Abstract
Monoclonal antibodies were raised against homogeneous Ro and La antigens, two proteins associated with Ro and La ribonucleoproteins (RNPs). The specificity of the monoclonal antibodies was proven by immunoblot analysis and by immunoprecipitation. The anti-Ro antibody reacted with a Mr 95,000 protein in a mouse lymphoma cell extract and with a Mr 60,000 polypeptide in extracts from human spleen. The anti-La antibody recognized a Mr 50,000 polypeptide in the mouse L5178y cell extract. The two monoclonal antibodies precipitated RNPs that contained the typical RNA species of Ro or La RNPs. The localization of Ro and La antigen was performed by direct immunofluorescence microscopy. It was found that the anti-Ro antibody reacted with a fibrous network that behaves like cytokeratin, one of the intermediate filament systems. The anti-La antibody reacted with nuclear structures that gave a speckled-type pattern.
Full text
PDF

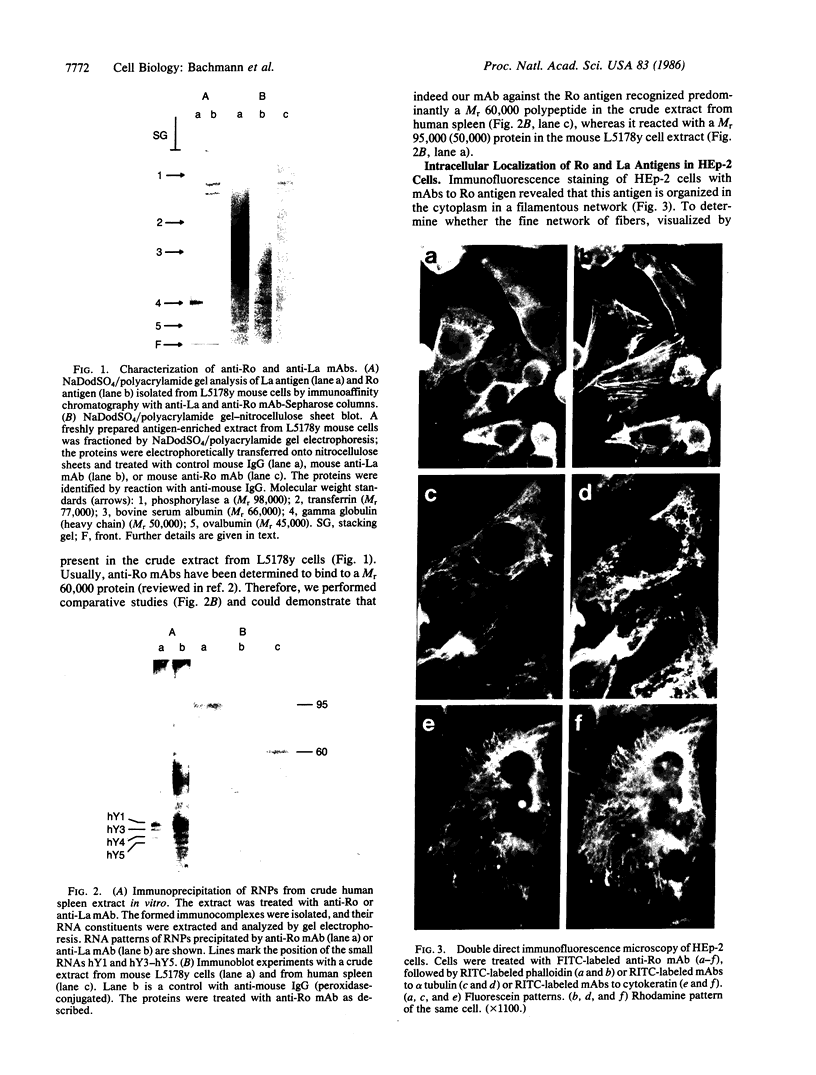


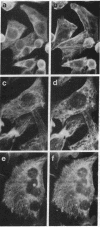
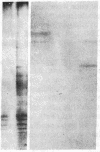
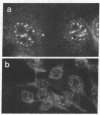
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann M., Messer R., Trautmann F., Müller W. E. 12 S small nuclear ribonucleoprotein-associated acidic-and pyrimidine-specific endoribonuclease from calf thymus and L5178y cells. Biochim Biophys Acta. 1984 Oct 5;783(1):89–99. doi: 10.1016/0167-4781(84)90082-4. [DOI] [PubMed] [Google Scholar]
- Bachmann M., Trautmann F., Messer R., Zahn R. K., Meyer zum Büschenfelde K. H., Müller W. E. Association of a polyuridylate-specific endoribonuclease with small nuclear ribonucleo-proteins which had been isolated by affinity chromatography using antibodies from a patient with systemic lupus erythematosus. Eur J Biochem. 1983 Nov 15;136(3):447–451. doi: 10.1111/j.1432-1033.1983.tb07762.x. [DOI] [PubMed] [Google Scholar]
- Bachmann M., Zahn R. K., Müller W. E. Purification and properties of a novel pyrimidine-specific endoribonuclease termed endoribonuclease VII from calf thymus that is modulated by polyadenylate. J Biol Chem. 1983 Jun 10;258(11):7033–7040. [PubMed] [Google Scholar]
- Barak L. S., Nothnagel E. A., DeMarco E. F., Webb W. W. Differential staining of actin in metaphase spindles with 7-nitrobenz-2-oxa-1,3-diazole-phallacidin and fluorescent DNase: is actin involved in chromosomal movement? Proc Natl Acad Sci U S A. 1981 May;78(5):3034–3038. doi: 10.1073/pnas.78.5.3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollum F. J. Antibody to terminal deoxynucleotidyl transferase. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4119–4122. doi: 10.1073/pnas.72.10.4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervera M., Dreyfuss G., Penman S. Messenger RNA is translated when associated with the cytoskeletal framework in normal and VSV-infected HeLa cells. Cell. 1981 Jan;23(1):113–120. doi: 10.1016/0092-8674(81)90276-2. [DOI] [PubMed] [Google Scholar]
- Chaly N., Bladon T., Setterfield G., Little J. E., Kaplan J. G., Brown D. L. Changes in distribution of nuclear matrix antigens during the mitotic cell cycle. J Cell Biol. 1984 Aug;99(2):661–671. doi: 10.1083/jcb.99.2.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkon K. B., Jankowski P. W. Fine specificities of autoantibodies directed against the Ro, La, Sm, RNP, and Jo-1 proteins defined by two-dimensional gel electrophoresis and immunoblotting. J Immunol. 1985 Jun;134(6):3819–3824. [PubMed] [Google Scholar]
- Fey E. G., Wan K. M., Penman S. Epithelial cytoskeletal framework and nuclear matrix-intermediate filament scaffold: three-dimensional organization and protein composition. J Cell Biol. 1984 Jun;98(6):1973–1984. doi: 10.1083/jcb.98.6.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francoeur A. M., Mathews M. B. Interaction between VA RNA and the lupus antigen La: formation of a ribonucleoprotein particle in vitro. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6772–6776. doi: 10.1073/pnas.79.22.6772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramzow M., Bachmann M., Uhlenbruck G., Dorn A., Müller W. E. Identification and further characterization of the specific cell binding fragment from sponge aggregation factor. J Cell Biol. 1986 Apr;102(4):1344–1349. doi: 10.1083/jcb.102.4.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin J. A., Mimori T. Autoantibodies to ribonucleoproteins. Clin Rheum Dis. 1985 Dec;11(3):485–505. [PubMed] [Google Scholar]
- Hendrick J. P., Wolin S. L., Rinke J., Lerner M. R., Steitz J. A. Ro small cytoplasmic ribonucleoproteins are a subclass of La ribonucleoproteins: further characterization of the Ro and La small ribonucleoproteins from uninfected mammalian cells. Mol Cell Biol. 1981 Dec;1(12):1138–1149. doi: 10.1128/mcb.1.12.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe J. G., Hershey J. W. Translational initiation factor and ribosome association with the cytoskeletal framework fraction from HeLa cells. Cell. 1984 May;37(1):85–93. doi: 10.1016/0092-8674(84)90303-9. [DOI] [PubMed] [Google Scholar]
- Kalb V. F., Jr, Bernlohr R. W. A new spectrophotometric assay for protein in cell extracts. Anal Biochem. 1977 Oct;82(2):362–371. doi: 10.1016/0003-2697(77)90173-7. [DOI] [PubMed] [Google Scholar]
- Kurata N., Tan E. M. Identification of antibodies to nuclear acidic antigens by counterimmunoelectrophoresis. Arthritis Rheum. 1976 May-Jun;19(3):574–580. doi: 10.1002/art.1780190309. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lerner M. R., Boyle J. A., Hardin J. A., Steitz J. A. Two novel classes of small ribonucleoproteins detected by antibodies associated with lupus erythematosus. Science. 1981 Jan 23;211(4480):400–402. doi: 10.1126/science.6164096. [DOI] [PubMed] [Google Scholar]
- Mattioli M., Reichlin M. Heterogeneity of RNA protein antigens reactive with sera of patients with systemic lupus erythematosus. Description of a cytoplasmic nonribosomal antigen. Arthritis Rheum. 1974 Jul-Aug;17(4):421–429. doi: 10.1002/art.1780170413. [DOI] [PubMed] [Google Scholar]
- Mattioli M., Reichlin M. Physical association of two nuclear antigens and mutual occurrence of their antibodies: the relationship of the SM and RNAprotein (MO) systems in SLE sera. J Immunol. 1973 May;110(5):1318–1324. [PubMed] [Google Scholar]
- McNeilage L. J., Whittingham S., Jack I., MacKay I. R. Molecular analysis of the RNA and protein components recognized by anti-La(SS-B) autoantibodies. Clin Exp Immunol. 1985 Dec;62(3):685–695. [PMC free article] [PubMed] [Google Scholar]
- McNeilage L. J., Whittingham S. Use of the Bio-Rad silver stain to identify gel purified RNA components of small nuclear ribonucleoprotein antigens. J Immunol Methods. 1984 Feb 10;66(2):253–260. doi: 10.1016/0022-1759(84)90336-3. [DOI] [PubMed] [Google Scholar]
- Rinke J., Steitz J. A. Precursor molecules of both human 5S ribosomal RNA and transfer RNAs are bound by a cellular protein reactive with anti-La lupus antibodies. Cell. 1982 May;29(1):149–159. doi: 10.1016/0092-8674(82)90099-x. [DOI] [PubMed] [Google Scholar]
- Salden M. H., Van Eekelen C. A., Habets W. J., Vierwinden G., Van de Putte L. B., Van Venrooy W. J. Anti-nuclear matrix antibodies in mixed connective tissue disease. Eur J Immunol. 1982 Sep;12(9):783–786. doi: 10.1002/eji.1830120915. [DOI] [PubMed] [Google Scholar]
- Smith P. R., Williams D. G., Venables P. J., Maini R. N. Monoclonal antibodies to the Sjögren's syndrome associated antigen SS-B (La). J Immunol Methods. 1985 Feb 28;77(1):63–76. doi: 10.1016/0022-1759(85)90184-x. [DOI] [PubMed] [Google Scholar]
- Tan E. M. Autoantibodies to nuclear antigens (ANA): their immunobiology and medicine. Adv Immunol. 1982;33:167–240. doi: 10.1016/s0065-2776(08)60836-6. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Eijk H. G., Van Noort W. L. Isolation of rat transferrin using CNBr-activated sepharose 4B. J Clin Chem Clin Biochem. 1976 Oct;14(10):475–478. doi: 10.1515/cclm.1976.14.1-12.475. [DOI] [PubMed] [Google Scholar]
- Walter P., Blobel G. Signal recognition particle contains a 7S RNA essential for protein translocation across the endoplasmic reticulum. Nature. 1982 Oct 21;299(5885):691–698. doi: 10.1038/299691a0. [DOI] [PubMed] [Google Scholar]
- Wolin S. L., Steitz J. A. Genes for two small cytoplasmic Ro RNAs are adjacent and appear to be single-copy in the human genome. Cell. 1983 Mar;32(3):735–744. doi: 10.1016/0092-8674(83)90059-4. [DOI] [PubMed] [Google Scholar]
- Wolin S. L., Steitz J. A. The Ro small cytoplasmic ribonucleoproteins: identification of the antigenic protein and its binding site on the Ro RNAs. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1996–2000. doi: 10.1073/pnas.81.7.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumbé A., Stähli C., Trachsel H. Association of a Mr 50,000 cap-binding protein with the cytoskeleton in baby hamster kidney cells. Proc Natl Acad Sci U S A. 1982 May;79(9):2927–2931. doi: 10.1073/pnas.79.9.2927. [DOI] [PMC free article] [PubMed] [Google Scholar]