Abstract
Type 1 diabetes is characterized by infiltration of pancreatic islets with immune cells, leading to insulin deficiency. Although infiltrating immune cells are traditionally considered to negatively impact β-cells by promoting their death, their contribution to proliferation is not fully understood. Here we report that islets exhibiting insulitis also manifested proliferation of β-cells that positively correlated with the extent of lymphocyte infiltration. Adoptive transfer of diabetogenic CD4+ and CD8+ T cells, but not B cells, selectively promoted β-cell proliferation in vivo independent from the effects of blood glucose or circulating insulin or by modulating apoptosis. Complementary to our in vivo approach, coculture of diabetogenic CD4+ and CD8+ T cells with NOD.RAG1−/− islets in an in vitro transwell system led to a dose-dependent secretion of candidate cytokines/chemokines (interleukin-2 [IL-2], IL-6, IL-10, MIP-1α, and RANTES) that together enhanced β-cell proliferation. These data suggest that soluble factors secreted from T cells are potential therapeutic candidates to enhance β-cell proliferation in efforts to prevent and/or delay the onset of type 1 diabetes.
Introduction
Type 1 diabetes (T1D) is a chronic T-cell–mediated autoimmune disease characterized by selective destruction of β-cells, resulting in hyperglycemia (1). A major limitation to successful therapy has been a lack of complete understanding of the precise pathways and mechanisms that trigger T1D compounded by the polygenic nature of the disease and the influence of environmental and/or stochastic factors (2).
Studies using the nonobese diabetic (NOD) mice have identified roles for CD4+ and CD8+ T cells and macrophages in β-cell destruction. Other cell types, including B cells, natural killer (NK) cells, NKT cells, and the dendritic cell subsets, have also been detected in the pancreatic infiltrate and draining lymph nodes and could contribute to β-cell death (3).
Although immune cells are generally considered to promote β-cell death, some studies argue that they also enhance their replication. For example, Sreenan et al. (4) have reported increased β-cell proliferation in NOD mice that exhibit infiltration of pancreatic islets prior to the onset of diabetes. In addition, von Herrath et al. (5) reported that nondiabetic RIP-LCMV x SV129 mice, where the numbers and effector functions of autoaggressive CD4+ and CD8+ lymphocytes were not decreased, have increased β-cell regeneration compared with nondiabetic C57BL/6 controls. In other studies, Sherry et al. (6) suggested the increased β-cell proliferation that occurs after arresting the autoimmune process is secondary to effects of the inflammatory infiltrate. The latter study also showed that reversal of infiltration by anti-CD3 monoclonal antibody (mAb) or regulatory T-cell therapy was associated with reduced β-cell proliferation. A notable study that partially addressed the mechanism is that by Dor and colleagues (7), who reported that the use of standard immunosuppression drugs abolished β-cell proliferation and recovery from diabetes. Recent studies have also reported that humans with T1D exhibit persistent mature β-cells in the pancreas that may be secondary to protective factors that prevent their destruction (8,9). An understanding of how these β-cells survive and/or regenerate is an exciting and timely area of interest.
Notwithstanding the scant information on the ability of human β-cells to replicate (10,11), studies in rodent models indicate that β-cell proliferation is increased in physiologic conditions, pathophysiologic states, and injury models (7,12–15). In these models, glucose, insulin, IGFs, growth hormone, glucagon-like peptide 1, adipokines such as leptin, hepatocyte growth factor, and lactogens such as prolactin have all been implicated in regulating β-cell proliferation (16).
In addition to the factors noted above, cytokines derived from the inflammatory response itself have been reported to stimulate islet cell replication (17,18), and treatment with interleukin-4 (IL-4) or IL-10 has been reported to inhibit the development and prevent the recurrence of T1D in NOD mice (19,20).
In this study, we tested the hypothesis that one or more lymphocytes, or their secretions, promote β-cell regeneration in vivo. We report, for the first time to our knowledge, that CD4+ and CD8+ T-cell subsets, but not B cells, secrete soluble factors and are potential novel targets that can be harnessed to promote β-cell proliferation to counter the progression of T1D.
Research Design and Methods
Mice
Female NOD/shiLTJ mice, 20 weeks of age, were used as splenocyte donors, and NOD.RAG1−/− mice, 5–6 weeks of age, were used as recipients for adoptive transfer studies and islet donors for splenocyte-islet coculture experiments. Male C57BL/6J (B6) mouse islets, 5–6 weeks of age, were used for recombinant protein treatments. Blood glucose was measured under ad libidum conditions, and mice were considered diabetic when two consecutive measurements of blood glucose exceeded 200 mg/dL.
Adoptive Transfer of Diabetes and Depletion of Splenocytes
A total of 107 splenocytes were purified from NOD mice with diabetes and injected intravenously into a single NOD.RAG1−/− mouse. To obtain splenocyte preparations devoid of B cells and CD4+ and CD8+ T cells, they were incubated with anti–B220-PE, anti–CD4-PE, and anti–CD8a-PE (BioLegend), respectively. The cells were washed in PBS and resuspended in magnetic-activated cell sorter (MACS) buffer and anti-PE Microbeads and run on the autoMACS system (Miltenyi Biotec). Samples from the B-cell–, CD4+-, and CD8+-depleted splenocyte aliquots were stained with anti-mouse CD19-PE, anti–CD4-Pacific Blue, and anti–CD8a-FITC (BioLegend), respectively, analyzed with a FACSAria (BD Biosciences), and determined to be >98% depleted (data not shown). For CD4+ and CD8+ double depletion, fractionated depleted cells were injected into NOD.RAG1−/− mice. We also used in vivo depletion by injecting 0.5 mg of anti-CD4, anti-CD8, or both mAbs into NOD.RAG1−/− mice every 3 days after depleted splenocyte transfer. Three weeks postinjection of total or depleted splenocytes, pancreas was harvested and prepared for β-cell morphometry. To track lymphocyte homing to host pancreatic islets in adoptively transferred mice, we used NOD.Raspberry splenocytes from mice generated by microinjection of a β-actin/mRaspberry construct in the pronucleus of fertilized NOD mouse eggs.
Streptozotocin Injection and Insulitis Scoring
Eighteen female NOD mice 6 weeks of age were injected intraperitoneally with streptozotocin (STZ) (Sigma-Aldrich) at a concentration of 75 or 100 mg/kg/body weight (BW). Day 0 was defined as the first day of injection, and pancreas was harvested from three mice every other day starting at day 1 until day 7. Insulitis was evaluated as reported (21).
Immunohistochemistry
Pancreata were harvested, fixed, and embedded in paraffin 6 h postinjection with BrdU (100 mg/kg/BW). Sections were stained using antibodies to BrdU, Ki67, phosphohistone H3, or insulin and appropriate secondary antibodies and counterstained with DAPI. At least 1,000–2,000 β-cell nuclei were counted per animal, and data were expressed as percentage of BrdU+, Ki67+, or pHH3+ β-cells. Cell death was detected by TUNEL assay (ApopTag S7100; Chemicon). Frozen sections were coimmunostained for insulin and a DSRed polyclonal antibody (Clontech) to detect mRaspberry protein followed by appropriate secondary antibodies.
Islet Isolation and Mixed Lymphocyte-Islet Cell Culture
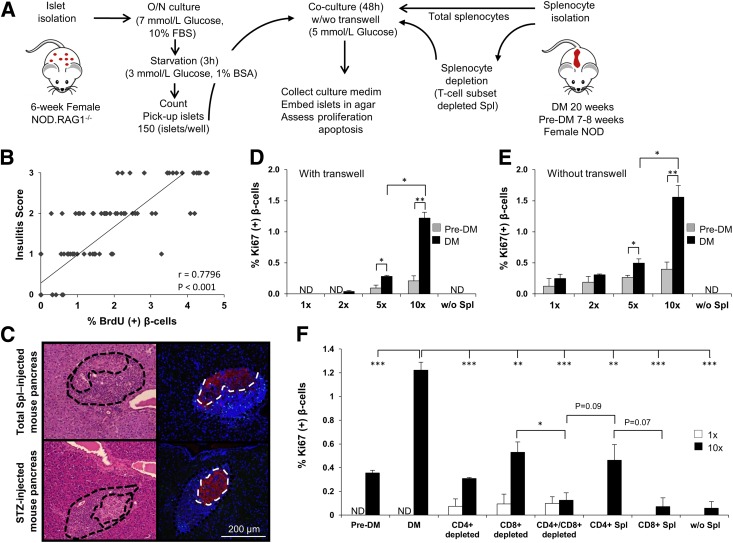
Islets were isolated from 5–6-week-old NOD.RAG1−/− or B6 mice and cultured as described previously (22). In parallel, we prepared depleted or total lymphocyte cell suspensions from 20-week-old diabetic (DM) or 7–8-week-old prediabetic (pre-DM) female NOD mice (23). After starvation, 150 size-matched islets were cocultured with splenocytes or treated with recombinant proteins in 5 mmol/L glucose. Contact between islets and splenocytes placed above the transwell membrane was prevented by using a 0.4-µm transwell insert (Corning Life Sciences). Forty-eight hours after coculture, medium was collected for Luminex assay and islets were embedded in agar for β-cell morphometry. At least 1,000–2,000 β-cell nuclei were counted for quantifying proliferation and apoptosis.
Islet Dispersion and Cell Sorting
Overnight cultured islets were dispersed and β-cells were sorted as described previously (24). Sorted β-cells were washed and stained with anti-CD45 (eBioscience) followed by fixation, permeabilization, and staining with anti-BrdU (BrdU Staining Kit-APC; eBioscience). Cells were analyzed with BD LSR II analyzer.
Recombinant Protein Treatment
One hundred fifty handpicked islets isolated from B6 mice were cultured in the absence or presence of IL-2 (5 or 500 pg/mL), IL-6 (200 pg/mL or 200 ng/mL), IL-10 (4 or 400 pg/mL), macrophage inflammatory protein 1α (MIP-1α) (10 pg/mL or 10 ng/mL), RANTES (5 or 500 pg/mL), a low-dose combination of all the cytokines/chemokines, or 15% FBS (positive control). Low doses were selected from our Luminex assay results, and high doses were based on manufacturer recommendations (R&D Systems). To determine whether the proliferative effects are direct, islets treated with either recombinant proteins or total DM splenocytes were cultured in the presence of specific inhibitors/neutralizing molecules: Ro 26-4550 (IC50 = 3 μmol/L), anti–IL-6 (ND50 = 0.005 μg/mL), anti–IL-10 (ND50 = 0.045 μg/mL), maraviroc (IC50 = 3.3 nmol/L), and maraviroc (IC50 = 5.2 nmol/L) to block IL-2, IL-6, IL-10, MIP-1α, and RANTES, respectively
Statistics
Data are expressed as means ± SEM after a two-tailed Student t test and considered significant at P value ≤0.05.
Study Approval
All animal experiments were conducted after approval by the Institutional Animal Care and Use Committee of the Joslin Diabetes Center in accordance with National Institutes of Health (NIH) guidelines.
Results
Adoptive Transfer of Diabetogenic Splenocytes Promotes β-Cell Replication in NOD.RAG1−/− Mice
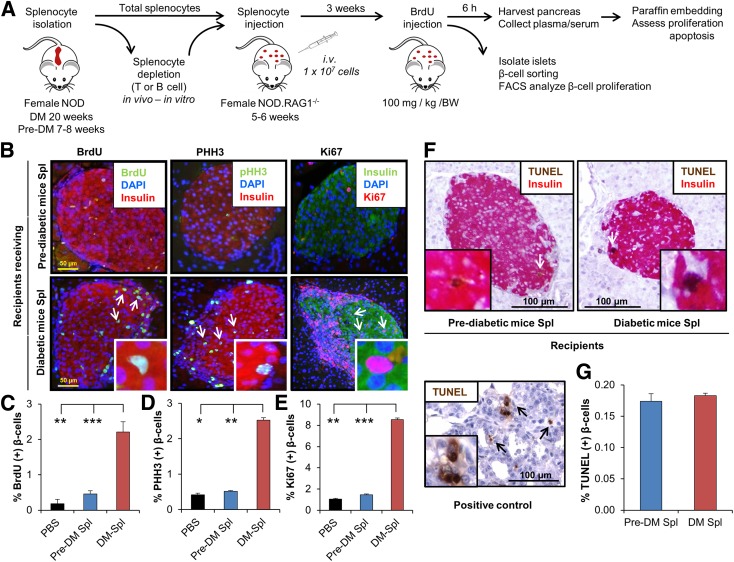
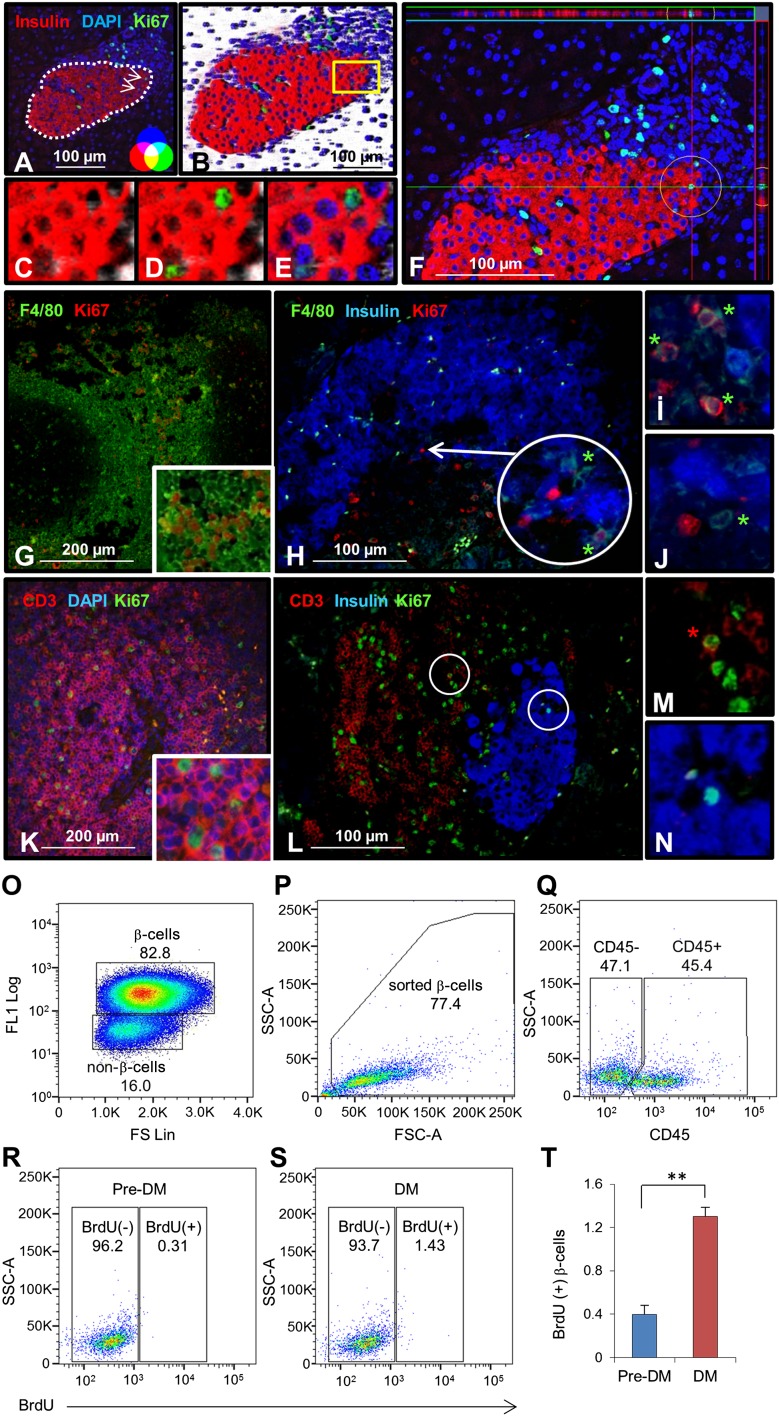
To directly examine whether splenocytes induce proliferation of β-cells, we performed adoptive transfer experiments (6,25). We used 20-week-old hyperglycemic (DM) or 7–8-week-old normoglycemic NOD mice (pre-DM) as splenocyte donors. Since the kinetics of disease transfer are dependent on the age of the mice when the splenocytes are transferred (26) and most islets in prediabetic animals have only peri-insulitis (Supplementary Fig. 1A), we considered pre-DM as the control cohort. To confirm that splenocytes after adoptive transfer target host pancreatic islets, we injected splenocytes derived from hyperglycemic NOD.Raspberry mice congenically marked with mRaspberry fluorescent protein, a far red protein that is generally preferred for in vivo imaging, into female NOD mice. We visualized that marked cells accumulate and infiltrate into host islets (Supplementary Fig. 1B). Intravenous injection of freshly isolated total DM splenocytes or control pre-DM splenocytes into 5–6-week-old immune-deficient NOD.RAG1−/− mice (Fig. 1A) showed a significant increase in β-cell mitosis in the group injected with DM splenocytes compared with controls. To ascertain that we were counting only proliferating β-cells and not overlapping immune cells, we used confocal microscopy z-stack, three-dimensional (3D), and orthographic imaging and double staining for insulin and CD3, a T-cell receptor marker, or F4/80, a common macrophage marker (Fig. 2A–N). A fivefold increase in BrdU incorporation indicated β-cells in the S phase of the cell cycle (Fig. 1B and C), whereas an augmentation in pHH3 immunostaining suggested progression into the G2 or M phases (Fig. 1B and D). The enhanced mitosis was confirmed using Ki67 (Fig. 1B and E). To confirm our immunohistological findings, we performed fluorescence-activated cell sorter analysis of dispersed islets for β-cell sorting according to autofluorescence and size (Fig. 2O and P). We used CD45 staining to gate out immune cells and quantified BrdU+ β-cells (Fig. 2Q). We examined BrdU immunostaining in the CD45+ cell population as an internal control (Supplementary Fig. 1C–F). The increase in proliferating β-cells was consistent with the immunohistological data (Fig. 2R–T). In addition, coimmunostaining for BrdU and insulin or GLUT2 (β-cell membrane marker) confirmed the identity of the cells (Supplementary Fig. 2A and B). TUNEL immunostaining did not reveal significant differences in β-cell apoptosis between groups (Fig. 1F and G). Together these data indicate that injection of splenocytes isolated from DM promotes β-cell replication in vivo.
Figure 1.
Adoptive transfer of diabetes stimulates β-cell proliferation in NOD.RAG1−/− recipients. A: Experimental strategy showing total splenocyte (DM or pre-DM) or depleted splenocyte (diabetic mice) transfer (1 × 107 cells) into NOD.RAG1−/− mice. BrdU (100 mg/kg/BW) was injected 3 weeks post‐transfer, and 6 h later, the pancreases were harvested for immunohistochemical analyses. B: Paraffin-embedded sections of pancreas from mice receiving DM or pre-DM splenocytes, costained with proliferation markers BrdU, pHH3, or Ki67 with insulin and DAPI. Scale bar, 50 µm. Arrows indicate proliferating β-cells (BrdU+/insulin+). Insets show magnified view of representative proliferating β-cells. Quantification of data shown in B for BrdU (C), pHH3 (D), and Ki67 (E) (n = 4–16 mice in each group). *P < 0.05; **P < 0.01; ***P < 0.001 (Student t test). F: TUNEL staining of paraffin-embedded sections of pancreatic tissues obtained from recipient mice receiving DM or pre-DM splenocytes for apoptosis detection. Scale bar, 100 µm. Arrows indicate TUNEL+/β-cell+ cells undergoing apoptosis. Inset shows a magnified representative image of TUNEL+ β-cell. Lower image represents positive control of TUNEL staining in rat tumor tissue. G: Quantification of data in F. n = 4–6 mice in each group. Data are expressed as means ± SEM. FACS, fluorescence-activated cell sorting; Spl, splenocyte.
Figure 2.
Confirmation of proliferating β-cells. Two-dimensional (A) and three-dimensional (B) confocal microscopy view of pancreatic section derived from total diabetic NOD mouse splenocyte–injected animal. Dotted line in A represents the border between islet and immune cells. Magnified area highlighted from B shows three-dimensional imaging of insulin (red) (C), Ki67 (green) (D), and DAPI (blue) (E). Scale bar, 100 µm. Arrows indicate proliferating β-cells. F: Orthographic image of the same pancreatic section in A shows the horizontal and vertical view of a proliferating β-cell in a circle (ZEN-2009). G: Mouse spleen section stained as positive control for F4/80 (green), common macrophage marker, and Ki67 (red). H: Total diabetic splenocyte–injected NOD.RAG1−/− pancreatic section stained for F4/80 (green), insulin (blue), and Ki67 (red). Scale bar, 100 µm. Arrow indicates proliferating β-cells. Magnified view of proliferating (I) and nonproliferating (J) macrophages from H. K: Mouse spleen section stained as positive control for CD3 (red), T-cell marker, and Ki67 (green). L: Total diabetic splenocyte–injected NOD.RAG1−/− pancreatic section stained for CD3 (red), insulin (blue), and Ki67 (green). Scale bar, 100 µm. Magnified view of proliferating T cell (M) and β-cell (N) from L. O: Sorting of β- and non–β-cells from dispersed islets from mice after adoptive transfer of pre-DM or DM splenocytes by flow cytometry based on size (FS Lin) and autofluorescence (FL1). P: Sorted β-cells stained for CD45 and BrdU. Dot plot showing gated-out CD45+ cells (Q) and BrdU+ β-cells from pre-DM (R) or DM (S) splenocyte-transferred mice. T: Quantification of data in R and S. n = 3 each group. *Proliferating macrophages or T cells. **P < 0.01. Experiment was performed in triplicate. Data are expressed as means ± SEM. FSC-A, forward scatter detector A; SSC-A, side scatter detector A.
T Cells, but Not B Cells, Are the Dominant Players in β-Cell Proliferation
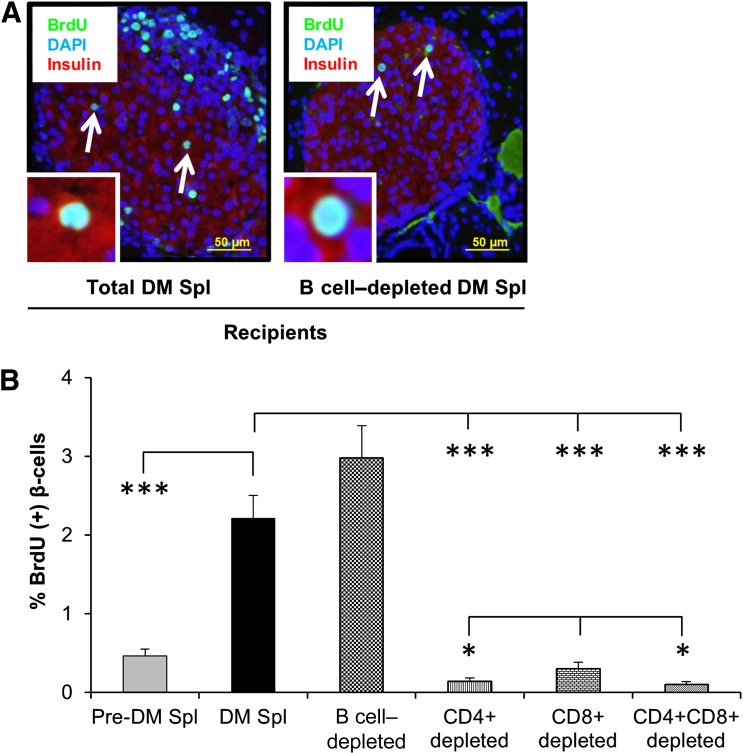
Previous studies have reported that T1D is primarily a T-cell–mediated autoimmune disease (27–29). To evaluate the effect of B cells on β-cell proliferation, we injected total (107) or B-cell–depleted (6.4 × 106) cell populations intravenously into female NOD.RAG1−/− mice (Fig. 1A) followed, 3 weeks later, by harvesting of pancreas, liver, and epididymal fat. Coimmunostaining of BrdU and insulin in pancreas sections from animals receiving total splenocytes from diabetic animals and animals administered B-cell–depleted splenocytes showed no significant difference in β-cell replication (Fig. 3A and B). Furthermore, coimmunostaining of pancreas sections for PDX1 (pancreatic and duodenal homebox-1), a β-cell transcription factor, and BrdU did not reveal differences between groups (Supplementary Fig. 2C). The β-cell specificity of the effects on proliferation was confirmed by a virtual lack of proliferation in hepatocytes or adipocytes (Supplementary Fig. 2D and E). These data demonstrate that B cells are unlikely to contribute to β-cell proliferation in this model.
Figure 3.
T-cell subsets play a major role in β-cell proliferation. A: Paraffin-embedded sections of pancreatic tissues derived from recipient mice receiving total diabetic or B-cell–depleted diabetic NOD mouse splenocytes, costained for the proliferation marker BrdU (green) with insulin (red) and DAPI (blue). Scale bar, 50 µm. Arrows indicate proliferating β-cells (BrdU+/insulin+). Insets show a magnified representative image of a proliferating β-cell. B: Quantification of proliferating β-cells in pancreatic sections obtained from mice receiving total (DM or pre-DM) or B‐cell–, CD4+-, CD8+-, and CD4+/CD8+–double-depleted diabetic NOD splenocytes. n = 4–6 mice each group. *P < 0.05; ***P < 0.001 (Student t test). Data are expressed as means ± SEM. Spl, splenocyte. (A high-quality color representation of this figure is available in the online issue.)
We next evaluated the relative importance of T cells for β-cell proliferation using a similar approach. We considered CD4+ and CD8+ T cells to be likely candidate(s) because they are the major T-cell subsets infiltrating in or around the islets and are the final executors of β-cell destruction (30). In addition to in vitro depletion, we injected NOD.RAG1−/− mice receiving in vitro depleted splenocytes with anti-CD4, anti-CD8, or both mAbs to promote in vivo depletion. The groups receiving the individual CD4+- and CD8+-depleted splenocytes as well as the CD4/CD8–double-depleted splenocytes exhibited dramatically decreased β-cell proliferation compared with the groups injected either with whole splenocytes from diabetic animals or B-cell–depleted splenocytes. Moreover, mice that received CD8+-depleted splenocytes showed a slightly greater β-cell proliferation compared with animals administered CD4+ or double-depleted splenocytes (P < 0.05) (Fig. 3B). Apoptosis tended to be higher in the total DM splenocyte– and B-cell–depleted splenocyte–administered animals but did not reach statistical significance (Supplementary Fig. 5A). These results suggest that CD4+ and CD8+ T cells act together to stimulate β-cell replication in animals injected with diabetogenic splenocytes.
β-Cell Proliferation Is Positively Correlated With Islet Infiltration
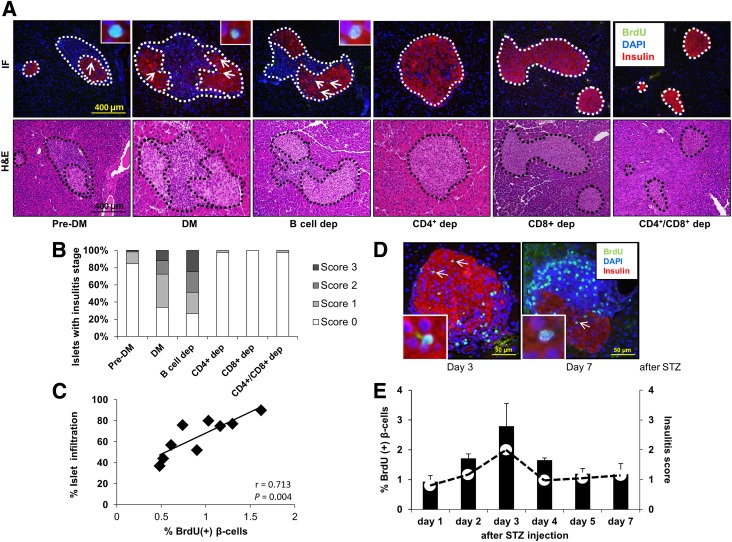
Infiltration in the pancreatic islets with mononuclear inflammatory cells is a key feature in T1D in NOD mice. An interesting observation during the analyses of sections for β-cell proliferation in the different groups discussed above was a striking difference in the percentage of infiltrated cells (Fig. 4A). Scoring for insulitis revealed that whereas the animals receiving total DM splenocytes and B-cell–depleted DM splenocytes contained islets with moderate to severe insulitis, the number of affected islets was significantly reduced in animals receiving pre-DM splenocytes. In contrast, all the groups that received T-cell subtype(s)–depleted splenocytes were virtually free of insulitis, with a few scattered islets exhibiting minimal infiltration (Fig. 4A and B). We observed a linear and significant correlation between the islets manifesting insulitis and β-cell proliferation (r = 0.71; P = 0.004) (Fig. 4C). To confirm this finding, we used an alternative model that promotes infiltration in islets, namely the STZ-induced diabetic NOD mouse. Examination of pancreas sections in mice that receive intraperitoneal injection of a single dose (75 or 100 mg/kg/BW) of STZ again revealed a positive correlation between β-cell proliferation and mononuclear cell infiltration beginning on day 1 and peaking on day 5 or day 3 after injection (Fig. 4D and E). The lack of significant alterations in blood glucose and insulin levels at the peak of the proliferation effect suggested that the proliferation was independent of the effects of glycemia or insulin (Supplementary Fig. 3A and B). The mice that were subjected to the adoptive transfer experiments over the 3-week period after splenocyte injection were also normoglycemic (Supplementary Fig. 3C). The virtual absence of mononuclear immune cell infiltration in liver and adipose confirmed the β-cell specificity (Supplementary Fig. 3D). Together these results suggest that β-cell proliferation occurs soon after immune cell infiltration, prior to the onset of diabetes, and is independent of the effects of glucose and insulin.
Figure 4.
Pancreatic islet infiltration positively correlates with β-cell proliferation. A: Immunofluorescence (IF) and hematoxylin and eosin (H&E) staining of consecutive pancreatic sections harvested from NOD.RAG1−/− mice 3 weeks after receiving total (DM or pre-DM) or B-cell–, CD4+-, CD8+-, and CD4+/CD8+–double-depleted diabetic NOD splenocytes. Scale bar, 400 µm. Pancreatic islets are outlined with dotted lines for ease of comprehension. B: Pancreatic islets showing insulitis expressed as a percentage in the treated groups in A. C: Linear regression of islet infiltration and BrdU+ β-cells in pancreas sections harvested from NOD.RAG1−/− mice transferred with total diabetic splenocytes. Each square represents a mouse (n = 9) scored for insulitis in at least 20 islets (n = 9). r = 0.713; P = 0.004. D: Pancreatic sections harvested from STZ-induced diabetic NOD mice at day 3 and day 7, costained for the proliferation marker BrdU (green), with insulin (red) and DAPI (blue). Scale bar, 50 µm. Arrows indicate proliferating β-cells (BrdU+/insulin+). Insets show a magnified view of a representative proliferating β-cell. E: Quantification of β-cell proliferation (bars) and insulitis scores (red dots) in the pancreatic islets in STZ-injected NOD mice at 1–7 days post-STZ administration (n = 3 mice for each time point). Data are expressed as means ± SEM. dep, depleted.
Soluble Factors Secreted by Lymphocytes Promote β-Cell Proliferation
Our in vivo data indicated that increasing numbers of infiltrating lymphocytes positively correlated with β-cell proliferation. To determine whether this direct effect is observed in vitro, we designed mixed lymphocyte-islet culture experiments to examine whether splenocytes (total or T-cell depleted) from diabetic NOD mice promoted β-cell proliferation. In brief, islets were isolated from NOD.RAG1−/− mice and cultured overnight; in parallel, we isolated aliquots of diabetogenic total or T-cell–depleted splenocytes (Fig. 5A).
Figure 5.
β-cell proliferation is stimulated by infiltrating lymphocytes via soluble factors. A: Experimental strategy showing total splenocytes (DM or pre-DM) or depleted splenocytes (diabetic mice), cocultured with 5–6-week-old NOD.RAG1−/− mouse islets (150 islets/condition) for 48 h in 5 mmol/L glucose in the presence or absence of transwell inserts. B: Linear regression of insulitis score and BrdU+ β-cells in single pancreatic islets harvested from NOD.RAG1−/− mice transferred with total diabetic splenocytes. Each square represents a single islet out of 120 analyzed islets. r = 0.80; P < 0.001. C: Representing pancreatic sections derived from total splenocyte– or STZ-injected mice used for determining the ratio between infiltrating immune cells vs. insulin+ β-cells. Islets are indicated by dotted lines in the right panel. The area of infiltration is shown around the islet in the left panels. β-cell proliferation in agar-embedded NOD.RAG1−/− islets cocultured with total splenocytes from DM or pre-DM at a ratio of 1:1, 1:2, 1:5, or 1:10 or without splenocytes in the presence (D) or absence (E) of transwell inserts (n = 3–4). *P < 0.05; **P < 0.01 (Student t test). F: Quantification of proliferating β-cells in pancreatic islets cocultured with total (DM or pre-DM), negatively, or positively selected DM splenocytes at 1:1 or 1:10 ratio with transwell conditions (n = 3–6 for each condition). *P < 0.05; **P < 0.01; ***P < 0.001 (Student t test). Data are expressed as means ± SEM. ND, not detected; O/N, overnight; Spl, splenocyte.
Prior to coculture of lymphocytes with islets, we hypothesized that a specific ratio of islet cells to lymphocytes is critical to promote proliferation of β-cells and that soluble factors mediate the proliferation. Analyses of β-cell proliferation in single islets showed a positive correlation with lymphocyte infiltration (Fig. 5B) that was similar to the in vivo studies (Fig. 4C). To determine the ratio between infiltrating immune cells versus insulin+ β-cells, pancreas sections from total splenocyte– or STZ-injected mice were examined for both cell types; the infiltrating cells were between 4 and 10 times greater than β-cells in the islets that exhibited proliferation (Fig. 5C). Therefore, we cocultured freshly isolated splenocytes with 150 islets in varying ratios for 48 h followed by embedding the islets in agar for immunohistochemical analyses (Fig. 5A). To address whether the effects are mediated by soluble factors or by direct contact, we cocultured the lymphocytes with the islets either in the presence or absence of microporous transwell inserts, which prevent direct splenocyte-islet contact while allowing soluble factors to diffuse across (Fig. 5D). We first assessed the capacity of total DM and pre-DM splenocytes to stimulate β-cell proliferation at an increasing islet cell to splenocyte ratio (1:1, 1:2, 1:5, and 1:10). Forty-eight hours after coculture, we observed that total splenocytes from diabetic mice, at a ratio of 1:5 and 1:10, significantly induced β-cell proliferation in a dose-dependent manner compared with islets cocultured with pre-DM splenocytes in transwell conditions, suggesting a role for soluble factors secreted from lymphocytes isolated from DM in β-cell proliferation (Fig. 5D). On the other hand, nontranswell conditions that permitted cell-cell contact also revealed an effect on β-cell proliferation that was slightly higher compared with the transwell studies likely due to the increased cell-cell contact (Fig. 5E).
Next, we performed a second set of coculture experiments to examine the effects of selected T-cell subtype(s) on β-cell proliferation in vitro (Fig. 5F). A 1:1 and 1:10 islet cell to splenocyte ratio in the transwell system revealed that islets cocultured with total DM splenocytes promoted a 3–10-fold higher β-cell proliferation at the 1:10 ratio compared with pre-DM splenocytes or splenocytes that are depleted for T-cell subtype(s). The proliferation was either very low or undetectable (ND) at the 1:1 ratio. Among the groups treated with splenocytes that are depleted of the T-cell subtype(s), the CD8+-depleted group revealed statistically significant higher β-cell proliferation compared with CD4+ only or CD4+/CD8+–double-depleted (P < 0.05) groups. Moreover, in positive selection experiments, islets cultured with only CD4+ cells (1:10 ratio of islet cells to CD4+ cells) showed higher (P = 0.07) β-cell proliferation compared with CD8+-only cocultured groups (Fig. 5F) and was consistent with our negative selection experiments exhibiting low proliferation in the CD4+-depleted group. Evaluation of β-cell death by TUNEL assay did not reveal significant differences between groups (Supplementary Fig. 5B). These results support our in vivo findings that CD4+ and CD8+ T cells act together and that CD4+ T cells are likely more effective in stimulating β-cell proliferation by secreting soluble factors independent of cell-cell contact.
Effects of Cytokines on β-Cell Proliferation
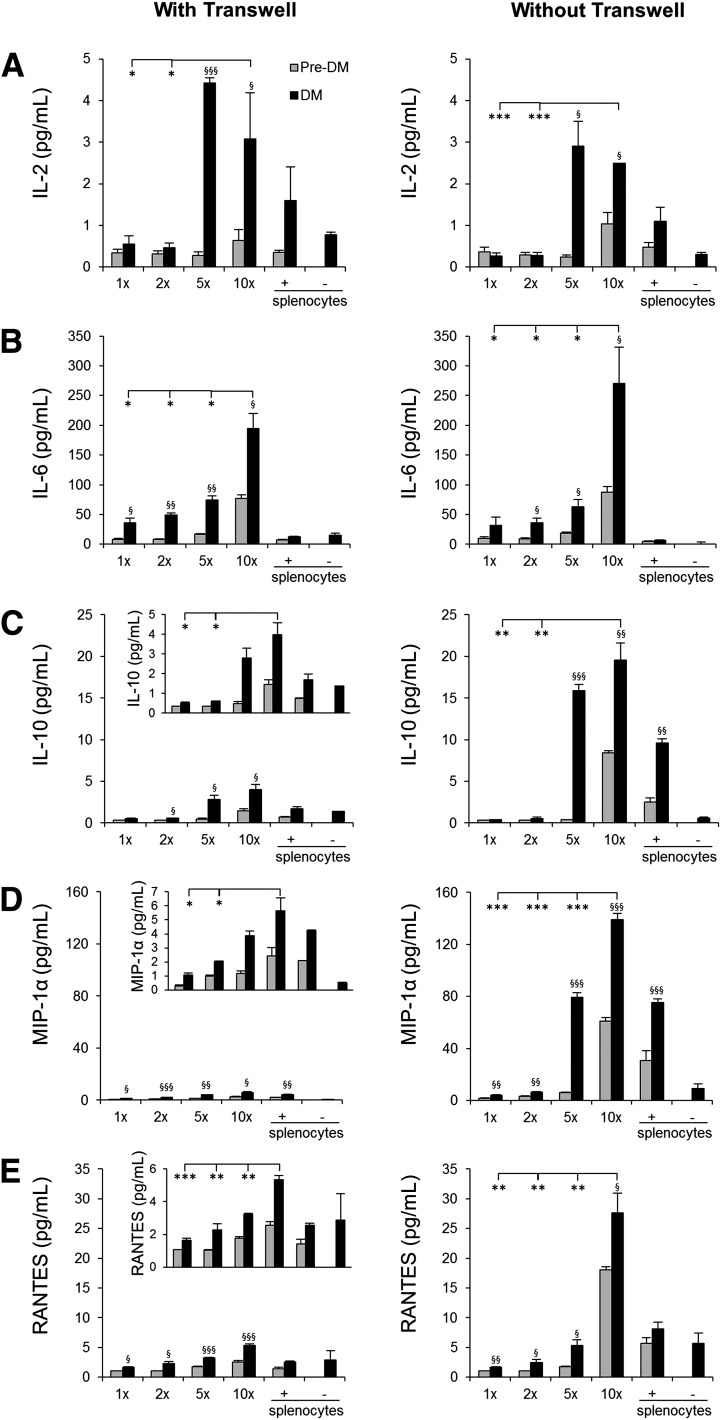
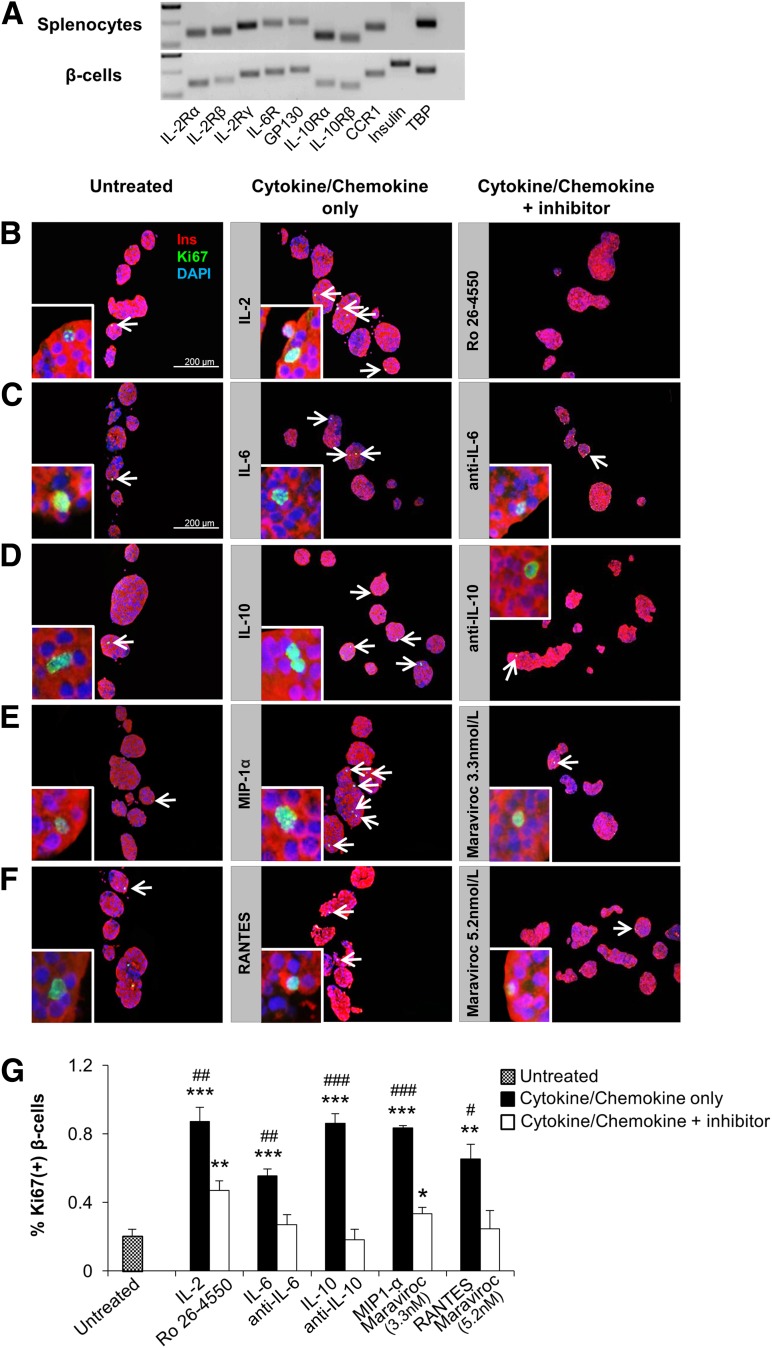
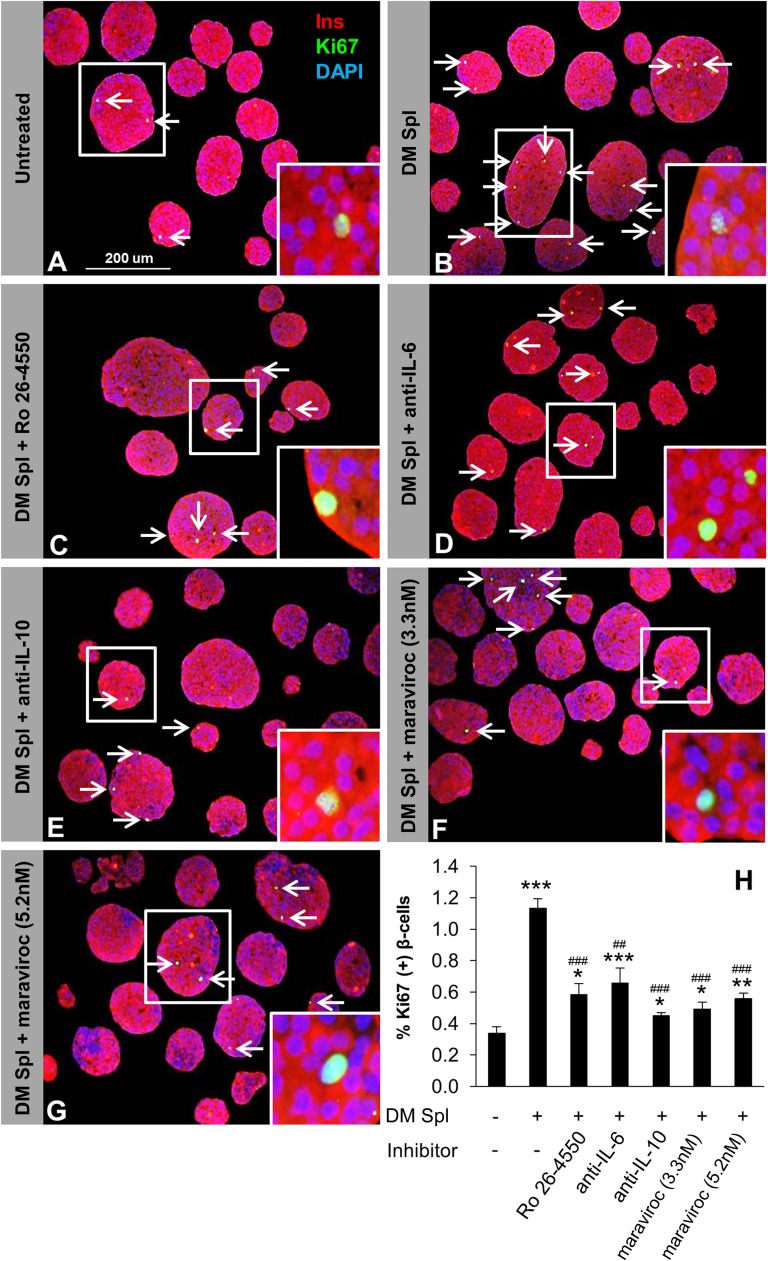
To identify the soluble factor(s) that drive β-cell proliferation in pancreatic islets, we analyzed media in the coculture experiments to detect potential cytokines/chemokines/growth factors released by the splenocytes. We ranked the cytokines/chemokines from 1 to 4 according to their significant differences between DM and pre-DM splenocyte treatments in the two transwell conditions (Supplementary Table 1). Among them, group 1 cytokines/chemokines included candidate molecules (IL-2, IL-6, IL-10, MIP-1α, and RANTES) that showed a dose-dependent higher concentration in the group treated with splenocytes from DM compared with mice treated with splenocytes from pre-DM in both coculture conditions (Fig. 6A–E). We ruled out IP-10 as a candidate since it is not expressed on lymphocytes, is a known chemoattractant for immune cells, and has been shown to be produced by the β-cells. The candidate molecules in the other groups (groups 2–4) were not significantly increased between DM and pre-DM splenocyte–treated mice at least in one or both coculture conditions and were therefore not studied in detail. Whereas some of the candidates (IL-2 and IL-6) were increased in both “with” and “without” transwell conditions, others (IL-10, MIP-1α, and RANTES) were higher in the “without” transwell condition probably due to direct cell-cell contact, which potentially allows immune cells to respond rapidly via multiple pathways. In support of a potential proliferative role for each of the five cytokines/chemokines on β-cells, we first confirmed expression of their receptors on sorted β-cells. (Fig. 7A). Second, we investigated the effects of the individual cytokines/chemokines on pancreatic islets isolated from B6 mice in the presence or absence of specific inhibitors and/or neutralizing antibodies over a range of concentrations (18,31,32). Low doses of IL-6 (200 pg/mL) strongly induced β-cell proliferation and increased up to 10-fold at the higher dose (200 ng/mL) (P < 0.05) (Supplementary Fig. 4B). Moreover, IL-2, IL-10, and MIP-1α demonstrated significantly higher proliferation even at low levels, with eight-, four-, and threefold increases, respectively (P < 0.05) (Supplementary Fig. 4A, C, and D). On the other hand, treatment with RANTES resulted in β-cell proliferation at lower doses (5 and 500 pg/mL), but the effect was surprisingly reduced at 50 ng/mL (Supplementary Fig. 4E). To determine specificity, we examined the effects of specific inhibitors or neutralizing antibodies in islets treated with low-dose cytokines/chemokines. In all cases, we observed neutralization of the proliferative effects of the respective cytokine/chemokine (Fig. 7B–F). In some cases (IL-2 inhibitor and maraviroc 3.3 nmol/L), the neutralization was not complete and is likely due to variable IC50s of the compounds (Fig. 7G). Cytokines and/or chemokines are known to be secreted by macrophages or dendritic cells to impact T-cell function, and, conversely, secretions from T cells can also impact macrophages (27,33). To examine whether the cytokines/chemokines act synergistically to enhance β-cell proliferation, we compared the individual effects versus a combination (Supplementary Fig. 4F). In addition to their significant individual effects on proliferation, a combination of the cytokines/chemokines, at doses used in the individual treatments, showed a significant increase (P < 0.001) but was not dramatically different from the individual effects likely because some of the cytokines share common downstream pathways to stimulate proliferation. Finally, to confirm the recombinant protein treatment findings, we undertook an independent experiment wherein total DM splenocytes served as the source of cytokines/chemokines. Islet cells and splenocytes were cultured in a 1:10 ratio in a transwell system in the presence or absence of inhibitory/neutralizing antibodies against the candidate factors. Whereas splenocytes alone significantly increased β-cell proliferation compared with untreated islets (Fig. 8A and B), adding the inhibitory/neutralizing molecule reversed this effect. Consistent with our previous observations, these data suggested that each candidate has a potential to induce β-cell regeneration (Fig. 8C–H). In summary, cytokines/chemokines that are secreted from lymphocytes in close proximity to islet cells promote detectable β-cell proliferation.
Figure 6.
Lymphocyte-secreted soluble factors that drive β-cell proliferation. Luminex assay results from culture medium obtained 48 h after coculturing NOD.RAG1−/− islets with NOD splenocytes (DM or pre-DM) at a ratio of 1:1, 1:2, 1:5, or 1:10 or only splenocytes at 10× in the presence or absence of transwell for IL-2 (A), IL-6 (B), IL-10 (C), MIP-1α (D), and RANTES (E) (n = 3–4 for each condition). *, §P < 0.05; **, §§P < 0.01; ***, §§§P < 0.001 (Student t test). *, diabetic vs. diabetic; §, diabetic vs. prediabetic. Data are expressed as means ± SEM.
Figure 7.
Effect of soluble factors on β-cell proliferation is reversed by inhibitory/neutralizing antibody treatment in vitro. A: Detection of the cytokine/chemokine receptor subunit mRNAs by real-time PCR from sorted β-cells and splenocytes harvested from C57BL/6 mice. Tata-box-binding protein (TBP) was used as reference. B–F: Agar-embedded pancreatic islets from C57BL/BJ mouse treated in the absence (control) or presence of low-dose recombinant proteins with or without inhibitory/neutralizing molecules (as described in research design and methods) for 48 h (150 islets/condition, three to four replicates). Representative sections are shown. Islets were costained for the proliferation marker Ki67 (green) with insulin (red) and DAPI (blue). Arrows indicate proliferating β-cells (Ki67+/insulin+). Scale bar, 200 µm. Insets show a magnified image of a representative proliferating β-cell. G: Quantification of data in B–F (n = 3–4 in each group). *, #P < 0.05; **, ##P < 0.01; ***, ###P < 0.001 (Student t test). *, untreated vs. cytokine/chemokine or inhibitory/neutralizing antibody treated; #, cytokine/chemokine treated vs. inhibitory/neutralizing antibody treated. Data are expressed as means ± SEM. CCR1, C-C chemokine receptor type 1; GP130, glycoprotein 130; IL-2R, IL-2 receptor.
Figure 8.
Lymphocyte-secreted soluble factors enhance β-cell proliferation in vitro. One hundred fifty agar-embedded islets harvested from C57BL/6 mice cocultured without (untreated control) (A) or with total DM splenocytes in the absence (treated control) (B) or presence of inhibitory/neutralizing molecule Ro 26-4550 (C), anti–IL-6 (D), anti–IL-10 (E), maraviroc 3.3 nmol/L (F), or maraviroc 5.2 nmol/L (G). Islets were costained for the proliferation marker Ki67 (green), insulin (red), and DAPI (blue). Arrows indicate proliferating β-cells (Ki67+/insulin+). Scale bar, 200 µm. Insets show magnified image of a representative proliferating β-cell. H: Quantification of proliferating β-cells in A–G (n = 3–4 in each group). *P < 0.05; **, ##P < 0.01; ***, ###P < 0.001 (Student t test). *, untreated vs. DM splenocyte treated; #, DM splenocyte treated vs. inhibitory/neutralizing antibody treated. Data are expressed as means ± SEM. Ins, insulin; Spl, splenocyte.
Discussion
Although immune cells have been implicated in the proliferation of β-cells during the progression of T1D (4,6), the cell type(s) and mechanisms that control β-cell regeneration remain unknown. Here we report that soluble factors secreted from CD4+ and CD8+ T cells directly stimulate β-cell proliferation.
NOD.RAG1−/− mice injected with DM splenocytes exhibited a significant elevation in all markers of β-cell proliferation compared with controls. Adoptive transfer of diabetes to immunocompromised syngenic recipients can be achieved only when a combination of splenic CD4+ and CD8+ T cells from donor NOD mice is used and not by either T-cell subsets alone (34). Injecting CD4+-, CD8+-, or CD4+/CD8+–double-depleted splenocytes into NOD.RAG1−/− animals resulted in a dramatic decrease in β-cell proliferation compared with the animals that received total DM splenocytes. In addition, we also observed that animals with depleted T-cell subtype(s) exhibited minimal pancreatic islet infiltration compared with animals receiving total splenocytes or B-cell–depleted splenocytes. Considering that the development of T1D requires the presence of both CD4+ and CD8+ T cells, depleting one or both of these cells would impact the inflammatory response and alter β-cell replication. This possibility was supported by a linear and significant correlation between β-cell proliferation and immune cell infiltration in our studies. Among the T-cell subtypes, both CD8+ T-cell–depleted splenocyte injection (in vivo) and islet coculture (in vitro) studies demonstrated higher proliferation compared with CD4+-depleted or CD4+/CD8+–double-depleted cohorts. Similar results were observed when islets were cultured with CD4+ T-cell subset alone, signifying their role in β-cell proliferation. We did not detect a significant difference in β-cell apoptosis between the groups because the duration after injection of splenocytes is not sufficient to promote significant apoptosis and/or because it is often difficult to detect dead β-cells due to their rapid engulfment and disposal (35).
Although our studies point to CD4+ and CD8+ T cells as critical for β-cell proliferation during T1D progression, we cannot exclude the potential contribution of macrophages or dendritic cells and their secreted products (36). Our data suggest that B cells are unlikely to play a role in β-cell replication. Depletion of macrophages prevents T1D development (37), and macrophages have been reported to impact β-cells by producing proinflammatory cytokines (38). One possible role for macrophages in β-cell proliferation is that T-cell subtypes, especially CD4+, recruit additional CD4+, CD8+, or granulocytes into the infiltrate and contribute to local secretion of cytokines and chemokines. Indeed, T cells secrete soluble factors such as granulocyte macrophage–colony-stimulating factor (39) to influence leukocytes and recruit them to inflammatory sites during inflammation (40,41). Thus, in addition to their direct effect on β-cell proliferation by cytokine secretion, it is possible that T cells act indirectly by triggering mononuclear cells to secrete soluble factors, via the classical or nonclassical pathways, that can in turn enhance β-cell proliferation.
Although earlier studies implicate a T-cell–dependent proliferative effect on aortic smooth muscle (42) and orbital fibroblasts (43), the mechanism(s) remains unclear. Careful analyses of our data from the transwell experiments indicate that CD4+ and CD8+ lymphocytes secrete IL-2, IL-6, IL-10, MIP-1α, and RANTES, each of which showed a dose-dependent effect on β-cell proliferation. Some of these factors have been associated with proliferation of other cell types. For example, IL-2 regulates the growth and function of T cells (44), and IL-6 stimulates α- and β-cell proliferation in vitro (18) and is known to reinforce the effects of IL-2 in promoting the differentiation of CD4+ cells into type 2 T-helper cells (45). RANTES acts with IL-2 to induce the proliferation and activation of NK cells to form chemokine-activated killer cells (46). Since β-cells themselves have been reported to produce inflammatory cytokines such as IL-1β (47), we do not exclude the β-cell as a source of some of these molecules. Cytokines, such as IL-1β and interferon-γ, when used in combination, can induce de-differentiation of newly generated β-cells mediated by re-expression of the Notch-Delta pathway (48). Whether the soluble factors detected in our experiments are also involved in similar pathways to modulate β-cell mass warrants further investigation.
Although the capacity to proliferate is strikingly different between rodents and humans, the observations that β-cells can regenerate in humans have prompted studies to investigate safe approaches to enhance their functional mass. Whether the candidate molecules identified in our study can be used either individually or in combination with an appropriate immunosuppressive regimen to preserve β-cell mass requires further research. In the context of cytokines, IL-6 has been reported to stimulate human islet proliferation (18). Although attempts at expansion ex vivo resulted in a change in the β-cell phenotype, lineage-tracing studies suggest that de-differentiated human β-cells are able to survive and replicate in vitro (49). Thus, testing the candidates we have identified in ex vivo conditions can be a first step to evaluate their ability to expand human β-cells. However, a role for these molecules as “therapeutic” agents has to be viewed with caution due to their well-established roles in the immune network. For example, rapamycin, an immunosuppressant drug used to protect rejection in organ transplantations, inhibits lymphocyte proliferation by inhibiting their response to IL-2 (50). Despite IL-2 being important in lymphocyte activation, it also contributes to the development and expansion of CD4+ CD25+ regulatory T cells, which promote self-tolerance by suppressing T-cell responses in vivo. Thus, extensive and careful dosing studies are necessary to examine the potential of the candidate cytokines/chemokines for β-cell expansion.
We propose that some of the pro- and anti-inflammatory cytokines/chemokines secreted in the islet microenvironment during insulitis have the potential to promote proliferation by activating diverse signaling cascades (e.g., JAK/STAT, mitogen-activated protein kinase, or phosphatidylinositol 3-kinase/AKT). This potential beneficial effect triggers the islets to secrete chemoattractant molecules (e.g., eotaxin [CCL11], IP-10 [CXCL-10], and MCP-1 [CCL2]), which in turn amplify the recruitment of mononuclear cells and the release of multiple cytokines/chemokines. The detection of increased levels of chemotactic molecules in our experiments, especially IP-10 and eotaxin, likely amplifies the number of immune cells that secrete soluble factors in the inflamed area to further promote β-cell proliferation and prevent progression of diabetes.
In summary, we report that CD4+ and CD8+ T cells secrete soluble factors that promote β-cell replication in the NOD model of T1D. Therapeutic targeting of one or a combination of these soluble factors may prove useful to delay and/or counter the progression of T1D by enhancing functional β-cell mass.
Article Information
Acknowledgments. The authors thank G.C. Weir (Joslin Diabetes Center and Harvard Medical School) and F. Urano (Washington University School of Medicine, St. Louis, MO) for discussions and advice, O. Lovegreen (Joslin Diabetes Center) for editorial assistance, and the Specialized Assay Core (Joslin Diabetes Center) for hormone and metabolite analyses.
Funding. This work was supported by a Juvenile Diabetes Research Foundation Fellowship (3-2010-474) and Turkish Diabetes, Obesity and Nutrition Association Novartis Science Award to E.D. Some of the reagents were supported by NIH Grant RO1-DK-67536 to R.N.K., and the Joslin Diabetes Center Flow Cytometry Cores were supported by Diabetes Research Center Grant NIH P30-DK-036836 and NIH PO1-AI-054904 to D.M.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. E.D. conceived the idea, designed the experiments, performed all experiments, analyzed the data, and wrote the manuscript. S.K. contributed to animal maintenance and assisted in islet isolation. W.J. assisted in the adoptive transfer experiments. A.E.O. assisted in islet isolation. D.F.D.J. contributed to animal maintenance. A.K.K.T. assisted in the real-time PCR experiments. J.H. and D.K. assisted in the immunohistochemical experiments. J.L.G. contributed to the flow cytometry analysis and NOD.Raspberry studies. D.M. contributed to designing the experiments, troubleshooting, and the NOD.Raspberry studies. R.N.K. conceived the idea, designed the experiments, supervised the project, and wrote the manuscript. R.N.K. and E.D. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. This study was presented at the 73rd Scientific Sessions of the American Diabetes Association, Chicago, Illinois, 21–25 June 2013.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db13-0204/-/DC1.
References
- 1.Mathis D, Vence L, Benoist C. beta-Cell death during progression to diabetes. Nature 2001;414:792–798 [DOI] [PubMed] [Google Scholar]
- 2.Redondo MJ, Fain PR, Eisenbarth GS. Genetics of type 1A diabetes. Recent Prog Horm Res 2001;56:69–89 [DOI] [PubMed] [Google Scholar]
- 3.Turley S, Poirot L, Hattori M, Benoist C, Mathis D. Physiological beta cell death triggers priming of self-reactive T cells by dendritic cells in a type-1 diabetes model. J Exp Med 2003;198:1527–1537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sreenan S, Pick AJ, Levisetti M, Baldwin AC, Pugh W, Polonsky KS. Increased beta-cell proliferation and reduced mass before diabetes onset in the nonobese diabetic mouse. Diabetes 1999;48:989–996 [DOI] [PubMed] [Google Scholar]
- 5.von Herrath MG, Wolfe T, Möhrle U, Coon B, Hughes A. Protection from type 1 diabetes in the face of high levels of activated autoaggressive lymphocytes in a viral transgenic mouse model crossed to the SV129 strain. Diabetes 2001;50:2700–2708 [DOI] [PubMed] [Google Scholar]
- 6.Sherry NA, Kushner JA, Glandt M, Kitamura T, Brillantes AM, Herold KC. Effects of autoimmunity and immune therapy on beta-cell turnover in type 1 diabetes. Diabetes 2006;55:3238–3245 [DOI] [PubMed] [Google Scholar]
- 7.Nir T, Melton DA, Dor Y. Recovery from diabetes in mice by beta cell regeneration. J Clin Invest 2007;117:2553–2561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keenan HA, Sun JK, Levine J, et al. Residual insulin production and pancreatic β-cell turnover after 50 years of diabetes: Joslin Medalist Study. Diabetes 2010;59:2846–2853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meier JJ, Bhushan A, Butler AE, Rizza RA, Butler PC. Sustained beta cell apoptosis in patients with long-standing type 1 diabetes: indirect evidence for islet regeneration? Diabetologia 2005;48:2221–2228 [DOI] [PubMed] [Google Scholar]
- 10.Lee YC, Nielsen JH. Regulation of beta cell replication. Mol Cell Endocrinol 2009;297:18–27 [DOI] [PubMed] [Google Scholar]
- 11.Meier JJ, Butler AE, Saisho Y, et al. Beta-cell replication is the primary mechanism subserving the postnatal expansion of beta-cell mass in humans. Diabetes 2008;57:1584–1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finegood DT, Scaglia L, Bonner-Weir S. Dynamics of beta-cell mass in the growing rat pancreas. Estimation with a simple mathematical model. Diabetes 1995;44:249–256 [DOI] [PubMed] [Google Scholar]
- 13.Karnik SK, Chen H, McLean GW, et al. Menin controls growth of pancreatic beta-cells in pregnant mice and promotes gestational diabetes mellitus. Science 2007;318:806–809 [DOI] [PubMed] [Google Scholar]
- 14.Michael MD, Kulkarni RN, Postic C, et al. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol Cell 2000;6:87–97 [PubMed] [Google Scholar]
- 15.Peshavaria M, Larmie BL, Lausier J, et al. Regulation of pancreatic beta-cell regeneration in the normoglycemic 60% partial-pancreatectomy mouse. Diabetes 2006;55:3289–3298 [DOI] [PubMed] [Google Scholar]
- 16.Dirice E, Kulkarni RN. Pathways underlying β-cell regeneration in type 1, type 2 and gestational diabetes. In Islet Cell Growth Factors. Kulkarni RN, Ed. Texas, Landies Bioscience, 2011, p. 1–15 [Google Scholar]
- 17.Maedler K, Schumann DM, Sauter N, et al. Low concentration of interleukin-1beta induces FLICE-inhibitory protein-mediated beta-cell proliferation in human pancreatic islets. Diabetes 2006;55:2713–2722 [DOI] [PubMed] [Google Scholar]
- 18.Ellingsgaard H, Ehses JA, Hammar EB, et al. Interleukin-6 regulates pancreatic alpha-cell mass expansion. Proc Natl Acad Sci USA 2008;105:13163–13168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cameron MJ, Strathdee CA, Holmes KD, Arreaza GA, Dekaban GA, Delovitch TL. Biolistic-mediated interleukin 4 gene transfer prevents the onset of type 1 diabetes. Hum Gene Ther 2000;11:1647–1656 [DOI] [PubMed] [Google Scholar]
- 20.Pennline KJ, Roque-Gaffney E, Monahan M. Recombinant human IL-10 prevents the onset of diabetes in the nonobese diabetic mouse. Clin Immunol Immunopathol 1994;71:169–175 [DOI] [PubMed] [Google Scholar]
- 21.Flodström-Tullberg M, Yadav D, Hägerkvist R, et al. Target cell expression of suppressor of cytokine signaling-1 prevents diabetes in the NOD mouse. Diabetes 2003;52:2696–2700 [DOI] [PubMed] [Google Scholar]
- 22.Kulkarni RN, Winnay JN, Daniels M, et al. Altered function of insulin receptor substrate-1-deficient mouse islets and cultured beta-cell lines. J Clin Invest 1999;104:R69–R75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haxhinasto S, Mathis D, Benoist C. The AKT-mTOR axis regulates de novo differentiation of CD4+Foxp3+ cells. J Exp Med 2008;205:565–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.King AJ, Fernandes JR, Hollister-Lock J, Nienaber CE, Bonner-Weir S, Weir GC. Normal relationship of beta- and non-beta-cells not needed for successful islet transplantation. Diabetes 2007;56:2312–2318 [DOI] [PubMed] [Google Scholar]
- 25.Gonzalez A, Andre-Schmutz I, Carnaud C, Mathis D, Benoist C. Damage control, rather than unresponsiveness, effected by protective DX5+ T cells in autoimmune diabetes. Nat Immunol 2001;2:1117–1125 [DOI] [PubMed] [Google Scholar]
- 26.Fuchtenbusch M, Larger E, Thebault K, Boitard C: Transfer of diabetes from prediabetic NOD mice to NOD-SCID/SCID mice: association with pancreatic insulin content. Horm Metab Res 2005;37:63–67 [DOI] [PubMed]
- 27.Lehuen A, Diana J, Zaccone P, Cooke A. Immune cell crosstalk in type 1 diabetes. Nat Rev Immunol 2010;10:501–513 [DOI] [PubMed] [Google Scholar]
- 28.Adorini L, Gregori S, Harrison LC. Understanding autoimmune diabetes: insights from mouse models. Trends Mol Med 2002;8:31–38 [DOI] [PubMed] [Google Scholar]
- 29.Bluestone JA, Herold K, Eisenbarth G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature 2010;464:1293–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Belle TL, Coppieters KT, von Herrath MG. Type 1 diabetes: etiology, immunology, and therapeutic strategies. Physiol Rev 2011;91:79–118 [DOI] [PubMed] [Google Scholar]
- 31.Crawley JB, Rawlinson L, Lali FV, Page TH, Saklatvala J, Foxwell BM. T cell proliferation in response to interleukins 2 and 7 requires p38MAP kinase activation. J Biol Chem 1997;272:15023–15027 [DOI] [PubMed] [Google Scholar]
- 32.Hanifi-Moghaddam P, Kappler S, Seissler J, et al. Altered chemokine levels in individuals at risk of type 1 diabetes mellitus. Diabet Med 2006;23:156–163 [DOI] [PubMed]
- 33.Eizirik DL, Colli ML, Ortis F. The role of inflammation in insulitis and beta-cell loss in type 1 diabetes. Nat Rev Endocrinol 2009;5:219–226 [DOI] [PubMed] [Google Scholar]
- 34.Phillips JM, Parish NM, Raine T, et al. Type 1 diabetes development requires both CD4+ and CD8+ T cells and can be reversed by non-depleting antibodies targeting both T cell populations. Rev Diabet Stud 2009;6:97–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scaglia L, Cahill CJ, Finegood DT, Bonner-Weir S. Apoptosis participates in the remodeling of the endocrine pancreas in the neonatal rat. Endocrinology 1997;138:1736–1741 [DOI] [PubMed] [Google Scholar]
- 36.Hu CY, Rodriguez-Pinto D, Du W, et al. Treatment with CD20-specific antibody prevents and reverses autoimmune diabetes in mice. J Clin Invest 2007;117:3857–3867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jun HS, Yoon CS, Zbytnuik L, van Rooijen N, Yoon JW. The role of macrophages in T cell-mediated autoimmune diabetes in nonobese diabetic mice. J Exp Med 1999;189:347–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dahlén E, Dawe K, Ohlsson L, Hedlund G. Dendritic cells and macrophages are the first and major producers of TNF-alpha in pancreatic islets in the nonobese diabetic mouse. J Immunol 1998;160:3585–3593 [PubMed] [Google Scholar]
- 39.Khajah M, Millen B, Cara DC, Waterhouse C, McCafferty DM. Granulocyte-macrophage colony-stimulating factor (GM-CSF): a chemoattractive agent for murine leukocytes in vivo. J Leukoc Biol 2011;89:945–953 [DOI] [PubMed] [Google Scholar]
- 40.Codarri L, Gyülvészi G, Tosevski V, et al. RORγt drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat Immunol 2011;12:560–567 [DOI] [PubMed] [Google Scholar]
- 41.Huffnagle GB, Lipscomb MF, Lovchik JA, Hoag KA, Street NE. The role of CD4+ and CD8+ T cells in the protective inflammatory response to a pulmonary cryptococcal infection. J Leukoc Biol 1994;55:35–42 [DOI] [PubMed] [Google Scholar]
- 42.Rolfe BE, Campbell JH, Smith NJ, Cheong MW, Campbell GR. T lymphocytes affect smooth muscle cell phenotype and proliferation. Arterioscler Thromb Vasc Biol 1995;15:1204–1210 [DOI] [PubMed] [Google Scholar]
- 43.Feldon SE, Park DJ, O’Loughlin CW, et al. Autologous T-lymphocytes stimulate proliferation of orbital fibroblasts derived from patients with Graves’ ophthalmopathy. Invest Ophthalmol Vis Sci 2005;46:3913–3921 [DOI] [PubMed] [Google Scholar]
- 44.Liou HC, Smith KA. The roles of c-rel and interleukin-2 in tolerance: a molecular explanation of self-nonself discrimination. Immunol Cell Biol 2011;89:27–32 [DOI] [PubMed] [Google Scholar]
- 45.Diehl S, Rincón M. The two faces of IL-6 on Th1/Th2 differentiation. Mol Immunol 2002;39:531–536 [DOI] [PubMed] [Google Scholar]
- 46.Maghazachi AA, Al-Aoukaty A, Schall TJ. CC chemokines induce the generation of killer cells from CD56+ cells. Eur J Immunol 1996;26:315–319 [DOI] [PubMed] [Google Scholar]
- 47.Maedler K, Sergeev P, Ris F, et al. Glucose-induced beta cell production of IL-1beta contributes to glucotoxicity in human pancreatic islets. J Clin Invest 2002;110:851–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Darville MI, Eizirik DL. Notch signaling: a mediator of beta-cell de-differentiation in diabetes? Biochem Biophys Res Commun 2006;339:1063–1068 [DOI] [PubMed] [Google Scholar]
- 49.Russ HA, Bar Y, Ravassard P, Efrat S. In vitro proliferation of cells derived from adult human beta-cells revealed by cell-lineage tracing. Diabetes 2008;57:1575–1583 [DOI] [PubMed] [Google Scholar]
- 50.Kay JE, Kromwel L, Doe SE, Denyer M. Inhibition of T and B lymphocyte proliferation by rapamycin. Immunology 1991;72:544–549 [PMC free article] [PubMed] [Google Scholar]