formed during the first trimester. Since arachnoid granulations do not appear until just before birth, it is unlikely that CSF reabsorption via the adult route of circulation is the major pathway during infancy. In fact, there is some evidence that arachnoid granulations continue to develop well into the second decade, and so the ‘infantile’ route of circulation may be significant in childhood.
Intracranial pressure
ICP is the pressure of CSF inside the cerebral ventricles, which is often approximated with cerebral intraparenchymal pressure measured using a fiber-tipped microsensor. In health, ICP is determined by CBF and CSF circulation. The Davson equation describes this relationship and states that ICP is the sum of sagittal sinus pressure and the product of CSF formation rate and resistance to CSF outflow (4). Normal values for sagittal sinus pressure, CSF formation rate, and resistance to CSF outflow are 5 to 8 mm Hg, 0.3 to 0.4 mL/min, and 6 to 10 mm Hg/ mL/min, respectively. In most clinical situations, sagittal sinus pressure stays constant or is coupled to central venous pressure. In practice, measured ICP is often greater than the value calculated using the equation. The difference is due to a vascular component, which is probably a result of pulsation in the arterial bed and determined by the interaction between pulsatile arterial inflow and venous outflow curves, cardiac function, and cerebral vasomotor tone.
Under usual conditions, ICP in the remains less than 15 mm Hg and reflects the volume of three compartments: brain parenchyma (1200–1600 ml in the adult human), extracellular or CSF (100–150 ml) and CBV (100–150 ml). Since the intracranial vault is fixed in volume, increases in the size of one component of the intracranial contents must be compensated by removal of an equivalent amount of another intracranial component or ICP will increase. The point at which perfusion-compromising ICP elevation occurs is dependent on brain elastance and potential displacement of intracranial contents.
Cerebrovascular autoregulation and vascular homeostasis to changes in blood gases
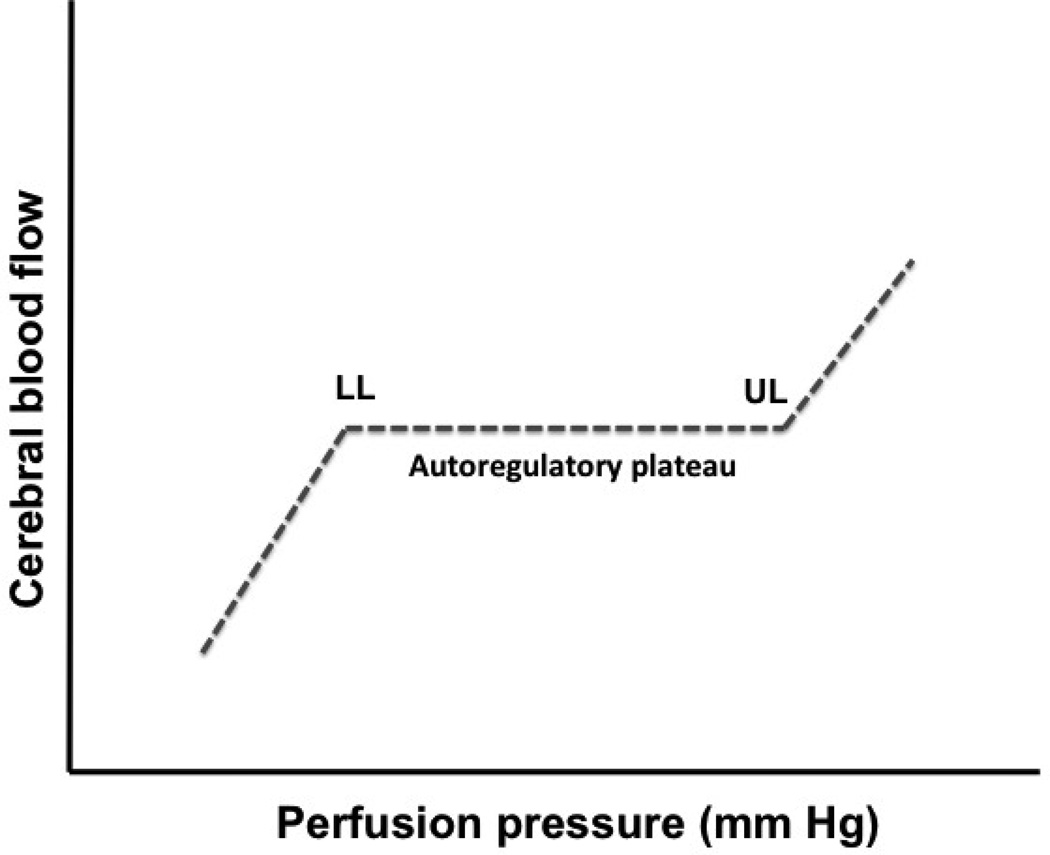
Cerebral resistance arteries dilate during reductions in CPP and constrict during increases in pressure. As a result, CBF remains relatively constant over a fairly broad range of arterial pressure defined as the autoregulatory plateau (Figure 2). The lower and upper limits of the autoregulatory plateau in adults have been determined as approximately 50–60 mm Hg and 150–160 mm Hg, respectively. The lower limit of autoregulation refers to the point at which CBF starts to decrease and not to the point at which cerebral resistance arteries are fully vasodilated. Cerebral resistance arteries may continue to dilate to some degree, even after the lower limit of CBF autoregulation is exceeded. Passive reductions in diameter occur only at very low levels of CPP. Thus the lower limit of cerebral vasodilation does not match precisely the lower limit of CBF autoregulation.
Figure 2. CBF autoregulation.
Graph showing the relationship between perfusion pressure and CBF; the autoregulatory curve describes near constant CBF despite changes in perfusion pressure. The lower limit (LL) and upper limit (UL) of autoregulation denote pressure-passive states
Reductions of CPP below the lower limit of autoregulation result in hypoperfusion in the brain. In an attempt to compensate for reductions in CBF, the extraction coefficient of O2 from the blood increases. No clinical symptoms are observed until reductions in CPP exceed the ability of increased O2-extraction to satisfy metabolic demands of cerebral tissues. The mechanisms responsible for CBF autoregulation are not yet clearly understood. Possibilities that have been considered include neurogenic, myogenic, metabolic and endothelial factors.
Cerebrovascular regulation in development
In normal term infants, several days are required for the maturation of vascular responses. Laboratory experimental studies have shown that pressure autoregulation, although present, is poorly developed at birth. Studies in preterm human infants have shown that CBF increases over the first 3 postnatal days. Cerebral autoregulation may be disturbed in ill preterm and full-term infants suffering from cerebral hypoxia-ischemia (5). If pressure-flow autoregulation is absent, then increased CPP is expected to result in increased CBF – and vice versa.
The lower limit of cerebral autoregulation of 29 mm Hg has been described in nonanesthetized preterm infants less than 30 weeks gestational age. There are no available data on which to determine whether the cerebral autoregulation curve for expremature infants mimics that of term infants. There is also evidence that autoregulatory reserve is less in older infants than in children and adults, and that the physical state of the patient matters (6, 7). The most conservative approach, given the lack of clinical data, would be to assume a very narrow autoregulatory range around baseline blood pressure (BP).
Cerebrovascular response to carbon dioxide
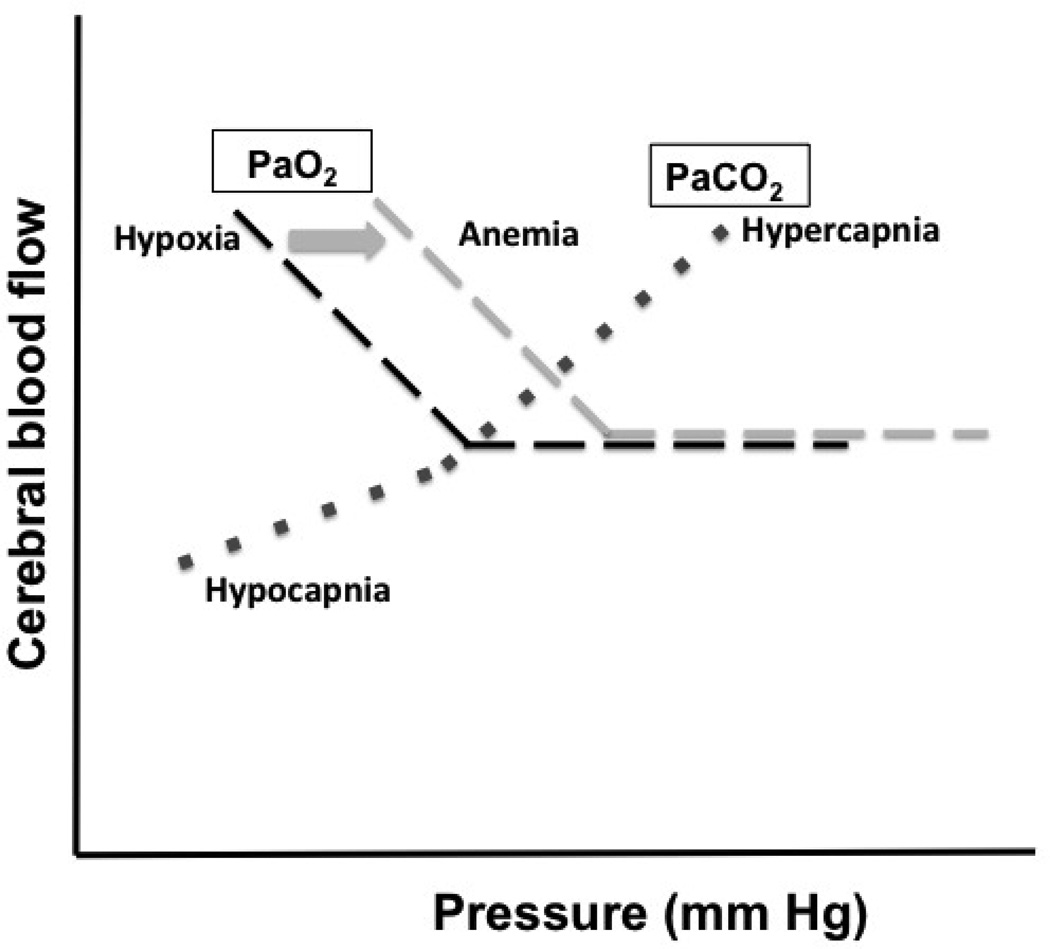
Under steady state conditions, over a period of 10 minutes, inhalation of 5–7% CO2 results in a 75% increase in CBF (Figure 3). Inhalation of 10% O2 increases CBF by 35%. Both hypercapnia and hypoxia lead to a decrease in CVR indicating that the increase in CBF is a consequence of vasodilation.
Figure 3. CBF changes with PaO2 and PaCO2.
Graphs showing the change in CBF with Hyper- and hypocapnia (dotted line) and hypoxia (dashed line); during conditions of anemia, the hypoxia curve is shifted to the right, as indicated by the arrow.
In 1948 Kety and Schmidt (8) described a curvilinear relationship between arterial partial pressure of CO2 (PaCO2) and CBF. A reduction of PaCO2 from 40 to 20 mm Hg decreased CBF but not to the same extent as the increase in CBF when PaCO2 is increased from 40 to 60 mm Hg. In these studies CMRO2 did not change during this degree of hypercapnia or hypoxia. The increase in CBF without any increase in CMRO2 resulted in a decrease in the AVDO2, i.e., reduced O2-extraction. The hypercapnia-induced increase in CBF is ~6% per mm Hg change in PaCO2, and hypocapnia decreases CBF by ~3% per mm Hg change in PaCO2. The relation between CBF and CBV (including arterial, capillary and venous blood volume) during changes in PaCO2 has also been investigated in humans. The increase in CBV during hypercapnia is less than that in CBF and that the degree of decrease in CBV during hypocapnia is less than that in CBF (Equation 5, Table 1). According to Poiseuille’s law, CBV increases proportionally to the square of the diameter, yielding the relation CBV = c × CBF0.5, which is in good agreement with the relation during changes in PaCO2.
The question of whether alteration in PaCO2 changes CBF equally in all brain regions is somewhat controversial. Also, whether both gray and white matter behaves the same. There appears to be a developmental difference in CBF response to CO2, although in all age groups, CBF increases with increasing PaCO2. In both fetus and newborn, gray matter CBF increases at PaCO2 greater than 40 mm Hg, but changes little at lower PaCO2 levels. Also, it has been demonstrated that the change in CBF per mm Hg change in PaCO2 is higher in newborn than in the fetus, and this suggests that the cerebrovascular response to CO2 is not completely developed at birth. This depressed CO2 response in the fetus may be correlated to a difference in CMRO2 (i.e., when CBF responses are normalized for CMRO2, the increase in CBF is greatest in newborns, smaller in adults and even smaller in fetuses).
Hypocapnia does not alter the lower limit of cerebral autoregulation in mature animals but does lead to lower CBF per unit change in CPP (9). At lower BP, below the level of cerebral autoregulation, there is an attenuation of the slope of the CBF/CPP graph suggesting that the cerebral vascular response is attenuated with hypotension. CBF in preterm infants is dependent on PaCO2 (10). The cerebral vascular response to PaCO2 is less in the first day of life and increases with gestational age. It is also attenuated, but not eliminated, in hypotensive infants. This reactivity is present even in preterms; it is estimated to be about 4% per mmHg PCO2 (11). Intraoperatively, it is also preserved in children (18 months to 7 years of age) anesthetized with sevoflurane (12). Hypocapnia may, therefore, result in cerebrovascular vasoconstriction and reduction of CBF.
In preterm infants, even mild hypocapnia (PaCO2 < 35 mm Hg) is associated with cerebral palsy and leucomalacia (13). In term infants with congenital diaphragmatic hernia it has been shown that there is positive correlation between intelligence quotient on follow-up and postoperative PaCO2 (14). There is also a positive association between the neurocognitive outcome of term infants with hypoxic-ischemic encephalopathy and hypocapnia (15).
Cerebrovascular response to oxygen
Hypoxia elevates CBF. CBF does not change in response to small deviations of the PaO2 around normal levels. Rather, when PaO2 falls to ~50 mm Hg regional CBF begins to rise. As PaO2 is further reduced below this threshold CBF increases exponentially. It may reach over 400% of basal flow at lowered PaO2 in an attempt to maintain O2 delivery. There is no significant change in CMRO2 over the range of PaO2 23 to 100 mm Hg.
The responses of the cerebral circulation to hypoxia relate to hemoglobin oxygen saturation (SaO2). At a PaO2 > 70 mm Hg SaO2 is 100%. However, when the PaO2 reaches about 50 mm Hg SaO2 is 85%. Under conditions of reduced availability of O2 (e.g., anemia) the regional CBF/PaO2 curve is shifted to the right. Hypoxic elevations in regional CBF are not associated with changes in metabolic rate. However, the vasodilation is additive with that produced by metabolic signals, particularly acidosis and hypercapnia.
Clinical application of vascular and hydrodynamic physiology
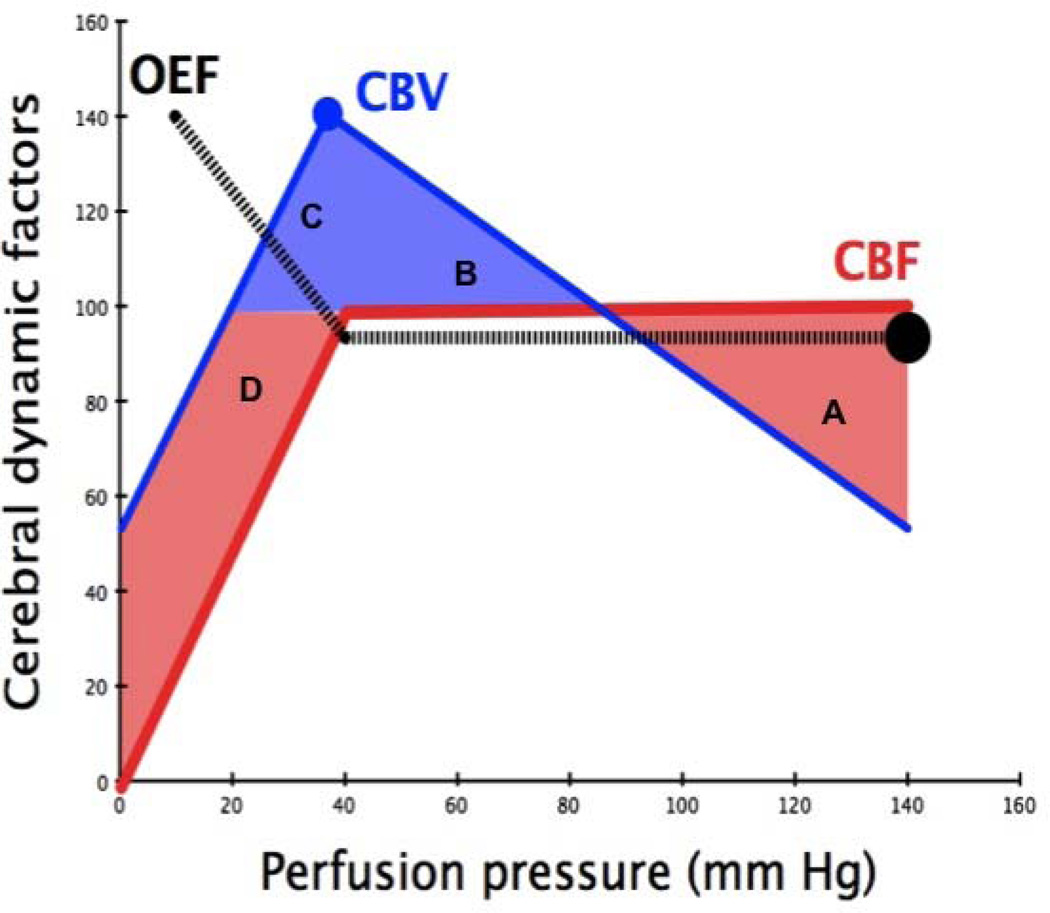
Figure 4 provides a summary of the changes in CBV and O2-extraction fraction (OEF) around the lower limit of autoregulation when it is intact. Throughout childhood regional brain OEF is relatively constant and given by Equation 6 (Table 1). By rearranging Equations 1 and 6, we derive Equation 7 (Table 1). Constant OEF therefore reflects the coupling between regional CMRO2 and regional CBF. In some circumstances CMRO2-CBF coupling may be disrupted, and the effect on OEF will reflect the predominant abnormality. For example: ischemia is present when CMRO2 exceeds O2-delivery via CBF; hyperemia occurs when O2-delivery via CBF exceeds tissue requirement or CMRO2. Figure 4 depicts four zones of physiology: A, a state of normal CBF and AVDO2 with reduced CBV due to increased CVR; B, a state of normal CBF and AVDO2 with reduced CBV due to reduced CVR; C, a state of falling CBF with raised CBV and AVDO2 at a time when cerebrovascular vasodilatation is maximal; and D, a state of low CBF and CBV with raised AVDO2. States C and D have the potential for causing brain injury – it is a matter of duration and intensity of insult (16).
Figure 4. Changes in oxygen extraction fraction (OEF), CBV and CBF as perfusion pressure falls.
See text for details.
In the perioperative critically ill infant the clinician is often faced with the problem of trying to achieve neuroprotection. What this means in regard to bedside physiology can be phrased more simply. For example, how low can we let the patient’s hemoglobin fall before we should consider the detrimental effect of decreased blood O2 content and a shift in the CBF/PaO2 curve to the right? In some cases, we may need to consider how high can we let the PaCO2 go before we have concerns about risk of increased CBV and change in intracranial hydrodynamics? Alternatively, in almost all critically ill perioperative cases, we have to address the question of how low can we let the blood pressure (BP) drop before we should be concerned about CPP? In this regard, the BP target that we should use in the perioperative infant is far from clear. There are few data and there are too many factors to consider – not least is the fact that we aren’t really interested in the measured variable (driving pressure) but flow, which can only be inferred; that said, BP is the best surrogate that we have.
During the first day of postnatal life, average systolic- and diastolic-BP (s- and d-BP) is 62.6/38.9 mmHg for term infants and rises in the first week and month of life. The average BP in females from >8 days of life to <1 month is 81.7/50.7 and in males 82.0/50.3 mm Hg (17). This change represents an almost 30% rise in s-BP from the first day of postnatal life compared with the last 3 weeks of the first month after birth. Expremature infants have normative BP data that are generally higher than that of term infants. The definition of hypotension in non-anesthetized neonates is debated and is usually defined as any value that falls below the 5th or 10th percentile for gestational and postnatal age. The perioperative target for BP, for age, is unknown: whether to aim for the normal range for age or whether to target the patient’s baseline BP, or whether to tolerate some “allowable” drop in BP of 20–25%. The Pediatric Advanced Life Support definition of hypotension – s-BP<60 mmHg for infants – represents a drop in s-BP of 27% from the expected s-BP in infants older than one week (18).
These questions about hemoglobin, CO2 and BP, and their respective effects on CBV, CBF and AVDO2 are often the topics discussed at the bedside. In the future we may have better data to assist our understanding and management (19, 20).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schulman RG, Rothman DL, editors. Brain Energetics & Neuronal Activity: applications to fMRI and medicine. Hoboken, NJ: John Wiley & Sons, Inc.; 2004. [Google Scholar]
- 2.Harris JJ, Reynell C, Attwell D. The physiology of developmental changes in BOLD functional imaging signals. Dev Cogn Neurosci. 2011;1:199–216. doi: 10.1016/j.dcn.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang BY, Castillo M. Hypoxic-ischemic brain injury: imaging findings from birth to adulthood. Radiographics. 2008;28:417–439. doi: 10.1148/rg.282075066. [DOI] [PubMed] [Google Scholar]
- 4.Davson H. Physiology of the cerebrospinal fluid. London, UK: J &A Churchill Ltd.; 1967. [Google Scholar]
- 5.Greisen G. Autoregulation of cerebral blood flow in newborn babies. Early Hum Dev. 2005;81:423–428. doi: 10.1016/j.earlhumdev.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 6.Vavilala MS, Lee LA, Lam AM. The lower limit of cerebral autoregulation in children during sevoflurane anesthesia. J Neurosurg Anesthesiol. 2003;15:307–312. doi: 10.1097/00008506-200310000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Chock VY, Ramamoorthy C, Van Meurs KP. Cerebral autoregulation in neonates with hemodynamically significant patent ductus arteriosus. J Pediatr. 2012;160:936–942. doi: 10.1016/j.jpeds.2011.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kety SS, Schmidt CF. The effects of altered arterial tensions of carbon dioxide and oxygen on cerebral blood flow and cerebral oxygen consumption of normal young men. J Clin Invest. 1948;27:484–492. doi: 10.1172/JCI101995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Artru AA, Katz RA, Colley PS. Autoregulation of cerebral blood flow during normocapnia and hypocapnia in dogs. Anesthesiology. 1989;70:288–292. doi: 10.1097/00000542-198902000-00018. [DOI] [PubMed] [Google Scholar]
- 10.Vanderhaegen J, Naulaers G, Vanhole C, et al. The effect of changes in tPCO2 on the fractional tissue oxygen extraction – as measured by near-infrared spectroscopy – in neonates during the first days of life. Eur J Paediatr Neurol. 2009;13:128–134. doi: 10.1016/j.ejpn.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 11.Pryds O, Andersen GE, Friis-Hansen B. Cerebral blood flow reactivity in spontaneously breathing, preterm infants shortly after birth. Acta Paediatr Scand. 1990;79:391–396. doi: 10.1111/j.1651-2227.1990.tb11482.x. [DOI] [PubMed] [Google Scholar]
- 12.Rowney DA, Fairgrieve R, Bissonnette B. Cerebrovascular carbon dioxide reactivity in children anaesthetized with sevoflurane. Br J Anaesth. 2002;88:357–361. doi: 10.1093/bja/88.3.357. [DOI] [PubMed] [Google Scholar]
- 13.Collins MP, Lorenz JM, Jetton JR, Paneth N. Hypocapnia and other ventilationrelated risk factors for cerebral palsy in low birth weight infants. Pediatr Res. 2001;50:712–719. doi: 10.1203/00006450-200112000-00014. [DOI] [PubMed] [Google Scholar]
- 14.Frisk V, Jakobson LS, Unger S, Trachsel D, O'Brien K. Long-term neurodevelopmental outcomes of congenital diaphragmatic hernia survivors not treated with extracorporeal membrane oxygenation. J Pediatr Surg. 2011;46:1309–1318. doi: 10.1016/j.jpedsurg.2010.12.023. [DOI] [PubMed] [Google Scholar]
- 15.Pappas A, Shankaran S, Laptook AR, et al. Hypocarbia and adverse outcome in neonatal hypoxic-ischemic encephalopathy. J Pediatr. 2011;158:752–758. e1. doi: 10.1016/j.jpeds.2010.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hou X, Ding H, Teng Y, et al. Research on the relationship between brain anoxia at different regional oxygen saturations and brain damage using near-infrared spectroscopy. Physiol Meas. 2007;28:1251–1265. doi: 10.1088/0967-3334/28/10/010. [DOI] [PubMed] [Google Scholar]
- 17.Report of the Second Task Force on Blood Pressure Control in Children - 1987. Task Force on Blood Pressure Control in Children. National Heart, Lung, and Blood Institute, Bethesda, Maryland. Pediatrics. 1987;79:1–25. [PubMed] [Google Scholar]
- 18.Guidelines 2000 for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Part 10: pediatric advanced life support. The American Heart Association in collaboration with the International Liaison Committee on Resuscitation. Circulation. 2000;102:I291–I342. [PubMed] [Google Scholar]
- 19.Liem KD, Greisen G. Monitoring of cerebra haemodynamics in newborn infants. Early Hum Dev. 2010;86:155–158. doi: 10.1016/j.earlhumdev.2010.01.029. [DOI] [PubMed] [Google Scholar]
- 20.Wong FY, Silas R, Hew S, Samarasinghe T, Walker AM. Cerebral oxygenation is highly sensitive to blood pressure variability in sick preterm infants. PLoS One. 2012;8:e43165. doi: 10.1371/journal.pone.0043165. [DOI] [PMC free article] [PubMed] [Google Scholar]