Abstract
KIF20A (RAB6KIFL) belongs to the kinesin superfamily of motor proteins, which play critical roles in the trafficking of molecules and organelles during the growth of pancreatic cancer. Immunotherapy using a previously identified epitope peptide for KIF20A is expected to improve clinical outcomes. A phase I clinical trial combining KIF20A-derived peptide with gemcitabine (GEM) was therefore conducted among patients with advanced pancreatic cancer who had received prior therapy such as chemotherapy and/or radiotherapy. GEM was administered at a dose of 1000 mg/m2 on days 1, 8, and 15 in a 28-day cycle. The KIF20A-derived peptide was injected subcutaneously on a weekly basis in a dose-escalation manner (doses of 0.5, 1, and 3 mg/body; 3 patients/cohort). Safety and immunologic parameters were assessed. No severe adverse effects of grade 3 or higher related to KIF20A-derived peptide were observed. Of the 9 patients who completed at least one course of treatment, interferon-γ (IFN-γ)-producing cells were induced in 4 of 9 patients (P2, P3, P6, and P7), and IFN-γ-producing cells were increased in 4 of the 9 patients (P1, P5, P8, and P9). Four of the 9 patients achieved stable disease. The disease control rate was 44%. The median survival time after first vaccination was 173 days and 1-year survival rate was 11.1%. IFN-γ-producing cells were induced by the KIF20A-derived peptide vaccine at a high rate, even in combination with GEM. These results suggest that this combination therapy will be feasible and promising for the treatment of advanced pancreatic cancer.
Key Words: pancreatic cancer, peptide, KIF20A, phase I, immunotherapy
Pancreatic cancer is the fourth leading cause of cancer mortality in the world. The prognosis for patients with pancreatic cancer is extremely poor, with an overall 5-year survival of only 5%.1 The primary reason for this high mortality rate is the aggressive nature of the malignancy in the absence of early detection. There are few (if any) symptoms that offer an early indication of pancreatic cancer growth; therefore, most such cancers are diagnosed in the advanced stage. As a result, the majority of pancreatic cancers are unresectable. Other therapies, including radiation and chemotherapy, have limited effects in terms of increased survival. Consequently, median survival time (MST) after the diagnosis of pancreatic cancer is measured in months rather than years.2,3 Gemcitabine (GEM) is currently one of the standard therapies for advanced pancreatic cancer, although many chemotherapeutic agents have been used in clinical trials over the past 2 decades.4–6 Among these chemotherapeutic agents, GEM is clinically more effective, but the MST is still <6–9 months. The development of new treatment modalities, including specific immunotherapies, is thus required. Recent advances in molecular biology and cellular immunology in the field of tumor immunology have resulted in the identification of a large number of antigens and epitopes recognized by human leukocyte antigen (HLA) class I restricted cytotoxic T lymphocytes (CTL) from melanomas and epithelial cancers.7–12 Using cDNA microarray technology coupled with laser microdissection, we recently identified novel HLA-A24-restricted epitope peptides as targets for cancer vaccination for patients with pancreatic cancer.13–15 KIF20A (RAB6KIFL) belongs to the kinesin superfamily of motor proteins, which have critical functions in the trafficking of molecules and organelles.16 Immunotherapy using a new epitope peptide for KIF20A is expected to improve clinical outcomes. A phase I clinical trial combining KIF20A-derived peptide with GEM was therefore conducted for patients with advanced pancreatic cancer who had received prior therapy such as chemotherapy and/or radiotherapy.
MATERIALS AND METHODS
Peptides
The KIF20A-10-66 peptide (KVYLRVRPLL) was synthesized by BCN Peptides (Barcelona, Spain) according to a standard solid-phase synthesis method, thereafter purified by reversed-phase high-performance liquid chromatography (HPLC). The purity (>90%) and identity of peptides were determined by analytical HPLC and mass spectrometry analysis, respectively. Endotoxin levels and the bioburden of these peptides were tested and determined to be within acceptable levels as Good Manufacturing Practice grade for vaccines.
Patient Eligibility
The institutional review board at Yamaguchi University approved this clinical protocol. Complete written informed consent was obtained from all patients at the time of enrollment. According to the protocol, patients were required to show positive results for HLA-A*2402. Nine patients diagnosed with metastatic and/or unresectable pancreatic cancer who had received prior therapy such as chemotherapy and/or radiotherapy were enrolled in this trial between January and December 2009 at Yamaguchi University Hospital. Eligibility criteria were as follows: age ≥20 years; life expectancy ≥3 months; and adequate hepatic, renal, and bone marrow function (serum creatinine level, <2.0 mg/dL; bilirubin level, <3.0 g/dL; platelet count, ≥75,000/mL; total white blood cell count ≥3000/mL and ≤15,000/mL). All patients were untreated for ≥4 weeks before enrolling into the study and had to have an Eastern Cooperative Oncology Group performance status of 0-2 at the time of enrollment.
Study Design and End-points
This study was a nonrandomized, open-label, phase I clinical trial with dose escalation of the KIF20A-derived peptide combined with GEM for patients with advanced unresectable pancreatic cancer. The primary end-point in this trial was the safety of peptide vaccination combined with GEM. Secondary end-points were clinical outcome, immunologic responses, and determination of the optimal dose of peptide for further clinical trials. The MST is calculated as time after first vaccination. Immunologic responses were assessed by measuring levels of interferon (IFN)-γ production from antigen-specific T cells responding to the KIF20A-derived peptide.
Adverse Events and Clinical Responses
Adverse events were monitored according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0 (CTCAE). Dose-limiting toxicity was defined as a hematological toxicity of ≥grade 4 and nonhematological toxicity of ≥grade 3. Clinical response was evaluated based on clinical observations and radiologic findings. All known sites of disease were evaluated on a monthly basis by computed tomography (CT) or magnetic resonance imaging before vaccination and after each course. Tumor size was estimated by direct measurement of the region of abnormal enhancement observed on CT or magnetic resonance imaging. Patients were assigned a response category according to the Response Evaluation Criteria in Solid Tumors. Overall survival (OS) was estimated from the date of initial vaccination to the date of death.
Treatment Protocol
Dose was escalated from 0.5 to 1 to 3 mg/body of the vaccinated peptide. The KIF20A-derived peptide was administered emulsified with incomplete Freund’s adjuvant (Montanide ISA-51VG; SEPPIC, Paris, France) by subcutaneous injection on days 1, 8, 15, and 22 in a 28-day treatment course. GEM was administered intravenously at a dose of 1000 mg/m2 on days 1, 8, and 15. Administration of KIF20A and GEM was performed repeatedly for at least one course until satisfying the criteria for treatment cessation. We injected peptide vaccine biweekly after 8 times weekly injection (2 courses) to avoid the risk of exhaustion of the immune response and we chose right inguinal lesion or left inguinal lesion alternately as injection site.
Enzyme-linked ImmunoSpot (ELISPOT) Assay
Antigen-specific T-cell response was estimated by ELISPOT assay following in vitro sensitization.17 Immunologic response of all cases is shown in Table 3. Representative data are shown in Figure 1. Frozen peripheral blood mononuclear cells (PBMCs) derived from the patient were thawed at the same time, and viability was confirmed as >90%. PBMCs (5×105/mL) were cultured with 10 μg/mL of the candidate peptide and 100 IU/mL of interleukin (IL)-2 (Novartis, Emeryville, CA) at 37°C for 2 weeks. Peptide was added into the culture on days 0 and 7. Following CD4+ cell depletion using a Dynal CD4-positive isolation kit (Invitrogen, Carlsbad, CA), IFN-γ ELISPOT assay was performed with vaccinated peptide-pulsed or HIV-Env peptide-pulsed (as the control) HLA-A*2402-positive TISI cells (IHWG Cell and Gene Bank, Seattle, WA) using Human IFN-γ ELISpot PLUS kit (MabTech, Cincinnati, OH) and MultiScreen-IP 96-plate (Millipore, Bedford, MA). Briefly, HLA-A*2402-positive TISI cells were incubated overnight with 20 μg/mL of respective peptides; thereafter, residual peptides in the media were washed out to prepare peptide-pulsed TISI cells as stimulator cells. Prepared CD4− cells were cultured overnight with peptide-pulsed stimulator cells (2×104 cells/well) at 1:1, 1:2, 1:4, and 1:8 mixture ratios of responder cells to stimulator cells (R/S ratio) on 96-well plates (Millipore) at 37°C. To confirm IFN-γ productivity, responder cells were stimulated overnight with phorbol 12-myristate 13-acetate (66 ng/mL) and ionomycin (3 μg/mL), then applied to IFN-γ ELISPOT assay (2.5×103 cells/well) without stimulator cells. All ELISPOT assays were performed in triplicate wells. Plates were analyzed using an automated ELISPOT reader, ImmunoSPOT S4 (Cellular Technology, Shaker Heights, OH), and ImmunoSpot Professional Software version 5.0 (Cellular Technology). The number of peptide-specific spots was calculated by subtracting the spot number in the control well from the spot number of a well with vaccinated peptide-pulsed stimulator cells. Antigen-specific T-cell response was classified into 4 grades (−, +, ++, or +++) according to the algorithm flow chart described in our previous report (+++: IFN-γ-producing cell is contained >0.2% , ++: IFN-γ-producing cell is contained 0.02%–0.2%, +: IFN-γ producing cell is contained 0.01%–0.02%, −: IFN-γ producing cell is contained <0.01% in the sample applied for ELISPOT).18 Sensitivity of our ELISPOT assay was estimated as approximately average level by the ELISPOT panel of the Cancer Immunotherapy Consortium [CIC (http://www.cancerresearch.org/consortium/assay-panels/)].19
TABLE 3.
Immunologic Response
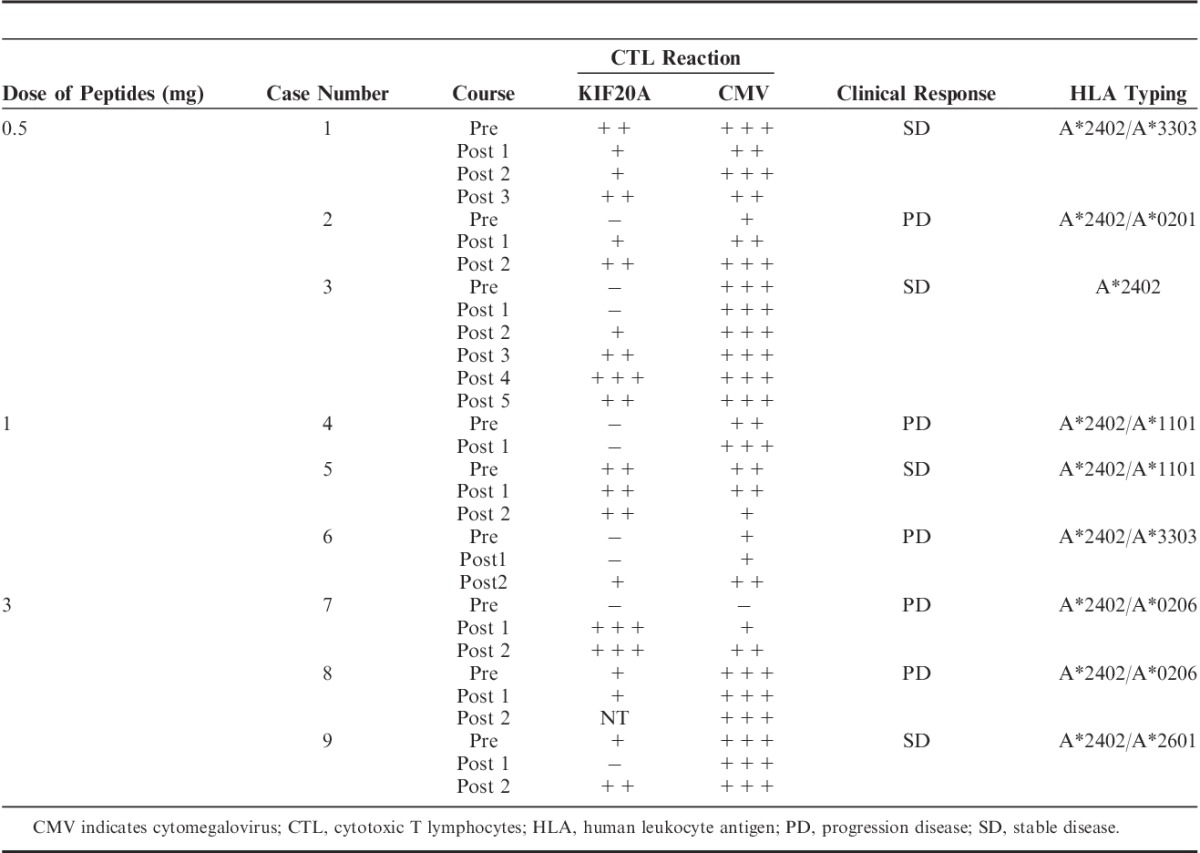
FIGURE 1.
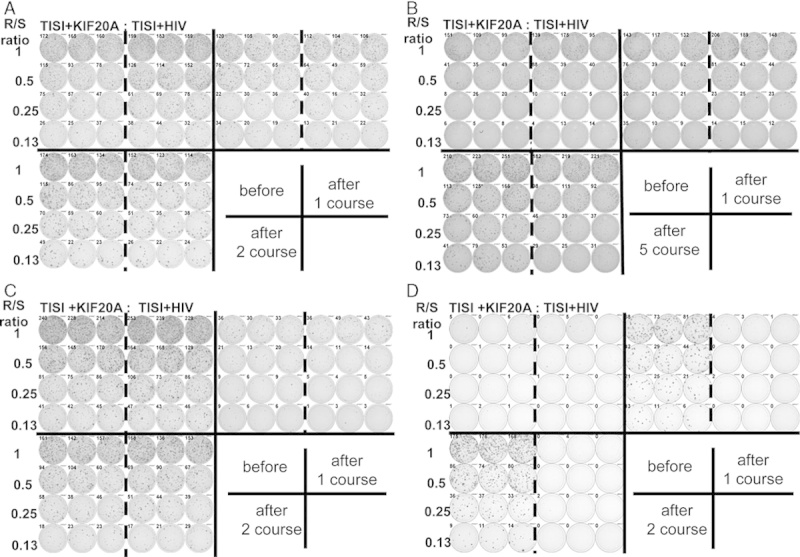
Representative immunologic monitoring assays detecting antigen-specific T-cell responses in patient 2 (A), 3 (B), 6 (C), and 7 (D), which were induced interferon-γ (IFN-γ)-producing cells. Positivity of antigen-specific T-cell response was quantitatively defined according to the evaluation tree algorithm.18 In brief, the peptide-specific spots (SS) were the average of triplicates by subtracting the HIV peptide-pulsed stimulator well from the immunized peptide-pulsed stimulator well. The %SS means the percentage of SS among the average spots of the immunized peptide-pulsed stimulator well. The positivity of antigen-specific T-cell response were classified into four grades (−, +, ++, and +++) depending on the amounts of peptide-specific spots and invariability of peptide-specific spots at different responder/stimulator ratios.
Statistical Analysis
Statistical analysis was performed using the unpaired Student t test for the ELISPOT assay. A value of P<0.05 was considered statistically significant. OS curves were estimated using Kaplan-Meier methodology. Any correlations with clinical outcomes were estimated using the Wilcoxon rank sum test.
RESULTS
Feasibility and Adverse Reactions
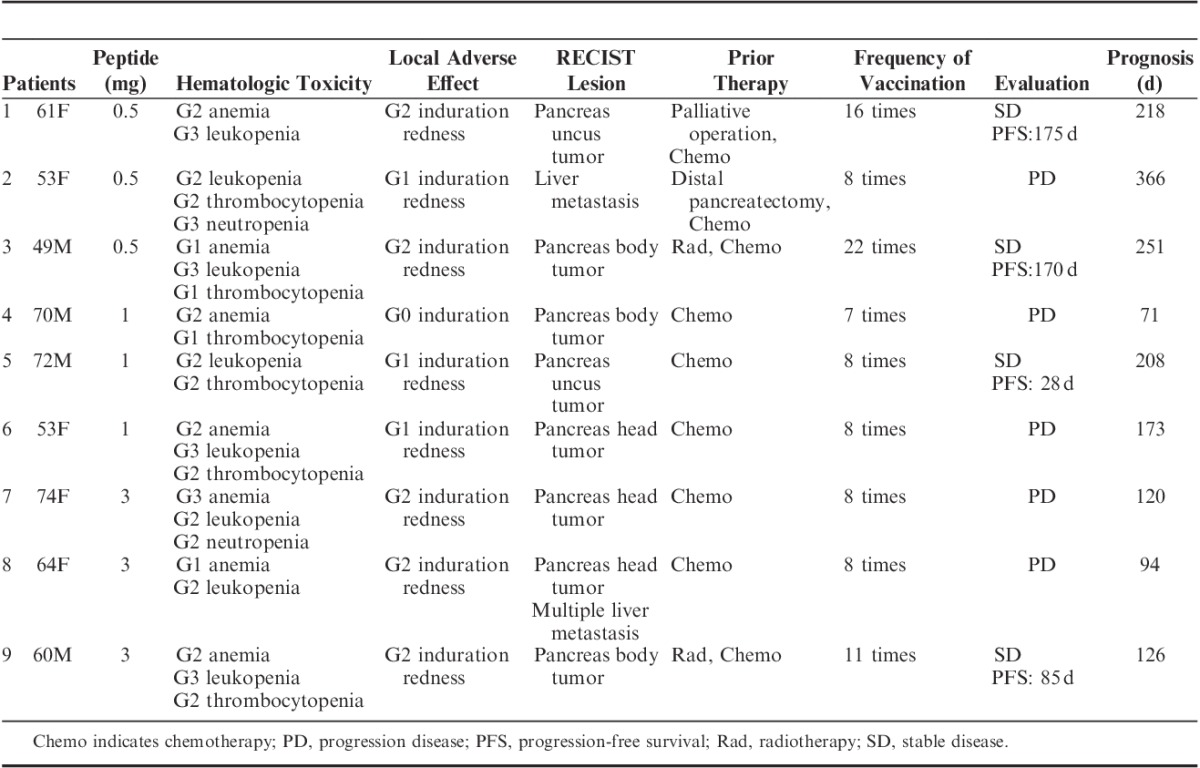
No severe adverse effects of grade 4 or higher were observed. Nine patients satisfying the eligibility criteria were enrolled in this study. Patient characteristics are shown in Table 1. All patients developed grade 1 or 2 local skin reactions with redness and induration at the injection sites. In particular, all 9 patients completed at least 1 course of treatment and all 9 developed immunologic reactions at the injection sites. G2/G3 leukopenia and neutropenia and G1/G2 thrombocytopenia appeared to be caused by GEM itself. G1–G3 anemia appeared attributable to the progression of pancreatic cancer, although GEM is known to cause anemia as well. No febrile neutropenia was recorded during the course of this study. High-grade fever, fatigue, diarrhea, headache, rash, and itching were not observed in any patients. No hematologic, cardiovascular, hepatic, or renal toxicity was observed during or after vaccination (Table 2). The vaccination protocol was well tolerated in all patients enrolled.
TABLE 1.
Patients’ Characteristics
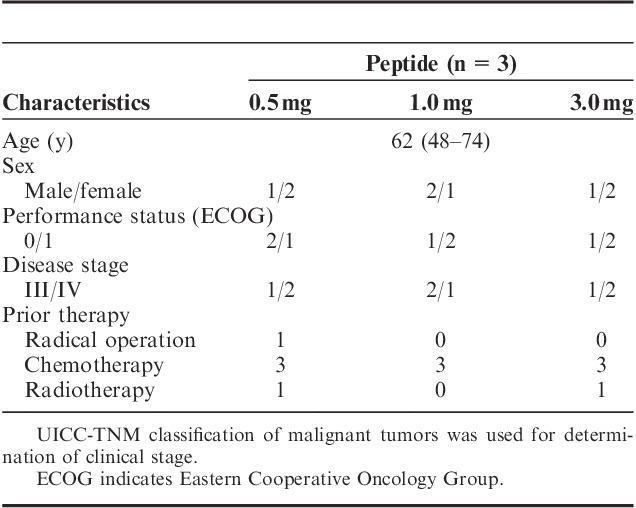
TABLE 2.
Patients’ Toxicity Assessment and Clinical Outcome
Immunologic Monitoring
The KIF20A-specific T-cell (IFN-γ-producing cells) response was determined using the IFN-γ ELISPOT assay. Representative antigen-specific T-cell responses are shown in Figure 1. In which, PBMC from patients 2, 3, 6, and 7 produced higher level of IFN-γ after vaccine than the level of pre-vaccination (Fig. 1). Positive antigen-specific T-cell (IFN-γ producing cells) responses specific to the vaccinated peptide were determined as described in the Materials and methods section. IFN-γ-producing cells were induced in 4 of 9 patients (P2, P3, P6, and P7), and IFN-γ producing cells were increased in 4 of the 9 patients (P1, P5, P8, and P9) (Table 3). Antigen-specific T-cell responses were seen in all 3 patients receiving 0.5 mg vaccination; in 2 of the 3 patients receiving 1 mg; and in all 3 patients receiving 3 mg. Antigen-specific T-cell response (IFN-γ-producing cells) could therefore be induced by the KIF20A peptide vaccine at a high rate, even in combination with GEM.
Clinical Responses and OS
Four of the 9 patients achieved stable disease (SD), with the other 5 patients showing progression disease (PD). The disease control rate was 44%. Achievement of SD was seen in 2 of the 3 patients receiving 0.5 mg vaccination, 1 of 3 patients receiving 1 mg, and 1 of 3 patients receiving 3 mg (Table 2). Images from CT of a patient with SD are shown in Figure 2. All 4 patients who achieved SD showed induction of the antigen-specific T-cell responses at a level of 2+ or more (++ or +++) for the KIF20A peptide (Table 3). In contrast, 3 of the 5 patients who showed PD displayed induction of antigen-specific T-cell responses from negative (−) to reaction (+). No relationship between peptide doses and the antigen-specific T-cell responses or clinical outcome was identified. The MST calculated as time after first vaccination was 173 days and 1-year survival rate was 11.1% (Fig. 3). The MST calculated as time after first diagnosis was 18 months and 1-year survival rate was 78%.
FIGURE 2.
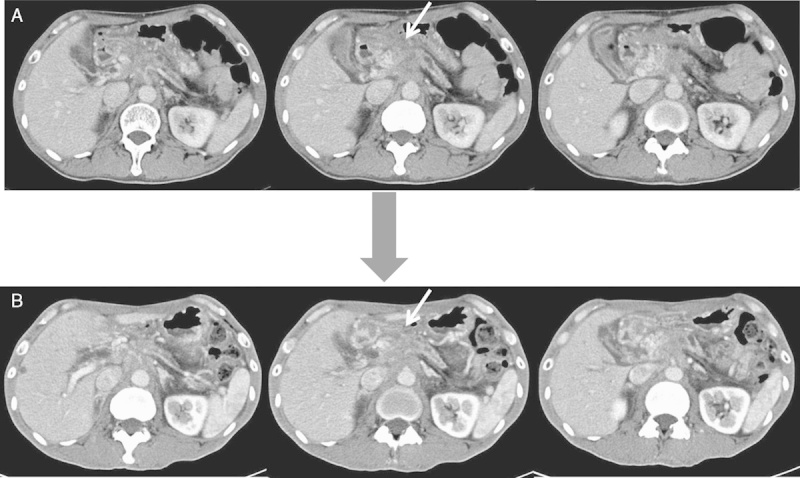
Axial contrast-enhanced computed tomography (CT) scans of patient 3 who showed SD. A, Axial contrast-enhanced CT showing locally advanced tumor of the pancreatic body before vaccination (arrow). B, Axial contrast-enhanced CT after 4 months shows SD of the pancreatic body mass (arrow). SD indicates stable disease.
FIGURE 3.
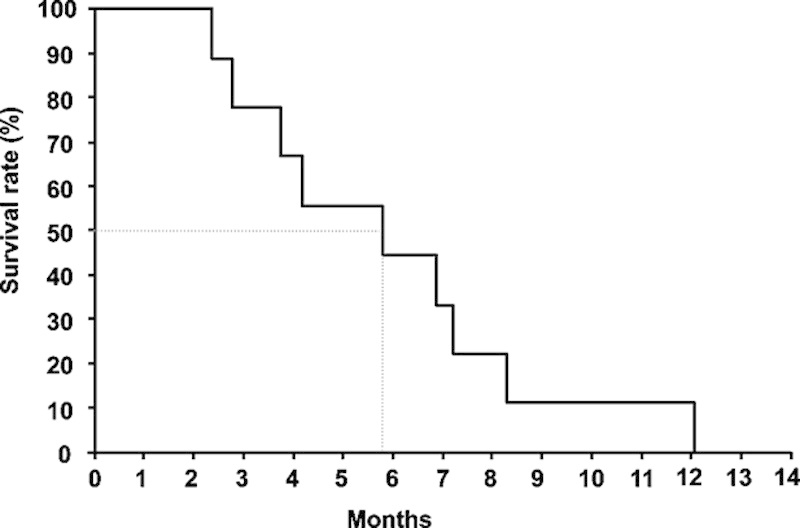
Overall survival measured using the Kaplan-Meier method. The median survival time after first vaccination was 173 days. One-year survival rate was 11.1%.
DISCUSSION
The only cure for pancreatic cancer is surgical resection, although this malignancy is difficult to detect early. At the time of diagnosis, approximately 60% of patients are already beyond the possibility of surgical resection.20–23 GEM is currently used as the standard therapy for unresectable pancreatic cancer. Noninferiority of S-1 compared with GEM was shown in GEST study conducted in Japan, but the superiority of the combination of GEM and S-1 over GEM monotherapy has not yet been conclusively proven.24 The establishment of combination therapy with GEM has been performed many times to date. One large randomized controlled phase III trial with erlotinib showed significantly prolonged survival time (P=0.038),25 but the difference was only about 10 days. In another study, MST was 11.1 months for the FOLFIRINOX group, compared with 6.8 months in the GEM group, showing a significant difference (P<0.001). However, markedly more adverse events were noted in the FOLFIRINOX group.26 Taking into account toxicity and economic aspects, the development of new drugs for advanced pancreatic cancer is urgently required.
The present study investigated a novel cancer vaccine therapy for pancreatic cancer using a KIF20A-derived peptide in combination with GEM. To the best of our knowledge, this is the first report to use the KIF20A-derived peptide in a clinical trial. We observed no severe adverse events related to the treatment in this trial (Table 2). Specific adverse events caused by this vaccine treatment were local redness and induration at the injection site; however, no events >grade 3 were observed. In several papers we have examined—their authors show that the intradermic administration of vaccine has proven superior to subcutaneous administrations.27
We tried to administer the KIF20A-derived peptide emulsified with incomplete Freund’s adjuvant as close as possible to the dermis—so as to activate the dendritic cells.
Because the volume was 2 mL, it was too much to inject the intradermic administration. We think the data of this study were able to prove that IFN-γ-producing cells could be enhanced by this message. Immunologic responses in this trial were measured by local redness and induration at the injection site and antigen-specific T-cell responses against the vaccinated peptide. No dose-limiting toxicity was observed in any dose cohort. We injected peptide vaccine biweekly after 8 times weekly injection (2 courses) to avoid the risk of exhaustion of the immune response. We chose right inguinal lesion or left inguinal lesion alternately as injection site. Local redness and induration as CTCAE grade 2 at the injection site were observed in all 3 patients with the 3 mg vaccination (Table 2). However, achievement of SD was seen in 2 of the 3 patients receiving 0.5 mg vaccination, 1 of 3 patients receiving 1 mg, and 1 of 3 patients receiving 3 mg (Table 2). In this study, we consider that the optimum peptide dosage for future clinical trials could be set at a level of at least 0.5 mg or more.
As a point of immunologic monitoring, IFN-γ-producing cells were induced in 4 of 9 patients (P2, P3, P6, and P7), and IFN-γ-producing cells were increased in 4 of the 9 patients (P1, P5, P8, and P9). Patient 4 in whom IFN-γ-producing cells response was absent was suffering from acute cholangitis during vaccination. Prior to vaccination, the proportion of lymphocyte in this patient was only 13%. Yamamoto et al28 previously reported that peptide-reactive cellular and humoral responses to vaccinated peptides in postvaccination PBMCs and sera were lower for advanced pancreatic cancer patients than for patients with other solid cancers. They commented that these results suggest that immunity in advanced pancreatic cancer is more depressed than in other epithelial cancers. Alternatively, a more suitable peptide repertoire might be provided for pancreatic cancer patients. Miyazawa et al29 reported that VEGFR2-169 peptide-specific positive CTL responses were observed in 11 of 18 patients who received at least one course of vaccination. Ishikawa et al30 reported URLC10-177 peptide-specific positive CTL responses in 4 of 7 patients. KIF20A peptide vaccine therefore induced or further increased peptide-specific T-cell responses at a higher rate compared with these reports. Four of the 9 patients achieved SD, whereas the other 5 patients showed PD (Table 2). Achievement of SD was seen in 2 of the 3 patients receiving 0.5 mg vaccination, 1 of 3 patients receiving 1 mg, and 1 of 3 patients receiving 3 mg (Table 2). There is no evidence that the SD was mediated by the vaccine. This could simply be the natural history of this disease, but it is interesting to note that all 4 patients who achieved SD showed antigen-specific T-cell response of ++ or +++ reactions for KIF20A peptide. In contrast, 3 of the 5 patients who experienced PD showed antigen-specific T-cell response from negative to 1+ reaction. A tendency toward a correlation between antigen-specific T-cell response and clinical outcome was suggested, but no significant relationship was proved (P=0.074). However, the population was too small to be evaluated in this clinical trial. Many prior peptide vaccine studies have demonstrated significant immunogenicity against the peptides utilized in the vaccine without translating into significant clinical benefits. This will be our next focus but prior to that the important thing is to identify a new peptide that possesses high immunogenicity. This protocol was well tolerated, and peptide-specific IFN-γ-producing cells were found to be induced or increased by the KIF20A-derived peptide vaccine at a high rate, even in combination with the anticancer agent, GEM. Although safety and immunogenicity are promising, no conclusions can be made about efficacy at this level of study. We are proceeding on to conduct a phase II clinical trial among patients with advanced pancreatic cancer by combining KIF20A-derived peptide with GEM as the first line. Therefore, additional efficacy data would be required before committing to a large randomized controlled trial.
ACKNOWLEDGMENTS
The authors thank Prof. Yusuke Nakamura, Dr Takuya Tsunoda, Dr Koji Yoshida, Laboratory of Molecular Medicine, Human Genome Center, Institute of Medical Science, The University of Tokyo, for their excellent advice and cooperation and providing all the peptides.
CONFLICTS OF INTEREST/FINANCIAL DISCLOSURES
All authors have declared there are no financial conflictsof interest with regard to this work.
Footnotes
Reprints: Masaaki Oka, Departments of Digestive Surgery and Surgical Oncology (Surgery II), Yamaguchi University Graduate School of Medicine, Minami-Kogushi, Ube, Yamaguchi 755-8505, Japan. (e-mail: 2geka-1@yamaguchi-u.ac.jp).
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008.CA Cancer J Clin. 2008;58:71–96 [DOI] [PubMed] [Google Scholar]
- 2.Sener SF, Fremgen A, Menck HR, et al. Pancreatic cancer: a report of treatment and survival trends for 100,313 patients diagnosed from 1985–1995 using the National Cancer Database.J Am Coll Surg. 1999;189:1–7 [DOI] [PubMed] [Google Scholar]
- 3.Bramhall SR, Allum WH, Jones AG, et al. Treatment and survival in 13,560 patients with pancreatic cancer, and incidence of the disease, in the West Midlands: an epidemiological study.Br J Surg. 1995;82:111–115 [DOI] [PubMed] [Google Scholar]
- 4.Rothenberg ML, Moore MJ, Cripps MC, et al. A phase II trial of gemcitabine in patients with 5-FU-refractory pancreas cancer.Ann Oncol. 1996;7:347–353 [DOI] [PubMed] [Google Scholar]
- 5.Burris HA, I, Moore MJ, Andersen J, et al. Improvement in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreatic cancer: a randomized trial.J Clin Oncol. 1997;15:2403–2413 [DOI] [PubMed] [Google Scholar]
- 6.Berlin JD, Catalano P, Thomas JP, et al. Phase III study of gemcitabine combination with fluorouracil versus gemcitabine alone in patients with advanced pancreatic carcinoma: Eastern Cooperative Oncology Group Trial E2297.J Clin Oncol. 2002;20:3270–3275 [DOI] [PubMed] [Google Scholar]
- 7.van der Bruggen P, Traversari C, Chomez P, et al. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma.Science. 1991;254:1643–1647 [DOI] [PubMed] [Google Scholar]
- 8.Kawakami Y, Eliyahu S, Sakaguchi K, et al. Identification of the immunodominant peptides of the MART-1 human melanoma antigen recognized by the majority of HLA-A2-restricted tumor infiltrating lymphocytes.J Exp Med. 1994;180:347–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shichijo S, Nakao M, Imai Y, et al. A gene encoding antigenic peptides of human squamous cell carcinoma recognized by cytotoxic T lymphocytes.J Exp Med. 1998;187:277–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salgaller ML, Afshar A, Marincola FM, et al. Recognition of multiple epitopes in the human melanoma antigen gp100 by peripheral blood lymphocytes stimulated in vitro with synthetic peptides.Cancer Res. 1995;55:4972–4979 [PubMed] [Google Scholar]
- 11.Rosenberg SA, Yang JC, Schwartzentruber DJ, et al. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma.Nat Med. 1998;4:321–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suzuki N, Maeda Y, Tanaka S, et al. Detection of peptide-specific cytotoxic T-lymphocyte precursors used for specific immunotherapy of pancreatic cancer.Int J Cancer. 2002;98:45–50 [DOI] [PubMed] [Google Scholar]
- 13.Okabe H, Satoh S, Kato T, et al. Genome-wide analysis of gene expression in human hepatocellular carcinomas using cDNA microarray: identification of genes involved in viral carcinogenesis and tumor progression.Cancer Res. 2001;61:2129–2137 [PubMed] [Google Scholar]
- 14.Lin YM, Furukawa Y, Tsunoda T, et al. Molecular diagnosis of colorectal tumors by expression profiles of 50 genes expressed differentially in adenomas and carcinomas.Oncogene. 2002;21:4120–4128 [DOI] [PubMed] [Google Scholar]
- 15.Hasegawa S, Furukawa Y, Li M, et al. Genome-wide analysis of gene expression in intestinal-type gastric cancers using a complementary DNA microarray representing 23,040 genes.Cancer Res. 2002;62:7012–7017 [PubMed] [Google Scholar]
- 16.Taniuchi K, Nakagawa H, Nakamura T, et al. Down-regulation of RAB6KIFL/KIF20A, a kinesin involved with membrane trafficking of discs large homologue 5, can attenuate growth of pancreatic cancer cell.Cancer Res. 2005;65:105–112 [PubMed] [Google Scholar]
- 17.Okuno K, Sugiura F, Hida JI, et al. Phase I clinical trial of a novel peptide vaccine in combination with UFT/LV for metastatic colorectal cancer.Exp Ther Med. 2011;2:73–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kono K, Iinuma H, Akutsu Y, et al. Multicenter, phase II clinical trial of cancer vaccination for advanced esophageal cancer with three peptides derived from novel cancer-testis antigens.J Transl Med. 2012;10:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Janetzki S, Panageas KS, Ben-Porat L, et al. Results and harmonization guidelines from two large-scale international Elispot proficiency panels conducted by the Cancer Vaccine Consortium (CVC/SVI).Cancer Immunol Immunother. 2008;57:303–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsuno S, Egawa S, Shibuya K, et al. Pancreatic cancer: current status of treatment and survival of 16071 patients diagnosed from 1981-1996, using Japanese National Pancreatic Cancer Database.Int J Clin Oncol. 2000;5:153–157 [Google Scholar]
- 21.Pantalone D, Ragionieri I, Nesi G, et al. Improved survival in small pancreatic cancer.Dig Surg. 2001;18:41–46 [DOI] [PubMed] [Google Scholar]
- 22.Casper ES, Green MR, Kelsen DP, et al. Phase II trial of gemcitabine (2,2′-difluorodeoxycytidine) in patients with adenocarcinoma of the pancreas.Invest New Drugs. 1994;12:29–34 [DOI] [PubMed] [Google Scholar]
- 23.Carmichael J, Fink U, Russell RC, et al. Phase II study of gemcitabine in patients with advanced pancreatic cancer.Br J Cancer. 1996;73:101–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ueno H, Ioka T, Ikeda M, et al. Randomized phase III study of gemcitabine plus S-1, S-1 alone, or gemcitabine alone in patients with locally advanced and metastatic pancreatic cancer in Japan and Taiwan: GEST study.J Clin Oncol. 2013;31:1640–1648 [DOI] [PubMed] [Google Scholar]
- 25.Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group.J Clin Oncol. 2007;25:1960–1966 [DOI] [PubMed] [Google Scholar]
- 26.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer.N Engl J Med. 2011;364:1817–1825 [DOI] [PubMed] [Google Scholar]
- 27.Launay O, Surenaud M, Desaint C, et al. Long-term CD4(+) and CD8(+) T-cell responses induced in HIV-uninfected volunteers following intradermal or intramuscular administration of an HIV-lipopeptide vaccine (ANRS VAC16).Vaccine. 2013;31:4406–4415 [DOI] [PubMed] [Google Scholar]
- 28.Yamamoto K, Mine T, Katagiri K, et al. Immunological evaluation of personalized peptide vaccination for patients with pancreatic cancer.Oncol Rep. 2005;13:875–883 [PubMed] [Google Scholar]
- 29.Miyazawa M, Ohsawa R, Tsunoda T, et al. Phase I clinical trial using peptide vaccine for human vascular endothelial growth factor receptor 2 in combination with gemcitabine for patients with advanced pancreatic cancer.Cancer Sci. 2010;101:433–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ishikawa H, Imano M, Shiraishi O, et al. Phase I clinical trial of vaccination with URLC10-derived peptide for patients with advanced esophageal cancer.Esophagus. 2012;9:105–112 [Google Scholar]


