Abstract
The association of chronic glycemia, measured by HbA1c, with long-term complications of type 1 diabetes has been well established in the Diabetes Control and Complications Trial (DCCT) and other studies. The role of intermediate-term and acute glycemia and of glucose variability on microvascular and cardiovascular disease (CVD) is less clear. In order to examine the interrelationships among long-term, intermediate-term, and acute measures of glucose and its daily variability, we compared HbA1c, glycated albumin (GA), and seven-point glucose profile concentrations measured longitudinally in a case-cohort subpopulation of the DCCT. HbA1c and GA were closely correlated with each other and with the mean blood glucose (MBG) calculated from the seven-point profile. The associations of glucose variability and postprandial concentrations with HbA1c and GA were relatively weak and were further attenuated when MBG was included in multivariate models. In the case-cohort analyses, HbA1c and GA had similar associations with retinopathy and nephropathy, which were strengthened when both measures were considered together. Only HbA1c was significantly associated with CVD. The demonstrated interrelationships among different measures of glycemia will need to be considered in future analyses of their roles in the development of long-term complications of type 1 diabetes.
Introduction
The Diabetes Control and Complications Trial (DCCT) showed that intensive therapy aimed at near-normal levels of HbA1c substantially reduced the risk of microvascular complications (1), and that mean HbA1c was the principal determinant of risk of complications (2). Moreover, the difference in risk between the intensive and conventional treatment groups was virtually completely explained by the difference in levels of HbA1c during the DCCT (3). The reductions in risk of microvascular complications with intensive therapy persisted during the Epidemiology of Diabetes Interventions and Complications (EDIC) observational follow-up study despite negligible differences in HbA1c since the end of the DCCT—a phenomenon named “metabolic memory” (4–6). Extended follow-up of the DCCT cohort also revealed a major effect of intensive therapy and lower levels of HbA1c on cardiovascular outcomes, with a 58% reduction in fatal and nonfatal myocardial infarctions and stroke (7). The DCCT results have been used to set targets for diabetes management based on desired levels of HbA1c.
HbA1c, the result of the nonenzymatic glycation of hemoglobin, reflects average glycemia over the preceding 8–12 weeks (8). Whether average glycemia as reflected by HbA1c is the major glycemic determinant of complications and whether other measures of glycemia contribute, and to what extent, are unknown. Glycemic variability, potentially through an independent effect on oxidative stress, has been suggested to be an additional mediator of complications (9,10), but studies have not been consistent in supporting this hypothesis (11,12). Other measures of shorter-term glycemia, such as fructosamine or glycated albumin (GA), have also been associated with long-term complications (13,14). In addition, GA has been suggested to be a better index of glucose variability than HbA1c (15). For evaluation of the contributions of different indices of glycemia to the risk of developing complications, understanding the relationships among them is necessary.
During the DCCT, HbA1c and quarterly seven-point daily glucose profiles were obtained from its 1,441 subjects, and outcomes were assessed systematically over an average of 6.5 years. The results herein are based on a substudy of 497 subjects in whom shorter-term glycemia (GA) was measured annually from frozen specimens. We describe the association between HbA1c and GA levels and their relationships with mean blood glucose (MBG), pre- and postprandial glucose concentrations, and measures of within-profile glucose variation—all derived from the seven-point profile. We then evaluate whether and to what extent GA levels contribute to the risk relationship between HbA1c and retinopathy, nephropathy, and cardiovascular disease (CVD) events. Analyses relating glucose variation to clinical outcomes will be the focus of a future study based on all 1,441 subjects in the cohort.
Research Design and Methods
The DCCT cohort and study design have previously been described in detail (1). In brief, the DCCT enrolled 1,441 subjects 13–39 years of age who were assigned to receive intensive or conventional therapy. Seven hundred and twenty-six were members of the primary prevention cohort with no preexisting retinopathy, albumin excretion rate (AER) <40 mg/24 h, and 1–5 years of diabetes duration, and 715 were members of the secondary intervention cohort with minimal to moderate nonproliferative retinopathy, AER <200 mg/24 h, and 1–15 years’ duration. Retinopathy severity was assessed every 6 months by centrally read seven-field fundus photographs, and AER was measured centrally from an annual 4-h timed collection. Patients were followed for an average of 6.5 years at DCCT study end in 1993. Thereafter, all conventional group subjects were instructed in intensive therapy, and all patients referred to their personal physicians for care. EDIC annual observational follow-up was initiated in 1994, and 96% of the surviving DCCT cohort chose to participate. During the DCCT, mean HbA1c levels were ~2% lower in the intensive than in the conventional treatment group, while during EDIC, HbA1c levels between the original treatment groups converged and were no longer different (16).
Case Definitions
For the current case-control study, retinopathy was defined as a sustained three-step progression on the final Early Treatment Diabetic Retinopathy Study (ETDRS) scale (17) during DCCT. Nephropathy was defined as the onset of microalbuminuria (AER ≥40 mg/24 h) during the DCCT among those free of microalbuminuria at baseline. CVD was defined as the occurrence of any of the following during the DCCT or during the first 16 years of follow-up in EDIC: fatal or nonfatal myocardial infarction or stroke, death judged to be due to CVD, subclinical myocardial infarction present on an annual electrocardiogram, angina confirmed by ischemic changes on exercise tolerance testing or by clinically significant obstruction on coronary angiography, or revascularization with angioplasty or coronary artery bypass (7).
Laboratory and Clinical Analyses
HbA1c was measured quarterly during the DCCT and annually during EDIC using a high-precision, high-performance liquid chromatography assay with long-term control subjects to monitor assay stability as previously described (18). Interassay coefficients of variation (CVs) have remained <2%. GA was measured using the LUCICA GA-L kit (Asahi Kasei Pharma, Tokyo, Japan) with a Roche Modular P Chemistry Analyzer (Roche Diagnostics) on stored, frozen (−70°C) sera collected at each DCCT annual visit between 1983 and 1993. Interassay CVs have remained ~2%. The assay has proven to be very stable for samples stored at –70°C for as many as 23 years (19). GA levels are not affected by albumin and hemoglobin levels (20), and the assay is not influenced by the physiologic concentrations of ascorbic acid, bilirubin, and up to 1,000 mg/dL glucose (21). The quarterly seven-point capillary glucose samples were collected in capillary tubes, placed in diluent, and assayed in the central laboratory as previously described (1).
The pooled within-subject CVs computed from all repeated measures over time were 12% for GA and 9% for HbA1c. Each measures the sum of laboratory variation and within-subject variation over time.
Case-Cohort Design
The detailed description of statistical considerations is presented in the Supplementary Data. Briefly, prior Cox proportional hazards analyses of progression of retinopathy (2) showed a 42% risk reduction per 10% lower updated mean HbA1c, with a lower confidence limit of a 36% risk reduction. With use of the methods of Lachin (22), a sample size of 145 case and 145 control subjects provided 90% power to detect a 36% risk reduction per 10% lower GA when adjusted for HbA1c or for blood glucose.
A case-cohort design (23) was used for each outcome: retinopathy, nephropathy, and CVD. A subcohort of 250 subjects was randomly selected from the DCCT cohort of 1,441 subjects. This included some case subjects of each outcome and some noncase subjects (control subjects) for each outcome type. This provided >145 control subjects for each outcome, specifically, 186 for retinopathy, 184 for nephropathy, and 225 for CVD. For each outcome, additional case subjects were drawn at random from the remainder of the cohort (1,441 − 250) to provide a total of 145 retinopathy and 145 nephropathy cases. All 127 CVD cases (all that were known to exist at that time) were selected. Note that a given subject could be included as a case or control subject in more than one of the three case cohorts. All analyses used the survey weights equal to the inverse of the sampling probabilities for the case and control subjects of each outcome.
Missing Data
The combination of the case cohorts for the three outcomes comprised 497 subjects. However, 4.1% of the expected GA values and 12.4% of the individual blood glucose values from the seven-point profiles were missing, whereas the HbA1c data were nearly complete. To address the missing data, we employed the statistical technique of multiple imputation (24) to provide 10 estimates of each missing value, yielding 10 complete datasets. A given analysis was then repeated using each of the 10 complete datasets, and the results were averaged using the methods of Rubin and Schenker (25). The resulting confidence limits and P values accounted for the overall extent of the original missing data.
Statistical Analysis
From each seven-point blood glucose profile, we computed the overall mean, SD, mean amplitude of glycemic excursion (MAGE) (26), and mean of the pre- and postprandial values separately. The updated mean of each measure at each year was computed from the annual posttreatment values, with the baseline being the initial value.
Analyses of the association of the updated mean values with the risk of each outcome were conducted using a modification of the Cox proportional hazards model for application to a case-cohort design (27,28). The models for retinopathy and nephropathy also were adjusted for diabetes duration, and models for CVD were adjusted for age, with each baseline covariate being among the most strongly associated with each respective outcome. Analyses used the natural log of each glycemic measure that had a somewhat stronger association with the outcome (i.e., had better fit) than did the untransformed values. The effect of each measure (e.g., HbA1c) was described as the percent change in risk of the outcome per 10% higher value of the measure (2).
Results
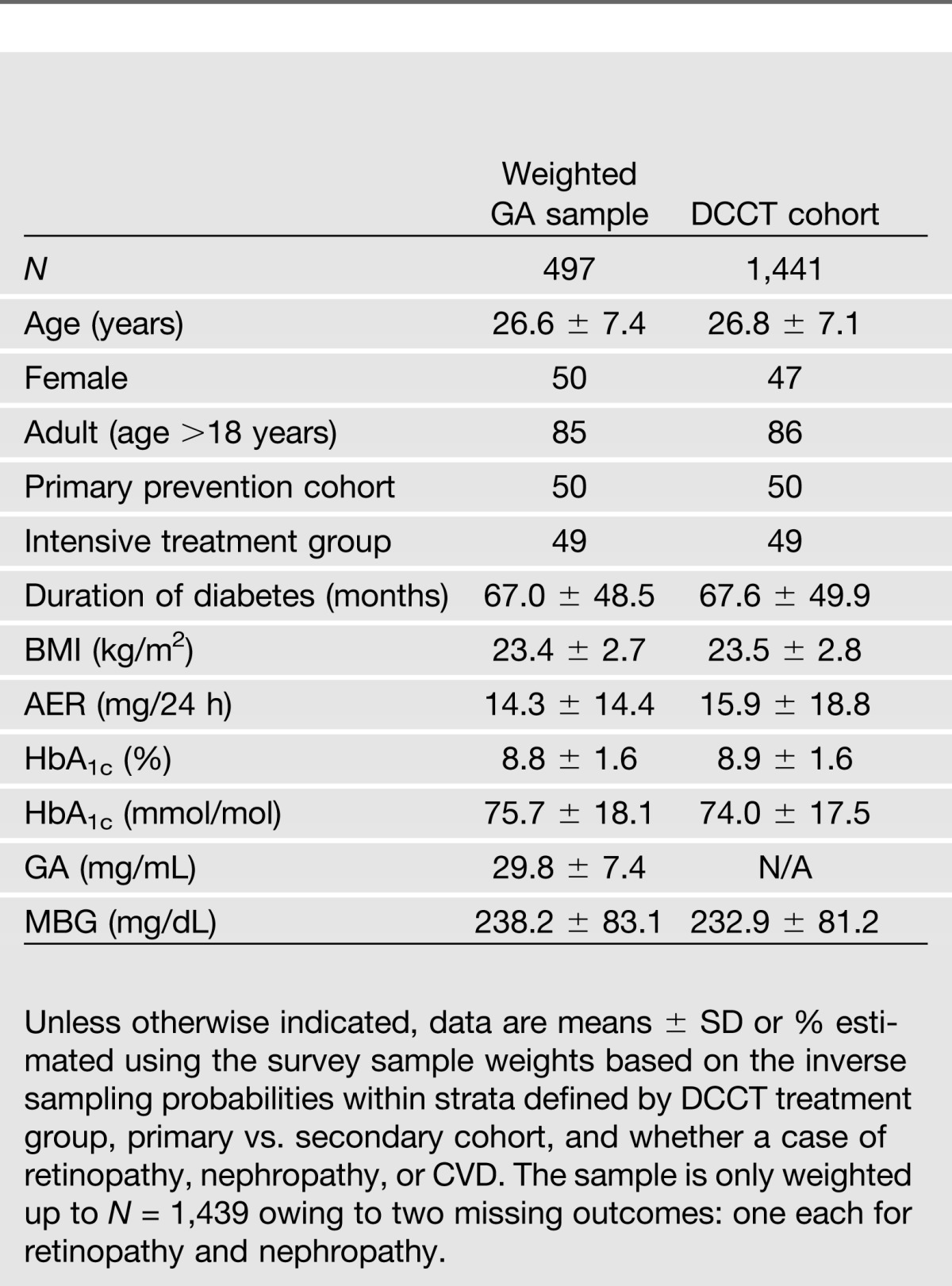
The weighted estimates of patient characteristics in the population corresponding to each case-cohort sample and the aggregate sample in which the GA was measured showed that the characteristics were similar among the cohorts and similar to those in the original complete DCCT cohort (N = 1,441) (Table 1).
Table 1.
Baseline characteristics* from the weighted GA case-cohort and from the full DCCT cohort
Interrelationships Among Measures of Glycemia
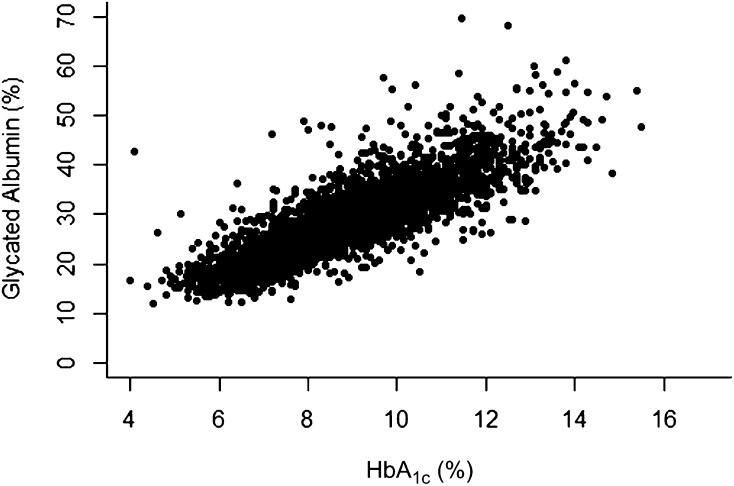
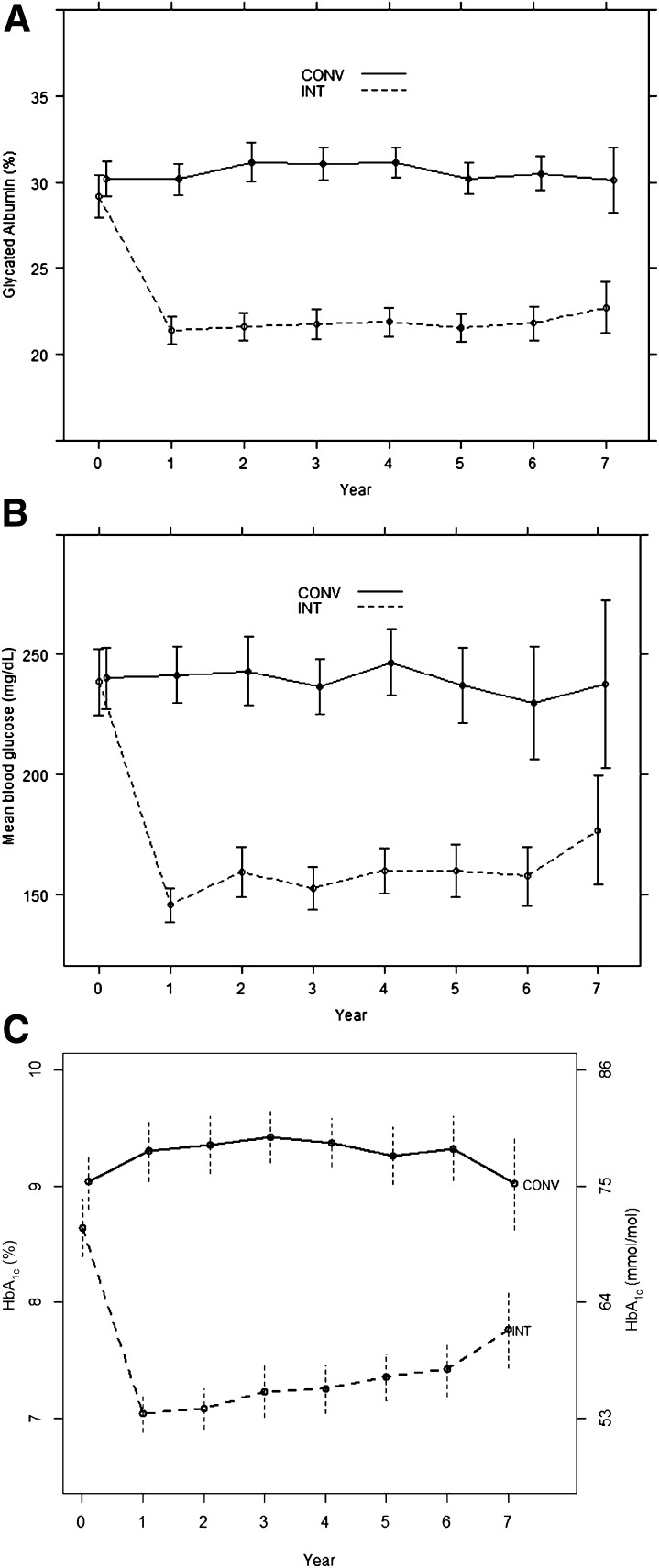
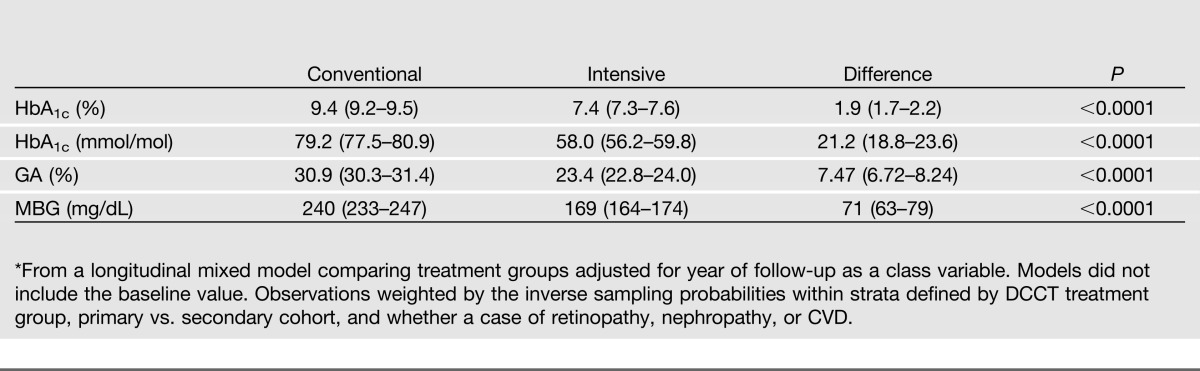
The scatterplot of the associations between HbA1c and GA values at all visits combined showed a strong linear relationship with an overall R2 of 0.75 (Fig. 1). The estimated mean values of each measure within the intensive and conventional groups over time are shown in Fig. 2 and over all 7 years of follow-up in Table 2. In this subsample of the full cohort, the intensive group estimated mean HbA1c tended to track along with that observed in the full cohort, except for the final visit at which there was a rise among the small number of subjects (n = 90) followed to that visit. The MBG and GA values paralleled those of HbA1c.
Figure 1.
Scatterplot of all GA vs. HbA1c values. The slope is a 3.37 increase in GA % per unit increase in HbA1c % (SE = 0.118; P < 0.0001), with R2 = 0.746.
Figure 2.
Weighted estimates of the mean ± 95% CI of measures of glycemia at each annual visit during the DCCT in the aggregate cohort (N = 497) obtained from a longitudinal model with separate estimates for each group at each visit. A: GA. B: MBG. C: HbA1c. Computed as the average of the values at each visit from the 10 imputed datasets with no missing values. Sample sizes at each year of 497, 497, 496, 494, 492, 446, 332, and 214. Observations weighted by the inverse sampling probabilities within strata defined by DCCT Treatment Group, primary vs. secondary cohort, and whether a case of retinopathy, nephropathy, or CVD. CONV, conventional group; INT, intensive group.
Table 2.
Mean* values and 95% CIs for HbA1c, GA, and MBG over 1–7 years of treatment and follow-up within each treatment group
The regression values of the annual HbA1c and GA values (including baseline) on measures of glycemia and glycemic variation derived from the corresponding annual blood glucose profiles are shown in Table 3. As might be expected from the shorter period of turnover of albumin compared with hemoglobin (resulting in a shorter period of glycemia reflected by GA than with HbA1c), GA had a slightly stronger association with measures derived from the single-day blood glucose profiles (which were performed on the same day as the blood sampling) than the associations of HbA1c with glucose concentrations in all models. The mean concentration of daily blood glucose (model 1) was more strongly associated with the HbA1c and GA than was the within-profile SD or the MAGE (models 2 and 3). When the SD and mean were both used in the model (model 4), SD was no longer statistically significant. Similar results applied to models with the MBG and the MAGE in which the MAGE was no longer significantly associated with HbA1c or GA (data not shown).
Table 3.
Association of the annual HbA1c and GA values with glycemic measures derived from the annual seven-point blood glucose profiles in separate weighted longitudinal models*
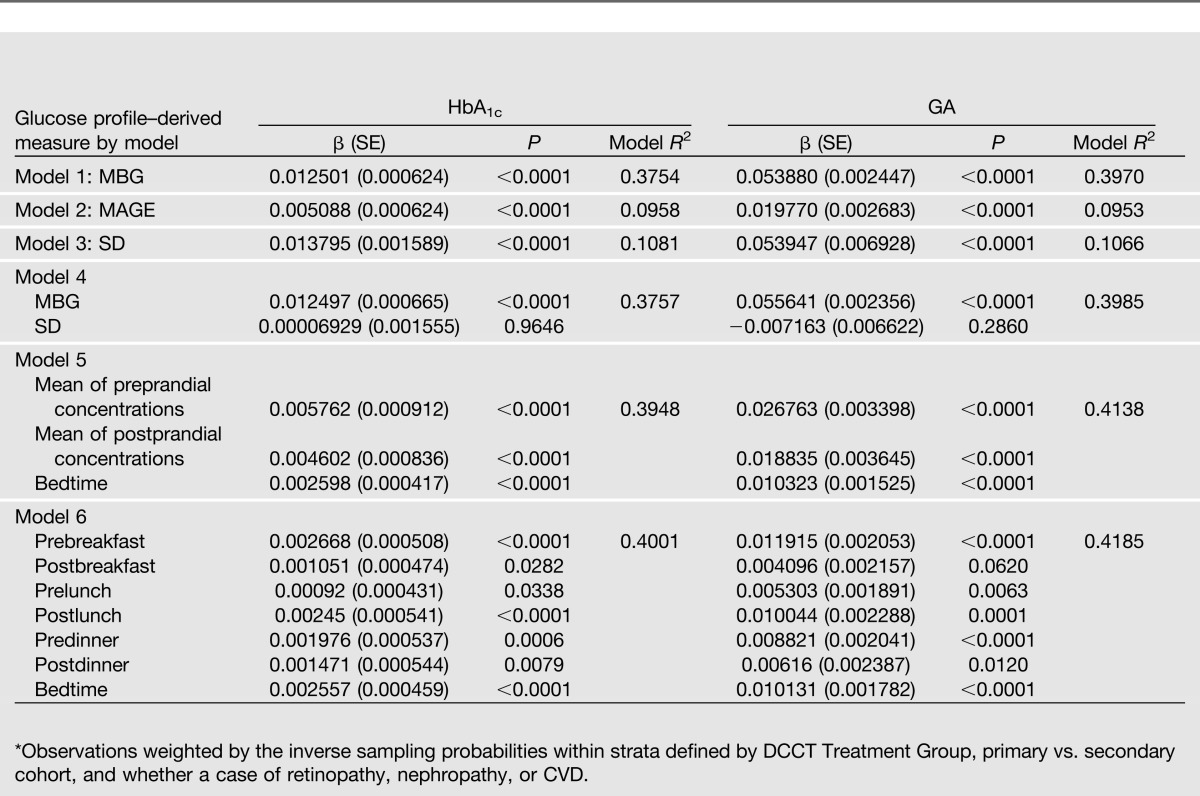
The associations of both HbA1c and GA with the mean preprandial, mean postprandial, and the value at bedtime glucose concentrations (model 5) were slightly stronger than with the overall mean, and both the pre- and postprandial means were approximately equally significant with similar coefficients and SEs. The effect of the bedtime glucose was also significant, but the coefficient was smaller. The final model 6 shows the association of all seven-point blood glucose values in the profile jointly with HbA1c and GA. Both glycated products were more strongly correlated with the fasting (prebreakfast) and bedtime values than with the other time points, all but one of which were also nominally significant. Since the MBG model R2 was only slightly less than that using all seven values together, only the mean glucose was used in analyses in relation to other outcomes.
Retinopathy
The weighted Cox proportional hazards models examining the association of the measures of glycemia with the risk of sustained three-step retinopathy progression were conducted for each of the three measures of glycemia (HbA1c, GA, and MBG) separately, then for two at a time, and then using all three (Table 4). The average model χ2 value (over the 10 imputations) was used as a measure of the strength of the association.
Table 4.
Association of measures of glycemia individually and in combination with the risk of sustained three-step progression of retinopathy among 145 case vs. 186 control subjects in weighted Cox proportional hazards models*
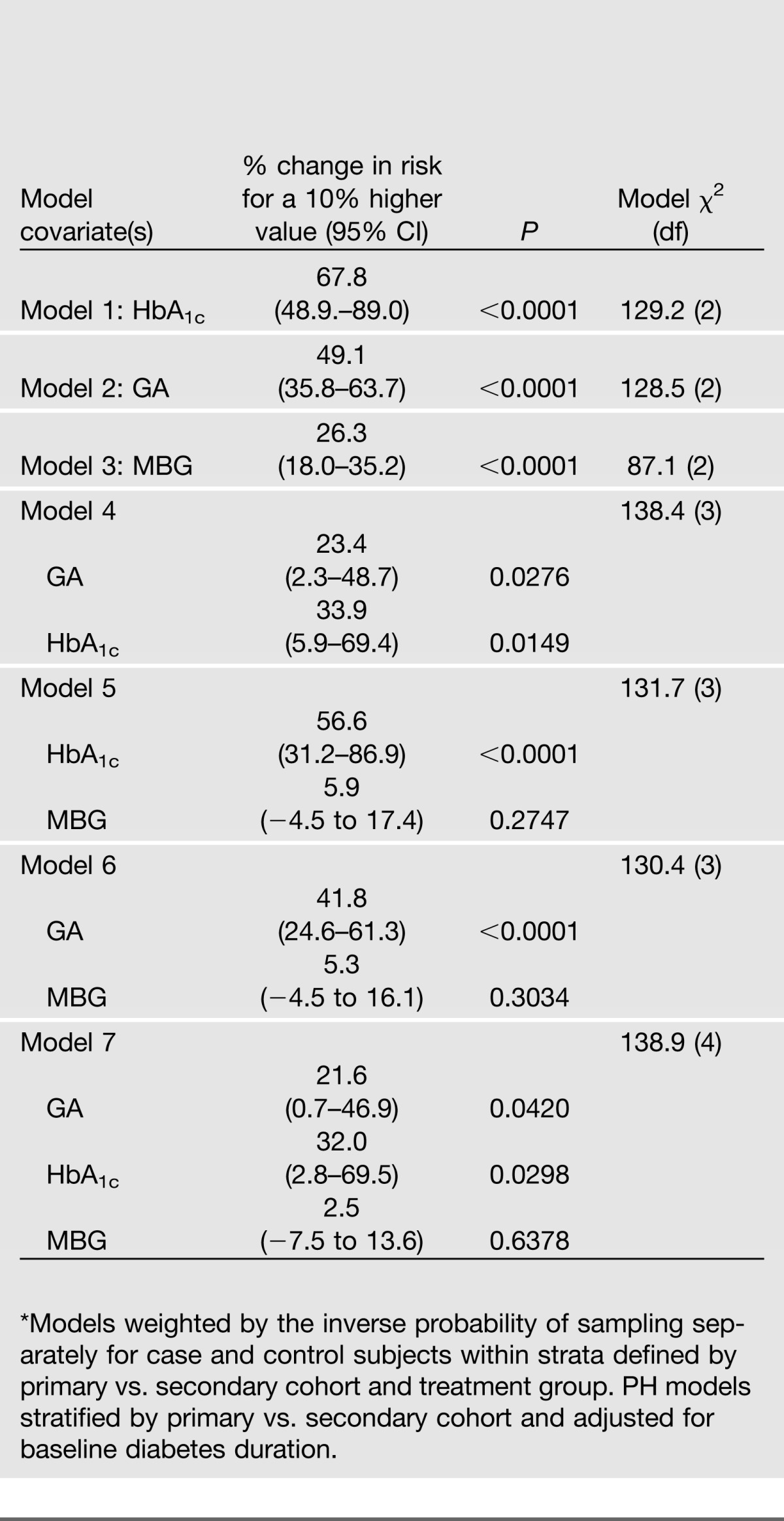
MBG concentrations derived from the seven-point profile had a weaker, albeit still significant (P < 0.0001), effect than either of the glycated products (shown by the χ2 [models 1–3]). When used jointly with either glycation product (models 5–7), the MBG was no longer significant. When HbA1c and GA were considered together, the HbA1c had a slightly smaller P value without or with adjustment for the MBG (models 4 and 7). Based on the model χ2 value, adding HbA1c to GA (model 4 vs. 2) or GA to HbA1c (model 4 vs. 1) each resulted in a substantial increase. Adding the MBG to the two glycation products (model 7 vs. 4) resulted in a trivial increase.
Nephropathy
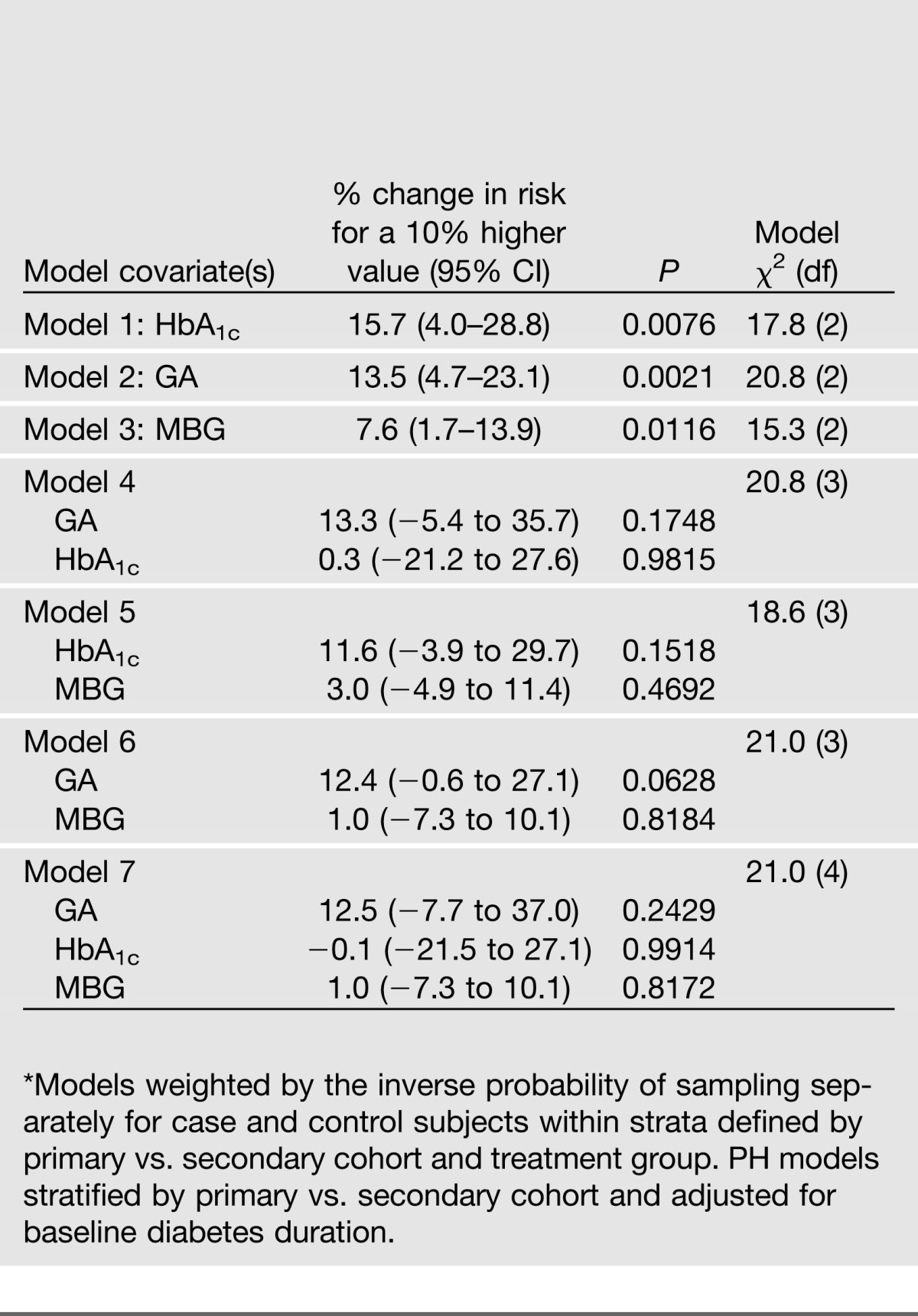
The associations of the measures of glycemia with the risk of microalbuminuria (or worse) in weighted Cox proportional hazards models are shown in Table 5. As with retinopathy, the MBG alone had a weaker effect than either of the glycated products (models 1–3) and was not significant when adjusted for either glycation product (models 5–7). When HbA1c and GA were considered together, neither effect was significant without or with adjustment for the MBG (models 4 and 7).
Table 5.
Association of measures of glycemia individually and in combination with the risk of microalbuminuria (AER ≥40 mg/24 h) or worse among 145 case vs. 184 control subjects in weighted Cox proportional hazards models*
CVD
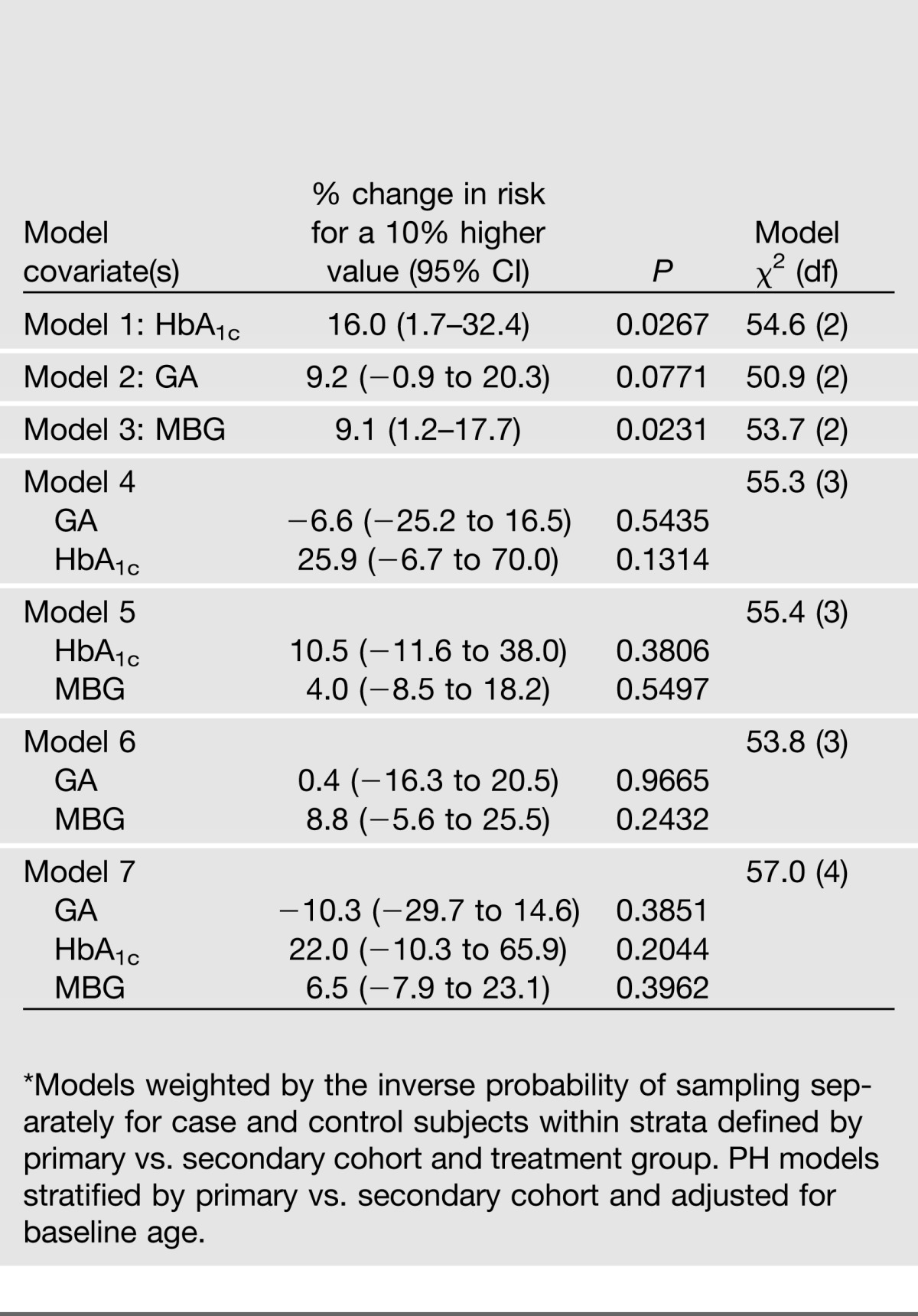
The association of the measures of glycemia with the risk of developing CVD (time to the first event) is shown in Table 6. Unlike retinopathy and nephropathy, where all 145 cases of progression were observed during the DCCT (maximum 9 years of follow-up), 113 of the 127 clinical CVD cases were observed during the EDIC follow-up study with the mean event time being 14.5 years from randomization. HbA1c alone was significant (P = 0.027), although not in any combination with either GA or blood glucose. GA was not significant either alone or in combination with other factors. MBG alone was also a significant predictor (P = 0.024) but not in combination with other glycemic factors.
Table 6.
Association of measures of glycemia individually and in combination with the risk of CVD among 127 case vs. 225 control subjects in weighted Cox proportional hazards models
Discussion
The availability in DCCT/EDIC of longitudinal measurements of glycemia (including indices of chronic [HbA1c] and intermediate [GA] glycemia) and of acute glucose concentrations and glucose variability from a seven-point profile allowed us to examine the relationships among the different measures and their association with clinical outcomes. Initial analyses examined the association of features of the blood glucose profile with HbA1c and GA (Table 3). As expected, HbA1c and GA were highly correlated. Both were strongly associated with the MBG value, but with a lesser association with the within-profile SD or the MAGE measures of glucose variation. Furthermore, the SD had no significant association with the level of HbA1c or GA beyond the association between MBG and HbA1c or GA. This finding directly contradicts prior assertions (29). In fact, the mean of the preprandial values, the mean of the postprandial values, and the value at bedtime all had significant effects on both HbA1c and GA when used together in a model. When the seven-point profile blood glucose values were used jointly, the bedtime and fasting blood glucose, spanning the longest interval in the day (about 8 h), had a somewhat stronger association with both glycation products than the other individual pre- or postprandial values.
Further analyses then assessed the relationship of the MBG, GA, and HbA1c with outcomes. These analyses did not incorporate measures of glucose variation, since the principal objective was to assess whether GA had independent effects above and beyond the MBG and HbA1c. Analyses of the association of glucose variation with outcomes will be conducted using data from all 1,441 DCCT subjects rather than from the subset of 497 subjects in the current study who had GA measurements.
Individually, the HbA1c and GA had a strong association with progression of retinopathy, both in terms of the percent change in risk per percent change in the factor and the model χ2 value. The strongest association with retinopathy was observed when GA and HbA1c were included together, both effects being nominally significant at the 0.05 level, supporting a potential independent effect of each measurement as a risk factor. Both HbA1c and GA remained significant when also adjusted for the MBG.
HbA1c and GA each separately had similar associations with nephropathy, but when the two were used together, the HbA1c had no independent contribution above the GA. Neither was significant when adjusted for the MBG. The differential effects of HbA1c and GA on retinopathy versus nephropathy suggest that the two complications may respond to different aspects of the history of hyperglycemia. Although the measurement of HbA1c may be problematic and GA preferred in the setting of advanced renal disease (30), virtually none of the DCCT cohort had such advanced disease during the period of these analyses. The similar correlations of HbA1c and GA with retinopathy and nephropathy provide support to the cross-sectional study of the Atherosclerosis Risk in Communities (ARIC) population in which GA was at least as powerful a predictor of these complications as HbA1c (13).
For both retinopathy and nephropathy, the relationships with MBG were substantially weaker and were attenuated once GA or HbA1c levels were also considered, indicating that the measurements of long(er)-term glycemia prevail as risk factors for microvascular complications. Intermittent measurements of blood glucose profiles to capture MBG did not provide as strong an association with long-term complications as did HbA1c or GA. Variability of HbA1c levels over time, measured as the intraindividual SD of values measured over ~6 years, has been suggested to be an independent risk factor for nephropathy and CVD events (10).
HbA1c had a significant association with CVD outcomes, as did MBG. On the other hand, the association of GA with CVD, although with a risk profile qualitatively similar to that of HbA1c, was not significant. No index of chronic glycemia had a significant relationship with CVD when adjusted for another index of glycemia. Previously, we showed that the DCCT mean HbA1c as a time-dependent covariate was significantly associated with the risk of CVD in the 83 subjects who had developed this outcome at the time of the prior publication (7).
The associations of both glycation products with retinopathy and nephropathy were in general stronger than observed with CVD events. Since retinopathy and nephropathy are more specific to diabetes than CVD, the stronger correlation of chronic hyperglycemia with the microvascular complications is not surprising. Another reason may be that retinopathy was assessed every 6 months in relation to the annual HbA1c values during the DCCT that were used for this study and nephropathy annually; in contrast, only 14 CVD cases were observed during the DCCT, when the GA and HbA1c levels were measured. The majority of the CVD cases occurred many years after the close of the DCCT, and the analyses herein related the mean value of the glycation products (and MBG) up to the end of the DCCT (mean 6.5 years) with CVD events that occurred many years thereafter. Thus, weaker associations should be expected.
The objective of these analyses was to replicate the major findings from DCCT/EDIC that included the effects of HbA1c on microvascular complications during the DCCT, and on CVD during DCCT and EDIC combined, with the addition of GA results. Thus the retinopathy and nephropathy case and control subjects were selected based on outcomes during the DCCT only. It would also have been desirable to assess the association of GA levels during the DCCT with further progression of retinopathy and nephropathy during EDIC—the so-called “metabolic memory” phenomenon that was previously shown to be associated with the DCCT levels of HbA1c. Unfortunately, it is not possible to replicate either analysis with the case-cohort design used for the present analysis.
However, the associations with CVD do include an assessment of metabolic memory for CVD since the analysis included cases observed over both the DCCT and EDIC with the overwhelming majority being observed in EDIC (113 of 127). As in our prior analyses (7), the mean HbA1c over the period of the DCCT was strongly associated with the risk of CVD over the DCCT and EDIC combined. However, the effect of the DCCT mean GA was weaker than that of the HbA1c and nonsignificant. This may in part be a result of the lag of up to 16 years from the end of the DCCT to the last of the EDIC cases observed herein. It is possible that GA values measured during the EDIC, which would have been more proximate to the time of the CVD event, would have had a much stronger association with CVD risk.
Additional limitations of the current study include our reliance on seven-point glucose profiles measured quarterly, but with only the annual measure included in these analyses, to capture MBG concentrations and variability over time. Such infrequent measurements may lead to sampling errors and are not ideal to capture mean glycemia or provide a true measure of variability. Also, the profiles rely on participant collection, a potentially significant source of error. Although a modest fraction (∼12%) of the profile measurements was missing, which is another potential limitation, we used sturdy imputation methods to “fill in the blanks.” These provide a more complete picture of the glucose profiles. Even with the potential weakness of the seven-point profiles, the blood glucose concentrations were measured in a central laboratory, which provides greater accuracy and precision than would be found in self-monitored or continuous glucose monitoring results. The DCCT/EDIC is the only type 1 diabetes study that has the long-term longitudinal measurements of complications and glycemia necessary to examine the relationships we have examined herein.
Finally, our analyses only used the annual HbA1c and seven-point blood glucose values to match the annual GA values, and the current analyses are restricted to a subsample of case and control subjects from the full cohort. Thus, the HbA1c results reported herein differ somewhat from those previously published, which were based on the quarterly HbA1c measurements in the entire cohort in relation to each outcome.
In conclusion, we have examined the intercorrelation of three different measures of glycemia and demonstrated similar relationships among MBG, HbA1c, and GA. The strongest correlations were between MBG and GA and HbA1c, with measures of glucose variability, including postprandial glycemia, contributing little to the GA and HbA1c values. Both HbA1c and GA were similarly associated with microvascular complications, but only HbA1c was associated with the cardiovascular complications. Combining both glycemic measures appeared to strengthen the relationships with microvascular but not with cardiovascular complications.
Article Information
Funding. The DCCT/EDIC has been supported by U01 Cooperative Agreement grants (1982–1993 and 2011–2016), contracts (1982–2011) with the Division of Diabetes, Endocrinology, and Metabolic Diseases of the National Institute of Diabetes and Digestive and Kidney Diseases, and support from the National Eye Institute, the National Institute of Neurological Disorders and Stroke, the Genetic Clinical Research Centers Program (1993–2007), and the Clinical Translational Science Center Program (2006–present), Bethesda, Maryland. D.M.N. was supported in part by the Charlton Foundation for Innovative Diabetes Research.
Industry contributors have provided free or discounted supplies or equipment to support participants’ adherence to the study: Abbott Diabetes Care (Alameda, CA), Animas (Westchester, PA), Bayer Diabetes Care (North America Headquarters, Tarrytown, NY), Becton Dickinson (Franklin Lakes, NJ), CanAm (Atlanta, GA), Eli Lilly (Indianapolis, IN), LifeScan (Milpitas, CA), Medtronic Diabetes (Minneapolis, MI), Omron (Shelton, CT), OmniPod Insulin Management System (Bedford, MA), Roche Diabetes Care (Indianapolis, IN), and Sanofi (Bridgewater, NJ). The current ancillary project received support from Asahi Kasei Pharma Corporation, Tokyo, Japan, in the form of donated assay kits.
Industry contributors have had no role in the DCCT/EDIC study. Asahi Kasei Pharma Corporation also had no role in the design, conduct, or reporting of this study.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. D.M.N. and P.M. wrote the manuscript and participated in the design and conduct of the study. M.W.S. critically reviewed and edited the study and participated in the design and conduct of the study. J.M.L. wrote the manuscript and participated in the design and conduct of the study. J.M.L. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db13-0782/-/DC1.
A complete list of the members of the DCCT/EDIC Research Group is provided in the supplementary appendix published in the article by the DCCT/EDIC Research Group, de Boer IH, Sun W, Cleary PA, et al. Intensive diabetes therapy and glomerular filtration rate in type 1 diabetes. N Engl J Med 2011;365:2366–2376.
See accompanying commentary, p. 45.
References
- 1.The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 2.DCCT Research Group The relationship of glycemic exposure (HbA1c) to the risk of development and progression of retinopathy in the Diabetes Control and Complications Trial. Diabetes 1995;44:968–983 [PubMed] [Google Scholar]
- 3.Lachin JM, Genuth S, Nathan DM, Zinman B, Rutledge BN, DCCT/EDIC Research Group Effect of glycemic exposure on the risk of microvascular complications in the diabetes control and complications trial—revisited. Diabetes 2008;57:995–1001 [DOI] [PubMed] [Google Scholar]
- 4.The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. N Engl J Med 2000;342:381–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Genuth S, Lachin J, Cleary P, Writing Team for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group Effect of intensive therapy on the microvascular complications of type 1 diabetes mellitus. JAMA 2002;287:2563–2569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Albers JW, Herman WH, Pop-Busui R, et al. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group Effect of prior intensive insulin treatment during the Diabetes Control and Complications Trial (DCCT) on peripheral neuropathy in type 1 diabetes during the Epidemiology of Diabetes Interventions and Complications (EDIC) Study. Diabetes Care 2010;33:1090–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nathan DM, Cleary PA, Backlund JY, et al. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nathan DM, Kuenen J, Borg R, Zheng H, Schoenfeld D, Heine RJ, A1c-Derived Average Glucose Study Group Translating the A1C assay into estimated average glucose values. Diabetes Care 2008;31:1473–1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirsch IB, Brownlee M. Beyond hemoglobin A1c--need for additional markers of risk for diabetic microvascular complications. JAMA 2010;303:2291–2292 [DOI] [PubMed] [Google Scholar]
- 10.Wadén J, Forsblom C, Thorn LM, Gordin D, Saraheimo M, Groop PH, Finnish Diabetic Nephropathy Study Group A1C variability predicts incident cardiovascular events, microalbuminuria, and overt diabetic nephropathy in patients with type 1 diabetes. Diabetes 2009;58:2649–2655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Service FJ, O’Brien PC. The relation of glycaemia to the risk of development and progression of retinopathy in the Diabetic Control and Complications Trial. Diabetologia 2001;44:1215–1220 [DOI] [PubMed] [Google Scholar]
- 12.Kilpatrick ES, Rigby AS, Atkin SL. The effect of glucose variability on the risk of microvascular complications in type 1 diabetes. Diabetes Care 2006;29:1486–1490 [DOI] [PubMed] [Google Scholar]
- 13.Selvin E, Francis LMA, Ballantyne CM, et al. Nontraditional markers of glycemia: associations with microvascular conditions. Diabetes Care 2011;34:960–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furusyo N, Koga T, Masumi A, et al. Plasma glycated albumin level and atherosclerosis: results from the Kyushu and Okinawa Population Study. Int J Cardiol 2012;167:2066–2072 [DOI] [PubMed] [Google Scholar]
- 15.Yoshiuchi K, Matsuhisa M, Katakami N, et al. Glycated albumin is a better indicator for glucose excursion than glycated hemoglobin in type 1 and type 2 diabetes. Endocr J 2008;55:503–507 [DOI] [PubMed] [Google Scholar]
- 16.Nathan DM, Zinman B, Cleary PA, et al. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Research Group Modern-day clinical course of type 1 diabetes mellitus after 30 years’ duration: the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications and Pittsburgh Epidemiology of Diabetes Complications experience (1983-2005). Arch Intern Med 2009;169:1307–1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Early Treatment Diabetic Retinopathy Study research group. Arch Ophthalmol 1985;103:1796–1806 [PubMed] [Google Scholar]
- 18.Steffes M, Cleary P, Goldstein D, et al. Hemoglobin A1c measurements over nearly two decades: sustaining comparable values throughout the Diabetes Control and Complications Trial and the Epidemiology of Diabetes Interventions and Complications study. Clin Chem 2005;51:753–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nathan DM, Steffes MW, Sun W, Rynders GP, Lachin JM. Determining stability of stored samples retrospectively: the validation of glycated albumin. Clin Chem 2011;57:286–290 [DOI] [PubMed] [Google Scholar]
- 20.Kouzuma T, Uemastu Y, Usami T, Imamura S. Study of glycated amino acid elimination reaction for an improved enzymatic glycated albumin measurement method. Clin Chim Acta 2004;346:135–143 [DOI] [PubMed] [Google Scholar]
- 21.Nagamine Y, Mitsui K, Nakao T, Matsumoto M, Fujita C, Doi T. Evaluation of the enzymatic method for glycated albumin with liquid type reagent (Lucia GA-L). Jpn J Med Pharm Sci 2004;51:737–745 [in Japanese] [Google Scholar]
- 22.Lachin JM. Sample size evaluation for a multiply matched case-control study using the score test from a conditional logistic (discrete Cox PH) regression model. Stat Med 2008;27:2509–2523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prentice R. A case-cohort design for epidemiologic cohort studies and disease prevention trials. Biometrika 1986;73:1–11 [Google Scholar]
- 24.Raghunathan TE, Lepkowski JM, Hoewyk JV, Solenberger P. A multivariate technique for multiply imputing missing values using a sequence of regression models. Surv Methodol 2001;27:85–95 [Google Scholar]
- 25.Rubin DB, Schenker N. Multiple imputation for interval estimation from simple random samples with ignorable nonresponse. J Am Stat Assoc 1986;81:366–374 [Google Scholar]
- 26.Molnar GD, Gastineau CF, Rosevear JW, Moxness KE. Quantitative aspects of labile diabetes. Diabetes 1965;14:279–288 [DOI] [PubMed] [Google Scholar]
- 27.Therneau TM, Li H. Computing the Cox model for case cohort designs. Lifetime Data Anal 1999;5:99–112 [DOI] [PubMed] [Google Scholar]
- 28.Barlow WE. Robust variance estimation for the case-cohort design. Biometrics 1994;50:1064–1072 [PubMed] [Google Scholar]
- 29.McCarter RJ, Jr, Hempe JM, Chalew SA. Mean blood glucose and biological variation have greater influence on HbA1c levels than glucose instability: an analysis of data from the Diabetes Control and Complications Trial. Diabetes Care 2006;29:352–355 [DOI] [PubMed] [Google Scholar]
- 30.Peacock TP, Shihabi ZK, Bleyer AJ, et al. Comparison of glycated albumin and hemoglobin A(1c) levels in diabetic subjects on hemodialysis. Kidney Int 2008;73:1062–1068 [DOI] [PubMed] [Google Scholar]