Abstract
Purpose
Radiation-induced heart disease (RIHD) is a chronic severe side effect of radiotherapy of intrathoracic and chest wall tumors. The heart contains a dense network of sensory neurons that are not only involved in monitoring of cardiac events such as ischemia/reperfusion, but also play a role in cardiac tissue homeostasis, preconditioning, and repair. The purpose of this study was to examine the role of sensory nerves in RIHD.
Methods and Materials
Male Sprague-Dawley rats were administered capsaicin to permanently ablate sensory nerves, two weeks before local image-guided heart X-ray irradiation with a single dose of 21 Gy. During the 6-months follow up time, heart function was assessed with high resolution echocardiography. At 6 months after irradiation, cardiac structural and molecular changes were examined with histology, immunohistochemistry, and Western-Blots.
Results
Capsaicin-pretreatment blunted the effects of radiation on myocardial fibrosis and mast cell infiltration and activity. On the other hand, capsaicin-pretreatment caused a small but significant reduction in cardiac output at 6 months after irradiation. Capsaicin did not alter the effects of radiation on cardiac macrophage number or indicators of autophagy and apoptosis.
Conclusions
These results suggest that sensory nerves, while playing a predominantly protective role in radiation-induced cardiac function changes, may eventually enhance radiation-induced myocardial fibrosis and mast cell activity.
Introduction
Exposure of the heart to ionizing radiation may lead to radiation-induced heart disease (RIHD). RIHD presents several years after cardiac radiation exposure and involves accelerated atherosclerosis, conduction defects, and/or pericardial and myocardial fibrosis (1). Although thoracic radiotherapy has greatly improved over the last decade, and most patients no longer receive high doses of radiation to the whole heart, some patients with Hodgkin’s disease, lung cancer, esophageal and proximal gastric cancer still receive either a high dose of radiation to a small part of the heart or a lower dose to the whole heart (2–4). Recent evidence has led to a reduction of the estimated tolerance dose of the heart (5,6). Moreover, RIHD may be exacerbated by concomitant therapy with anthracyclines or other cardiotoxic chemotherapeutic agents (7). Biological mechanisms of RIHD are largely unknown. Hence, our research aims to understand biological mechanisms of RIHD in an effort to identify potential targets for intervention.
The heart contains a dense epicardial network of sensory nerve fibers, and sensory neurons are also found in the myocardium (8).The cardiac sensory nervous system is not only involved in the sensing and monitoring of cardiac events such as ischemia/reperfusion, but is now also known to play a complex role in cardiac tissue homeostasis, preconditioning, and repair. Cardiac sensory nerves release neuropeptides such as calcitonin gene related peptide (CGRP), neuropeptide Y, and substance P that regulate vascular tone and have chronotropic and inotropic effects on the heart (9,10).
Neurons closely interact with the immune system, and mast cells are considered one of the main cell types in these neuroimmune interactions (11). In many organs including the heart, mast cells are found in close proximity to nerve terminals or axons (12), and there is a two-way communication through the secretion of molecules by both neurons and mast cells (11,13). Moreover, mast cells interact with neurons via specific adhesion molecules that may resemble synaptic contacts (14). In a rat model of RIHD, the absence of mast cells reduced radiation-induced myocardial degeneration, but enhanced cardiac function loss and cardiac fibrosis (15).
Capsaicin, the pungent ingredient in chilli peppers, activates the transient receptor potential vanilloid type 1 (TRPV1), one of the main pain sensing receptors expressed by sensory nerves. When administered at high enough doses, capsaicin causes degeneration of a subset of sensory nerves, thereby permanently depleting them. Permanent depletion of sensory nerves by capsaicin in small animals is used as a tool to study the role of sensory nerves in tissue repair and remodeling in several organs including the heart (16–18). To determine the role of sensory nerves in RIHD, we investigated the effects of capsaicin-induced sensory nerve depletion on cardiac function, microvascular damage, inflammatory infiltration, mast cell activity, and myocardial fibrosis in a rat model of local heart irradiation.
Methods and Materials
Animals
This study conformed to the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and was approved by the Institutional Animal Care and Use Committee. Male Sprague-Dawley rats (Harlan Laboratories, Indianapolis, IN) were housed in the Division of Laboratory Animal Medicine on a 12:12 light-to-dark cycle with free access to food and water. After two weeks of acclimatization, rats were administered capsaicin to ablate their sensory nerves. Two weeks after the end of capsaicin administration, rats were exposed to local heart irradiation. The experiment contained four experimental groups: vehicle-pretreated sham-irradiated, vehicle-pretreated irradiated, capsaicin-pretreated sham-irradiated, and capsaicin-pretreated irradiated (n=12 in each group). Animals were observed for 6 months after irradiation.
Capsaicin administration
At a weight of 230–330 g., rats were administered capsaicin or vehicle as described before (18). Rats were anesthetized with 3% isoflurane inhalation and injected subcutaneously with capsaicin (Sigma-Aldrich, St Louis, MO) dissolved in 10% ethanol, 10% Tween 80, 80% saline at increasing doses of 10, 25, 40, and 50 mg/kg, or vehicle only, on 4 consecutive days. Because capsaicin administration may lead to instant respiratory distress, as a precaution terbutaline sulfate (Sigma-Aldrich, 0.2 mg/kg in saline) was given intramuscularly immediately after each capsaicin injection. Starting one week after capsaicin administration, and once a month thereafter, adequacy of sensory nerve ablation was ascertained by verifying the absence of a behavioral response of the rats to application of capsaicin (0.1 mg/ml in saline) to the eye or mouth, indicating that the animals had no pain sensation.
Local heart irradiation
Image-guided local heart irradiation was performed with the Small Animal Conformal Radiation Therapy Device our institution as described before (19). Rats were anesthetized with 3% isoflurane inhalation and placed vertically in a cylindrical Plexiglas holder that was cut out such that no material was in between the radiation beam and the chest. The heart was irradiated with three 19 mm-diameter fields (anterior-posterior and two lateral fields) to 7 Gy each (225 kV, 13 mA, 0.5 mm Cu-filtration, 1.92 Gy/min at 1 cm tissue depth). Before each exposure, the location of the heart was verified with the X-ray imager (70 kV, 5 mA, <1 cGy) and, when necessary, the position of the rat was adjusted with the robotic arm. Dosimetry was performed as described previously (19).
Echocardiography
At 1, 3, and 6 months after irradiation, echocardiography was performed with a Vevo 2100 imaging system (VisualSonics, Toronto, Canada) as described in supplementary methods (available online at www.redjournal.org).
Histology and immunohistochemistry
Animals were anesthetized with 3% isoflurane inhalation, and hearts were isolated, briefly rinsed via retrograde perfusion, fixed in 10% buffered formalin or methanol Carnoy’s solution (60% methanol, 30% chloroform, 10% acetic acid) and embedded in paraffin. Longitudinal sections of 5 μm were used for histology or immunohistochemistry. Histology and immunohistochemistry are described in supplementary methods (available online at www.redjournal.org).
Western-Blots
Animals were anesthetized with 3% isoflurane inhalation, and LV tissue was isolated, snap-frozen and stored at -80°C, and subsequently u sed for Western-Blot analysis as described in supplementary methods (available online at www.redjournal.org).
Statistical analysis
Data are presented as average ± standard error of the mean (SEM). Statistical analysis was performed with the software package NCSS 8. Echocardiography data were analyzed with repeated-measures ANOVA, followed by Newman-Keuls individual comparisons. Histological scores for vWf were tested with a Chi-Square test. All other data were evaluated with two-way ANOVA, followed by Newman-Keuls individual comparisons. The criterion for significance was p<0.05.
Results
Capsaicin pretreatment significantly inhibited cardiac sensory neurons
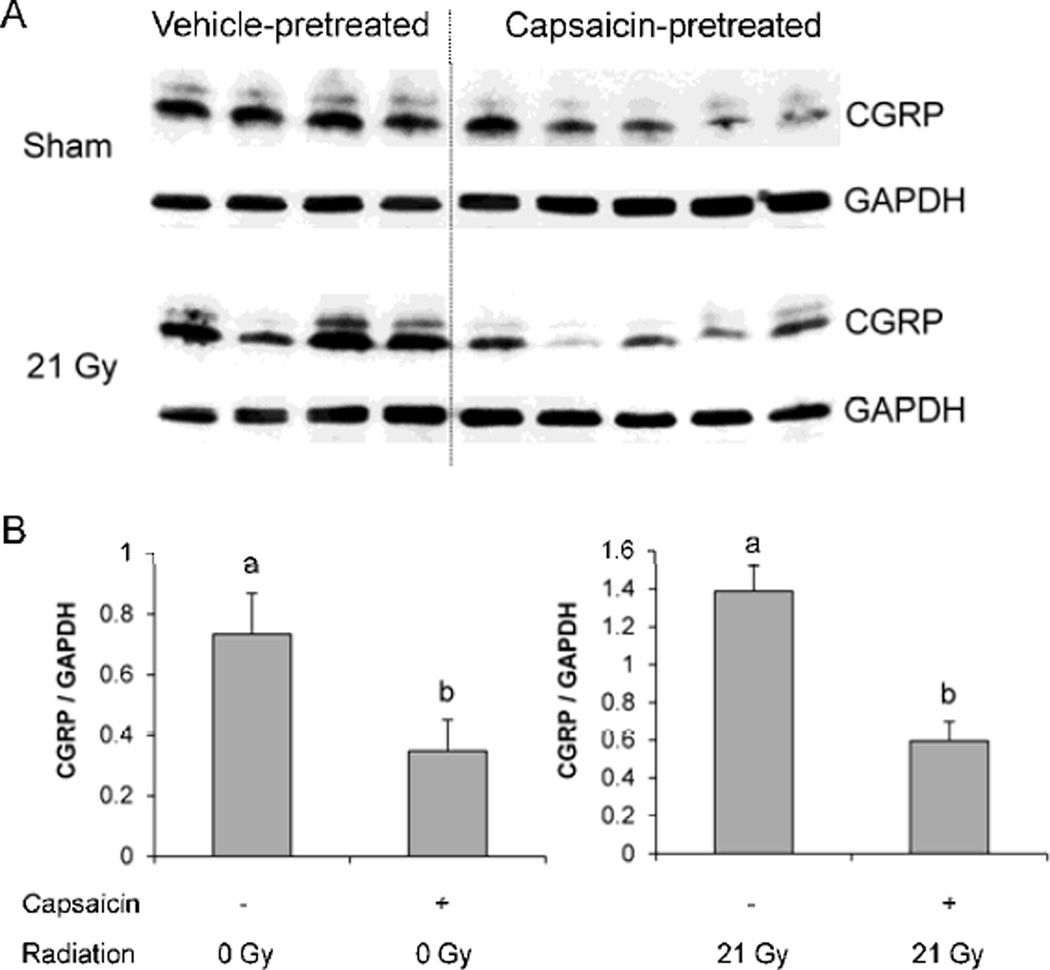
To confirm that capsaicin pretreatment inhibited sensory neurons in the heart, Western-Blots were performed to measure CGRP expression in LV samples at 6 months after (sham-)irradiation, 6.5 months after capsaicin administration. Capsaicin pretreatment significantly reduced LV CGRP in both sham-irradiated and irradiated animals (Figure 1), indicating that long-term inhibition of sensory neurons had occurred with capsaicin pretreatment.
Figure 1. LV protein expression of CGRP at 6 months after local heart irradiation.
Capsaicin pretreatment caused a significant reduction in LV CGRP in sham-irradiated and irradiated rats. Representative blot (A) and densitometry analysis (B). Average ± SEM, n=4-5. Different characters indicate statistically significant differences (p<0.05).
Heart weight to body weight ratio did not alter in any of the groups
Although local heart irradiation caused a significant reduction in heart weight and body weight in vehicle-pretreated animals, heart to body weight ratio did not significantly change in any of the groups (Table 1).
Table 1.
Heart and body weights at 6 months after irradiation. Average ± SEM, n=12.
| Heart weight (g) | Body weight (g) | Heart/body weight ratio |
|
|---|---|---|---|
| Vehicle, 0 Gy | 1.6 ± 0.08a | 498.4 ± 12.9a | 3.2 ± 0.09 |
| Vehicle, 21 Gy | 1.4 ± 0.05b | 465 ± 11.4b | 3.3 ± 0.1 |
| Capsaicin, 0 Gy | 1.5 ± 0.05 | 473 ± 9.5 | 3.3 ± 0.1 |
| Capsaicin, 21 Gy | 1.4 ± 0.03 | 465 ± 8.7 | 3.1 ± 0.1 |
Different characters indicate statistically significant differences (p<0.05).
Sensory denervation may have worsened cardiac dysfunction in irradiated animals
Throughout the study one of the capsaicin pretreated sham-irradiated rats had an enlarged heart with M-mode functional parameters that were outside the normal physiological range. This animal was excluded from further analysis. Capsaicin had no effect on cardiac function in sham-irradiated animals.
At one month, radiation did not significantly change M-mode parameters in the vehicle-pretreated group or in the capsaicin-pretreated group (data not shown). At 3 months, radiation significantly reduced LV mass in the vehicle-pretreated group, but not in the capsaicin-pretreated group (Supplementary Table e1, available online at www.redjournal.org).
At 6 months after irradiation, 4 out of 12 vehicle-pretreated irradiated animals showed bradycardia (heart rate <250 bpm) and arrhythmia. Capsaicin-pretreatment did not significantly alter the effect of radiation on bradycardia and arrhythmia (5 out of 12 animals). Bradycardia and arrhythmia were not observed in sham-irradiated groups.
In vehicle-pretreated animals, radiation caused a significant reduction in the thickness of the LV walls, a reduction in LV mass, and an increase in stroke volume. Of these alterations, capsaicin-pretreatment blunted the effect of radiation on LV mass only (Supplementary Table e1, available online at www.redjournal.org). Moreover, capsaicin-pretreatment exacerbated radiation-induced increased LV inner diameter and volume, and reduced the size of the aortic outflow tract. While both vehicle-pretreated and capsaicin-pretreated rats showed a trend towards a reduced cardiac output after irradiation, the change in cardiac output was significant only in capsaicin-pretreated rats.
Sensory denervation blunted myocardial fibrosis but did not alter endothelial injury or infiltration of CD2 and CD68 positive cells
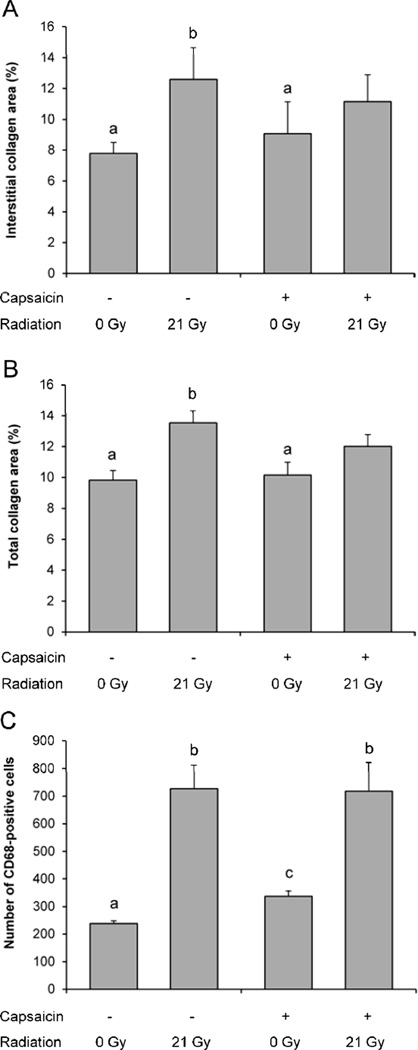
In vehicle-pretreated animals, at 6 months after local heart irradiation, significant increases were observed in ventricular deposition of interstitial collagen and total (interstitial and perivascular) collagen and vWf, a marker of endothelial injury. Capsaicin pretreatment inhibited the effects of radiation on collagen deposition (Figure 2), but did not significantly alter scores for vWf (Table 2).
Figure 2. Ventricular collagen and numbers of CD68 positive cells.
Radiation caused a significant increase in ventricular interstitial collagen (A) and ventricular total (interstitial and perivascular) collagen (B) in vehicle-pretreated rats but not in capsaicin-pretreated rats. Capsaicin caused a small increase in total number of CD68 positive cells in sham-irradiated animals, but did not alter the effect of radiation on CD68 positive cells (C). Average ± SEM, n=7. Different characters indicate statistically significant differences (p<0.05).
Table 2.
Scores for vWf deposition (on a scale from 0 to 3, n=7), at 6 months after irradiation.
| vWf scores | |
|---|---|
| Vehicle, 0 Gy | 0,0,0,0,0,0,0a |
| Vehicle, 21 Gy | 0,1,2,2,3,3,3b |
| Capsaicin, 0 Gy | 0,0,0,0,0,0,0a |
| Capsaicin, 21 Gy | 1,1,2,2,2,3,3b |
Different characters indicate statistically significant differences (p<0.05).
In vehicle-pretreated animals, radiation significantly enhanced the numbers of CD68 positive cells (monocytes/macrophages, Figure 2). Capsaicin pretreatment caused a small but significant increase in CD68 positive cells in sham-irradiated animals, but did not affect the number of CD68 positive cells in irradiated animals. No changes were found in the numbers of CD2 positive cells (T-cells/natural killer cells) in any of the groups (data not shown).
Sensory denervation inhibited myocardial mast cell activity
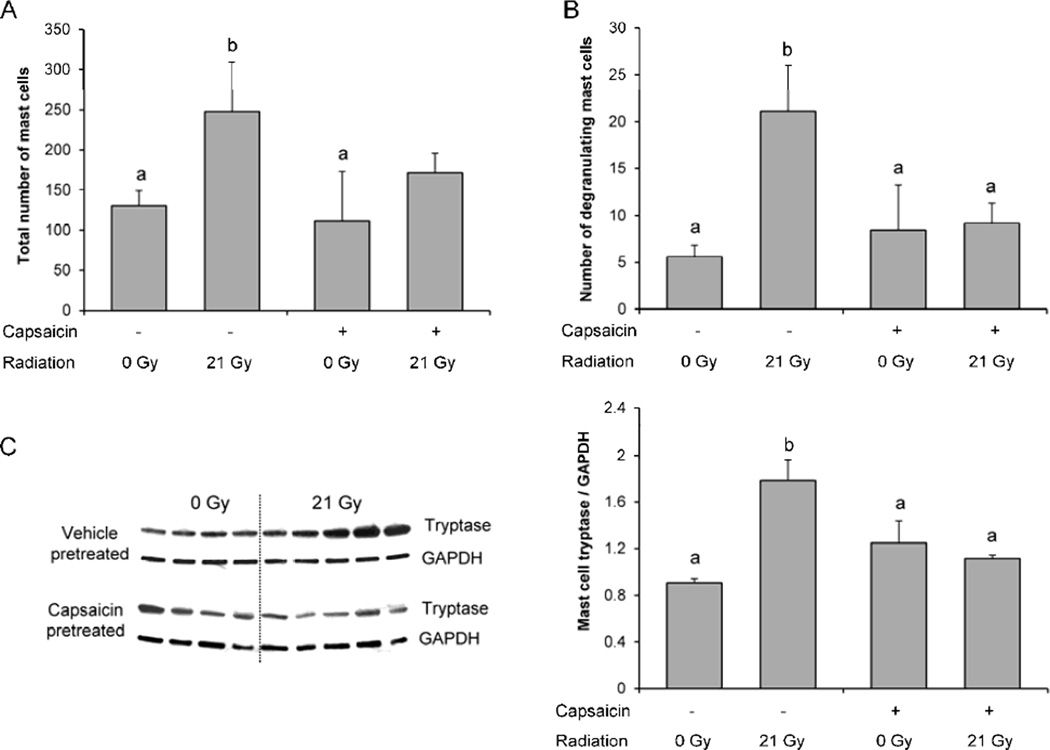
In our rat model of local heart irradiation, mast cell numbers closely correlate with myocardial fibrosis. Hence, at 6 months after irradiation, a significant increase was observed in total mast cell number (Figure 3A). The Toluidine Blue staining used to visualize mast cells reveals when mast cells are degranulating. Radiation caused an increase in the number of degranulating cells (Figure 3B), which suggests increased mast cell activity. We therefore tested LV mast cell tryptase content as a marker for mast cell activity (Figure 3C). Capsaicin pretreatment blunted the effects of radiation on total mast cell number and on cardiac mast cell activity.
Figure 3. Total ventricular numbers of mast cells, degranulating mast cells, and tryptase levels.
Radiation caused a significant increase in total number of mast cells (A), number of degranulating mast cells (B), and left ventricular levels of the mast cell enzyme tryptase (C) in vehicle-pretreated rats only. Average ± SEM, n=4-5. Different characters indicate statistically significant differences (p<0.05).
Sensory denervation abrogated the effects of radiation on nuclear receptor subfamily 4, group A (NR4A) 1 and 2
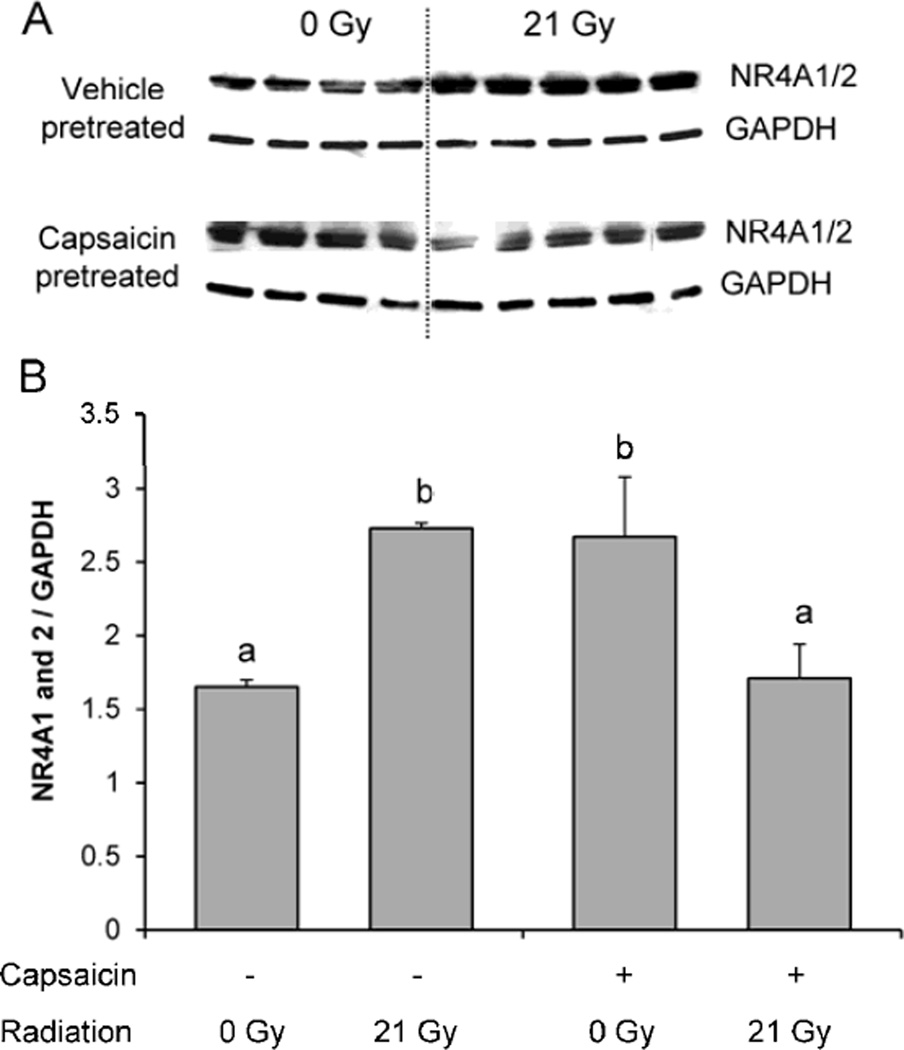
NR4A1 and NR4A2 are closely related nuclear orphan receptors whose expression increases upon cardiomyocyte injury. In previous studies, we had determined that LV levels of NR4A1/2 increased as early as 24 hours after irradiation and stayed upregulated from then on (unpublished data). We here showed that capsaicin-pretreatment caused a significant increase in NR4A1 and 2 in sham-irradiated rats, but prevented the increase in expression of these proteins in response to radiation (Figure 4).
Figure 4. LV protein expression of nuclear receptor subfamily 4, group A (NR4A) 1/2 at 6 months after local heart irradiation.
Capsaicin pretreatment prevented a radiation-induced increase in left ventricular protein levels of NR4A1/2. Representative blot (A) and densitometry analysis (B). Average ± SEM, n=4-5. Different characters indicate statistically significant differences (p<0.05).
NR4A1/2 may play a role in the induction of both autophagy and apoptosis in cardiomyocytes. However, no changes were seen in LV protein levels of the autophagy indicators LC3A I and II (Supplementary Figure e1, available online at www.redjournal.org), or in the numbers of apoptotic nuclei in any of the experimental groups (data not shown).
Discussion
This study examined the role of sensory nerves in the development of chronic manifestations of RIHD, by testing the effects of sensory denervation by capsaicin on cardiac effects of local heart irradiation in the rat.
Permanent inhibition of small animal sensory nerves by systemic administration of capsaicin is a common tool to study the role of sensory nerves in tissue repair and remodeling in several organs including the heart (16–18). One of the proposed mechanisms by which capsaicin causes sensory neuron degeneration involves an influx of sodium through capsaicin-induced activation of TRPV1, causing swelling and ultimately neuronal cell death (20). In our study, capsaicin pretreatment significantly reduced LV levels of CGRP, indicating that cardiac sensory neurons were indeed inhibited. Nonetheless, some CGRP was still detected in the heart samples. We therefore cannot exclude that some sensory neuron function may have remained in the capsaicin-pretreated rats. Moreover, we cannot exclude that capsaicin caused other long-term changes in the heart. For instance, it is uncertain whether capsaicin-induced changes in the number of CD68 positive cells and LV levels of NR4A1 and 2 in sham-irradiated rats was due to sensory nerve inhibition. Future studies that involve sensory depletion with more selective TRPV1 agonists may provide more insight into the specificity of capsaicin and the effects of sensory denervation in unirradiated rats.
Sensory denervation with capsaicin before local heart irradiation abrogated the effects of radiation on LV mass, LV expression of NR4A1/2, myocardial fibrosis, and mast cell activity. On the other hand, sensory denervation did not affect radiation-induced infiltration of CD68 positive cells and worsened a few of the parameters of cardiac structure and function at 6 months. Previous preclinical studies have shown that the heart has a strong capacity to compensate for structural damage in the myocardium and cardiac microvasculature, to maintain cardiac output (21). A plausible explanation for the data provided by the current study is that capsaicin-pretreatment reduced this ability of the heart to compensate for radiation-induced morphological injury. These results are in accordance with previous studies showing that cardiac sensory nerves and their neuropeptides play a protective role in models of acute cardiac injury (22), possibly due to positive inotropic or vasodilatory effects of the sensory neuropeptides (10,23). Nonetheless, because only a subset of echocardiography parameters was affected by capsaicin, future studies have to provide definite evidence for the nature of the role of sensory neurons in radiation-induced changes in cardiac function.
Capsaicin may have short-term effects on the cardiac sinoatrial node (24). However, in our studies, capsaicin-pretreatment did not modify the ECGs of sham-irradiated rats and did not alter the number of irradiated rats that showed bradycardiac and arrhythmia, suggesting that the conduction system was not significantly affected by capsaicin.
Our experiments suggest that sensory nerves enhance radiation-induced cardiac fibrosis. Few previous studies have examined the role of sensory nerves and sensory neuropeptides in adverse cardiac remodeling. Sensory nerves may reduce myocardial fibrosis in some models of acute heart disease (22). Capsaicin pretreatment, on the other hand, reduced chronic collagen deposition in a rat model of local intestine irradiation (18). This previous study and the current study both suggest that sensory nerves enhance chronic radiation-induced normal tissue fibrosis.
In our rat model of local heart irradiation, mast cell numbers closely correlate with myocardial fibrosis. Although the interaction between sensory nerves and mast cells in the heart has not been studied extensively, some studies have shown that cardiac sensory nerves may locally activate mast cells (25), thereby causing changes such as a local activation of the local renin-angiotensin system (26). While mast cells may somewhat enhance myocardial degeneration, mast cells reduce cardiac fibrosis and may protect against a radiation-induced cardiac function loss in the rat (15). Here, we show that sensory nerves are involved in the recruitment and activation of cardiac mast cells in the irradiated heart, suggesting that cardiac sensory nerves modulate RIHD at least in part by altering mast cell activity. Interestingly, the effects of capsaicin pretreatment on intestinal radiation injury were blunted in the absence of mast cells, indicating that sensory nerve – mast cell interactions indeed play a role in normal tissue radiation injury (17).
Nuclear hormone receptors play an important role in the pathophysiology of cardiovascular diseases. Among these receptors, NR4A1/2 have no known ligand and are considered early response genes that are induced by cellular injury and in response to cytokines and growth factors (27). NR4A1/2 are known to induce the expression of certain genes as homodimers and heterodimers in the nucleus, and can translocate to the cytoplasm to cause other cellular effects (28). Although all roles of NR4A1/2 in the heart have yet to be elucidated, these proteins are known to regulate apoptosis and autophagy, both by altering gene expression and by modifying the mitochondrial membrane (28). Even though capsaicin pretreatment blunted the effects of radiation on LV mass as measured with echocardiography, we did not find other evidence of increased apoptosis or autophagy at 6 months after local heart irradiation. We speculate that NR4A1/2 play other roles in RIHD, for instance through their regulation of metabolism and inflammation (27,29).
The radiation-induced upregulation of NR4A1/2 was abrogated by sensory nerve ablation, indicating a role for sensory nerves in the induction of these nuclear receptors. The neuropeptide substance P has been shown to induce NR4A1 expression and activation in cells expressing the substance P receptor (30). Interestingly, the expression of NR4A1/2 in the heart is also induced by β-adrenergic stimulation (31,32). Local heart irradiation alters β-adrenergic signaling in the rat (33,34). Moreover, in the heart there is cross-talk between cardiac sensory and adrenergic nerves (35). Future studies will have to address the role of NR4A1/2 in RIHD, and the mechanisms by which cardiac sensory nerves cause their induction.
In conclusion, these results suggest that sensory nerves may play a dual role in the development of RIHD. Sensory nerves may enhance cardiomyocyte expression of the injury-related nuclear receptors NR4A1/2 and enhance radiation-induced myocardial fibrosis, but may also enhance mast cell activity and play a predominantly protective role in radiation-induced cardiac function changes. Future studies will have to elucidate the mechanisms by which sensory neurons may modulate RIHD.
Supplementary Material
Summary.
Radiation-induced heart disease (RIHD) is a serious chronic side effect of radiotherapy of thoracic tumors. To test the hypothesis that sensory nerves may regulate the development of RIHD, structural and functional effects of local heart irradiation were studied in sensory denervated rats. This study shows that sensory nerves may play a dual role in RIHD, by enhancing radiation-induced myocardial fibrosis and mast cell activity, but ameliorating changes in cardiac output.
Acknowledgments
Grant support: National Institutes of Health (CA148679, CA71382) and American Cancer Society (RSG-10-125-01-CCE).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: none
References
- 1.Adams MJ, Hardenbergh PH, Constine LS, et al. Radiation-associated cardiovascular disease. Crit Rev Oncol Hematol. 2003;45:55–75. doi: 10.1016/s1040-8428(01)00227-x. [DOI] [PubMed] [Google Scholar]
- 2.Chera BS, Rodriguez C, Morris CG, et al. Dosimetric comparison of three different involved nodal irradiation techniques for stage II Hodgkin's lymphoma patients: conventional radiotherapy, intensity-modulated radiotherapy, and threedimensional proton radiotherapy. Int J Radiat Oncol Biol Phys. 2009;75:1173–1180. doi: 10.1016/j.ijrobp.2008.12.048. [DOI] [PubMed] [Google Scholar]
- 3.Kole TP, Aghayere O, Kwah J, et al. Comparison of Heart and Coronary Artery Doses Associated with Intensity-Modulated Radiotherapy Versus Three- Dimensional Conformal Radiotherapy for Distal Esophageal Cancer. Int J Radiat Oncol Biol Phys. 2012;83:1580–1586. doi: 10.1016/j.ijrobp.2011.10.053. [DOI] [PubMed] [Google Scholar]
- 4.Wu WC, Chan CL, Wong YW, et al. A study on the influence of breathing phases in intensity-modulated radiotherapy of lung tumours using four-dimensional CT. Br J Radiol. 2009;83:252–256. doi: 10.1259/bjr/33094251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schultz-Hector S, Trott KR. Radiation-induced cardiovascular diseases: Is the epidemiologic evidence compatible with the radiobiologic data? Int J Radiat Oncol Biol Phys. 2007;67:10–18. doi: 10.1016/j.ijrobp.2006.08.071. [DOI] [PubMed] [Google Scholar]
- 6.Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368:987–998. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 7.Shapiro CL, Hardenbergh PH, Gelman R, et al. Cardiac effects of adjuvant doxorubicin and radiation therapy in breast cancer patients. J Clin Oncol. 1998;16:3493–3501. doi: 10.1200/JCO.1998.16.11.3493. [DOI] [PubMed] [Google Scholar]
- 8.Zahner MR, Li DP, Chen SR, et al. Cardiac vanilloid receptor 1-expressing afferent nerves and their role in the cardiogenic sympathetic reflex in rats. J Physiol. 2003;551:515–523. doi: 10.1113/jphysiol.2003.048207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anand IS, Gurden J, Wander GS, et al. Cardiovascular and hormonal effects of calcitonin gene-related peptide in congestive heart failure. J Am Coll Cardiol. 1991;17:208–217. doi: 10.1016/0735-1097(91)90729-s. [DOI] [PubMed] [Google Scholar]
- 10.Holdright DR, Clarke D, Fox K, et al. The effects of intracoronary substance P and acetylcholine on coronary blood flow in patients with idiopathic dilated cardiomyopathy. Eur Heart J. 1994;15:1537–1544. doi: 10.1093/oxfordjournals.eurheartj.a060427. [DOI] [PubMed] [Google Scholar]
- 11.Williams RM, Bienenstock J, Stead RH. Mast cells: the neuroimmune connection. Chem Immunol. 1995;61:208–235. [PubMed] [Google Scholar]
- 12.Arizono N, Matsuda S, Hattori T, et al. Anatomical variation in mast cell nerve associations in the rat small intestine, heart, lung, and skin. Similarities of distances between neural processes and mast cells, eosinophils, or plasma cells in the jejunal lamina propria. Lab Invest. 1990;62:626–634. [PubMed] [Google Scholar]
- 13.Skaper SD, Pollock M, Facci L. Mast cells differentially express and release active high molecular weight neurotrophins. Brain Res Mol Brain Res. 2001;97:177–185. doi: 10.1016/s0169-328x(01)00314-x. [DOI] [PubMed] [Google Scholar]
- 14.Ito A, Oonuma J. Direct interaction between nerves and mast cells mediated by the SgIGSF/SynCAM adhesion molecule. J Pharmacol Sci. 2006;102:1–5. doi: 10.1254/jphs.cpj06014x. [DOI] [PubMed] [Google Scholar]
- 15.Boerma M, Wang J, Wondergem J, et al. Influence of mast cells on structural and functional manifestations of radiation-induced heart disease. Cancer Res. 2005;65:3100–3107. doi: 10.1158/0008-5472.CAN-04-4333. [DOI] [PubMed] [Google Scholar]
- 16.Holzer P. Capsaicin as a tool for studying sensory neuron functions. Adv Exp Med Biol. 1991;298:3–16. doi: 10.1007/978-1-4899-0744-8_1. [DOI] [PubMed] [Google Scholar]
- 17.Deidentified reference for the purpose of a blinded manuscript. Full reference will be included after acceptance of the mansucript [Google Scholar]
- 18.Katona M, Boros K, Santha P, et al. Selective sensory denervation by capsaicin aggravates adriamycin-induced cardiomyopathy in rats. Naunyn Schmiedebergs Arch Pharmacol. 2004;370:436–443. doi: 10.1007/s00210-004-0985-7. [DOI] [PubMed] [Google Scholar]
- 19.Deidentified reference for the purpose of a blinded manuscript. Full reference will be included after acceptance of the mansucript [Google Scholar]
- 20.Pecze L, Blum W, Schwaller B. Mechanism of capsaicin receptor TRPV1- mediated toxicity in pain-sensing neurons focusing on the effects of Na+/Ca2+ fluxes and the Ca2+-binding protein calretenin. Biochim Biophys Acta. 2013;1833:1680–1691. doi: 10.1016/j.bbamcr.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 21.Seemann I, Gabriels K, Visser NL, et al. Irradiation induced modest changes in murine cardiac function despite progressive structural damage to the myocardium and microvasculature. Radiother Oncol. 2012;103:143–150. doi: 10.1016/j.radonc.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 22.Li JZ, Peng J, Xiao L, et al. Reversal of isoprenaline-induced cardiac remodeling by rutaecarpine via stimulation of calcitonin gene-related peptide production. Can. J Physiol Pharmacol. 2010;88:949–959. doi: 10.1139/y10-067. [DOI] [PubMed] [Google Scholar]
- 23.Katori T, Hoover DB, Ardell JL, et al. Calcitonin gene-related peptide in vivo positive inotropy is attributable to regional sympatho-stimulation and is blunted in congestive heart failure. Circ Res. 2005;96:234–243. doi: 10.1161/01.RES.0000152969.42117.ca. [DOI] [PubMed] [Google Scholar]
- 24.Yan-Ping C, Yi-He W, Li-Ping C, et al. Electrophysiologic effects of capsaicin on pacemaker cells in sinoatrial nodes of rabbits. Acta Pharmacol Sin. 2003;24:826–830. [PubMed] [Google Scholar]
- 25.Imamura M, Smith NC, Garbarg M, et al. Histamine H3-receptor-mediated inhibition of calcitonin gene-related peptide release from cardiac C fibers. A regulatory negative-feedback loop. Circ Res. 1996;78:863–869. doi: 10.1161/01.res.78.5.863. [DOI] [PubMed] [Google Scholar]
- 26.Morrey C, Brazin J, Seyedi N, et al. Interaction between sensory C-fibers and cardiac mast cells in ischemia/reperfusion: activation of a local renin-angiotensin system culminating in severe arrhythmic dysfunction. J Pharmacol Exp Ther. 2010;335:76–84. doi: 10.1124/jpet.110.172262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao Y, Bruemmer D. NR4A orphan nuclear receptors: transcriptional regulators of gene expression in metabolism and vascular biology. Arterioscler Thromb Vasc Biol. 2010;30:1535–1541. doi: 10.1161/ATVBAHA.109.191163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheng Z, Volkers M, Din S, et al. Mitochondrial translocation of Nur77 mediates cardiomyocyte apoptosis. Eur Heart J. 2011;32:2179–2188. doi: 10.1093/eurheartj/ehq496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McMorrow JP, Murphy EP. Inflammation: a role for NR4A orphan nuclear receptors? Biochem Soc Trans. 2011;39:688–693. doi: 10.1042/BST0390688. [DOI] [PubMed] [Google Scholar]
- 30.Cottrell GS, Padilla BE, Amadesi S, et al. Endosomal endothelin-converting enzyme-1: a regulator of beta-arrestin-dependent ERK signaling. J Biol Chem. 2009;284:22411–22425. doi: 10.1074/jbc.M109.026674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Myers SA, Eriksson N, Burow R, et al. Beta-adrenergic signaling regulates NR4A nuclear receptor and metabolic gene expression in multiple tissues. Mol Cell Endocrinol. 2009;309:101–108. doi: 10.1016/j.mce.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 32.Soker T, Godecke A. Expression of the murine Nr4a1 gene is controlled by three distinct genomic loci. Gene. 2013;512:517–520. doi: 10.1016/j.gene.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 33.Schultz-Hector S, Bohm M, Blochel A, et al. Radiation-induced heart disease: morphology, changes in catecholamine synthesis and content, betaadrenoceptor density, and hemodynamic function in an experimental model. Radiat Res. 1992;129:281–289. [PubMed] [Google Scholar]
- 34.Wondergem J, Franken NA, van der Laarse A, et al. Changes in cardiac performance and sympathetic stimulation during and after fractionated radiotherapy in a rat model. Radiother Oncol. 1996;38:33–40. doi: 10.1016/0167-8140(95)01161-7. [DOI] [PubMed] [Google Scholar]
- 35.Seyedi N, Maruyama R, Levi R. Bradykinin activates a cross-signaling pathway between sensory and adrenergic nerve endings in the heart: a novel mechanism of ischemic norepinephrine release? J Pharmacol Exp Ther. 1999;290:656–663. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.