Abstract
Purpose of review
Recent WHO guidelines recommend immediate initiation of lifelong antiretroviral therapy (ART) in all children below 5 years, irrespective of immune/clinical status, to improve access to paediatric ART. Interim trial results provide strong evidence for immediate ART during infancy because of high short-term risk of mortality and disease progression, but there is wider debate regarding the potential risks and benefits of immediate ART in asymptomatic children aged above 1 year. Concerns include long-term toxicities and treatment failure, particularly in resource-constrained settings with limited paediatric treatment options.
Recent findings
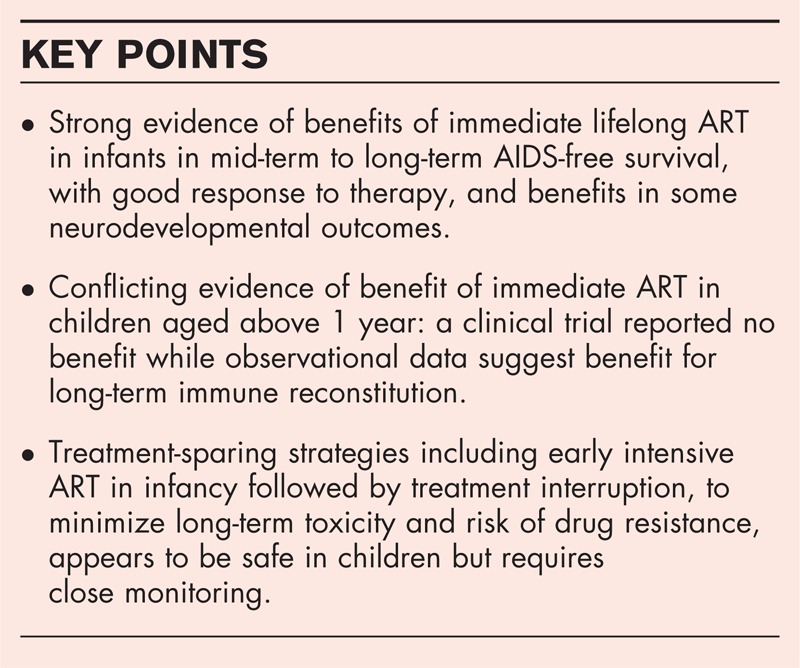
Benefits of immediate ART among infants appear to be maintained in the mid-term to long-term, with low risk of treatment failure, and better neurodevelopmental outcomes. In contrast, a trial reported no benefits of immediate versus deferred ART in asymptomatic children aged above 1 year. However, observational studies suggest that ART initiation at older ages and lower CD4 reduces the probability of immune reconstitution, with unclear implications on risk of clinical events or treatment change. A recent trial on treatment interruption following early intensive ART suggest that this may be a safe alternative approach.
Summary
Although there are clear benefits of immediate ART among infants, there remains conflicting evidence on the benefits for older children.
Keywords: antiretroviral therapy, children, immune response, neurodevelopment, survival
INTRODUCTION
In 2011, an estimated 3.3 million children aged below 15 years were living with HIV, of whom more than 90% lived in sub-Saharan Africa, and the vast majority were infected through mother-to-child transmission [1]. Despite the rapid scale up of interventions for prevention of mother-to-child transmission of HIV, coverage in the top 21 Global Plan priority countries remains highly variable, ranging from 9% to more than 95% [2]. Within these priority countries, there were an estimated 210 000 children newly infected in 2012 alone [3]. Without antiretroviral therapy (ART), up to 50% of children will die by 2 years of age in resource-limited settings [4,5▪]. Yet, the global coverage of ART among children continues to lag behind at 28% of those in need of treatment, as compared to 58% in adults [6].
The WHO recommendations on when to start ART in children have been revised four times over the past decade, each time relaxing the eligibility criteria to higher CD4 levels, and more recently increasing the age for immediate ART from all those below 2 years to all below 5 years, irrespective of clinical or immune status [7] (Table 1) [7–11]. Older children are recommended to start ART when CD4 is less than 500 cells (an increase from <350 cells), in alignment with adult guidelines, and following studies which showed similar risk of disease progression in children aged above 5 years as young adults [12].
Table 1.
Changes in World Health Organization recommendations on immunological threshold of when to start antiretroviral therapy by age from 2003 to 2013
| Age | <12 months | 12–23 months | 24–35 months | 36–59 months | ≥60 months | |
| WHO guidelines | ||||||
| 2003 [8] | CD4% | <20% | <15% | |||
| Absolute CD4 | NA | NA | ||||
| 2006 [9] | CD4% | <25% | <20% | <15% | <15% | |
| Absolute CD4 | <1500 cells/mm3 | <750 cells/mm3 | <350 cells/mm3 | <200 cells/mm3 | ||
| 2008 [10] | CD4% | All | <20% | <20% | <15% | |
| Absolute CD4 | All | <750 cells/mm3 | <350 cells/mm3 | <200 cells/mm3 | ||
| 2010 [11] | CD4% | All | All | ≤25% | NA | |
| Absolute CD4 | All | All | <750 cells/mm3 | ≤350 cells/mm3 | ||
| 2013 [7] | CD4% | All | All | All | All | NA |
| Absolute CD4 | All | All | All | All | ≤500 cells/mm3 | |
In contrast, the United States and European Paediatric European Network for Treatment of AIDS (PENTA) paediatric guidelines only recommend immediate ART for all infants below 12 months, whereas older children are recommended to initiate ART based on clinical and immune status [13,14].
Box 1.
no caption available
However, the WHO recommendations are based on a public health approach, taking into account programmatic issues in resource-limited settings, such as difficulties in provision of CD4 testing and high rates of loss-to-follow-up among children not eligible for treatment [15,16,17▪]. Furthermore, recent data suggest that a large number of children still present late for treatment, when severely immunocompromised and at advanced disease stage [18], with high risk of mortality during screening and in the first year of ART [15], especially in those aged below 2 years [19,20].
With the aim to minimize the risk of disease progression and mortality, under the consolidated WHO guidelines HIV-infected children would initiate lifelong ART from infancy. In this review, we focus on the most recent evidence on the risks and benefits of earlier initiation of ART in three main areas: survival and AIDS-free survival; immune restoration, as this is closely associated with risk of disease progression; and neurodevelopmental outcomes, because of their important implications on quality of life. Lastly, we briefly review the latest evidence on treatment-sparing strategies. This is critical to minimize long-term toxicities and to preserve future treatment options, particularly in resource-limited settings wherein often only first and second-line regimens are available [21].
WHEN TO START ANTIRETROVIRAL THERAPY: SURVIVAL AND AIDS-FREE SURVIVAL
Up until 2008, the WHO recommendations on when to initiate ART in children were largely based on observational data which demonstrated younger age and poorer immune status as the main predictors of risk of disease progression and death, as well as higher risk in untreated children in sub-Saharan Africa compared with Europe and the USA [22,23]. To date, there have only been two clinical trials assessing when to start ART in children, both conducted in low or middle-income countries.
The Children with HIV early antiretroviral therapy (CHER) trial in South Africa randomized 377 asymptomatic HIV-infected infants (CD4 >25% at median 7 weeks of age) to immediate initiation of ART before 12 weeks of age or deferred ART based on WHO 2006 clinical or CD4 criteria. After a median of only 40 weeks, there was a 76% [95% confidence interval (CI), 49–89%, P <0.001] reduction in mortality and 75% reduction in disease progression (95% CI, 59–85%, P <0.001) in the early treatment arm [24], and these results were confirmed in the final analysis at 4 years of follow-up [25▪▪] (Table 2) [25▪▪,26,27▪,28▪▪,29▪].
Table 2.
Summary of recent trials on in HIV-infected children
| Study and setting | Population | Methods | Results | Summary and comment |
| CHER trial, South Africa (a) Interim analysis [26] | HIV-infected asymptomatic infants aged 6–12 weeks with CD4 >25% | 377 children randomized to deferred ART based on CD4/clinical criteria (Def-ART, n = 125); immediate ART (early-ART, n = 252); primary outcome: death or treatment failure (immunological, virological or clinical) | At randomization, median age and CD4% was 7.4 weeks and 35%; at 40 weeks, 76% reduction in death and 75% reduction in disease progression to CDC severe stage B or stage C event in the early-ART arm (P < 0.001); enrolment in the Def-ART arm was stopped after the interim analysis | Results led to change in international guidelines to recommend immediate ART in infants |
| (b) Neurocognitive sub-study [27▪] | As above, with additional HEU and HU controls | Cross-sectional sub-study; examination using GMDS | 90 HIV-infected children (63 early-ART and 26 Def-ART), 155 HEU and 164 HU controls were included; median age of 11 months; significantly lower general and locomotor scores in the Def-ART versus; early-ART arm; children in early-ART arm had similar scores to HIV-uninfected controls except for locomotor score | Early ART was associated with better neurological outcomes at 11 months; cross-sectional design, so unclear if the outcome changes over time on ART; children in the Def-ART arm had higher incidence of illness and hospitalization, which may have contributed to their poorer outcome |
| (c) Final analysis [25▪▪] | As in (a) | Children in the early-ART strategy were randomized to TI after 40 weeks (ART-40weeks, n = 126) or 96 weeks (ART-96 weeks, n = 126); ART was reinitiated if CD4 < 25% in infancy; otherwise, CD4 < 20% or CDC B/ C event; children in the Def-ART arm were on continuous therapy; primary outcome: composite endpoint of time failure to first-line ART (immunological, virological or clinical) or death | Median follow-up was 4.8 years, 9% lost to follow-up; overall mortality was 16.8% in the Def-ART arm and 9% in the early ART arms; 38% of children in the Def-ART arm, 25% in ART-40 weeks, and 21% in ART-96 weeks group reached the primary endpoint (P ≤ 0.02); time to reinitiation of ART was 33 weeks (IQR, 26–45) in the ART-40 weeks and 70 weeks (35–109) in ART-96 weeks arm; at end of the trial, 19 and 32% of patients remained off ART, respectively; very few children switched to second-line across all arms | Long-term survival benefit of early ART as compared to deferred ART, with good long-term outcomes despite TI; early time-limited ART appears to be a safe strategy but requires close clinical and CD4 monitoring; there was no early continuous ART arm with which to compare the outcomes of the two TI arms; insufficient power to detect difference in outcomes between the two TI arms |
| PREDICT study, Cambodia and Thailand (a) Final analysis [28▪▪] | HIV-infected children aged 1–12 years with CD4 of 15–24% and no history of AIDS illness | Children randomized to deferred ART based on CD4/clinical criteria (Def-ART, n = 150); immediate ART (early-ART, n = 150); primary outcome: AIDS-free survival | At randomization, median age and CD4% was 6.4 years and 20%; at 144 weeks, very low rate of events and no difference in AIDS-free survival across the two arms: 98.7% (95% CI, 94.7–99.7) in Def-ART and 98.9% (95% CI, 93.7–99.3) in early-ART, P = 0.6; also no difference in secondary outcomes of CDC B/C events, hospitalization or immune response | No difference in AIDS-free survival in children aged ≥1 year, but because of the low rate of event was underpowered to detect a difference; potential limitation of survival bias, as the study was mainly composed of older children, which may have contributed to the low rate of events; findings may not be generalizable to younger children <5 years |
| (b) Neurodevelopmental sub-study [29▪] | As above, plus HEU and HU controls. | Repeat examinations over follow-up time for HIV-infected children; cross-sectional design for HIV-uninfected controls; assessments include: Berry Visual Motor Integration, Purdue Pegboard, Colour Trials and Child Behavioural Checklist | 284 HIV-infected children (139 early-ART and 145 Def-ART), 155 HEU and 164 HU controls were included; median age of 9 years at last examination; among HIV+ children, cognitive function and neurodevelopmental outcomes did not differ between the early versus deferred ART arms; HIV-infected children performed worse than uninfected controls | No difference in outcomes between early or deferred ART; HIV-infected children performed worse than uninfected controls; included few young children <3 years so may not be generalizable to all age groups |
ART, antiretroviral therapy; CDC, Centres of Disease Control and Prevention; GMDS, Griffiths Mental Development Scales; HEV, HIV exposed uninfected; HU, HIV unexposed; TI, treatment interruption.
The majority of excess deaths observed in the deferred treatment arm was among untreated children who died before meeting the clinical/immune criteria for ART. Following the interim result of this trial, the WHO, United States and PENTA guidelines were all revised in 2008 to recommend immediate ART in all HIV-infected infants under 12 months, irrespective of clinical or immune status [10]. In the WHO 2010 guidelines, this was extended to all children under 24 months [11], for programmatic reasons and also in recognizing that observational data indicate the higher risk of AIDS and death extend to young children below 2 years [22,23]. Importantly, final results of the CHER trial showed that very few children in the early ART arms switched to second line (4 of 252), although this may have been partly because of the treatment-sparing strategy in which infants on early ART were randomized to treatment interruption after 40 or 96 weeks of ART (see later section) [25▪▪].
The Paediatric Randomized Early vs. Deferred Initiation in Cambodia and Thailand (PREDICT) study, randomized 284 children, aged 1–12 years with CD4 of 15–24% and no AIDS-defining illness to immediate ART or deferred treatment until CD4 less than 15% or Centres for Disease Control and Prevention (CDC) stage C. At 3 years of follow-up, there was no difference in the AIDS-free survival: 97.9% (95% CI, 93.7–99.3) and 98.7% (95%CI, 94.7–99.7, P = 0.6) in the early and deferred arms, respectively [28▪▪]. The rate of events was very low (one death and five CDC C events), and the authors concluded that the study was underpowered to detect a difference between the two strategies. However, there were also no differences between the two arms in any secondary outcomes: rate of hospitalization, CDC stage B or C events, virological suppression, immunological response or neurodevelopmental outcomes, suggesting no significant benefit of earlier treatment in asymptomatic children above 1 year. However, it is important to note that children in the deferred treatment arm received CD4 assessments every 3 months and prompt ART initiation at higher median CD4% as compared to other studies in comparable routine care settings [30,31]. Furthermore, this trial included mainly older surviving children, with a median age of 6.4 years at randomization (only 26% were aged <3 years and 6% aged 1–2 years); therefore, the findings may not be generalizable to all children below 5 years.
In the absence of clinical trial data, the South Africa International Epidemiologic Database to Evaluate AIDS collaboration conducted a causal modelling analysis, using data from the their large cohort of approximately 3000 children aged 2 to below 5 years at first presentation [32▪]. The model estimated mortality over 3 years if the 2013 guidelines for immediate ART was implemented as compared to starting ART when CD4 declined to less than 25% or less than 750 cell counts as per routine practice within the cohort. The model showed no significant benefit in mortality from the immediate treatment strategy, most likely because of the relatively low risk of mortality in children aged 2–5 years with CD4 more than 25%.
In summary, there is limited evidence to support immediate ART in children aged 2–5 years in terms of survival or AIDS-free survival. This is partly reflected in the WHO recommendations, which state that priority for immediate ART should be given to children aged less than 2 years or those aged 2–5 years with advanced disease stage [7].
IMMUNOLOGICAL RECONSTITUTION
As most children on ART are now surviving into adolescence and young adulthood, there is a need to study the impact of earlier treatment initiation on long-term response and morbidity. Numerous studies have shown that children who initiate ART at low CD4 or older age are less likely to achieve immune reconstitution to near normal levels, often defined as CD4 at least 25% [33–35] or at least 30% [36], which represents the upper threshold for start of treatment in children above 2 years. Children who fail to reach this threshold may be vulnerable to disease progression. However, most studies have been limited to follow-up of 2–5 years on ART, and the long-term trajectory of immune response is unclear.
To address that, one novel study by Lewis et al.[37▪▪] used data from the PENTA-5 clinical trial, of 127 European/South American children in an immunological sub-study, to model the CD4 trajectories of children on ART into adulthood. As expected, the long-term CD4 z-scores were significantly lower for children starting ART at older ages and those with lower pre-ART CD4. However, children starting with lower CD4 cell count at younger ages had greater capacity for immune reconstitution as compared to older children, possibly due to increased thymic activity and a shorter duration of infection [37▪▪]. The same model was also applied to data from the Antiretroviral research for Watoto (ARROW) trial, of 1206 children randomized to clinical-based or clinical and laboratory-based monitoring in Uganda and Zimbabwe, with 4 years of follow-up on ART [38▪]. Similar results were observed, whereby higher long-term CD4 cell counts were predicted for children starting ART at younger ages and with higher CD4 cell counts (P <0.0001). Interestingly, children starting ART aged above 10 years were unlikely to ever normalize CD4 cell counts, irrespective of their CD4 at start of therapy.
These findings are consistent with recent results from an observational study in Thailand. Among 507 children followed up for a median of 7 years, 22% failed to achieve immune reconstitution defined as confirmed CD4 of at least 25%, of whom over half had sustained viral suppression less than 400 copies throughout their follow-up time [39]. Age above 7 years and very low CD4 less than 5% at start of ART were associated with poor immune reconstitution, although the impact on clinical events was not assessed. In contrast, in the PREDICT trial, composed of similarly older children, there were no differences in long-term immune response in the immediate versus deferred ART arms, and it was unclear if there was an effect of age at start of therapy [28▪▪].
In summary, observational studies suggest that delayed initiation of ART until age above 7 years or when severely immunosuppressed significantly reduces the probability of immune reconstitution, although the clinical implications of this remains unclear. In adult studies, nonimmune response despite viral suppression has been associated with increased risk of severe clinical events [40] and death [41], whereas prolonged periods of immune reconstitution (defined as CD4 ≥ 500 cells/mm3) have been associated with improved life expectancy, comparable to the general population [42,43]. No equivalent study has yet been conducted in perinatally infected patients, mainly because of lack of data on long-term clinical outcomes; this highlights the need for continued follow-up after children transfer to adolescent and adult clinics.
NEURODEVELOPMENTAL OUTCOMES
There were two recent reviews on neurodevelopmental outcomes in perinatally HIV-infected children and adolescents [44▪▪,45]. AIDS-defining illness and low CD4 have been associated with poorer neurodevelopmental outcomes in resource-rich and resource-limited settings [46▪,47▪]. However, the debate on whether earlier treatment initiation improves neurodevelopmental outcomes among children without advanced disease stage continues. In the CHER trial's neurological sub-study, children in the early ART group had significantly higher General and Locomotor scores at 11 months of age as compared to the deferred ART group, and were comparable to HIV-uninfected controls [27▪]. However, differences between the groups were relatively small, and the mean General and Locomotor score in the deferred arm remained within the normal developmental range.
In the PREDICT trial sub-study, with a median age 9 years at last examination, there was no difference in the neurocognitive performance in the early versus deferred ART groups, despite repeat measurements throughout the study. Both groups had poorer outcomes as compared to HIV-exposed uninfected controls on key measures [29▪]. However, it is important to note that these measures were based on the methods developed in the USA or Europe and have not been standardized for these other settings. Also comparisons with HIV-exposed uninfected controls may be subject to unmeasured confounders, such as differences in the family settings and access to care, which may affect outcomes. Also the magnitude of differences in outcomes and their impact on daily function, learning capabilities and quality of life are unknown.
OTHER CONSIDERATIONS: TOXICITIES, VIROLOGIC FAILURE AND TREATMENT-SPARING STRATEGIES
As with adults, earlier initiation of lifelong ART poses potential risks of long-term toxicities, increased risk of virological failure and limited treatment options. A fuller discussion of potential toxicities is beyond the scope of this review. Briefly, concerns have been raised regarding prolonged exposure to tenofovir (recommended first line for children >3 years) and protease inhibitors (lopinavir is recommended first line for children <3 years) among children and adolescents because of their impact on bone mineral density/kidney function and metabolic disease respectively, during critical periods of growth and development [48▪,49,50]. Such concerns are particularly important in resource-constrained settings wherein there is limited monitoring of such toxicities.
Recent results from the CHER and PREDICT trials show very good long-term virological suppression and low rates of switch to second line among children on immediate ART [25▪▪,26]. However, data from observational studies in routine care settings suggest much higher rates of virological failure in Asia and Africa (range from 19 to 25% at 3–5 years of ART in settings with routine virological monitoring) [51,52▪,53]. Also a recent meta-analysis reported higher levels of triple-class virological failure in European adolescents starting ART compared with 5–9-year olds [54]. In countries, where only first line and second-line treatment options are available, this presents a critical dilemma and has resulted in trials exploring treatment-sparing strategies.
The first is the Nevirapine Resistance Study (NEVEREST) trial, wherein children aged below 3 years started ART with lopinavir-based therapy and continued on this regimen or switched to nevirapine-based regimen after a prolonged period of virological suppression with the aim of preserving lopinavir for second line. Results suggested that those who switched to nevirapine had poorer virological outcomes but better growth and CD4 responses [55▪▪]. The other strategy was within the CHER trial, in which children on early ART had a treatment interruption at 40 or 96 weeks with CD4 and clinical-guided criteria for reinitiation of ART [25▪▪]. Up to a third of children in the 96 weeks arm remained off treatment at the end of the study with good CD4 profiles. The study showed that this strategy was well tolerated and superior when compared with deferred ART. However, the study was not powered to detect a difference in outcomes between the two treatment interruption arms, and there was no early ART continuous arm to compare with. These findings suggest that there may be a future role for early intensive treatment followed by periods of interruption, but, critically, rely on close clinical and laboratory monitoring, which is not available everywhere. Nonetheless, the findings confirm conclusions from previous studies that, unlike in adults, treatment interruption in children appears well tolerated without higher risk of mortality, serious clinical events [56] or negative impact on long-term immune/virological response after reinitiation of ART [57▪], although further study is needed.
CONCLUSION
With an estimated 300 000 children newly infected with HIV in 2012 alone, questions concerning when to start lifelong ART remain extremely relevant to paediatrics. Although there is strong evidence of wide ranging benefits of immediate ART in infants, there remains conflicting evidence on the benefits of early ART in asymptomatic children above 1 year. Modelling studies suggest that delayed ART initiation at older ages and lower CD4 reduces the probability of immune reconstitution with unknown implications for long-term disease progression, highlighting the need for continued follow-up of perinatally infected patients as they transfer to adolescent and adult clinics. Lastly, recent trial results suggest that treatment interruption following early intensive ART during infancy may be a well tolerated alternative strategy but requires close monitoring, which is not available in most of the affected regions.
Acknowledgements
We thank David Dunn for his comments on this article.
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
Footnotes
Correspondence to Intira J. Collins, MRC Clinical Trials Unit at UCL, Aviation House, 125 Kingsway, London WC2B 6NH E, UK. Tel: +44 20 7670 4767; e-mail: jeannie.collins@ucl.ac.uk
REFERENCES
- 1.UNAIDS: a progress report on the Global Plan towards the elimination of new HIV infections among children by 2015 and keeping their mothers alive; 2012. http://www.unaids.org/en/media/unaids/contentassets/documents/unaidspublication/2012/JC2385_ProgressReportGlobalPlan_en.pdf [Accessed 26 February 2013] [Google Scholar]
- 2.World Health Organization/UNICEF/UNAIDS WH: Global Update on HIV Treatment 2013: Results, Impact and Opportunities. 2013. http://apps.who.int/iris/bitstream/10665/85326/1/9789241505734_eng.pdf [Accessed 14 February 2013] [Google Scholar]
- 3.UNAIDS: 2013 Progress Report on the Global Plan towards the elimination of new HIV infections among children by 2015 and keeping their mothers alive. 2013. http://www.unaids.org/en/media/unaids/contentassets/documents/unaidspublication/2013/20130625_progress_global_plan_en.pdf [Accessed 15 September 2013] [Google Scholar]
- 4.Newell M-L, Coovadia H, Cortina-Borja M, et al. Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis. Lancet 2004; 364:1236–1243 [DOI] [PubMed] [Google Scholar]
- 5▪.Becquet R, Marston M, Dabis F, et al. Children who acquire HIV infection perinatally are at higher risk of early death than those acquiring infection through breastmilk: a meta-analysis. PLoS One 2012; 7:e28510. [DOI] [PMC free article] [PubMed] [Google Scholar]; A large pooled analysis of over 12 000 children, which showed poorer survival in perinatally infected as compared to postnatally infected children without ART.
- 6.Joint United Nations Programme on HIV/AIDS (UNAIDS): Global Report: UNAIDS report on the Global AIDS Epidemic.; 2012. http://www.unaids.org/en/media/unaids/contentassets/documents/epidemiology/2012/gr2012/20121120_UNAIDS_Global_Report_2012_with_annexes_en.pdf [Accessed 19 August 2013] [Google Scholar]
- 7.World Health Organization Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: Recommendations for a public health approach. 2013; http://www.who.int/hiv/pub/guidelines/arv2013/en/index.htmlhttp://www.who.int/hiv/pub/guidelines/arv2013/en/index.html [Accessed 14 August 2013] [PubMed] [Google Scholar]
- 8.World Health Organization: Scaling up Antiretroviral Therapy in Resource-Limited Settings: Treatment Guidelines for A Public Health Approach; 2003. http://www.who.int/hiv/pub/prev_care/en/arvrevision2003en.pdf [Accessed 30 August 2008] [Google Scholar]
- 9.World Health Organization Antiretroviral therapy of HIV infection in infants and children in resource-limited settings: towards universal access. Recommendations for a public health approach. 2006; http://www.who.int/hiv/pub/guidelines/paediatric020907.pdfhttp://www.who.int/hiv/pub/guidelines/paediatric020907.pdf [Accessed 30 August 2008] [Google Scholar]
- 10.World Health Organization: Paediatric HIV Antiretroviral Therapy and Care guideline review: Report of the WHO Technical Reference Group Paediatric HIV/ART Care Guideline Group Meeting. 2008. http://www.who.int/hiv/pub/paediatric/WHO_Paediatric_ART_guideline_rev_mreport_2008.pdf [Accessed 30 August 2008] [Google Scholar]
- 11.World Health Organization: Antiretroviral therapy of HIV infection in infants and children: Towards universal access. Recommendations for a public health approach. 2010. http://whqlibdoc.who.int/publications/2010/9789241599801_eng.pdf [Accessed 19 February 2013] [PubMed] [Google Scholar]
- 12.Dunn D, Woodburn P, Duong T, et al. Current CD4 cell count and the short-term risk of AIDS and death before the availability of effective antiretroviral therapy in HIV-infected children and adults. J Infect Dis 2008; 197:398–404 [DOI] [PubMed] [Google Scholar]
- 13.Panel on Antiretroviral Therapy and Medical Management of HIV-Infected Children: Guidelines for the Use of Antiretroviral Agents in Pediatric HIV Infection Edited by; 2012. Centres of Disease Control and Prevention; Developed by the HHS Panel on Antiretroviral Therapy and Medical Management of HIV-Infected Children—A Working Group of the Office of AIDS Research Advisory Council (OARAC) http://aidsinfo.nih.gov/contentfiles/lvguidelines/pediatricguidelines.pdf [Accessed 3 March 2013] [Google Scholar]
- 14.Welch S, Sharland M, Lyall EG, et al. PENTA 2009 guidelines for the use of antiretroviral therapy in paediatric HIV-1 infection. HIV Med 2009; 10:591–613 [DOI] [PubMed] [Google Scholar]
- 15.Okomo U, Togun T, Oko F, et al. Mortality and loss to programme before antiretroviral therapy among HIV-infected children eligible for treatment in The Gambia, West Africa. AIDS Res Ther 2012; 9:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sutcliffe C, van Dijk J, Munsanje B, et al. Risk factors for pretreatment mortality among HIV-infected children in rural Zambia: a cohort study. PLoS One 2011; 6:e29294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17▪.Mugglin C, Wandeler G, Estill J, et al. Retention in care of HIV-infected children from HIV test to start of antiretroviral therapy: systematic review. PLoS One 2013; 8:e56446. [DOI] [PMC free article] [PubMed] [Google Scholar]; A systematic review including 10 studies from Africa and Asia with more than 10 000 children showed that 63.2–90.7% of children met the eligibility criteria for ART and 39.5–99.4% of the eligible children started ART.
- 18.Avila D, Patel K, Chi B, Wools-Kaloustian K, Leroy V, Sohn A, Chimbetete C, Hazra R, Egger M, Davies MA. Severe Immunodeficiency in Children Starting ART in Low-, Middle- and High-income Countries (Poster #940). In 20th Conference on Retroviruses and Opportunistic Infections Edited by Georgia, USA; 2013 [Google Scholar]
- 19.Leroy V, Malateste K, Rabie H, et al. Outcomes of antiretroviral therapy in children in Asia and Africa: a comparative analysis of the IeDEA pediatric multiregional collaboration. J Acquir Immune Defic Syndr 2013; 62:208–219210.1097/QAI.1090b1013e31827b31870bf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kabue MM, Buck WC, Wanless SR, et al. Mortality and clinical outcomes in HIV-infected children on antiretroviral therapy in Malawi, Lesotho, and Swaziland. Pediatrics 2012; 130:e591–e599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.TREAT Asia Pediatric Observational Cohort (TApHOD) International Epidemiologic Databases to Evaluate AIDS (IeDEA) Southern Africa Paediatric Group A biregional survey and review of first-line treatment failure and second-line paediatric antiretroviral access and use in Asia and southern Africa. J Int AIDS Soc 2011; 14:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.HIV Paediatric Prognostic Markers Collaborative Study Group (HPPMCS) Short-term risk of disease progression in HIV-1-infected children receiving no antiretroviral therapy or zidovudine monotherapy: a meta-analysis. Lancet 2003; 362:1605–1611 [DOI] [PubMed] [Google Scholar]
- 23.Cross Continents Collaboration for Kids (3Cs4kids) Analysis and Writing Committee Markers for predicting mortality in untreated HIV-infected children in resource-limited settings: a meta-analysis. AIDS 2008; 22:97–105 [DOI] [PubMed] [Google Scholar]
- 24.Violari A, Cotton MF, Gibb DM, et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med 2008; 359:2233–2244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25▪▪.Cotton MF, Violari A, Otwombe K, et al. Early time-limited antiretroviral therapy versus deferred therapy in South African infants infected with HIV: results from the children with HIV early antiretroviral (CHER) randomised trial. Lancet 2013; http://www.thelancet.com/journals/lancet/article/PIIS0140-6736(13)61409-9/abstracthttp://www.thelancet.com/journals/lancet/article/PIIS0140-6736(13)61409-9/abstract [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]; Final results of the CHER trial, demonstrated better clinical and immunological outcomes in children initiated early time-limited ART as compared to deferred ART. Treatment interruption appeared well tolerated but needed close monitoring.
- 26.Violari A, Cotton MF, Gibb DM, et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med 2008; 359:2233–2244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27▪.Laughton B, Cornell M, Grove D, et al. Early antiretroviral therapy improves neurodevelopmental outcomes in infants. AIDS 2012; 26:1685–1690 [DOI] [PMC free article] [PubMed] [Google Scholar]; Sub-study of the CHER trial: cross-sectional study, which showed that infants on early ART had better neurodevelopmental outcomes as compared to deferred ART arm at 11 months of age, with comparable outcomes to uninfected controls.
- 28▪▪.Puthanakit T, Saphonn V, Ananworanich J, et al. Early versus deferred antiretroviral therapy for children older than 1 year infected with HIV (PREDICT): a multicentre, randomised, open-label trial. Lancet Infect Dis 2012; 12:933–941 [DOI] [PMC free article] [PubMed] [Google Scholar]; Trial in Cambodia and Thailand with 300 children aged 1–12 years with CD4 15–24% randomized to immediate versus deferred ART showed no difference in AIDS-free survival at 144 weeks.
- 29▪.Puthanakit T, Ananworanich J, Vonthanak S, et al. Cognitive function and neurodevelopmental outcomes in HIV-infected children older than 1 year of age randomized to early versus deferred antiretroviral therapy: the PREDICT neurodevelopmental study. Pediatr Infect Dis J 2013; 32:501–508 [DOI] [PMC free article] [PubMed] [Google Scholar]; Sub-study of the PREDICT trial, which showed no difference in neurodevelopmental outcomes in children (median age 9 years at last examination) who received early versus deferred ART, despite repeat measurements over 144 weeks of follow-up.
- 30.McConnell MS, Chasombat S, Siangphoe U, et al. National program scale-up and patient outcomes in a pediatric antiretroviral treatment program, Thailand 2000-2007. J Acquir Immune Defic Syndr 2010; 54:423–429 [DOI] [PubMed] [Google Scholar]
- 31.Collins IJ, Jourdain G, Hansudewechakul R, et al. Long-term survival of HIV-infected children receiving antiretroviral therapy in Thailand: a 5-year observational cohort study. Clin Infect Dis 2010; 51:1449–1457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32▪.M-A Davies, M Schomaker, T Gsponer, J Ndirangu, S Phiri, H Moultrie, K Technau, V Cox, J Giddy, C Chimbetete, et al. When to start ART in children aged 2-5 years? Causal modeling analysis of IeDEA southern Africa (TUPE313). In 7th IAS Conference on HIV Pathogenesis, Treatment and Prevention Edited by Kuala Lumpur, Malaysia; 2013 [Google Scholar]; Modelling study showed no benefit in survival with the application of immediate ART in children aged 2–5 years irrespective of immunological or clinical status.
- 33.Patel K, Hernan MA, Williams PL, et al. Long-term effects of highly active antiretroviral therapy on CD4+ cell evolution among children and adolescents infected with HIV: 5 years and counting. Clin Infect Dis 2008; 46:1751–1760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Puthanakit T, Kerr SJ, Ananworanich J, et al. Pattern and predictors of immunologic recovery in human immunodeficiency virus-infected children receiving non-nucleoside reverse transcriptase inhibitor-based highly active antiretroviral therapy. Pediatr Infect Dis J 2009; 28:488–492 [DOI] [PubMed] [Google Scholar]
- 35.Soh CH, Oleske JM, Brady MT, et al. Long-term effects of protease-inhibitor-based combination therapy on CD4 T-cell recovery in HIV-1-infected children and adolescents. Lancet 2003; 362:2045–2051 [DOI] [PubMed] [Google Scholar]
- 36.Walker AS, Doerholt K, Sharland M, Gibb DM. Collaborative HIV Paediatric Study CHIPS Steering Committee Response to highly active antiretroviral therapy varies with age: the UK and Ireland Collaborative HIV Paediatric Study. AIDS 2004; 18:1915–1924 [DOI] [PubMed] [Google Scholar]
- 37▪▪.Lewis J, Walker AS, Castro H, et al. Age and CD4 count at initiation of antiretroviral therapy in HIV-infected children: effects on long-term T-cell reconstitution. J Infect Dis 2012; 205:548–556 [DOI] [PubMed] [Google Scholar]; Novel modelling study using data from 127 children in a European trial to model the long-term CD4 trajectory of children, by age and CD4 at start of therapy, showing that children initiating ART at older ages and with low CD4 are less likely to achieve immune reconstitution.
- 38▪.ARROW Trial team Routine versus clinically driven laboratory monitoring and first-line antiretroviral therapy strategies in African children with HIV (ARROW): a 5-year open-label randomised factorial trial. Lancet 2013; 381:1391–1403 [DOI] [PMC free article] [PubMed] [Google Scholar]; A large trial of 1206 Ugandan and Zimbabwean children randomized to clinically driven monitoring or clinical and laboratory driven monitoring, which reported no difference in new WHO stage 4 event or death at 5 years of ART.
- 39.Collins I, Ngo-Giang-Huong N, Jourdain G, Chanta C, Puangsombat A, Kwanchaipanich R, Bunjongpak S, Cressey T, Le-Coeur S, Jaffar S, et al. Long-term immune response in HIV-infected children receiving highly active antiretroviral therapy in Thailand: outcomes at 7-years. In 7th IAS Conference on HIV Pathogenesis, Treatment and Prevention Kuala Lumpur, Malaysia Edited by (#TUPE314).; 2013 [Google Scholar]
- 40.Lapadula G, Cozzi-Lepri A, Marchetti G, et al. Risk of clinical progression among patients with immunological nonresponse despite virological suppression after combination antiretroviral treatment. AIDS 2013; 27:769–779710.1097/QAD.1090b1013e32835cb32747 [DOI] [PubMed] [Google Scholar]
- 41.Gilson R, Man SL, Copas A, et al. Discordant responses on starting highly active antiretroviral therapy: suboptimal CD4 increases despite early viral suppression in the UK Collaborative HIV Cohort (UK CHIC) Study∗. HIV Med 2010; 11:152–160 [DOI] [PubMed] [Google Scholar]
- 42.The Collaboration of Observational HIV Epidemiological Research Europe in EuroCoord All-cause mortality in treated HIV-infected adults with CD4 ≥500/mm3 compared with the general population: evidence from a large European observational cohort collaboration. Int J Epidemiol 2012; 41:433–445 [DOI] [PubMed] [Google Scholar]
- 43.Lewden C, Chêne G, Morlat P, et al. HIV-infected adults with a CD4 cell count greater than 500 cells/mm3 on long-term combination antiretroviral therapy reach same mortality rates as the general population. J Acquir Immune Defic Syndr 2007; 46:72–7710.1097/QAI.1090b1013e318134257a [DOI] [PubMed] [Google Scholar]
- 44▪▪.Laughton B, Cornell M, Boivin M, Van Rie A. Neurodevelopment in perinatally HIV-infected children: a concern for adolescence. J Int AIDS Soc 2013; 16:18603. [DOI] [PMC free article] [PubMed] [Google Scholar]; Insightful review of studies on neurodevelopment in HIV-infected children and adolescents in randomized trials and observational studies.
- 45.Le Doaré K, Bland R, Newell M-L. Neurodevelopment in children born to HIV-infected mothers by infection and treatment status. Pediatrics 2012; 130:e1326–e1344 [DOI] [PubMed] [Google Scholar]
- 46▪.Ruel TD, Boivin MJ, Boal HE, et al. Neurocognitive and motor deficits in HIV-infected Ugandan children with high CD4 cell counts. Clin Infect Dis 2012; 54:1001–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]; Cross-sectional study showed that HIV-infected ART-naive children in Uganda with CD4 more than 350 cells had poorer neurocognitive and motor scores as compared to uninfected controls despite having high CD4 (median >655 cells).
- 47▪.Smith R, Chernoff M, Williams PL, et al. Impact of HIV severity on cognitive and adaptive functioning during childhood and adolescence. Pediatr Infect Dis J 2012; 31:592–598510.1097/INF.1090b1013e318253844b [DOI] [PMC free article] [PubMed] [Google Scholar]; Cross-sectional study of HIV-infected youth (aged 7–16 years) in the USA, showed children with previous CDC C event had poorer cognitive performance, whereas children without CDC C events performed comparably to HIV-uninfected controls.
- 48▪.Barlow-Mosha L, Eckard AR, McComsey GA, Musoke PM. Metabolic complications and treatment of perinatally HIV-infected children and adolescents. J Int AIDS Soc 2013; 16:18600. [DOI] [PMC free article] [PubMed] [Google Scholar]; Comprehensive review of long-term treatment complications and toxicities of children and adolescents on ART as part of a special issue on perinatally HIV-infected adolescents.
- 49.Bhimma R, Purswani MU, Kala U. Kidney disease in children and adolescents with perinatal HIV-1 infection. J Int AIDS Soc 2013; 16:18596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Puthanakit T, Siberry GK. Bone health in children and adolescents with perinatal HIV infection. J Int AIDS Soc 2013; 16:18575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Davies MA, Moultrie H, Eley B, et al. Virologic failure and second-line antiretroviral therapy in children in South Africa: the IeDEA Southern Africa collaboration. J Acquir Immune Defic Syndr 2011; 56:270–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52▪.Lowenthal ED, Ellenberg JH, Machine E, et al. Association between efavirenz-based compared with nevirapine-based antiretroviral regimens and virological failure in HIV-infected children. JAMA 2013; 309:1803–1809 [DOI] [PMC free article] [PubMed] [Google Scholar]; Observational cohort of 804 children aged 3–16 years on ART and followed for median of 69 months in Botswana, showed higher rates of virological failure in those initiated on nevirapine versus efavirenz-based first-line regimens.
- 53.Collins I, Cairns J, Le Coeur S, et al. Five-year trends in antiretroviral usage and drug costs in HIV-infected children in Thailand. J Acquir Immune Defic Syndr 2013; 64:95–102110.1097/QAI.1090b1013e318298a318309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Castro H, Judd A, Gibb DM, et al. Risk of triple-class virological failure in children with HIV: a retrospective cohort study. Lancet 2011; 377:1580–1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55▪▪.Kuhn L, Coovadia A, Strehlau R, et al. Switching children previously exposed to nevirapine to nevirapine-based treatment after initial suppression with a protease-inhibitor-based regimen: long-term follow-up of a randomised, open-label trial. Lancet Infect Dis 2012; 12:521–530 [DOI] [PMC free article] [PubMed] [Google Scholar]; Final results of the NEVEREST trial showing children on continuous lopinavir-based regimens were more likely to achieve virological suppression as compared to those switched to nevirapine-based regimen after suppression on lopinavir. Most failures in the nevirapine arm were detected within 52 weeks after switch.
- 56.Paediatric European Network for Treatment of AIDS Response to planned treatment interruptions in HIV infection varies across childhood. AIDS 2010; 24:231–241210.1097/QAD.1090b1013e328333d328343 [DOI] [PubMed] [Google Scholar]
- 57▪.Bunupuradah T, Duong T, Compagnucci A, et al. Outcomes after reinitiating antiretroviral therapy in children randomized to planned treatment interruptions. AIDS 2013; 27:579–589510.1097/QAD.1090b1013e32835c31181 [DOI] [PubMed] [Google Scholar]; Results of 2-year posttrial follow-up of children participated in the PENTA-11 treatment interruption study, showing no clinical, immunological or virological consequences of treatment interruption.


