Abstract
Approaches to the diagnosis and management of hepatocellular carcinoma (HCC) are improving survival. In the Surveillance, Epidemiology and End Results-13 registries, HCC stage, histological confirmation and first course surgery were examined. Among 21,390 HCC cases diagnosed during examined during 1998-2008 there were 4,727 (22%) with reported first course invasive liver surgery, local tumor destruction, or both. The proportion with reported liver surgery or ablation was 39% among localized stage cases and only 4% among distant/unstaged cases. While 70% of cases had histologically confirmed diagnoses, the proportion with confirmed diagnoses was higher among cases with reported invasive surgery (99%) compared to cases receiving ablation (81%) or no reported therapy (65%). Incidence rates of histologically unconfirmed HCC increased faster than those of confirmed HCC from 1992 to 2008 (8% versus 3% per year). Two encouraging findings were that incidence rates of localized stage HCC increased faster than rates of regional and distant stage HCC combined (8% versus 4% per year) and that incidence rates of reported first course surgery or tumor destruction increased faster than incidence rates of HCC without such therapy (11% versus 7%). Between 1975-1977 and 1998-2007, 5-year cause-specific HCC survival increased from just 3% to 18%. Survival was 84% among transplant recipients, 53% among cases receiving radiofrequency ablation at early stage, 47% among cases undergoing resection, and 35% among cases receiving local tumor destruction. Asian or Pacific Islander cases had significantly better 5-year survival (23%) than white (18%), Hispanic (15%), or black cases (12%).
Conclusion
HCC survival is improving as more cases are diagnosed and treated at early stages. Additional progress may be possible with continued use of clinical surveillance to follow individuals at risk for HCC, enabling early intervention.
Hepatocellular carcinoma (HCC) incidence rates have been increasing in the United States among most racial and ethnic groups.1 The prognosis for HCC has improved in recent years, as higher proportions of cases are diagnosed at early stages,2 enabling them to benefit from interventions including potentially curative transplantation, resection or early stage radiofrequency ablation (RFA).3 Despite progress, the overall one-year cause-specific survival rate in Surveillance Epidemiology and End Results (SEER) registries was less than 50% among cases diagnosed in 2003-2005.2
A study of HCC treatment trends in SEER registries during 1998-2005 documented the increased use of local tumor destruction, or ablation, over time.4 One mode of ablation, readiofrequency ablation (RFA) is potentially curative and the treatment of choice for cases with early stage HCC tumors, less than 3 cm in size.5 Nonetheless, the clinicians that documented the increased use of ablation in SEER registries4 expressed concern that in some instances, ease of access to ablative therapy could limit referral of HCC cases to medical facilities that offer a range of therapeutic options including resection and transplantation.4 For this reason the authors4 recommended that HCC cases receive multidisciplinary assessment to enable delivery of services consistent with current clinical guidelines.5
Since 2000, in SEER registries, compared to liver cancer cases with other specified histologies, a lower proportion of HCC cases are histologically confirmed. This may reflect changing clinical practice guidelines in which biopsy is not indicated when imaging tests show typical features of HCC.5 Other factors that may contribute to the declining proportion of histologically confirmed HCC cases include patient co-morbidities or lack of cooperation,7,8 and the use of non-invasive therapies such as local tumor destruction.9 The present report describes simultaneous changes in HCC histologic confirmation, stage, treatment, and survival in the SEER-13 registries from 1992-2008.
Materials and Methods
HCCs were examined by site and histological confirmation status in the SEER-13 registries from 1992 through 2008 (SEER*Stat 7.0.4, IMS, Silver Spring, MD). The SEER-13 registries (Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco Bay Area, Utah, Seattle-Puget Sound, Atlanta, San Jose-Monterey, Los Angeles, Alaskan Native and Rural Georgia) provided coverage of 14% of the United States population. The SEER-13 catchment was selected for analysis over SEER-17 because it enabled analysis of trends over nearly twice as many years. Furthermore, data on first course primary site surgery was available in SEER-13 registries from 1998 with follow-up of vital status to 2008. Registries collected case data with a priori approval by appropriate Institutional Review Boards.
The definition of HCC was based on International Classification of Diseases for Oncology10-12 (ICD-O) topography codes=C22.0 and C22.1, liver and intrahepatic bile duct cancers, respectively and prior to 2001, histology code 8170 (HCC, not otherwise specified). With implementation of ICD-O-3 in 2001, morphology codes for HCC were expanded to include these histologies: 8171, 8172, 8173, 8174, and 8175 (fibrolamellar, scirrhous, spindle cell variant, clear cell type, and pleomorphic type HCC; respectively). Tumor histology was abstracted by cancer registrars. The first preference was to obtain this information from pathology reports, followed by other sources.
Stage, Confirmation, and First Course Therapy
Stage, histological confirmation, and first course primary site surgery data were all available for the period, 1998-2008. Incidence trends by stage and histological confirmation were examined for the years from 1992 through 2008. Linear regression models were used to fit trend data (Joinpoint software, version 3.3.1; IMS, Silver Spring, MD).13 Annual percent change in regression line slopes were considered statistically significant when the trend differed from zero (P-value less than 0.05). Incidence trends were examined by histological confirmation, stage, and reported first course surgical and ablative therapy.
HCC Survival
Five-year cause-specific survival was estimated during the most recent decade of surveillance with follow-up of vital status, 1998-2007. Cause-specific survival was selected because life-tables were unavailable for most racial and ethnic groups included in this analysis and because life tables may not reflect mortality differentials between HCC cases and the population related to screening, socioeconomic status, or health behavior.14 Cause of death was defined as cancer, with other causes of death censored at time of death.
Survival analyses were restricted to 16,020 of 18,894 reported HCC cases (85%) diagnosed in SEER-13 registries during the most recent decade of surveillance 1998-2007. Cases were excluded from survival analysis because HCC was a second or later primary cancer diagnosis (n=2409, 13%), case information was limited to death certificate or autopsy reports (n=418, 2%), or because the case was alive without information on survival time (n= 47, <0.5%). For historical context, 5-year cause-specific survival of HCC cases diagnosed in SEER-9 registries was calculated for the period, 1975-1977.
Overall, race- and ethnicity-specific survival and 95% confidence intervals (CIs) were estimated by first course therapies, in descending order of survival: liver transplantation, RFA of tumors less than 3 cm in size (potentially curative5), resection, local tumor destruction, all cases, and cases with no reported surgery. Stage distributions were presented by group based on “Reason no surgery performed,” “SEER historic stage A” and “First course primary site surgery.” Among 1,249 cases with local tumor destruction, 75 (6%) underwent resection. Their 38% 5-year survival was similar to all cases with local tumor destruction (35%). The groups were combined for analysis.
Results
Stage, Confirmation and Treatment
Of 21,390 HCCs diagnosed during 1998-2008 4,727 (22%) reported liver surgery or local tumor destruction (Table 1). The interventions were reported more often among localized (39%) than regional (16%) or distant/unstaged cases (4%). Furthermore, 14,900 HCC cases (70%) had histologically confirmed diagnoses varying with reported invasive surgery (99%), local tumor destruction (81%), and no intervention (65%).
Table 1.
First-Course Hepatic Surgery or Localized Tumor Destruction, Diagnostic Confirmation and Stage, Incident HCC Cases, SEER-13, 1998-2008
| Stage | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| First-Course Hepatic Surgery | All Cases | (%) | Confirmed Histology (%)* | Localized | (%) | Regional | (%) | Distant/Unstaged | (%) |
| All cases | 21,390 | 100 | 14,900 (70%) | 8,940 | 100 | 5,949 | 100 | 6,501 | 100 |
| No reported intervention | 16,166 | 76 | 10,443 (65%) | 5,435 | 61 | 4,977 | 84 | 5,754 | 89 |
| Reported intervention† | 4,727 | 22 | 4,372 (92%) | 3,492 | 39 | 946 | 16 | 289 | 4 |
| Transplant/resection | 2,980 | 14 | 2,955 (99%) | 2,263 | 25 | 561 | 9 | 156 | 2 |
| Local tumor destruction | 1,715 | 8 | 1,385 (81%) | 1,220 | 14 | 378 | 6 | 117 | 2 |
| Unspecified surgery | 32 | 0.1 | 32 (100%) | 9 | 0.1 | 7 | 0.1 | 16 | 0.2 |
Row percents are shown for histologic confirmation (confirmed/total). Column percents are shown for all cases and by stage (percent of total).
Intervention includes transplant, resection, local tumor destruction, and unspecified surgery
Reported surgery group excludes 497 cases (2%) with equivocal data regarding surgery.
Abbreviations: HCC, hepatocellular carcinoma; SEER, Surveillance, Epidemiology, and End Results
Incidence Trends
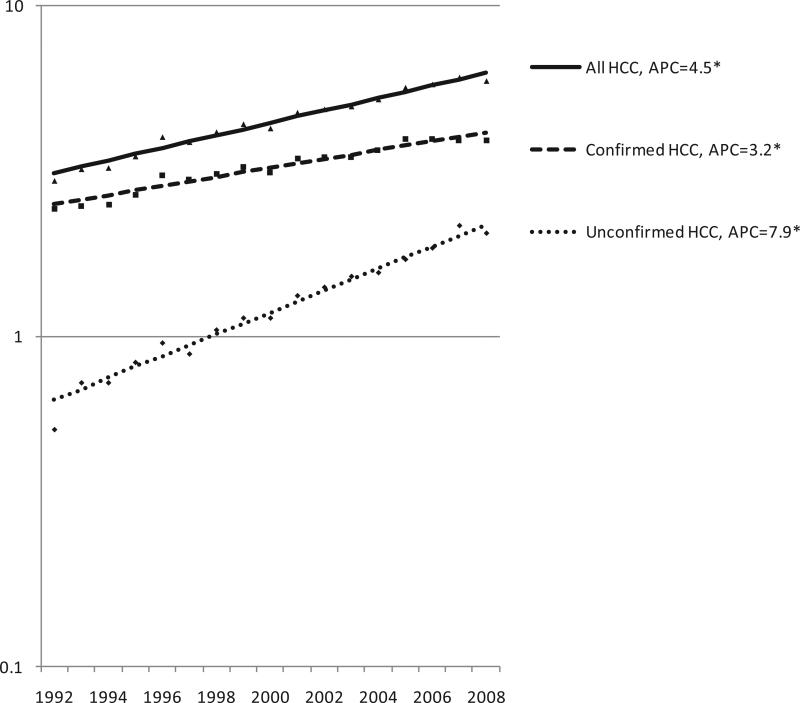
From 1992 to 2008, the incidence rate of histologically unconfirmed HCC increased 2.5 times more rapidly than the incidence rate of confirmed HCC (Fig. 1). The annual percent change (APC) in incidence rates for these two groups were 7.9 and 3.2, respectively (both statistically significant, P<0.05).
Fig. 1.
Incidence trends, all HCC cases, histologically confirmed cases, and unconfirmed cases, SEER-13, 1992-2008*† * APC: Annual percent change; Asterix: slope differs from zero, P<0.05 †Database: Incidence in SEER-13 Registries, Research Data, Nov 2010 Submission (1992-2008)
Stage Trends
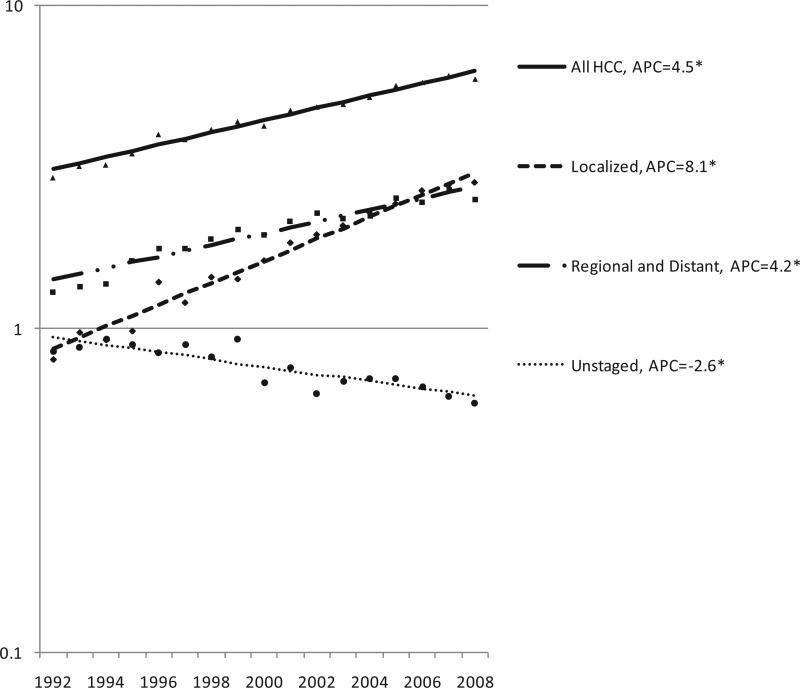
Between 1992 and 2008, incidence rates of localized stage HCC increased nearly twice as rapidly as rates of regional and distant stage HCC combined (Fig. 2), and surpassed regional and distant stage incidence rates during 2006-2008. The annual percent change (APC) in incidence rates for the two groups were 8.1 and 4.2, respectively (both statistically significant, P<0.05, as were APCs for regional and distant stage alone, data not shown). The APC for unstaged HCC was −2.6 (P<0.05).
Fig. 2.
Incidence trends, all HCC cases, cases diagnosed at localized stage, regional or distant stage, and unstaged cases, SEER-13, 1992-2008† * APC: Annual percent change; Asterix: slope differs from zero, P<0.05 †Database: Incidence in SEER-13 Registries, Research Data, Nov 2010 Submission (1992-2008)
Treatment Trends
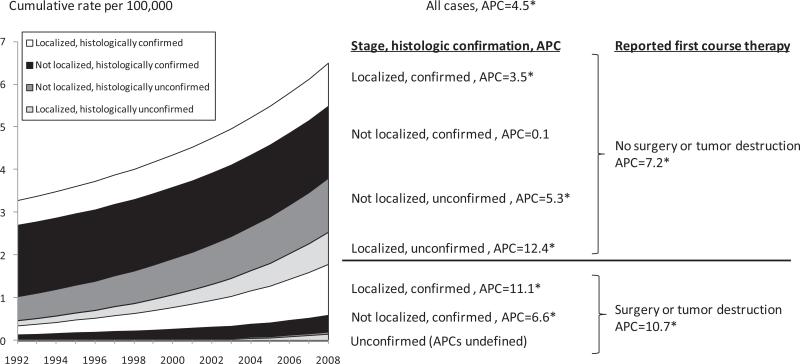
Incidence rates of reported invasive liver surgery or ablation (Fig. 3) increased significantly from 1992 to 2008 (APC=10.7, P<0.05). Incidence rates of HCC receiving no surgical intervention also increased significantly from 1992 to 2008 (APC=7.2, P<0.05).
Fig. 3.
Incidence trends, HCC cases by reported first course primary surgery/local tumor destruction, stage and histologic confirmation status. † * APC: Annual percent change (non-cumulative). Asterix indicates APC slope differs from zero, P<0.05. †Database: Incidence in SEER-13 Registries, Research Data, Nov 2010 Submission (1992-2008)
Stage and Treatment
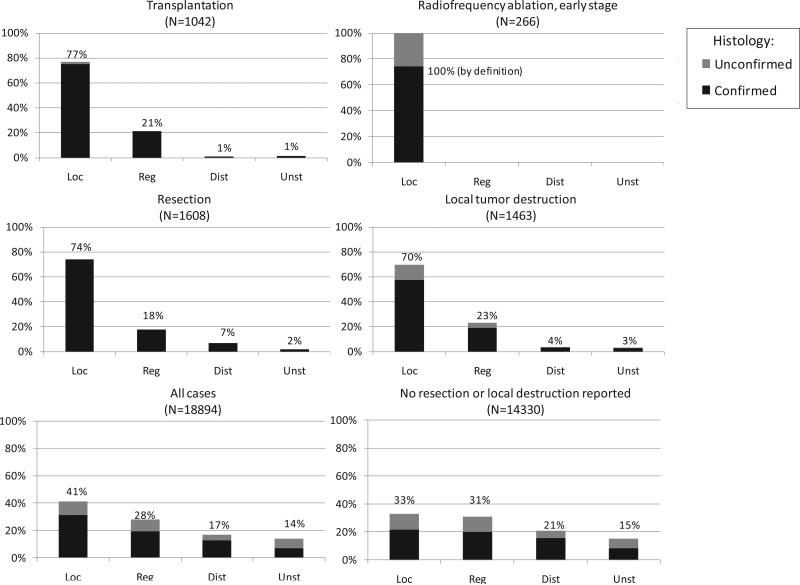
During 1998-2008, most HCC cases with reported first course surgery or ablation had localized stage HCC (Fig. 4), including cases receiving transplantation (77%), resection (75%), and local tumor destruction (70%). Overall, 41% of cases had localized stage HCC. One third of cases with no reported surgery or local tumor destruction had localized stage HCC.
Fig. 4.
HCC stage distribution and histologic confirmation status by reported first course surgery, diagnosis years 1998-2007*† * Transplant, resection, and local tumor destruction groups exclude 31 cases with unspecified surgery and 451 cases with equivocal data regarding surgery. †Database: Incidence in SEER-13 Registries, Research Data, Nov 2010 Submission (1998-2008)
Survival and Treatment
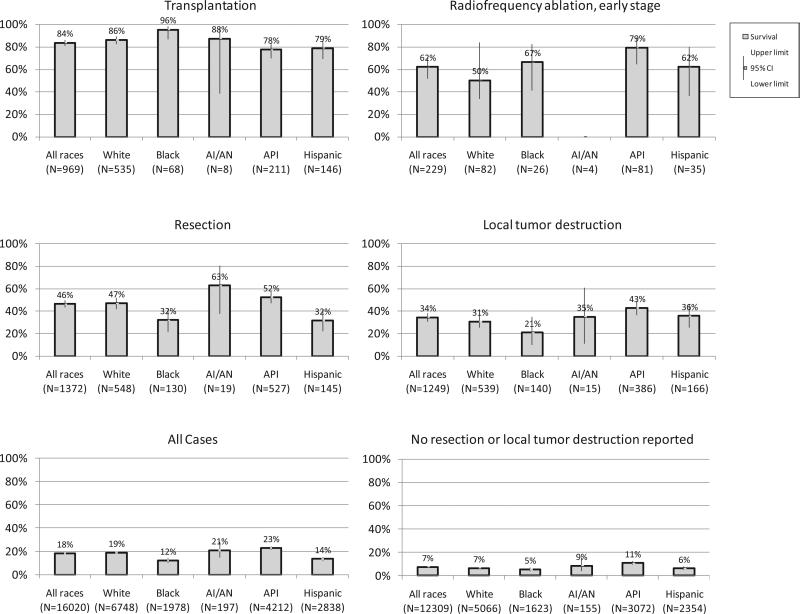
Among HCC cases diagnosed during 1998-2007 and followed for vital status through 2008, transplant recipients experienced 84% 5-year survival (Fig. 5). Cases with tumors less than 3 cm in size that received RFA had 5-year survival of 53%, while cases undergoing liver resection had 5-year survival of 47%. Among cases with reported local tumor destruction, 5-year survival was 35%. Compared to the 3% 5-year cause-specific survival in SEER-9 during 1975-1977 (data not shown), overall HCC survival during 1998-2007 was 18%, whereas survival was 7% among cases without reported invasive surgery or local tumor destruction.
Fig. 5.
HCC five-year cause-specific survival by reported first course surgery including by non-Hispanic race/ Hispanic ethnicity, diagnosis years 1998-2007.*† *Abbreviations: AI/AN=American Indian or Alaska Native, API=Asian or Pacific Islander. Note: Transplant, resection, and local tumor destruction groups exclude 24 cases with unspecified surgery and 121 cases with equivocal data regarding surgery. Data suppressed if fewer than 12 cases iden fied. †Database: Incidence in SEER-13 Registries, Research Data, Nov 2010 Submission (1998-2008)
Race, Treatment and Survival
5-year survival exceeded 75% among transplant recipients in all non-Hispanic racial and Hispanic ethnic groups with informative data (Fig. 5). Results were suppressed when there were fewer than 16 cases. Among cases with reported liver resection, Asians or Pacific Islanders experienced significantly better 5-year survival (52%) than Hispanics (35%) and blacks (33%). American Indian/Alaska Native cases had 63% survival following resection with wide CIs. 5-year survival following local tumor destruction was significantly higher among Asians or Pacific Islanders (43%) than black (21%) cases. Overall 5-year cause-specific survival among Asians or Pacific Islanders (23%) was significantly better than among white (18%), Hispanic (15%), or black cases (12%). White cases (18%) and American Indian/Alaska Native cases (20%) had significantly better survival than black cases (12%). Among cases with no reported surgery or ablation, Asian or Pacific Islander cases had better 5-year cause-specific survival (11%) than white (6%), Hispanic (7%) or black (5%) cases.
Discussion
This study indicates continuing improvement in stage distribution, treatment and survival for HCC cases in the SEER-13 registries. In the late 1970s, 5-year cause-specific HCC survival was 3%. Three decades later, HCC is increasingly detected at early stages when it is potentially curable.5 These improvements may be in part attributable to clinical surveillance of individuals with known risk factors for HCC.15 Despite the encouraging findings, overall 5-year survival remains less than 20%, and a majority of cases received neither surgical nor ablative therapy. Taken together the findings suggest that further improvements in HCC prognosis may be possible.
Potential may exist to improve survival through multi-disciplinary assessment in context of current clinical management guidelines.5 The best 5-year survival in this report was seen among cases that received liver transplantation (84%). Furthermore, RFA was potentially curative among cases with early stage HCC,16 associated with a 53% 5-year survival, similar to that of cases with reported resection (47%).4 Goals to improve overall cancer survival17 may be advanced by following patients at-risk for HCC to enable early-stage diagnosis and use of potentially curative therapy.15
In the present report, Asian or Pacific Islander HCC cases had better 5-year survival than white, Hispanic and black cases. Other reports also describe differences in overall HCC survival18-19 and treatment-specific survival between racial groups.20 In one study of localized stage cases that received invasive therapy, compared to whites,21 blacks had a 12% higher mortality rate while Asians or Pacific Islanders had a 16% lower mortality rates. The survival advantage among Asians or Pacific Islanders undergoing resection compared to Hispanics and whites could be explained by differences in risk factors or HCC prevention awareness between these racial groups. For example, a common risk-factor for HCC among Asians or Pacific Islanders is chronic HBV infection.18 While HBV DNA integrates into the host genome and is thought to be able to induce HCC without cirrhosis, HCV is an RNA virus that does not integrate into the host genome, with carcinogenesis mainly attributed to chronic inflammation and fibrosis.22 In a recent study of early stage HCC, compared to cases with HCV-associated HCC, HBV-associated HCC cases had better outcomes after resection.22 This finding was attributed to better liver reserve and less hepatic inflammation among the HBV-associated cases. Furthermore, because some Asian or Pacific Islander groups are known to be at high risk of HCC due to endemic HBV infection in parts of Asia, community-based screening programs within affected communities may facilitate detection of HCC at earlier stages.18 Other risk factors and co-morbidities might also affect survival across groups.23 Examples include but are not limited to liver failure, alcoholic liver disease, diabetes, obesity and non-alcoholic fatty liver disease.24 In addition, social factors such as income, education, employment, and access to health care may contribute to disparities in survival. For example, a United States study of area-level median household income found better survival among treated HCC patients from high- versus low-income counties.19
In this study, the proportion of HCC diagnoses that were histologically confirmed decreased with time. Possible reasons could include changes in etiology or co-morbidities of HCC cases.24 Histologic confirmation is also likely to be affected by guidelines affirming the use of non-invasive diagnostic5 and clinical management procedures under specified circumstances.5,8
This study has strengths including availability of trend data for stage and treatment as well as HCC survival data with sufficient counts to estimate survival within race and treatment subgroups. Caution is however recommended when interpreting the findings because of limited information on the method of diagnosis, as well as patient co-morbidities, HCC etiology and treatment. While the favorable survival associated with curative therapy in this report is thought to be meaningful, lead-time bias due to earlier diagnosis should not be dismissed as a contributory factor.25 Furthermore, the SEER-13 registries include only 14% of the United States population, with racial and economic attributes that differ in several meaningful ways from those of the entire nation. For example large Asian or Pacific Islander populations are found in the Hawaii, Seattle, and California registries of SEER-13 while Hispanic populations in Florida and Texas are not. The SEER-13 population is more urban than the United States. Although SEER-13 contains 14% of the nation's population, it contains 22% of U.S. liver transplant centers.26 Despite these limitations, the findings suggests that HCC diagnosis and management are changing in the SEER-13 population with favorable results on stage, treatment, and survival. In summary, the detection of early stage HCC among at-risk persons may enable the use of potentially curative therapies. This is likely to contribute to the improving survival described in this report. The small percent of cases receiving either liver surgery or ablation therapy (22%) suggests that the potential exists for further improvement in survival with ongoing implementation of the HCC control efforts already improving the prognoses of HCC cases.
Acknowledgements
We thank Carol Johnson, Leon Sun, Kathleen Cronin, Carol Kosary, Lynn Ries, Jennifer Ruhl, Susan Devesa, Nadia Howlader (critical review), Neil Neyman (coding), the U.S. Census Bureau (population data), SEER registries (population-based surveillance) and IMS, Inc. (data file).
This study was supported by National Cancer Institute, Division of Cancer Control and Population Sciences, Surveillance Research Program contracts with SEER Registries and Information Management Services and by the Intramural Research Program of the NIH, National Cancer Institute
Abbreviations
- APC
annual percent change
- CIs
confidence intervals
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- ICD-O
International Classification of Diseases for Oncology
- RFA
radiofrequency ablation
- SEER
Surveillance, Epidemiology and End Results
Footnotes
Potential conflict of interest: Nothing to report.
References
- 1.Ahmed F, Perz JF, Kwong S, Jamison PM, Friedman C, Bell BP. National trends and disparities in the incidence of hepatocellular carcinoma, 1998-2003. [May 17, 2011];Prev Chronic Dis. 2008 :5. http://www.cdc.gov/pcd/issues/2008/jul/07_0155.htm. [PMC free article] [PubMed]
- 2.Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27:1485–1491. doi: 10.1200/JCO.2008.20.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.El-Serag HB, Marrero JA, Rudolph L, et al. Diagnosis and treatment of hepatocellular carcinoma. Gastroenterology. 2008;134:1752–1763. doi: 10.1053/j.gastro.2008.02.090. [DOI] [PubMed] [Google Scholar]
- 4.Massarweh NN, Park JO, Farjah F, Yeung RS, Symons RG, Vaughan TL, et al. Trends in the utilization and impact of radiofrequency ablation for hepatocellular carcinoma. J Am Coll Surg. 2010;210:441–448. doi: 10.1016/j.jamcollsurg.2009.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Howlader N, Noone AM, Krapcho M, Neyman N, Aminou R, Waldron W, et al., editors. SEER Cancer Statistics Review, 1975-2008. National Cancer Institute; Bethesda, MD: 2011. [November 2, 2011]. Available at: http://seer.cancer.gov/csr/1975_2008/ [Google Scholar]
- 7.Talwalkar JA, Gores GJ. Diagnosis and staging of hepatocellular carcinoma. Gastroenterology. 2004;27(Suppl 1):S126–132. doi: 10.1053/j.gastro.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 8.Abrams P, Marsh JW. Current approach to hepatocellular carcinoma. Surg Clin North Am. 2010;90:803–816. doi: 10.1016/j.suc.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 9.Rahbari NN, Mehrabi A, Mollberg NM, Müller SA, Koch M, Büchler MW, et al. Hepatocellular carcinoma: current management and perspectives for the future. Ann Surg. 2011;253:453–469. doi: 10.1097/SLA.0b013e31820d944f. [DOI] [PubMed] [Google Scholar]
- 10.International Classification of Diseases for Oncology. World Health Organization; Geneva: 1976. [Google Scholar]
- 11.Percy C, Van Holten V, Muir C. International Classification of Diseases for Oncology. 2nd ed World Health Organization; Geneva: 1990. [Google Scholar]
- 12.Fritz A, Percy C, Jack A, Shanmugaratnam K, Sobin L, Parkin DM, et al. International Classification of Diseases for Oncology. 3rd ed. World Health Organization; Geneva: 2000. [Google Scholar]
- 13.Kim HJ, Fay MP, Feuer EJ, et al. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19:335–351. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 14.Howlader N, Ries LA, Mariotto AB, Reichman ME, Ruhl J, Cronin KA. Improved estimates of cancer-specific survival rates from population-based data. J Natl Cancer Inst. 2010;102:1584–1598. doi: 10.1093/jnci/djq366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sherman M. Hepatocellular carcinoma: epidemiology, surveillance, and diagnosis. Semin Liver Dis. 2010;30:3–16. doi: 10.1055/s-0030-1247128. [DOI] [PubMed] [Google Scholar]
- 16.Lencioni R. Loco-regional treatment of hepatocellular carcinoma in the era of molecular targeted therapies. Oncology. 2010;78(Suppl 1):107–112. doi: 10.1159/000315238. [DOI] [PubMed] [Google Scholar]
- 17.U.S. Department of Health and Human Services . Healthy People 2020. Washington, DC.: 2011. [November 2, 2011]. Available at: http://www.healthypeople.gov. [Google Scholar]
- 18.McClune AC, Tong MJ. Chronic Hepatitis B and Hepatocellular Carcinoma. Clinics in Liver Disease. 2010;14:461–476. doi: 10.1016/j.cld.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 19.Artinyan A, Mailey B, Sanchez-Luege N, Khalili J, Sun CL, Bhatia S, et al. Race, ethnicity, and socioeconomic status influence the survival of patients with hepatocellular carcinoma in the United States. Cancer. 2010;116:1367–1377. doi: 10.1002/cncr.24817. [DOI] [PubMed] [Google Scholar]
- 20.Wong RJ, Corley DA. Survival differences by race/ethnicity and treatment for localized hepatocellular carcinoma within the United States. Dig Dis Sci. 2009;54:2031–2039. doi: 10.1007/s10620-008-0661-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mathur AK, Osborne NH, Lynch RJ, Ghaferi AA, Dimick JB, Sonnenday CJ. Racial/ethnic disparities in access to care and survival for patients with early-stage hepatocellular carcinoma. 2010;145:1158–1163. doi: 10.1001/archsurg.2010.272. [DOI] [PubMed] [Google Scholar]
- 22.Kao WY, Su CW, Chau GY, Lui WY, Wu CW, Wu JC. A comparison of prognosis between patients with hepatitis B and C virus-related hepatocellular carcinoma undergoing resection surgery. World J Surg. 2011;35:858–867. doi: 10.1007/s00268-010-0928-z. [DOI] [PubMed] [Google Scholar]
- 23.Yang JD, Harmsen WS, Slettedahl SW, Chaiteerakij R, Enders FT, Therneau TM, et al. Factors that affect risk for hepatocellular carcinoma and effects of surveillance. Clin Gastroenterol Hepatol. 2011 doi: 10.1016/j.cgh.2011.03.027. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 24.London WT, McGlynn KA. Liver cancer, in Schottenfeld D. In: Fraumeni JF Jr, editor. Cancer Epidemiology and Prevention. ed 3 Oxford University Press; New York, NY: 2006. pp. 763–786. [Google Scholar]
- 25.El-Serag HB, Mason AC, Key C. Trends in survival of patients with hepatocellular carcinoma between 1977 and 1996 in the United States. Hepatology. 2001;33:62–65. doi: 10.1053/jhep.2001.21041. [DOI] [PubMed] [Google Scholar]
- 26.Organ Procurement and Transplantation Network [November 2, 2011];2011 Available at: http://optn.transplant.hrsa.gov.