Abstract
Several reviews and meta-analyses have demonstrated the incontrovertible benefits of statin therapy in patients with cardiovascular disease (CVD). But the role for statins in primary prevention remained unclear. The updated 2013 Cochrane review has put to rest all lingering doubts about the overwhelming benefits of long-term statin therapy in primary prevention by conclusively demonstrating highly significant reductions in all-cause mortality, major adverse cardiovascular events (MACE) and the need for coronary artery revascularization procedures (CARPs). More importantly, these benefits of statin therapy are similar at all levels of CVD risk, including subjects at low (<1% per year) risk of a MACE. In addition to preventing myocardial infarction (MI), stroke, and death, primary prevention with statins is also highly effective in delaying and avoiding expensive CARPs such as angioplasties, stents, and bypass surgeries. There is no evidence of any serious harm or threat to life caused by statin therapy, though several adverse effects that affect the quality of life, especially diabetes mellitus (DM) have been reported. Asian Indians have the highest risk of premature coronary artery disease (CAD) and diabetes. When compared with Whites, Asian Indians have double the risk of CAD and triple the risk of DM, when adjusted for traditional risk factors for these diseases. Available evidence supports the use of statin therapy for primary prevention in Asian Indians at a younger age and with lower targets for low-density lipoprotein cholesterol (LDL-C) and non-high density lipoprotein (non-HDL-C), than those currently recommended for Americans and Europeans. Early and aggressive statin therapy offers the greatest potential for reducing the continuing epidemic of CAD among Indians.
Keywords: Asian Indians, coronary artery disease, diabetes, myopathy, primary prevention, statin therapy
Introduction
Numerous large double blind randomized clinical trials (RCTs) with statins demonstrate significant benefit on cardiovascular disease (CVD) outcomes without any notable increase in non-CVD mortality1,2. Statins have become the first-line therapy for reducing the risk of CVD mortality and morbidity as well as the need for coronary artery revascularization procedures (CARP) in people who have suboptimal lipid profile, with or without other risk factors2,3. Statins inhibit the enzyme 3-hydroxy 3-methyglutaryl coenzyme A reductase (HMG-CoA reductase), which catalyzes the rate limiting step in cholesterol biosynthesis. The dose and potency of the statin used and individual responsiveness determine the magnitude of low density lipoprotein cholesterol (LDL-C) reduction and CVD benefit2. However, there is increasing concern about the reported increased risk of diabetes mellitus (DM) as well as muscle, liver, and kidney toxicity associated with statin therapy. The objective of this review is to examine the profound differences in magnitude of benefits and risks of statin therapy in primary prevention of CVD and the rationale for its expansive use at a younger age, especially in Asian Indians.
Global burden of CVD
Cardiovascular disease (CVD) is the number one cause of mortality, accounting for 30 per cent of all deaths worldwide. Annually, it causes 17 million deaths, including 7.6 million due to myocardial infarction (MI) and 5.7 million due to stroke with a cost of $863 billion1. Over 80 per cent of CVD deaths occur in low-and middle-income countries1. In developing countries, it causes twice as many deaths as HIV, malaria, and tuberculosis combined2,4,5. CVD is also a major contributor to morbidity, reduced economic activity and accounts for a large share of health service resources. India has a disproportionately higher burden of CAD than most developing countries. According to the official government projections, India will have 2.9 million CAD deaths in 2015, of which 1.16 million (40%) will be in people <45 yr of age5. In comparison, CAD claims only 400,000 lives annually in the United States with only one per cent of these deaths in Whites and 4 per cent among Blacks occurring in people <45 yr of age6,7.
Central role of total, LDL, and non-HDL cholesterol in atherosclerotic CVD
Atherosclerosis accounts for the vast majority of CVD and elevated serum cholesterol is the foremost risk factor for both8. The average cholesterol level within a population is the most important determinant of the CVD risk of the population. The differences in average cholesterol levels between populations are determined largely by differences in diet9. Populations with higher dietary intake of saturated fat and lower intake of polyunsaturated fat (lower ratio of polyunsaturated to saturated fatty acids) have higher cholesterol levels10. Since the relation between blood cholesterol level and CVD risk is continuous, there is no definite threshold to initiate treatment11. If a threshold for “high cholesterol” is set at over 147 mg/dl, it would account for 4.4 million deaths and 40.4 million disability-adjusted life years (DALYs) worldwide12.
Among the various components of total cholesterol, HDL is generally believed to be cardioprotective, whereas low and very low density lipoprotein (LDL, VLDL) and lipoprotein(a) [LP(a)] are highly atherogenic13. Elevated LDL-C plays a pivotal role in the development and progression of atheromatous plaque and its rupture causes most fatal and nonfatal MIs14. LDL-C level in human umbilical cord blood is low (25-30 mg/dl) and lower than the HDL-C level15. LDL-C is both a necessary and sufficient risk factor for atherosclerosis; necessary because atherosclerosis cannot be produced in experimental animals without some elevation in LDL-C and sufficient because advanced atherosclerosis with MI occurs in children with very high levels of LDL-C even in the absence of any other risk factors16. Moderate lifelong reduction in LDL-C level (<70 mg/dl) as seen in people with hypobetalipoproteinaemia is associated with substantial reductions in risk of CVD and MACE, even in the presence of highly prevalent non-lipid risk factors (smoking, hypertension, etc.)17,18,19.
Studies show that VLDL-C or remnant cholesterol is as much if not more atherogenic than LDL-C20,21,22. The combined risk from LDL-C and VLDL-C is best assessed by calculating non–HDL-C, which contains all the apo B containing atherogenic lipoproteins23. It is estimated by simply subtracting HDL-C from the total cholesterol and can be done even from non-fasting blood. High Non–HDL-C level has been shown to be a strong predictor of severity of coronary atherosclerosis and MACE, particularly in patients who have elevations in triglycerides24. Non-HDL-C target is 30 mg/dl higher than LDL-C target23.
Target goals for LDL-C non-HDL-C and Apo B
Numerous outcome trials of intensive statin therapy using atorvastatin 80 mg and rosuvastatin 40 mg have corroborated a causal role for LDL-C in atherogenesis and the safety and the benefits of lowering LDL-C to very low levels13. The optimum LDL-C level is currently standardized at 40 mg/dl25. Grundy et al25 have demonstrated a log-linear relationship between increased serum LDL-C levels and increased relative risk for CAD. The data plotted this way suggest that for every 1 mg/dl change in LDL-C, the relative CAD risk changes by 1 per cent25. Thus, an individual with an LDL-C of 70 mg/dl has a 30 per cent higher risk than one with an LDL-C of 40 mg/dl25. Regression studies have demonstrated that coronary plaques continue to accumulate when LDL-C is >60 mg/dl and substantial regression requires LDL-C closer to the optimum levels26.
Non–HDL-C is highly correlated with Apo B; hence, non–HDL-C is an acceptable surrogate marker for Apo B in clinical practice, especially for monitoring the response to statin therapy23. The optimum level of non-HDL-C level is 70 mg/dl. The non-HDL target is <100 mg/dl in very high risk patients with an LDL-C target of <70 mg/dl; the non-HDL-C target is <130 mg/dl in high-risk people whose LDL-C target is <100 mg/dl. It is worth highlighting that patients with LDL-C <100 mg/dl, but with non-HDL-C >130 mg/dl have a 34 per cent increased risk of CVD underscoring the clinical importance of non-HDL-C27. A 40 mg/dl decrease in non-HDL-C achieved with statin therapy confers a 35-40 per cent reduction in CAD risk (1:1 relationship between per cent non-HDL-C lowering and CAD risk reduction)22,24,28. Non HDL-C can be used as the poor man's test for apo B with non-HDL-C <100 mg/dl corresponding to Apo B <80 mg/dl. Apo B target is <90 mg/dl in high risk patients and <80 mg/dl in very high risk patients29,30,31,32.
Life-saving benefits of statin therapy in primary prevention of CAD
Cardiovascular disease is multi-factorial in its causation and lifestyle changes are the first line of any treatment strategy. Lowering LDL-C and non-HDL-C are important target for pharmacotherapy and statins are the first-choice agents for both32. Statins are also effective in halting the progression and even reversing coronary atherosclerosis, either alone or in combination with niacin26,33.
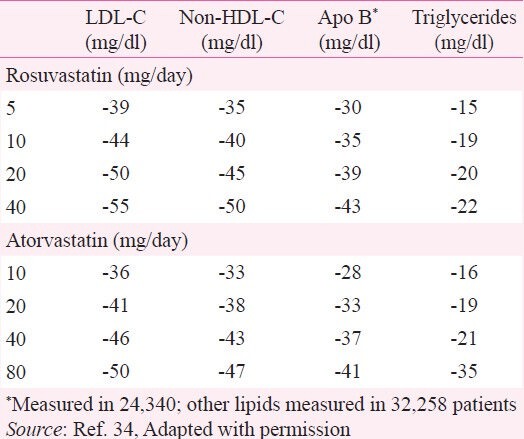
High doses of atorvastatin and rosuvastatin lowers LDL-C and non-HDL-C by 50 per cent or more (Table I)34. Evidence from clinical trials has demonstrated that statin therapy can reduce the risk of death, CVD events and the need for CARPs by 25-60 per cent, depending on the magnitude of LDL-C lowering achieved13. The 2012 Cholesterol Treatment Trials’(CTT) Collaboration demonstrated a consistent 21 per cent relative risk reduction in MACE with statins per 1 mmol/l (39 mg/dl) reduction in LDL-C, regardless of the baseline risk35. Although individuals without previous vascular disease are at lower absolute risk, more than two-thirds of MACE occur among them36. The CTT Collaboration has also published analyses focusing on the comparison between high and low doses of statins. It demonstrated that intensive lowering of LDL-C provides substantial additional CVD event reduction without any corresponding increase in non-vascular mortality2. Benefits of statins are seen across a broad spectrum of patients – men and women, young and old, and in people with and without diabetes or prior diagnosis of CVD13.
Table I.
Per cent reduction in lipid parameters with increasing statin doses
Several primary prevention trials (MEGA, WOSCOPS, ASCOT, and AFCAPS, JUPITER, etc.) have demonstrated a reduction in CVD outcome with statin therapy in people with LDL-C ranging from 108-190 mg/dl13. The JUPITER Trial involving 17,802 low-risk adults without CAD was terminated prematurely (in 19 months) due to a 44 per cent reduction in the primary end point37. Further analysis demonstrated a 65 per cent reduction in the primary end point among those who achieved LDL-C <50 mg/dl - a level considered unachievble and dangerous until that study38.
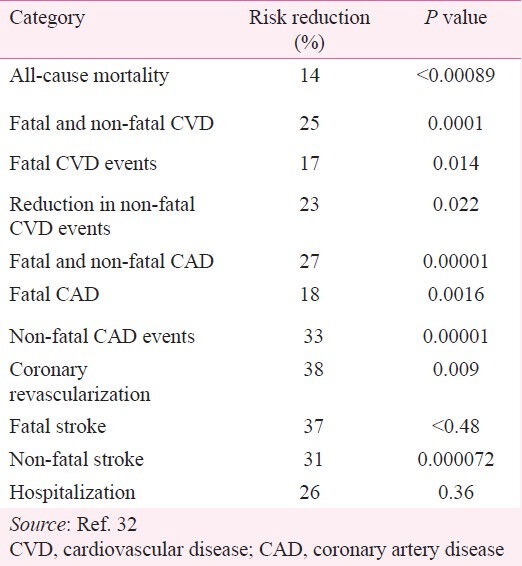
The Cochrane review32 updated the previous reviews by including all statin trials published since 2007 and reconciled the findings with those published by CTT collaboration35. A total of 56,934 participants in18 randomized trials included in the analysis showed consistent reductions in LDL-C that was associated with a significant reduction in mortality rates, CVD events, and interventions as shown in Table II32.
Table II.
Meta-analysis of statin outcome trials in primary prevention
Similar benefits were seen in statin trials that were stopped prematurely and in those running their planned course. After in-depth review of the safety profile, the researchers concluded that the benefits of statins far outweigh any risks of serious adverse effects. Specifically no excess of cancers was found and all-cause mortality was lower in those on statins32. No excess of combined adverse events, liver enzyme elevation, renal dysfunction or arthritis was found. However, an increase in the risk of DM, myopathy, rhabdomyolysis, and haemorrhagic stroke in people treated with statins was noted particularly among those treated with higher rather than lower doses of statins32.
Statin therapy and all-cause mortality
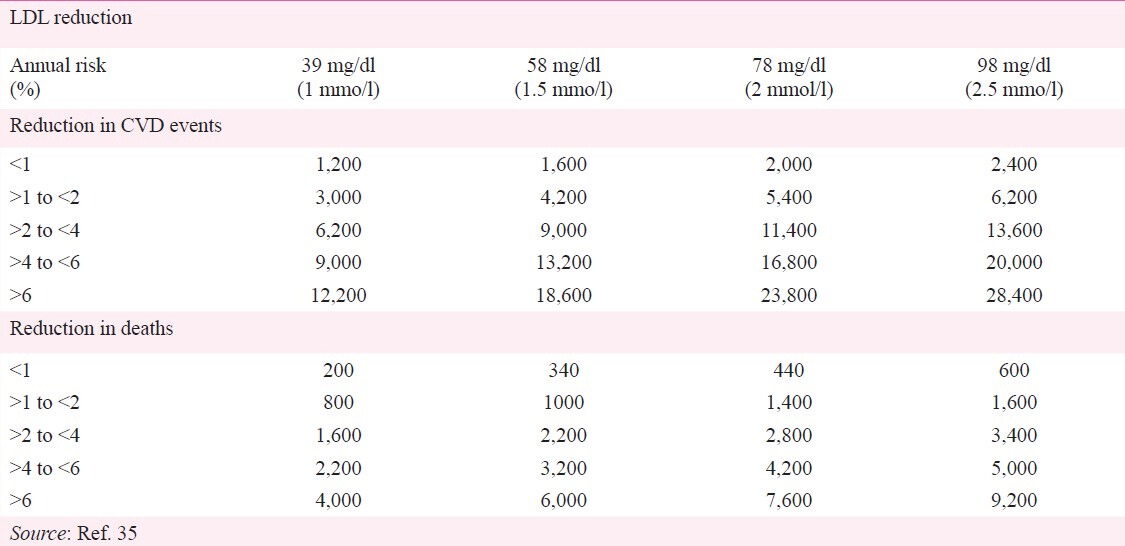
An earlier systematic review of five statin trials (N=30,817) found no evidence for increased rates of non-illness mortality (accidents, violence or suicide)39. The JUPITER trial had previously shown strong evidence of a reduction in total mortality37. Data on all-cause mortality were available for 13 trials involving 48,060 participants. During observation, 4.4 per cent died in the statin group compared with 5.1 per cent in the placebo group with a number needed to treat for five years of 9632. When the data were pooled, there was a 14 per cent reduction in all-cause mortality that favoured statin treatment. The number of deaths prevented with statin therapy varies from 200 to 9,200 per million person-years of treatment, depending upon the baseline risk and the degree of LDL-C reduction achieved (Table III)35.
Table III.
Absolute reduction in major adverse cardiovascular events and deaths per one million person-years of statin therapy in primary prevention, according to baseline risk status and degree of LDL-C reduction
Statin therapy and CAD events
Fourteen trials with 48,049 participants reported on combined fatal and non-fatal CAD events. The event rates were 4.6 per cent in the placebo group and 3.4 per cent in the statin group resulting in a 5-yr NNT (number needed to treat) of 56 and relative risk reduction of 27 per cent32. Statin therapy, on average, would reduce 11 MACE per 1,000 patients treated over 5 yr per 39 mg/dl reduction in LDL-C (2,200 per million person-years)35. However, the number of CVD events prevented with statin therapy varies from 1,200 to 28,400 per million person-years of treatment, depending upon baseline risk and the degree of LDL-C reduction achieved (Table III)35.
Statin therapy and stroke
Lipid abnormalities, especially elevated LDL-C are strong predictor and statin therapy is associated with significant reduction in first and recurrent stroke. International guidelines advise almost universal use of statins for secondary prevention. Statin therapy upon hospital discharge after a stroke not only lowers the recurrence of stroke but also decreases CAD events and improves survival40,41,42. Conversely, statin withdrawal in the hospital, even for a brief period, is associated with worsened survival43. Any possible excess of haemorrhagic stroke is greatly outweighed by the protective effect against ischaemic stroke and CAD events. A 31 per cent reduction in fatal and 37 per cent reduction in non-fatal stroke was observed in the recent Cochrane review32.
Statins and coronary artery revascularization procedures (CARPs)
Seven trials with 42,403 participants reported the need for CARPs and showed a 38 per cent reduction in the need for such procedures in the statin group32. The enormous costs of stents ( 200,000 to 500,000), especially in relation to the average annual income (
200,000 to 500,000), especially in relation to the average annual income ( 68,747 per year and 80 per cent living with <
68,747 per year and 80 per cent living with < 100/day), makes CARP beyond the reach of most Indians. Besides, only <10,000 coronary bypass surgeries and <100,000 angioplasties are done annually in a population of >30 million CAD patients due to lack of facilities44. Statin therapy offers an effective alternative to these expensive procedures as well as to reduce CVD morbidity and mortality, especially in India, where the annual cost of statin therapy is approximately
100/day), makes CARP beyond the reach of most Indians. Besides, only <10,000 coronary bypass surgeries and <100,000 angioplasties are done annually in a population of >30 million CAD patients due to lack of facilities44. Statin therapy offers an effective alternative to these expensive procedures as well as to reduce CVD morbidity and mortality, especially in India, where the annual cost of statin therapy is approximately  2000 to 3000.
2000 to 3000.
Statin therapy in low-risk people
A seminal finding in the updated Cochrane review32 was the conclusive demonstration of significant benefit of statin therapy among the low-risk individuals who would not qualify for statin therapy by the European and Australian guidelines (which have set an annual risk >2 per cent as the threshold risk for statin therapy)45. There was 43 per cent risk reduction in low-risk (annual risk <1%) and 39 per cent risk reduction in intermediate risk individuals (annual risk 1-2%) giving NNT values of 167 and 67, respectively. Treatment of 1000 people for five years would prevent six MACE (1,200 per million) in those with annual risk <1 per cent increasing to 15 MACE (3,000 per million) in those with annual risk of 1 to 2 per cent32. The NNT is well within the range considered worthwhile for other primary prevention strategies such as hypertension treatment32. The potential adverse effects of statins among people at low risk of CVD were a concern in earlier trials46,47. The meta-analyses conducted by the CTT Collaboration showed no excess risk of cancers, or non-vascular mortality35. The totality of evidence now suggests that the benefits of treatment in low-risk subjects far outweighs any possible hazards32.
Statin therapy in women
Women are generally considered to be at low risk, especially before 65 yr of age. Yet, women had equal and similar benefits as men in primary prevention32. A meta-analysis of 15 statin trials showed a MACE reduction of 19 per cent in women compared to 24 per cent among men. The CVD outcome was primarily driven by reductions in unstable angina and need for CARPs48. In addition to reducing MACE, statin use also reduces the risk of atrial fibrillation, gall stones and need for cholecystectomy in women49,50.
Statins therapy in people with elevated lipoprotein (a)
Lp(a), the most dangerous genetic variant of LDL-C, is believed to be the second causal factor of atherosclerotic CVD. Lp(a) alters the balance between intrinsic thrombotic and thrombolytic milieu more than any other risk factor. The risk of CVD in patients with elevated Lp(a) is markedly increased (12-14 fold) when LDL-C is also increased. Lowering LDL-C to target or preferably to near-optimal level with statins offers an opportunity to reduce the excess risk while effective therapy for lowering Lp(a) is being developed51.
Statin therapy for elevated triglycerides
Elevated triglyceride (TG) levels (usually accompanied by low HDL-C levels, except in patients with high alcohol consumption) are often due to obesity, insulin resistance, high glycaemic load, and physical inactivity8. Lifestyle modification and/or withdrawal of the offending agent may be the most appropriate risk reduction strategy. Pharmacologic therapy, however, is recommended when the TG serum levels remain >500 mg/dl, to prevent pancreatitis23,52.
Many physicians and medical practitioners are unaware of the TG lowering properties of statins; potent statins can lower TG to the same extent as it lowers LDL-C levels (1:1 ratio)53. Intensive statin therapy with atorvastatin 80 mg/day lowers LDL-C by 60 per cent, non-HDL-C by 53 per cent, and TG by 37 per cent, in patients with normal TG levels54. But in patients with elevated TG, atorvastatin 80 mg can decrease TG by 52 per cent (instead of 37%), VLDL-C by 62 per cent, and non-HDL-C by 52 per cent54. In addition, statin therapy produces very favourable changes in LDL and HDL particle size55. Most Asian Indians have combined dyslipidaemia (TG <500 mg/dl and non-HDL >130 mg/dl) and respond well to high dose atorvastatin or rosuvastatin with a 50 per cent reduction in LDL-C, non-HDL-C, and TG levels13.
While the combination of ezetimibe or fenofibrate with moderate dose-statins appears to be reasonably safe, outcome data are scant for statin-fibrate combination therapy13. The results of the ACCORD Trial provide some assurance of benefits with statin-fibrate combination therapy in men with DM and high TG levels, although potential harm was reported in women and non Whites56. Bile-acid sequestering agents are contraindicated in patients with high triglycerides.
Statin therapy in patients with chronic kidney disease (CKD)
The rates of CVD events and mortality progressively increase as the kidney function deteriorates57. Dialysis patients have up to 40-fold higher all-cause mortality and 15-fold higher CVD mortality than the general population58. A meta-analysis of statin therapy in 48,429 patients with CKD (6,690 major CVD events and 6,653 deaths) showed a 23 per cent reduction in major CVD events, 18 per cent reduction in CAD events, and 9 per cent reduction in CAD or all-cause deaths57. Subgroup analysis demonstrated that the relative CVD risk reduction with statin therapy decreased in parallel with the decrease in glomerular filtration rate (GFR), including those receiving dialysis (CKD stage 2-3:31%; CKD stage 4: 22%; CKD stage 5 non-dialysis:18%; CKD stage 5 dialysis: 7%). However, the absolute risk reductions were comparable (stage 2-3:NNT-24; stage 4: NNT-36; stage 5: NNT-46). There was no significant increase in adverse events or the development of renal failure57. In addition to dyslipidaemia, additional risk factors specific to CKD include: calcium and phosphorus dysregulation, anaemia, increased oxidative stress, and high levels of homocysteine and Lp(a)57. Thus, some of the excess CVD observed in people with advanced CKD may not be atherosclerotic in nature, and therefore, not amenable to be reduced with statins57. These data support the use of statins in all stages of CKD including dialysis patients57.
Statin therapy and metabolic syndrome
Metabolic syndrome (MS) in youth that persists into adulthood is associated with a 3-fold risk of subclinical atherosclerosis and a 12-fold risk of DM59. In the INTERHEART study60, patients with MS had 3-4 fold risk of first MI with greater risk noted in women. A post-hoc analysis of the TNT trial showed that patients with MS had a 44 per cent higher risk of CVD events, compared to those without MS. Those randomized to intensive statin therapy had a 29 per cent lower risk of MACE compared to standard therapy61. Although patients with MS have a higher risk of developing DM, statin therapy provides the greatest risk reduction in people with DM62,63.
Statin therapy in people with diabetes
Type 2 DM is associated with a 2 to 4-fold higher risk of CVD incidence and mortality compared to people without DM65,66. CVD accounts for 80 per cent of death and disability among patients with DM. Framingham Risk Score (FRS) and European SCORE prediction tools do not include DM. However, the SCORE and other data indicate that DM confers much greater risk than suggested by FRS with a relative risk of 5 in women and 3 in men45,67. Two of the three patients with multi-vessel disease or acute coronary syndrome (ACS) have known DM, undiagnosed DM or prediabetes68. In a study of Asian Indians with ACS (mean age 55 yr) 37 per cent had DM and 46 per cent had prediabetes with only 17 per cent having normal glucose tolerance69. Asian Indians have the highest rates of DM-3 times higher than Whites, after adjusting for obesity and other risk factors for DM70.
The CAD risk from DM is substantially greater among Asian Indians than in Whites. In an ongoing prospective study in the UK, nearly half of all CAD deaths among South Asians occurred in individuals with DM at baseline compared to only 13 per cent among Europeans71. Compared with those with no diabetes, DM increased CAD mortality nearly 3-fold among South Asians but only 1.5-fold among Europeans. Results of several studies show a 3 to 4-fold higher CAD mortality among South Asians with DM than Whites with DM (after adjustment for gender, age, educational level, smoking, hypertension, alcohol intake, and obesity)72,73,74. Thus, South Asians are markedly sensitive to the impact of DM on CAD risk. This increased risk of CAD among South Asians with DM is in sharp contrast to the 32 to 44 per cent lower risk observed among Blacks, Hispanics and other Asians75,76.
Hyperglycaemia is a weaker risk factor for CAD than high hypercholesterolaemia or high blood pressure. A meta-analysis of DM patients showed that a 1-percentage point increase in glycosylated haemoglobin level confers an 18 per cent risk of CVD77. The magnitude of the benefit from intensive glycaemic control is substantially lower than that reported with tight control of blood pressure and LDL-C78.
Randomized statin trials show that an 80 mg/dl reduction in LDL-C results in a decrease of CAD by 42 per cent, and stroke by 20 per cent2. Similarly, a 10 mm Hg decrease in systolic blood pressure results in 22 per cent reduction in CAD events and 41 per cent reduction in stroke79,80,81. In the CARDS Trial, atorvastatin 10 mg/day for four years in patients with DM reduced MACE by 36 per cent, CARP by 31 per cent, stroke by 48 per cent, and all-cause death by 27 per cent64. A meta-analysis involving 18,686 people with DM showed that statins would prevent 8,400 MACE per million person-years of therapy82. Statin therapy is now recommended for all diabetics >40 yr of age regardless of the baseline lipid levels13. The documented benefits of statin therapy is arguably more compelling than aspirin in primary prevention of CVD in patients with DM83. A systematic review of trials evaluating the benefit of aspirin therapy for primary prevention of CVD in 11,618 patients with diabetes showed no significant reduction in MACE84,85.
Legacy effect of statins
Long-term follow up of statin trials has shown that the absolute reductions in MACE increase while the statin treatment is continued2 and that these benefits persist for at least five years after the treatment has stopped, with no evidence of any adverse effects emerging with extended follow up86,87,88. The findings further suggest that the long-term persistent benefits are large enough to outweigh the small increase in the risk of DM and haemorrhagic stroke.
Benefits of lifelong reduction in LDL-C
The Mendelian randomization studies89,90 indicate that optimum LDL-C may be even lower than the 40mg/dl, currently set as optimal based on statin trials91, and the benefits of lifelong low LDL-C (as a result of genetic polymorphisms) is 3 times greater than those indicated by statin trials which typically last only for 5-6 years37,92,93. For example, in one land mark study, three per cent of the population had a nonsense mutation of PCSK9 gene. Whites with this mutation had a 15 per cent reduction in LDL-C but 47 per cent reduction in CAD. Blacks with this mutation had even greater impact -28 per cent reduction in LDL-C and 88 per cent reduction in CAD89. A lifelong 39 mg/dl decrease in LDL-C from a PCSK9 mutation has been shown to reduce CVD risk by 55 per cent90. Conversely, a lifelong 34 mg/dl increase in LDL-C doubles the risk of CAD. Other studies have also shown that a PCSK9 mutation is associated with a 60 per cent lower risk of premature MI94. Together, these studies indicate that a lifelong 1 mg/dl decrease in LDL-C confers 1.5 to 2 per cent reduction in CVD - far greater than the 0.5 per cent reduction observed in randomized clinical trials in middle aged people90. These data underscore the rationale for maintaining low LDL-C throughout life, including childhood and adolescence.
Pleiotropic benefits of statins beyond LDL-C reduction
In addition to improving lipid profile, statins have several beneficial biological, off-target or pleiotropic effects that contribute to their overall clinical benefit. These include reducing platelet aggregation and thrombus formation, improving fibrinolytic profile and lowering inflammation and C-reactive protein (CRP)95,96,97. In a meta-analysis involving a total of 863,805 patients, statin therapy significantly reduced the risk of venous thromboembolism by 19 per cent, whereas fibrate therapy significantly increased the risk by 58 per cent98.
Statin therapy was significantly associated with a decreased propensity for atrial and ventricular arrhythmia and venous thromboembolism99,100. A systematic review of randomized controlled trials with statins showed 77 per cent reduction in atrial fibrillation in secondary prevention and a 40 per cent reduction in new onset atrial fibrillation101. Statin therapy decreases risk of venous thromboembolism in a variety of patients including cancer102. Fibrate therapy was associated with increased risk of gallstones (39%) and cholecystectomy103. In sharp contrast, statin therapy decreases the risk of gallstones and cholecystectomy (36%), possibly by decreasing hepatic cholesterol biosynthesis and decreasing cholesterol concentration in bile104. These additional benefits are not currently included as clinical endpoints in outcome trials and may further increase the cost-effectiveness of statins. Further, clinical trials may have underestimated the benefit of statin by excluding second and third MACE which account for about 20 per cent of all MACE. A summary of the benefits of statin therapy in primary prevention is given in Table IV13,32,90.
Table IV.
Summary of the remarkable benefits and safety of statin therapy in primary prevention

Statin therapy - the foundation of preventive cardiology
The large body of knowledge gained over the past 50 years in the field of CVD suggests that more than 90 per cent of the CVD events and interventions, (at least before age 65), is due to failure of primary and primordial prevention, which could have prevented the development and growth of cholesterol plaque in the first place105. Regression studies indicate that plaques continue to accumulate when the LDL-C levels are >60 mg/dl and primary prevention trials indicate that 65 per cent event reduction can be achieved when LDL-C is lowered to <50 mg/dl26,38. Yet, unlike American guidelines, the various European guidelines limit statin therapy to those with annual risk >2 per cent (based primarily on what the society can afford)45,106,107.
The evidence of overwhelming benefits has led expert committees to promote the use of statins on a global scale106,108,109. Prescriptions and sale of statins have risen rapidly in the UK where the expenditure on statin increased from £20 million in 1993 to more than £500 million by 2006106.
Drastic reductions in prices of many potent satins arising from the generic availability have made them cost-effective even among individuals at annual CVD risk <1 per cent in Europe and additional cost saving in the US107,110. The recent Cochrane review32 clearly demonstrating the safety and effectiveness of statins in patients with annual CVD risk <2 per cent suggests that these guidelines might need to be reconsidered.
Statin therapy in children and young adults
Lowering LDL-C by 39 mg/dl with statins for five years in middle age-adults lowers the CVD risk by 20 per cent. A greater benefit is seen when statin therapy is initiated at a younger age. For example, a 39 mg/dl decrease in total cholesterol was associated with about 56 per cent lower CAD mortality at ages 40-49, in both sexes. But the risk reduction fell to 34 per cent at ages 50-69, and to 17 per cent at ages 70-89 yr, underscoring the need for lowering cholesterol levels at a younger age81,111. The data highlight the need and necessity of starting statin therapy earlier than the current practice.
Children with very high cholesterol levels develop atherosclerosis which progresses rapidly in young adulthood112. Elevated LDL-C and blood pressure in adolescence have been demonstrated to be predictive of coronary artery calcification (a marker of silent CAD) 3 decades later113. Mutations of genes that affect the LDL-receptor function or its ligand apo B, usually result in elevated LDL-C >400 mg/dl. Such individuals develop premature atherosclerotic CVD before 20 yr of age and death before 30 yr of age114,115,116. In fact, it was the investigation of a young brother and sister, ages 6 and 8 with advanced atherosclerosis and history of heart attacks that led to the discovery of LDL receptors and statin medications16,117. Increased cholesterol levels may affect the aortic valve and aortic regurgitation may be the earliest sign of accelerated atherosclerosis in children114.
The objective of statin therapy in children is to prevent the development and progression of the plaque and delay the development of CVD (rather than preventing an MI at this young age)13. Statin therapy has been shown to induce a significant regression of carotid atherosclerosis with no adverse effects on growth, sexual maturation, hormone levels, liver or muscle112,114. Children with dyslipidaemia should be treated with aggressive lifestyle modification from age two and medication from age 10 onwards (8 years if LDL-C is very high) if the lipid targets are not met with lifestyle modifications alone118. This is based on historic evidence of heightened risk of CAD in people with elevated LDL-C. Long-term outcome studies with statins are unlikely to be performed in children.
The threshold of pharmacological intervention with statin in children is an LDL-C ≥190 mg/dl in the absence of any other risk factors, or >160 mg/dl in the presence of a positive family history of premature CVD or two other risk factors118. Children with diabetes and LDL-C ≥130 mg/dl should be considered for drug therapy118. The minimal LDL goal is <130 mg/dl and ideal goal is <110 mg/dl. The threshold of intervention for LDL-C is 30 mg/dl lower for Indians than for Americans and Europeans119.
In summary, lowering LDL-C early in life is more effective in reducing CAD risk than the current practice of lowering LDL-C later in life when subclinical atherosclerosis (silent heart disease) is probably already present. A public health strategy that focuses on prolonged sustained reductions in LDL-C beginning early in life could potentially reduce the global burden of CAD118.
Rationale for early and intensive statin therapy for Asian Indians
A large verbal autopsy study of 48,000 urban and 32,000 rural adults (35-69 yr) in India has shown CVD death rates of 685 per 100,000 in men and 428 per 100,000 in women120. These rates are substantially higher than in the US, where the CVD mortality was 245 per 100,000 (men and women combined in 2008)121. The study also found that CVD accounted for 41 per cent of mortality in urban males and 37 per cent of mortality in urban females as well as 25 per cent of mortality in rural males and 22 per cent of mortality in rural females120. The CVD mortality ratios in urban India were similar to those reported for Indian Americans from the State of California (39% males and 32% for females)122. Prospective studies have shown that the incidence of and mortality from CAD among Asian Indians are at least 2-fold higher than Whites even when adjusted for major risk factors (smoking, blood pressure, cholesterol, insulin resistance, MS, DM, and socio-economic status)71,123,124. The increased risk of CAD is primarily due to Asian Indian dyslipidaemia which is characterized by the following125,126,127,128: (i) High levels of lipoprotein(a); (ii) High levels of Apo B and non-HDL-C; (iii) High levels of triglycerides; (iv) Borderline high levels of LDL-C; (v) Low levels of Apo A1and HDL-C; and (vi) High ratios of TC/HDL-C, TG/HDL-C, and apo B/apo A1.
In the INTERHEART study, Indians had the lowest HDL-C (32 mg/dl in men and 36 mg/dl in women) and the highest TC/HDL ratio and apo B/apo AI ratio - the two lipid measures with the highest predictive value for CAD risk and severity129. In fact, apo B/apo AI ratio had the highest population- attributable risk for MI (65%)130. At a given level of cholesterol, Indians have a lower LDL-C level due to high levels of triglycerides which artificially lowers LDL-C8. At a given LDL-C level, Indians have higher risk of CVD because of high levels of Lp(a), low levels of HDL-C and possibly dysfunctional HDL-C particles131. South Asian newborns have higher levels of E-selectin and Lp(a), which is in line with the hypothesis that endothelial dysfunction is present early in life132,133.
Many Asian Indians are in double jeopardy from nature and nurture - nature being the genetically determined Lp(a) excess, and nurture through an unhealthy lifestyle associated with affluence, urbanization, and mechanization131,134. The adverse effects of the modifiable risk factors related to lifestyle such as dyslipidaemia, smoking, hypertension, atherogenic diet, physical inactivity, abdominal obesity and DM are markedly magnified in those with Lp(a) excess135.
An analysis of LDL-C levels in a large cohort (n = 136, 905) of patients hospitalized for CAD found a mean LDL-C of 105 mg/dl TG 161 and HDL 39 mg/dl at admission. However, more than half the group had LDL-C >100 mg/dl and 82 per cent had LDL-C >70 mg/dl136. The average LDL-C was 125 mg/dl among Indians with acute MI, which is higher than those of US patients130, providing even further support for aggressive lowering of LDL-C before an acute MI137.
LDL-C is the principal target for treatment and non-HDL-C is the secondary target23. Non-HDL-C goal is set at 30 mg/dl higher than the LDL-C goal23. Evidence-based treatment for dyslipidaemia in Indians has been hindered by the lack of direct evidence in this population, but lower stricter blood pressure treatment targets in Blacks, than in Whites provide important insights for matching the intensity of treatment with severity of risks among Indians. High blood pressure, is more common, more dangerous and yet, poorly controlled in Blacks. The impact of higher blood pressure levels on stroke is three times higher for Blacks than for Whites. For example, for the same 10 mm Hg increase in systolic blood pressure, Blacks have 24 per cent increase in stroke risk, but only 8 per cent in Whites138. The updated consensus on the management of blood pressure in Blacks issued by the International society of hypertension recommends lower targets for Blacks than in Whites in recognition of this markedly elevated CVD risk from hypertension. Blood pressure goal is <135/85 mm Hg for low-risk Blacks without target organ damage and <130/80 mm Hg for high-risk Blacks with target organ damage139.
An analogous situation exists for the treatment of high cholesterol levels in Indians. At any given level of cholesterol, Indians have markedly increased risk of CAD. The impact of high cholesterol on CAD risk in Indians is much higher than that of high blood pressure on stroke in Blacks. Aarabi and Jackson124 have estimated that a cholesterol value of 108 mg/dl has to be added for Indians to compensate for the underestimation of CAD risk calculated by the Framingham Risk Score. For example, the CAD risk of an Indian with a cholesterol level of 192 mg/dl is similar to that of a European with a cholesterol level of 300 mg/dl124.
Because of the increased risk of CVD in Asian Indians, LDL-C and non-HDL-C targets are set at 30 mg/dl lower than that recommended by NCEP23,119. Accordingly, the LDL-C goal is <70 mg/dl and non-HDL-C <100 mg/dl for very high risk Indians [such as those with CVD, DM, MS, CKD or elevated levels of LP(a)]. The LDL-C goal is <100 mg/dl and non-HDL-C <130 mg/dl for Indians without these risk factors (Table V)23,119. Broader acceptance of this lower LDL-C and non-HDL-C targets and its implementation could reduce the CVD burden in the Indian population by 50 per cent in the next 25 years13. Moreover, these lipid targets more closely approximate the recent recommendations of the European Society of Cardiology45. For high-risk patients, the goals are TC <175 mg/dl and LDL-C <100 mg/dl with an option of <85 mg/dl if feasible; and TC <190 mg/dl and LDL-C <115 mg/dl for all Europeans (who are not at high risk).
Table V.
Recommendations of the Indo-US Health Summit with lower treatment target for selected risk factors
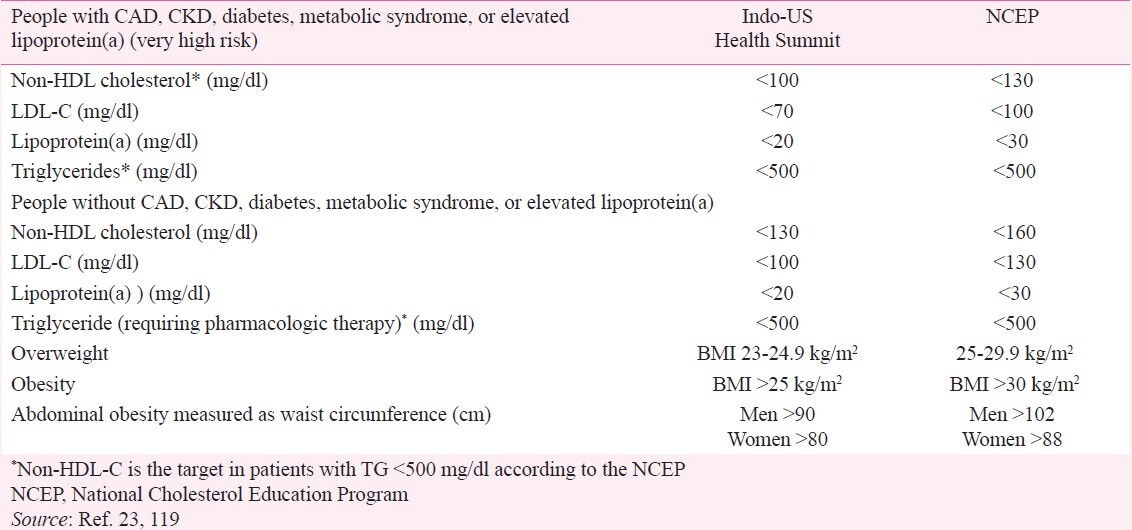
Indians have a high prevalence of CAD and its risk factors with a high risk for first and recurrent MACE with the following prevalence: CAD 12 per cent; DM 16-20 per cent; CKD 10-15 per cent; and high Lp(a) 35-40 per cent135,140. The prevalence of MS is 35-45 per cent in urban India with approximately half this rate in rural areas62,63. Thus, 60-80 per cent of Indians belong to the very high risk category with a non-HDL-C target of <100 mg/dl134. Besides, Indians have highest prevalence of elevated homocysteine (77%), and second highest prevalence of elevated Lp(a)(40%)141,142,143,144. The CVD risk is maekrdly increased when both Lp(a) and homocysteine levels are elevated51,145.
Intensive statin therapy is safe, well tolerated, and effective in decreasing LDL-C in South-Asians13. Intensive statin therapy with potent statins and at high doses is often required in patients who require a ≥ 50 per cent reduction in LDL-C13. Besides, most Indians with baseline LDL-C >160 mg/dl with a LDL-C target of <100 or baseline LDL >130 with a target of <70 mg/dl will require rosuvastatin 20-40 mg or atorvastatin 40-80 mg13. The percentage of patients who achieve the Indian targets at different doses of atorvastatin and rosuvastatin is given in Table VI13,34.
Table VI.
Percentage of patients achieving the LDL-C goals with various doses of atorvastatin and rosuvastatin
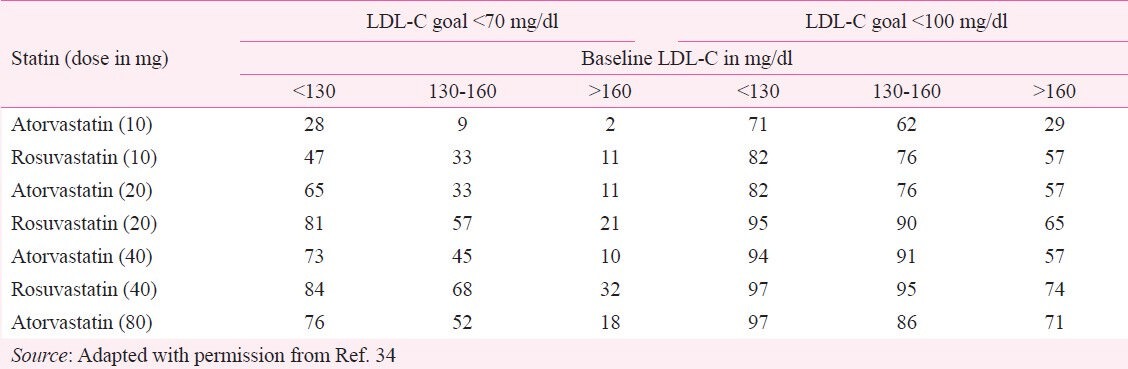
A higher starting dose of statin results in rapid achievement of the LDL-C goals with minimal titration146. Intensive lipid-lowering with atorvastatin 80 mg or rosuvastatin 40 mg in patients with CAD provides significant clinical benefit beyond that afforded by treatment with lower doses.
Treatment with a low dose of pravastatin reduced the risk of CAD in Japan by as much the same degree as higher doses have shown in Europe and the USA147. This does not appear to be the case among Indians8,13. The available data indicate that the efficacy and safety of statins among South Asians are no different from Whites, except for anecdotal information of increased myalgia8.
Adverse effects of statin therapy
Although there is no evidence of any increased risk of cancer or non-CVD deaths, several known or potential hazards need to be considered when estimating the net effects of statin therapy in people at lowest risk such as in primary prevention32. The avoidance of life-threatening or potentially disabling events in apparently healthy low-risk individuals is acceptable, provided that they are not accompanied by any definite hazard that is of comparable severity. The recent Cochrane review has conclusively demonstrated the absence of such a hazard32. This is in sharp contrast to the use of aspirin in primary prevention, where the risk outweighs the benefit in many cases. The adverse effects of statins include DM, haemorrhagic stroke, nephrotoxicity, hepatotoxicity, myopathy, rhabdomyolysis, and drug interactions. Unlike statin-induced LDL-C lowering, statin safety is not a class effect, because statins vary in their excretion and metabolism by other drugs.
Statin therapy and new onset diabetes (NOD)
Recent meta-analyses have suggested that statin therapy might be associated with a 9 per cent increase in the risk of NOD compared to placebo148,149,150. In terms of absolute risk, 255 people treated for four years with statin therapy would result in one case of NOD (or 980 NOD per million person-years of statin therapy)148,149,150. Intensive therapy is associated with a 12 per cent additional increase in NOD148,149,150. Moreover, the statin-associated risk of NOD appears to be a dose-dependent class effect.
Statin-related NOD is associated with four risk factors of MS – fasting blood glucose >100 mg/dl, fasting triglycerides >150 mg/dl, hypertension and obesity151. Moreover, these factors are also predictors of NOD in the general population (not on statin therapy)152,153,154. Post-hoc analysis of TNT and IDEAL (secondary prevention studies comparing intensive statin therapy versus standard statin therapy)155,156,157 has shown that NOD is limited to those with 2-4 of these risk factors. Compared with low-dose statin, atorvastatin 80 mg reduced the number of CVD events both in patients at low and high risk for NOD157. Among those at high-risk of NOD, atorvastatin 80 mg increased NOD by 24 per cent but was more than offset by greater CVD risk reduction. For example, among the 6,231 patients at high risk for NOD, treatment with atorvastatin 80 mg/day compared with a lower statin dose was associated with 80 more cases of NOD but 94 fewer major CVD events157. Moreover, many patients with CVD will die from another CVD event long before they develop complications from DM157.
Approximately 50-65 per cent of patients in these secondary prevention trials were at low risk of NOD with 0-1 risk factors157. No increased risk of NOD was seen in these low-risk patients who received atorvastatin 80 mg/day. The absence of increased risk of NOD with intensive statin therapy (atorvastatin 80 mg/day), while providing greater CVD event reduction, is a welcome reassurance for physicians treating patients at low risk for DM157. In the 1,501 TNT trial patients with established DM at baseline, those randomized to atorvastatin 80 mg/day experienced 25 per cent reduction in CVD events (14 vs 18%) compared to those randomized to atorvastatin 10 mg/day158.
New onset diabetes (NOD) in primary prevention: The risk of NOD should be viewed against the background of long established CVD benefits of statin therapy in patients with DM. In the CTT meta-analysis of 18,686 patients with DM in 14 randomized statin trials, a 39 mg/dl (1-mmol/l) reduction in LDL-C was associated with a 21 per cent reduction in MACE – a relative risk reduction nearly identical to that reported in all patients on statin therapy2.
A detailed analysis of the risk of NOD and benefits of CVD event reduction among the 17,603 men and women without previous CVD or DM in the JUPITER trial has been reported159. The subjects were randomly assigned to rosuvastatin 20 mg or placebo and followed for up to five years for the primary endpoint (cardiovascular death, MI, stroke, admission to hospital for unstable angina, CARP) and the protocol-prespecified secondary endpoints (venous thromboembolism, all-cause mortality, and incident physician-reported diabetes)159. The participants were stratified on the basis of having zero or ≥ 1 major risk factors for NOD: MS, fasting glucose ≥ 100 mg/dl, body-mass index ≥ 30 kg/m2, or glycated haemoglobin (A1c) ≥ 6 per cent159. Trial participants with ≥ 1 NOD risk factors (n=11,508) indeed had a 28 per cent higher risk of NOD than were those with no risk factor (n=6095). In individuals with ≥ 1 risk factors, statin therapy was associated with a 39 per cent reduction in the primary endpoint as well as a 36 per cent reduction in venous thromboembolism, and 17 per cent reduction in total mortality. For the 11,508 people with NOD risk factors, a total of 134 vascular events or deaths were avoided for every 54 new cases of DM diagnosed. For trial participants with no NOD risk factors, statin therapy was associated with a 52 per cent reduction in the primary endpoint, a 53 per cent reduction in venous thromboembolism, and a 22 per cent reduction in total mortality with no increase in DM. For these 6095 individuals, a total of 86 MACE or deaths were avoided with no diagnosis of NOD159.
A further analysis of the 486 participants who developed DM during follow-up (270 on rosuvastatin vs 216 on placebo) statin therapy was associated with 37 per cent CVD risk reduction – nearly similar to the 44 per cent risk reduction for the trial as a whole. By comparison with placebo, statins accelerated the average time to diagnosis of DM by five wk on rosuvastatin vs. 90 wk on placebo159.
In the updated recent Cochrane review32 among the 12,205 participants on statins therapy 342 (2.8%) developed diabetes compared with 290 out of 12,202 (2.4%) participants on placebo, with a relative risk of developing diabetes of 1.18 (95% CI 1.01 to 1.39)32. However, this higher risk of statin-related NOD was driven primarily by JUPITER trial which used rosuvastain 20 mg/day159. Although JUPITER trial showed higher risk of NOD, the CVD benefit was also high, resulting in premature termination of the trial.
The mechanism by which statins increase the risk of NOD has been the subject of speculation. Atorvastatin and rosuvastatin have been reported to increase insulin resistance. Nonetheless, the mechanism may only be operative in patients with multiple risk factors for DM159.
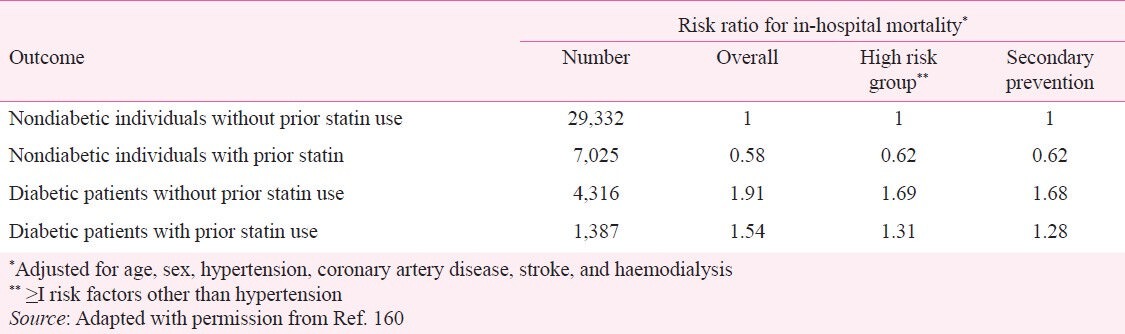
Balance of risk of NOD and benefit on CVD and mortality: A population-based study of nearly a million subjects from Taiwan, has also confirmed higher risk of NOD but and even higher CVD risk reduction with statin therapy. During a median follow up of 7.2 yr, NOD developed in 2.4 per cent of patients on statin therapy compared to 2.1 per cent not receiving statin therapy. This 14 per cent increase in NOD was more than offset by 18 per cent reduction in MACE and 39 per cent reduction in in-hospital mortality resulting in a highly favourable benefit risk ratio (Table VII)160. In terms of absolute numbers, statin therapy prevented one fatal event for every 202 subjects and led to one case of NOD for every 301 patients. However, the overall NOD incidence was high (21 per 1000 person-years) – four times higher than in the meta-analysis of studies conducted mostly in Whites148. The 14 per cent risk of NOD from statin therapy, however, was much smaller compared to 53 per cent NOD risk from diuretics and 40 per cent NOD risk from beta-blockers in the same study161. Many other studies have also shown much higher risk of NOD from diuretics (20-45%) and beta-blockers (20-36%) than from statins162,163. Thus, the magnitude of risk of NOD with statins appears to be about half than that of diuretics and beta-blockers widely used in the management of hypertension and CVD.
Table VII.
In-hospital mortality risk ratio according to diabetes status and prior statin use in real world, from a National Health Insurance Data
A Canadian study involving 143,630 Whites, 9529 South Asians, and 14,084 Chinese with newly diagnosed DM has also confirmed substantial reduction in mortality among those who were prescribed statins. Compared with no prescribing, statin prescribing was associated with mortality reduction of 31 per cent among South Asians, 40 per cent among Chinese and 35 per cent among Whites164.
A review of the literature indicates that the CVD benefits of statin therapy far outweighs the risks for statin-related DM and other risks165. A patient with CAD who develops DM faces several new challenges such as blood glucose monitoring requirements, increased dietary restrictions, and usually additional drug therapy. Besides, there are long-term threats of the macrovascular and microvascular complications of diabetes. However, the impact of NOD is relatively minor compared with the CVD events (fatal and nonfatal MI, fatal and nonfatal stroke, and death). Considering the balance between NOD and CVD event prevention, it is worth noting that the microvascular and macrovascular complications of diabetes are relatively uncommon during the first decade after diagnosis166.
In summary, NOD with statin therapy is confined to those with risk factors for DM and the CVD benefits far outweigh risk of NOD32,35,159. Even if statin- related NOD was associated with an immediate doubling in CVD risk, the expected effect would be approximately 40 fewer events avoided per million person-years167. This figure is 50 times smaller than the absolute benefit observed with statin therapy in such individuals (2,200 fewer MACE per million person-years per 39 mg/dl reduction in LDL-C)35.
Haemorrhagic stroke
International guidelines recommend statins in secondary prevention of stroke and advise objective assessment of CVD risk to determine the appropriateness of statins for primary prevention of stroke. Post-hoc analysis of SPARCL trial168 (4731 stroke survivors randomized to treatment with atorvastatin 80 mg/day or placebo, regardless of cholesterol level) has shown that those who achieved a >50 per cent LDL-C reduction had double the reduction in ischemic stroke compared to the overall cohort (33 vs. 16%) without any statistically significant increase in haemorrhagic stroke. More importantly, there was a 37 per cent reduction in MACE compared to those who had no change in LDL-C (possibly due to not taking the statin)168. Caution is advised in patients with a history of intracerebral haemorrhage169. Totality of data indicates that statins therapy may increase the risk of haemorrhagic stroke with an annual excess risk of 0.5 per 1000 people treated over five years (100 per million person-years) per 39 mg/dl LDL-C reduction35. The risk may be even higher in Asian populations, who have higher rates of stroke and higher proportion of haemorrhagic strokes than Whites170. This does not apply to Asian Indians who have very high risk of CAD and lower risk of stroke9.
Cognitive impairment and peripheral neuropathy
There are sporadic reports of cognitive impairment, primarily from observational data, but not from randomized trials. The data indicate that statin therapy has either a neutral or beneficial effect on cognition. Statin use and type were not associated with cognitive impairment after adjusting for known variables that affect cognition in a large cross-sectional analysis of 24 595 participants (7191 statin users and 17,404 nonusers age ≥45 yr), from a population-based national cohort study171. There is some speculation that statins that are hydrophilic (i.e. pravastatin and rosuvastatin) may be less likely to contribute to cognitive impairment (due to limited penetration across the blood-brain barrier). If statin-associated cognitive impairment is suspected, a trial discontinuation can reveal a temporal relationship. Switching from a lipophilic (e.g. simvastatin) to a hydrophilic statin (e.g. rosuvastatin) may resolve cognitive impairment. Given that the vascular benefits outweigh any potential risk of cognitive impairment associated with statin use, the current evidence does not support changing practice with respect to statin use172. A modest association between peripheral neuropathy and statin use has also been reported173,174. Evidence from four cohort studies and case reports suggests that the risk is small. Statin treatment is associated with increased self-reporting of reduced energy and fatigue on exertion but does not affect self-reported quality of life, mood, hostility, psychological well being, or anger expression175,176.
Liver toxicity and confounding by fatty liver
Moderate asymptomatic hepatic transaminase elevations (<3 times upper limit of normal or ULN) are common among patients taking statins, but serious liver damage is extremely rare. Asymptomatic transaminase elevations are also seen with all non-statin lipid-lowering therapies and believed to be the result of lowering LDL-C per se. Such elevations often resolve with continued statin treatment177,178,179,180,181. The incidence of significantly elevated liver transaminases (AST/ALT) (>3x ULN) levels is <1 per cent with moderate doses of statins. A pooled analysis of 18,696 patients treated with atorvastatin 80 mg/day showed an incidence of only 1.4 per cent which was much lower than the 5.3 per cent incidence in patients treated with fixed dose fenofibrate therapy182,183,184. There is no reason to suspect the incidence of minor transaminase elevations would be different among Indians.
Nearly 50 per cent of dyslipidaemia patients have coexisting non alcoholic fatty liver disease (NAFLD) – a condition well known for fluctuating transaminase levels185,186. Physicians should not withhold statin therapy from patients whose transaminase elevations have no clinical relevance or are attributable to known stable chronic conditions. Statins can be used in such patients and may actually improve the enzyme levels and liver histology187,188,189,190,191. This is also true in patients with obesity and MS who often have elevated transaminases from hepatic steatosis.
The post-hoc analysis of the GREACE trial has demonstrated that statin therapy is not only safe but also improves liver tests and reduces CVD morbidity in patients with mild-to-moderately abnormal liver enzymes that are potentially attributable to NAFLD192. Of the 437 patients with moderately abnormal liver function at baseline, (possibly associated with NAFLD), 227 were treated with a statin (mainly atorvastatin at a mean dose 24 mg per day) and 210 were not on statin therapy. Those who received statin had substantial improvement in liver enzymes whereas those who did not receive statin had further increase of liver enzyme levels. Among the patients with abnormal liver enzymes, CVD events occurred in 10 per cent of patients who received statin therapy and 30 per cent of those who did not receive statin. The 68 per cent CVD risk reduction with statin therapy was substantially higher than that observed in people with normal liver function. Further, only 1 per cent discontinued statin therapy because of liver-related adverse effects192. In any case, statin therapy should be discontinued in people with persistent transaminase elevation >3 x UNL.
Neither chronic liver disease nor compensated cirrhosis is a contraindication for statin therapy187,193. Statins have been successfully used in people with hepatitis B and C without adverse outcome. However, statin therapy should not be given to patients with progressively deteriorating liver function with decompensated cirrhosis, acute liver failure or active alcoholic liver disease. Statins should also be used with caution in people with excessive alcohol intake (>2 drinks/day for men and >1 drink/day for women). Heavy alcohol consumption and binge drinking (≥ 5 drinks in 24 h) increase the risk of hepatotoxicity. Alcohol abstinence or moderation can allow many patients to safely use statin medications.
No deaths due to liver failure have been reported to date despite the fact that more than a billion statin prescriptions have been filled worldwide during the past 26 years194,195. Serious liver damage or liver failure occurs at a rate of one per million person-years, which is similar to the incidence in the general population. The Food and Drug Administration (FDA) has finally acknowledged that liver damage with statins is rare and that routine transaminase tests are neither necessary nor effective in predicting or preventing severe hepatitis196.
Kidney toxicity
Statins do not cause kidney injury. The only clinical setting in which statins may be associated with acute renal failure is rhabdomyolysis. One meta-analysis compared renal effects of atorvastatin and rosuvastatin in 29,147 patients over 23 trials197. A significant decrease in renal function was detected in placebo as compared to either rosuvastatin or atorvastatin. No significant difference in GFR was detected in five head-to-head studies comparing atorvastatin to rosuvastatin. Likewise, there was no difference in proteinuria except a slight increase noted with rosuvastatin at 40 mg/dose. Atorvastatin and rosuvastatin have similar reno-protective effects in patients at high CVD risk, with comparable rates of new-onset proteinuria, when commonly used doses are considered197. Another meta-analysis with six trials involving 24,278 participants showed that both atorvastatin and rosuvastatin improved GFR, and atorvastatin appeared to be more effective in reducing proteinuria198. There is no dose restriction for atorvastatin in people with low GFR but the dose of rosuvastatin should be reduced to 10 mg when GFR is <30 ml.
Muscle toxicity (myalgia, myopathy, and rhabdomyolysis)
Severe muscle toxicity from statin therapy is rare199. However, vague muscle pain, creatine kinase (CK) elevation or both are fairly common among middle-aged and older people, even in the absence of statin therapy. Fatigue with or without pain has also been reported with the use of statins175,200,201. The definition of various muscle symptoms and toxicity is given in Table VIII199. In patients with myalgia, while receiving a statin, creatine kinase levels should be measured. Although most cases of the myalgia are not accompanied by elevated CK, asymptomatic CK elevation can occur without myalgia.
Table VIII.
Definition of myopathy, rhabdomyolysis, and CK elevation
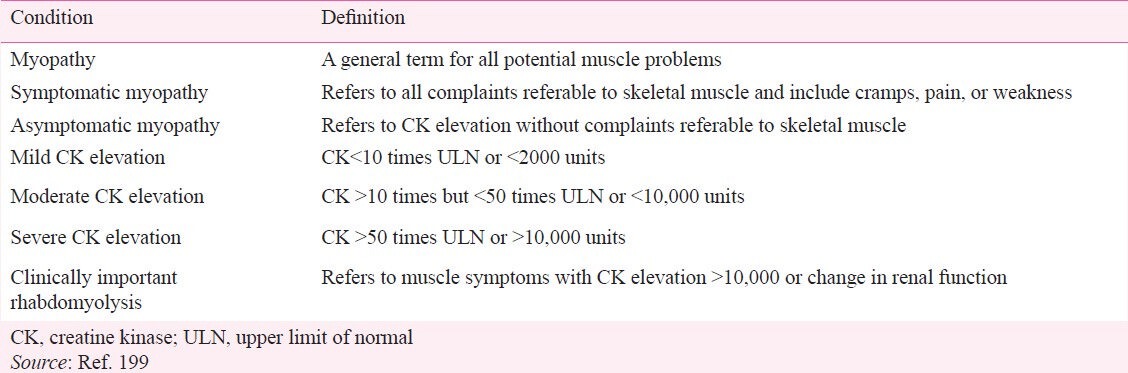
Myopathy: Myopathy is a general term for all potential muscle problems such as muscle pain or weakness and can occur with or without elevated CK199. Statin-related myopathy is usually symmetrical, involves large proximal muscle groups and resolves within two months of discontinuation of the medication199. It occurs in 5 per cent of the statin treated patients in clinical trials and 10 per cent of patients in clinical practice199. The risk of severe myopathy is very low with statin monotherapy and in primary prevention (excess incidence of about 0·5 per 1000 over 5 years or 100 per million person years)199.
Myopathy is a class effect of statins and the leading cause of non-adherence to statins202. The risk of myopathy is not related to the LDL-lowering efficacy, but the dose or the blood concentrations of the statin199. However, atorvastatin is a notable exception and has similar low rates of myalgia at all doses (10 to 80 mg/day)13,203. The patient characteristics that increase the risk of myopathy include low body mass index, female gender, uncontrolled hypothyroidism, collagen vascular disease, hepatic or renal dysfunction, age >80 yr, HIV infection, polypharmacy, and the use of medications that interact with statins204. Consumption of Chinese herbs containing red yeast rice and large quantities of alcohol also increase the risk205. The risk of myopathy varies markedly among the high potency statins with atorvastatin having the least and simvastatin having the most risk13. The prevalence of myopathy was 0.02 per cent or 240 per million among the almost 250,000 patients randomized into trials comparing atorvastatin 80 mg daily with various standard regimens of statins or placebo184.
Asymptomatic myopathy refers to CK elevation without any myalgia or weakness and is common among patients taking statins as well as placebo199. Mild CK elevations (> 2 to <10 x ULN) require careful monitoring. Clinically significant moderate or high CK elevation from statin monotherapy is rare. In a primary care practice, 1 per cent of patients taking statins was found to have significant elevation in both CK and transaminase levels but not attributable to statins upon careful evaluation206. African Americans have higher baseline CK levels. Other causes of CK elevation include: (i) hypothyroidism and hyperthyroidism; (ii) red rice yeast that contains lovastatin or use of large quantities of grape fruit juice that retards statin metabolism; (iii) muscle injury including intramuscular injection; and (iv) vigorous physical activity or seizure199,207. Most CK elevations are due to recent physical exertion which can increase the CK to >50,000204. The National Lipid Association (NLA) does not recommend baseline CK measurements before starting therapy, except for those who are at the high risk207.
Rhabdomyolysis: This is a rare but severe form of myopathy related to statin therapy. Breakdown of skeletal muscle results in release of myoglobin into the circulation and can produce acute renal failure, if not detected and treated early195. According to the NLA definition, the diagnosis of rhabdomyolysis is to be considered when CK is >10x ULN (>2000 units) together with elevation in serum creatinine. Treatment includes aggressive iv hydration therapy and in rare cases renal replacement therapy207. Myalgia is usually present but not an essential requirement.
The incidence of rhabdomyolysis is negligible – 34 per million person-years with statins and 18 per million with placebo in clinical trials195,208. Thus, the excess risk of rhabdomyolysis is estimated to be 16-20 per million person-years of statin therapy. Since the case fatality is about 10 per cent, excess deaths attributed to statin-related rhabdomyolysis is only 2 per million person-years1,195. Potential contributors to statin-related rhabdomyolysis are the same as myopathy plus surgery and trauma199.
The risk of rhabdomyolysis varies with different statins. It is four times more common with lovastatin, and simvastatin (metabolized by cytochrome 450 3A4 (P450 3A4) than fluvastatin and rosuvastatin (metabolized by cytochrome P450 2A9 (P450 2 A9) and pravastatin (not metabolized by P450 system)199. Rhabdomyolysis has been reported with hydrophilic statins such as pravastatin and rosuvastatin199,209. Pravastatin, simvastatin, and rosuvastatin, at doses double that is currently marketed, have been documented to produce unacceptable rates of muscle toxicity209,210,211. This increased risk of myopathy prompted the FDA to deny approval of rosuvastatin 80 mg dose and the withdrawal the 80 mg dose of simvastatin. The FDA has also imposed dose restrictions for simvastatin when used in combination with many commonly used medications (Tables IX, X)1,177,195,199,205,207,212,213. Although partially metabolized by P450 3A4, atorvastatin 80 mg/day has the most favorable risk-benefit ratio for rhabdomyolysis with no higher risk at 80 mg/day compared to 10 mg/day dose13,182.
Table IX.
Concomitant use of medications that may necessitate the use of statins with least drug interaction (rosuvastatin,*fluvastatin, pravastatin or pitavastatin)
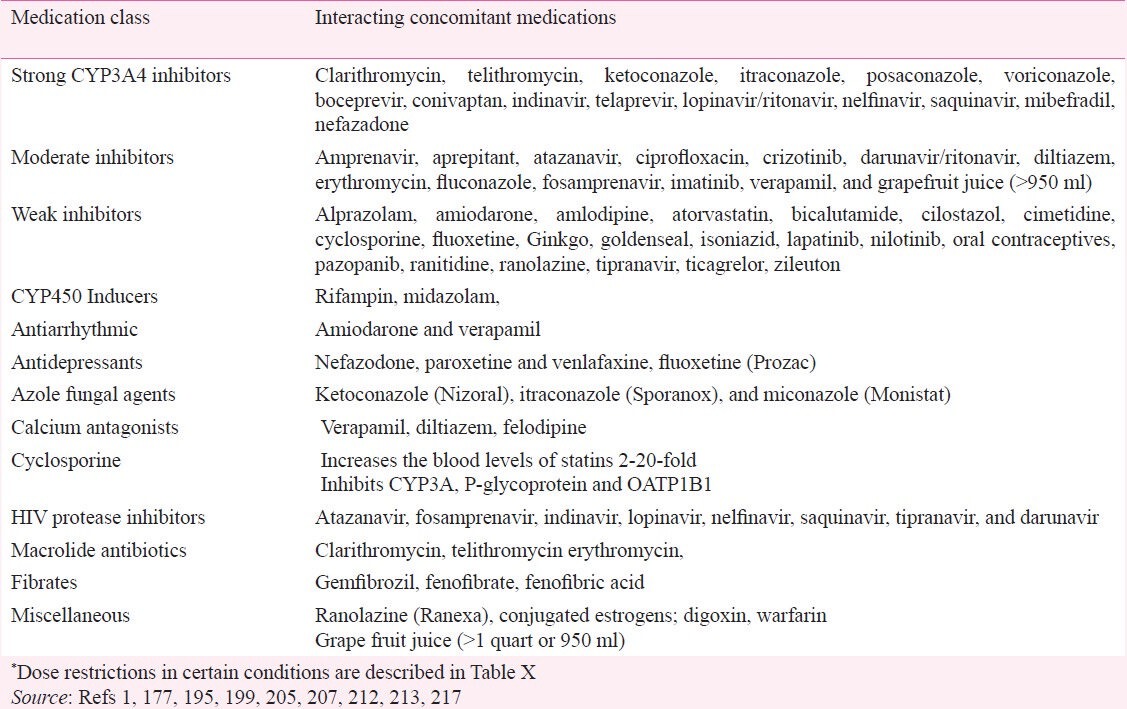
Table X.
Strategies to reduce muscle toxicity during lipid-optimizing therapy

If rhabdomyolysis is detected, statin treatment must be stopped immediately, followed by hospitalization and intravenous hydration. Approximately 90 per cent of patients recover fully and the usual mortality from rhabdomyolysis is 10 per cent. Full recovery usually occurs in a few weeks. If a particular interaction has been implicated, it may be appropriate to restart the statin without the interacting drug. Otherwise a lower dose or an alternative statin could be tried with careful monitoring13.
Medications that increase statin toxicity through drug interactions
More than 80 per cent of statin-associated myopathy and rhabdomyolysis are attributable to concomitant use of P450 3A4 inhibitors or gemfibrozil205. Approximately 20 per cent of patients taking statin also receive a drug that had a warning against co-prescribing with statin214. In a study of 245 co-prescriptions of statins with P450 3A4 inhibitors, simvastatin was prescribed in 134 and atorvastatin in 111 patients. Diltiazem (86), verapamil (72), erythromycin (48) and clarithromycin (29) were the other most common co-prescribed medications. The risk of rhabdomyolysis is particularly high in those with genetic SLCO1b1 variant215.
Pharmacokinetic differences in statins are major determinants of drug interactions. Nearly 80 per cent of all drugs including statins require biotransformation to hydrophilic metabolites for renal excretion, with about 50 per cent of these drugs undergoing metabolism by the cytochrome P450 3A4 isoenzyme. Concomitant use of drugs that are inhibitors of P450 3A4 results in increased concentrations of both drugs. The risk of myopathy and rhabdomyolysis is increased 7-fold when a statin is used concomitantly with a strong P450 3A4 inhibitor216. Some medications are strong and others are weak P450 3A4 inhibitors. Atorvastatin, simvastatin, lovastatin, and pitavastatin are relatively lipophilic (hydrophobic) compounds. Lipophilic statins are more susceptible to metabolism by the P450 3A4, except for pitavastatin, which undergoes limited metabolism via this pathway. Pravastatin and rosuvastatin are relatively hydrophilic and not significantly metabolized by P450 3A4 enzymes. Fluvastatin and rosuvastatin are metabolized primarily by P450 2C9205,220.
Concomitant drug-induced inhibition of P450 3A4 isoenzymes can result in marked increase in blood levels of simvastatin (most lipophilic) and lovastatin but not pravastatin and rosuvastatin. Atorvastatin is only partially (20%) metabolized by P450 3A4 and, therefore, elevation of blood levels with strong P450 3A4 inhibitors is mild. Pravastatin, rosuvastatin, and pitavastatin are excreted mainly unchanged, and their plasma concentrations are not significantly increased by P4503A4 inhibitors221. Strong P450 3A4 inhibitors increase the blood levels of rosuvastatin and fluvastatin by less than 2-fold but up to 20-fold with simvastatin and lovastatin217.
In patients requiring the concurrent use of statins and P4503A4 inhibitors, pravastatin, fluvastatin, and rosuvastatin carry the lowest risk of drug interactions and atorvastatin carries moderate risk. Simvastatin and lovastatin have the highest risk and should be avoided in patients taking concomitant CYP3A4 inhibitors222,223. For patients requiring long-term therapy with amiodarone, diltiazem, or verapamil, statins that are not metabolized by P4503A4 (rosuvastain, fluvastatin or pravastatin) are preferable. Although small doses of lovastatin and simvastatin can be used with moderately potent P4503A4 inhibitors (e.g. ranolazine, diltiazem and verapamil), rosuvastatin, fluvastatin, pravastatin, or pitavastatin (that are not metabolized by CYP 3A4) may be preferable for long-term use. Likewise, paroxetine and venlafaxine are preferable antidepressants for patients taking lovastatin and simvastatin. Rosuvastain is the most effective statin for dyslipidaemia associated with protease inhibitor therapy199,224.
Grape fruit juice >200 ml when given with felodipine increases the blood levels of simvastatin but safe up to 1.2 liters with atorvastatin. Since the duration of effect of grapefruit juice can last 24 h, repeated consumption can result in increase statin blood levels199. Statins and grape fruit juice ingestion should be separated by 2 h. Grape fruit juice inhibits intestinal but not hepatic 3A4 pathway but no report of myopathy has been published to date. Concomitant use of prescription and/or non-prescription medications including acetaminophen, herbal and alternative therapies that increases blood levels of statins should be evaluated in people with statin-related side effects225,226,227,228.
Management of myopathy and rhabdomyolysis
Muscular symptoms usually improve with withdrawal of the statin and recur with rechallenge177. If symptoms are tolerable and CK <10 x ULN, statin can be continued at the same or reduced dose and symptoms may be used as a guide to stop or continue therapy207. But statin should be discontinued if the symptoms are intolerable, regardless of CK, until the patient is asymptomatic199. Strategies for managing myalgia include: (i) a therapeutic trial of coenzyme Q10, 200-1200 mg/day; (ii) evaluation and correction of vitamin D deficiency if detected; and (iii) switching to atorvastatin which has less myalgia and myopathy (if applicable)199. Recurrent symptoms with multiple statins (despite concurrent and adequate doses of coenzyme Q10 and vitamin D supplements) require non-statin lipid therapy.
Vitamin D insufficiency
Vitamin D insufficiency appears to be a novel mechanism of statin-induced myalgia and two-thirds of patients who have myalgia while on statin therapy have low vitamin D levels229. Vitamin D deficiency (<30 ng/ml) is a highly prevalent condition, affecting approximately 30 to 50 per cent of the general population in the US230. Anecdotal evidence indicates that most Indians have very low levels. Low 25-hydroxyvitamin D levels are associated with CVD risk factors and adverse outcomes. Vitamin D deficiency activates the renin-angiotensin-aldosterone system leading to hypertension, left ventricular hypertrophy, insulin resistance, diabetes, and increased cardiovascular risk. Vitamin D supplementation is simple, safe, and inexpensive and often results in reduction in myalgia. Those with severe myalgia require a higher vitamin D level of 50-60 ng/ml. Those with severe vitamin deficiency and myalgia require 50,000 units per week or 8000 units per day for 8-12 wk followed by a lower maintenance dose231,232. Obese patients may also require higher doses of Vitamin D supplementation.
Coenzyme Q10: Coenzyme Q10 (CoQ10) functions as an electron carrier in the mitochondrial electron transport chain. It is carried in LDL-C and serves as an antioxidant, protecting the cell from free radical induced oxidation. HMG-CoA reductase inhibition blocks the production of farnestyl pyrophosphate (FPP) which is an intermediary for the production of CoQ10 (the predominant form of ubiquinone in man)199. Statin therapy lowers CoQ10 blood levels, (partly because CoQ10 is transported in the LDL-C particle)233,234,235,236. The resulting impairment of mitochondrial function may be exacerbated by exercise237. Serum and intramuscular CoQ10 levels are not correlated238. Myalgia, fatigue, dyspnoea, memory loss, and peripheral neuropathy often respond to CoQ10 supplements but may need doses of 400 to 1200 mg/day early in treatment239,240. CoQ10 supplementation offers an alternative to stopping treatment with these life saving medications237,241. There are no known risks to this supplement. Indians have lower levels of CoQ10 levels and higher incidence of myalgia while taking statins8,235,237.
Stain therapy and cancer
A meta-analysis of individual participant data from 27 randomized trials and 175,000 patients provides reassuring evidence that reducing LDL-C with statin therapy does not increase the risk of developing a new cancer or deaths from cancer. Specifically, there is no indication of any excess of particular types of cancer with prolonged or more intensive lowering of LDL-C, even among older people242. If low LDL-C concentration is a cause of cancer then one might expect to see a trend towards larger rate ratios among those with lower LDL-C before treatment. In fact, there were fewer cancers among participants with lower baseline LDL-C who were allocated statin or more intensive regimens242. For example, lowering LDL-C from 75 to 50 mg/dl was associated with a non-significant 8 per cent reduction in cancer incidence242. The data provide considerable reassurance about the safety of using intensive statin regimens to lower LDL-C levels substantially in patients who remain at high risk of major vascular events242.
Low cholesterol and high health risk – a case of reverse causality
Observational studies but not randomized clinical trials have shown an association between low cholesterol levels and increased risk of mortality from cancer, respiratory disease, liver disease and accidental/violent death32. This is mostly, or entirely, due to the fact that people with low cholesterol levels include a disproportionate number whose cholesterol has been reduced by illness - early cancer, respiratory disease, gastrointestinal disease and alcoholism, among others243,244. Besides, there was no evidence that lowering of LDL-C increased the risk of non-vascular death or of cancer, even when LDL-C was reduced to 50 mg/dl242. Thus, it appears that both low cholesterol and increased non CVD mortality are due to preexisting disease (reverse causality) and not vice versa10.
Balance of benefits and risks of statin therapy in primary prevention
Despite a doubling of obesity and diabetes, CAD mortality in the United States has declined by 70 per cent in the last 35-40 yr245,246. This decline is attributed to effective control of blood pressure and cholesterol along with reduction in smoking247. The benefits of lowering LDL-C and non-HDL-C are substantially greater in reducing CAD events than lowering blood pressure, which is more effective in preventing stroke rather than CAD. Statins are the most effective agents in lowering LDL-C and non-HDL-C by 50 per cent or 100 mg/dl or more32,34. Statins are also effective in halting the progression and even reversing coronary atherosclerosis, either alone or in combination with niacin26,33. The benefits of statin therapy is overwhelming whereas life threatening complication are extremely rare (Table XI)13,32,35,199,205. The benefit to risk ratio is much higher for statins than for fibrates or aspirin.
Table XI.
Summary of the balance of benefits and risk of statin therapy in primary prevention

Balance of benefits and risks of aspirin versus statin therapy in primary prevention
Aspirin has overwhelming benefit in secondary prevention with an absolute 1.5 per cent risk reduction in MACE which outweighs risk of major bleeding. However, evidence from the collaborative meta-analysis of individual participant data from randomized trials involving 660,000 person-years of follow-up and 3,554 serious MACE, has cast a serious doubt about the use of aspirin in primary prevention248. Both the relative and absolute benefits of aspirin are significantly lower than that of statins in primary prevention. For example, in the JUPITER trial37, people receiving rosuvastatin 20 mg/day had 44 per cent relative risk reduction in MACE which was 4-fold higher than the 12 per cent relative reduction reported in the meta-analysis of aspirin in primary prevention37. More importantly, the absolute risk reduction with rosuvastain was 0.59 per 100 person years, which is 8 times higher than with aspirin (0.07 per 100 person years) in primary prevention248.
In primary prevention of CVD, aspirin use, like alcohol consumption, is a double-edged sword. While physicians and public are well aware of the potential benefits, the risk of serious bleeding with aspirin has received less attention. These serious risks include major gastrointestinal bleeding (even in the absence of other agents that increase the risk) and haemorrhagic stroke. The risk of major bleeding is 1.2 per cent for women and 2.4 per cent for men 60-69 yr of age. The risk increases with increasing age of the patient and increasing dose of aspirin249,250. The risk is nearly double when aspirin is given concomitantly with clopidogrel and 3 times when warfarin is also added. Such triple therapy is common in patients with coronary stent and atrial fibrillation and can be avoided in the first place by starting statin early13.
A million person-years of treatment with aspirin would prevent 10,000 MACE in secondary prevention but only 1,300 MACE in primary pevention248,251. But aspirin therapy will also produce 14,800 bleeding complications including 2,300 major bleeding (requiring hospitalization and transfusion) and 100 haemorrhagic strokes. Accordingly, for every MACE prevented with statin therapy, 10 bleeding will be produced including two major bleedings251. Paradoxically, the benefits of aspirin in people who are already on a statin would be even smaller251. Statin therapy has been shown to reduce MACE by 44 per cent in the JUPITER trial37. Accordingly, the use of a statin before aspirin would reduce the number of MACE prevented to from 1300 to 728 per million, but the bleeding rates would remain the same. This would result in an even worse risk-benefit-ratio with aspirin, producing three major bleeding and 20 total bleeding for every MACE prevented.
Several meta-analyses of randomized controlled trials in patients with DM also failed to confirm a clear benefit of aspirin therapy in the primary prevention of major CVD events. This has led to the withdrawal of the previous recommendations for universal administration of aspirin in patients with DM84,252. It appears that statin therapy is 100 times safer than low dose aspirin (for life threatening complications)249,250. The accumulated evidence shows that statins should supplant aspirin as the first drug of choice in primary prevention of CVD with aspirin reserved for highly select individuals among whom the benefits clearly outweigh the risk.
Balance of benefits and risks of fibrates therapy
Unlike statins, most fibrate trials were conducted in patients with CVD, DM, and/or high triglycerides. None of the clinical trials showed clear benefit of reducing CVD risk from lowering triglycerides per se, although some benefit was noted in subgroups with high triglycerides and low HDL-C52,56,253. Currently the main indication for fibrates is for patients with high triglycerides of >500 mg/dl to prevent pancreatitis and not MI23. When the triglycerides levels are between 150 and 499 mg/dl, statin is the first line agent to achieve the primary target (LDL-C goal). When the LDL-C level is at goal but triglycerides levels are between 150 and 499 mg/dl, a fibrate is often used in combination with a statin to achieve the secondary target of non-HDL-C (but not necessarily to lower triglycerides)52. Fibrates lower triglycerides by as much as 50 per cent, but the reduction in non-HDL-C is only 20 per cent with monotherapy. Although combination of fibrate therapy with moderate dose statins such as 20 mg/day of rosuvastatin or 40 mg of atorvastatin can produce 42 per cent reduction in non-HDL-C and 37 per cent reduction in apo B, these values are lower than those achieved with high dose statin therapy alone. (47% reduction in non-HDL-C and 42 per cent reduction in apo B)52.
The risk of rhabdomyolysis with fibrate monotherapy was 6 times higher than the risk with statin monotherapy208,254. In a study of 252,460 patients treated with lipid-lowering agents, 24 cases of hospitalized rhabdomyolysis occurred during treatment255. Average incidence per million person-years for monotherapy was 44 for statins, 282 for fibrate and 598 for statin fibrate combination therapy. Per person-year of therapy, the number needed to produce one case of rhabdomyolysis was 22,727 for statin monotherapy compared to 484 for older patients with DM who were treated with statin-fibrate combination. However, no such harm was reported in the ACCORD Trial despite net harm was noted in women and non Whites56. It is worth highlighting that the incidence of significant elevation in transaminases elevation in transaminases (AST/ALT) is 5 per cent with fenofibrate – 3 times higher than all statins at the highest doses183. Other adverse effects of fibrates include cholelithiasis and increase in creatinine level56.
Barriers to statin use in clinical practice
Therapy directed at controlling both high blood pressure and high cholesterol can reduce the first or second CVD event by 80 per cent256. However, many patients stop taking medications that are intended to be taken lifelong or take it less than described (non adherence) reducing the potential benefits257.
Many patients fail to achieve the LDL-C and non-HDL-C treatment goals because of a combination of suboptimal prescription rates, failure to titrate statin dose and or premature cessation of therapy. Non-adherence to medications is common and as high as 50 per cent in primary prevention and 34 per cent in secondary prevention. This results in 260,000 avoidable CVD deaths and countless CVD events annually in the US257. Use of higher doses of potent statins such as atorvastatin or rosuvastain results in the greatest number of patients achieving therapeutic goals for all atherogenic lipoproteins [except for Lp(a)]34,51. The lack of any insurance coverage for prescription medications resulting in patient paying the entire costs is another major barrier in India.
Conclusions and recommendations
Previous arguments that statins do not reduce total mortality and that the benefits are limited to high risk individuals (with an annual risk of >2%) are no longer valid. The critical review of evidence clearly demonstrates that statin therapy provides significant overall benefit in primary prevention32,258. In primary prevention, statin therapy prevents between 1200 and 28,400 CVD events and between 200 and 9,200 deaths depending on the degree of LDL-C reduction and the patients risk status. The benefits of statin therapy far outweigh any possible serious adverse effects, even in people at very low risk of CVD events32,35. The risk of NOD is limited to those with known risk factors for DM and the magnitude of the risk is only about half that of diuretics and beta-lockers. The CVD risk reduction is 50 times larger with statins even among those who develop NOD. The excess risk of rhabdomyolysis with statins is 20 and fatal rhabdomyolysis is 2 per million person-years. There is no correlation between magnitude of LDL-C lowering and serious muscle or liver toxicity. This is in sharp contrast to the use of aspirin in primary prevention, where the net harm outweighs the benefit in many people, especially if they are already on a statin. Thus, statins should supplant aspirin as the drug of choice in primary prevention of CVD with aspirin reserved for highly select individuals among whom the benefits clearly outweigh the risk. Lowering LDL-C early in life is substantially more effective in reducing CAD risk than the current practice of lowering LDL-C later in life when subclinical atherosclerosis (silent heart disease) is probably already present. A public health strategy that focuses on prolonged sustained reductions in LDL-C beginning early in life could potentially reduce the global burden of CAD, especially in India. It is time to expand the widely accepted LDL-C dictum from “the lower the better” to “lower and earlier the better”.
References
- 1.Armitage J. The safety of statins in clinical practice. Lancet. 2007;370:1781–90. doi: 10.1016/S0140-6736(07)60716-8. [DOI] [PubMed] [Google Scholar]
- 2.Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–81. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robinson JG, Smith B, Maheshwari N, Schrott H. Pleiotropic effects of statins: benefit beyond cholesterol reduction? A meta-regression analysis. J Am Coll Cardiol. 2005;46:1855–62. doi: 10.1016/j.jacc.2005.05.085. [DOI] [PubMed] [Google Scholar]
- 4.Gaziano TA. Reducing the growing burden of cardiovascular disease in the developing world. Health Aff (Millwood) 2007;26:13–24. doi: 10.1377/hlthaff.26.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Indrayan A. Forecasting vascular disease cases and associated mortality in India. 2010. [accessed on March 5, 2013]. Available from: http://www. whoindia.org/LinkFiles/Commision_on_Macroeconomic_and_Health_Bg_P2_Forecasting_vascular_disease_cases_and_associated_mortality_in_India.pdf(197-215)
- 6.American Heart Association. Heart and Stroke Statistical Update. Circulation. 2012;125:188–97. doi: 10.1161/CIR.0b013e3182456d46. [DOI] [PubMed] [Google Scholar]
- 7.Jolly S, Vittinghoff E, Chattopadhyay A, Bibbins-Domingo K. Higher cardiovascular disease prevalence and mortality among younger blacks compared to whites. Am J Med. 2010;123:811–8. doi: 10.1016/j.amjmed.2010.04.020. [DOI] [PubMed] [Google Scholar]
- 8.Enas EA, Chacko V, Pazhoor SG, Chennikkara H, Devarapalli HP. Dyslipidemia in South Asian patients. Curr Atheroscler Rep. 2007;9:367–74. doi: 10.1007/s11883-007-0047-y. [DOI] [PubMed] [Google Scholar]
- 9. [accessed on May 5, 2013]. Available from: www.cadiresearch.org .
- 10.Smith GD, Shipley MJ, Marmot MG, Rose G. Plasma cholesterol concentration and mortality. The Whitehall Study. JAMA. 1992;267:70–6. [PubMed] [Google Scholar]
- 11.Chen Z, Peto R, Collins R, MacMahon S, Lu J, Li W. Serum cholesterol concentration and coronary heart disease in populations with low cholesterol concentrations. BMJ. 1991;303:276–82. doi: 10.1136/bmj.303.6797.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ezzati M, Lopez AD, Rodgers A, Vander Hoorn S, Murray CJ. Selected major risk factors and global and regional burden of disease. Lancet. 2002;360:1347–60. doi: 10.1016/S0140-6736(02)11403-6. [DOI] [PubMed] [Google Scholar]
- 13.Enas EA, Pazhoor HC, Kuruvila A, Vijayaraghavan K. Intensive Statin Therapy for Indians: Part I Benefits. Indian Heart J. 2011;63:211–27. [PubMed] [Google Scholar]
- 14.Ferrieres J. Effects on coronary atherosclerosis by targeting low-density lipoprotein cholesterol with statins. Am J Cardiovasc Drugs. 2009;9:109–15. doi: 10.1007/BF03256582. [DOI] [PubMed] [Google Scholar]
- 15.O’Keefe JH, Jr, Cordain L, Harris WH, Moe RM, Vogel R. Optimal low-density lipoprotein is 50 to 70 mg/dl: lower is better and physiologically normal. J Am Coll Cardiol. 2004;43:2142–6. doi: 10.1016/j.jacc.2004.03.046. [DOI] [PubMed] [Google Scholar]
- 16.Enas EA, Kuruvilla A. Retracing the heroic steps from lipid hypothesisi to aggressive tratment of blood cholesterol A revolution in preventive cadiology. In: Chopra HK, editor. Texttbook of cardiology. New Delhi: Jaypee Brothers Medical Publishers; 2012. [Google Scholar]
- 17.Welty FK, Lahoz C, Tucker KL, Ordovas JM, Wilson PW, Schaefer EJ. Frequency of ApoB and ApoE gene mutations as causes of hypobetalipoproteinemia in the framingham offspring population. Arterioscler Thromb Vasc Biol. 1998;18:1745–51. doi: 10.1161/01.atv.18.11.1745. [DOI] [PubMed] [Google Scholar]
- 18.Glueck CJ, Kelley W, Gupta A, Fontaine RN, Wang P, Gartside PS. Prospective 10-year evaluation of hypobetalipoproteinemia in a cohort of 772 firefighters and cross-sectional evaluation of hypocholesterolemia in 1,479 men in the National Health and Nutrition Examination Survey I. Metabolism. 1997;46:625–33. doi: 10.1016/s0026-0495(97)90004-4. [DOI] [PubMed] [Google Scholar]
- 19.Welty FK, Mittleman MA, Wilson PW, Sutherland PA, Matheney TH, Lipinska I, et al. Hypobetalipoproteinemia is associated with low levels of hemostatic risk factors in the Framingham offspring population. Circulation. 1997;95:825–30. doi: 10.1161/01.cir.95.4.825. [DOI] [PubMed] [Google Scholar]
- 20.Liu J, Sempos C, Donahue RP, Dorn J, Trevisan M, Grundy SM. Joint distribution of non-HDL and LDL cholesterol and coronary heart disease risk prediction among individuals with and without diabetes. Diabetes Care. 2005;28:1916–21. doi: 10.2337/diacare.28.8.1916. [DOI] [PubMed] [Google Scholar]
- 21.Bittner V, Hardison R, Kelsey SF, Weiner BH, Jacobs AK, Sopko G. Non-high-density lipoprotein cholesterol levels predict five-year outcome in the Bypass Angioplasty Revascularization Investigation (BARI) Circulation. 2002;106:2537–42. doi: 10.1161/01.cir.0000038496.57570.06. [DOI] [PubMed] [Google Scholar]
- 22.Pischon T, Girman CJ, Sacks FM, Rifai N, Stampfer MJ, Rimm EB. Non-high-density lipoprotein cholesterol and apolipoprotein B in the prediction of coronary heart disease in men. Circulation. 2005;112:3375–83. doi: 10.1161/CIRCULATIONAHA.104.532499. [DOI] [PubMed] [Google Scholar]
- 23.National Heart, Lung, and Blood Institute, National Institute of Health Bathesda; NIH Publication; 2001. Third Report of the Expert Panel on Detection, Evaluation, and Treatment of the High Blood Cholesterol in Adults (Adult Treatment Panel III): Executive Summary National Cholesterol Education Program. [Google Scholar]
- 24.Grundy SM, Vega GL, Tomassini JE, Tershakovec AM. Correlation of non-high-density lipoprotein cholesterol and low-density lipoprotein cholesterol with apolipoprotein B during simvastatin + fenofibrate therapy in patients with combined hyperlipidemia (a subanalysis of the SAFARI trial) Am J Cardiol. 2009;104:548–53. doi: 10.1016/j.amjcard.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 25.Grundy SM, Cleeman JI, Merz CN, Brewer HB, Jr, Clark LT, Hunninghake DB, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110:227–39. doi: 10.1161/01.CIR.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
- 26.Nicholls SJ, Ballantyne CM, Barter PJ, Chapman MJ, Erbel RM, Libby P, et al. Effect of two intensive statin regimens on progression of coronary disease. N Engl J Med. 2011;365:2078–87. doi: 10.1056/NEJMoa1110874. [DOI] [PubMed] [Google Scholar]
- 27.Renault BJ, Rana JS. Beyond low-Density Lipoprotein Cholesterol. J Am Coll Cardiol. 2010;55:35–41. doi: 10.1016/j.jacc.2009.07.057. [DOI] [PubMed] [Google Scholar]
- 28.Robinson J, Wang S, Smith BJ, Jacobson TA. Meta-analysis of the relationship between non-high-density lipoprotein cholesterol reduction and coronary heart disease risk. J Am Coll Cardiol. 2009;53:316–22. doi: 10.1016/j.jacc.2008.10.024. [DOI] [PubMed] [Google Scholar]
- 29.Charlton-Menys V, Betteridge DJ, Colhoun H, Fuller J, France M, Hitman GA, et al. Targets of statin therapy: LDL cholesterol, non-HDL cholesterol, and apolipoprotein B in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS) Clin Chem. 2009;55:473–80. doi: 10.1373/clinchem.2008.111401. [DOI] [PubMed] [Google Scholar]
- 30.Barter PJ, Ballantyne CM, Carmena R, Cabezas MC, Chapman MJ, Couture P, et al. Apo B versus cholesterol in estimating cardiovascular risk and in guiding therapy: report of the thirty-person/ten-country panel. J Intern Med. 2006;259:247–58. doi: 10.1111/j.1365-2796.2006.01616.x. [DOI] [PubMed] [Google Scholar]
- 31.Brunzell JD, Davidson M, Furberg CD, Goldberg RB, Howard BV, Stein JH, et al. Lipoprotein management in patients with cardiometabolic risk: consensus conference report from the American Diabetes Association and the American College of Cardiology Foundation. J Am Coll Cardiol. 2008;51:1512–24. doi: 10.1016/j.jacc.2008.02.034. [DOI] [PubMed] [Google Scholar]
- 32.Taylor F, Huffman MD, Macedo AF, Moore TH, Burke M, Davey Smith G, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013;1:1–97. doi: 10.1002/14651858.CD004816.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taylor AJ, Villines TC, Stanek EJ, Devine PJ, Griffen L, Miller M, et al. Extended-release niacin or ezetimibe and carotid intima-media thickness. N Engl J Med. 2009;361:2113–22. doi: 10.1056/NEJMoa0907569. [DOI] [PubMed] [Google Scholar]
- 34.Nicholls SJ, Brandrup-Wognsen G, Palmer M, Barter PJ. Meta-analysis of comparative efficacy of increasing dose of Atorvastatin versus Rosuvastatin versus Simvastatin on lowering levels of atherogenic lipids (from VOYAGER) Am J Cardiol. 2010;105:69–76. doi: 10.1016/j.amjcard.2009.08.651. [DOI] [PubMed] [Google Scholar]
- 35.Cholesterol Treatment Trialists’ (CTT) Collaborators. Mihaylova BEJ, Blackwell L, Keech A, Simes J, Barnes EH, Voysey M, et al. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. 2012;380:581–90. doi: 10.1016/S0140-6736(12)60367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kerr AJ, Broad J, Wells S, Riddell T, Jackson R. Should the first priority in cardiovascular risk management be those with prior cardiovascular disease? Heart. 2009;95:125–9. doi: 10.1136/hrt.2007.140905. [DOI] [PubMed] [Google Scholar]
- 37.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr, Kastelein JJ, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 38.Hsia J, Macfadyen JG, Monyak J, Ridker PM. Cardiovascular Event Reduction and Adverse Events Among Subjects Attaining Low-Density Lipoprotein Cholesterol <50 mg/dl With Rosuvastatin The JUPITER Trial (Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin) J Am Coll Cardiol. 2011;57:1666–75. doi: 10.1016/j.jacc.2010.09.082. [DOI] [PubMed] [Google Scholar]
- 39.Muldoon MF, Manuck SB, Mendelsohn AB, Kaplan JR, Belle SH. Cholesterol reduction and non-illness mortality: Meta-analysis of randomised clinical trials. BMJ. 2001;322:11–5. doi: 10.1136/bmj.322.7277.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Milionis H.J, Giannopoulos S, Kosmidou M, Panoulas V, Manios E, Kyritsis AP, et al. Statin therapy after first stroke reduces 10-year stroke recurrence and improves survival. Neurology. 2009;72:1816–22. doi: 10.1212/WNL.0b013e3181a711cb. [DOI] [PubMed] [Google Scholar]
- 41.Ni Chroinin D, Asplund K, Asberg S, Callaly E, Cuadrado-Godia E, Diez-Tejedor E, et al. Statin therapy and outcome after ischemic stroke: systematic review and meta-analysis of observational studies and randomized trials. Stroke. 2013;44:448–56. doi: 10.1161/STROKEAHA.112.668277. [DOI] [PubMed] [Google Scholar]
- 42.Athyros VG, Tziomalos K, Karagiannis A, Wierzbicki AS, Mikhailidis DP. Aggressive statin treatment, very low serum cholesterol levels and haemorrhagic stroke: is there an association? Curr Opin Cardiol. 2010;25:406–10. doi: 10.1097/HCO.0b013e3283393c1a. [DOI] [PubMed] [Google Scholar]
- 43.Flint AC, Kamel H, Navi BB, Rao VA, Faigeles BS, Conell C, et al. Statin use during ischemic stroke hospitalization is strongly associated with improved poststroke survival. Stroke. 2012;43:147–54. doi: 10.1161/STROKEAHA.111.627729. [DOI] [PubMed] [Google Scholar]
- 44.Kaul U, Bhatia V. Perspective on coronary interventions & cardiac surgeries in India. Indian J Med Res. 2010;132:543–8. [PMC free article] [PubMed] [Google Scholar]
- 45.Graham I, Atar D, Borch-Johnsen K, Boysen G, Durrington PN. European guidelines on cardiovascular disease prevention in clinical practice: executive summary: Fourth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (Constituted by representatives of nine societies and by invited experts) Eur Heart J. 2007;28:2375–414. doi: 10.1093/eurheartj/ehm316. [DOI] [PubMed] [Google Scholar]
- 46.Kashani A, Phillips CO, Foody JM, Wang Y, Mangalmurti S, Ko DT, et al. Risks associated with statin therapy: a systematic overview of randomized clinical trials. Circulation. 2006;114:2788–97. doi: 10.1161/CIRCULATIONAHA.106.624890. [DOI] [PubMed] [Google Scholar]
- 47.Silva MA, Swanson AC, Gandhi PJ, Tataronis GR. Statin-related adverse events: a meta-analysis. Clin Ther. 2006;28:26–35. doi: 10.1016/j.clinthera.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 48.Dale KM, Coleman CI, Henyan NN, Kluger J, White CM. Statins and cancer risk: a meta-analysis. JAMA. 2006;295:74–80. doi: 10.1001/jama.295.1.74. [DOI] [PubMed] [Google Scholar]
- 49.Tsai CJ, Leitzmann MF, Willett WC, Giovannucci EL. Statin use and the risk of cholecystectomy in women. Gastroenterology. 2009;136:1593–600. doi: 10.1053/j.gastro.2009.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pellegrini CN, Vittinghoff E, Lin F, Hulley SB, Marcus GM. Statin use is associated with lower risk of atrial fibrillation in women with coronary disease: the HERS trial. Heart. 2009;95:704–8. doi: 10.1136/hrt.2008.154054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Enas EA, Chacko V, Senthilkumar A, Puthumana N, Mohan V. Elevated lipoprotein(a) - a genetic risk factor for premature vascular disease in people with and without standard risk factors: a review. Dis Mon. 2006;52:1–50. doi: 10.1016/j.disamonth.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 52.Miller M, Stone NJ, Ballantyne C, Bittner V, Criqui MH, Ginsberg HN, et al. Triglycerides and cardiovascular disease: A scientific statement from the American Heart Association. Circulation. 2011;123:2292–333. doi: 10.1161/CIR.0b013e3182160726. [DOI] [PubMed] [Google Scholar]
- 53.Stein EA, Lane M, Laskarzewski P. Comparison of statins in hypertriglyceridemia. Am J Cardiol. 1998;81:66B–9B. doi: 10.1016/s0002-9149(98)00041-1. [DOI] [PubMed] [Google Scholar]
- 54.LIPITOR U.S. Patient Product Information. [accessed on October 1, 2013]. WWW.Pfizer LTD.
- 55.Karalis DG, Ishisaka DY, Luo D, Ntanios F, Wun CC. Effects of increasing doses of atorvastatin on the atherogenic lipid subclasses commonly associated with hypertriglyceridemia. Am J Cardiol. 2007;100:445–49. doi: 10.1016/j.amjcard.2007.03.043. [DOI] [PubMed] [Google Scholar]
- 56.Accord Study Group. Ginsberg HN, Elam MB, Lovato LC, Crouse JR, 3rd, Leiter LA, et al. Effects of Combination Lipid Therapy in Type 2 Diabetes Mellitus. N Engl J Med. 2010;362:1563–74. doi: 10.1056/NEJMoa1001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hou W, Lv J, Perkovic V, Yang L, Zhao N, Jardine MJ, et al. Effect of statin therapy on cardiovascular and renal outcomes in patients with chronic kidney disease: a systematic review and meta-analysis. Eur Heart J. 2013;34:1807–17. doi: 10.1093/eurheartj/eht065. [DOI] [PubMed] [Google Scholar]
- 58.Sarnak MJ, Levey AS. Cardiovascular disease and chronic renal disease: a new paradigm. Am J Kidney Dis. 2000;35(4 Suppl 1):S117–131. doi: 10.1016/s0272-6386(00)70239-3. [DOI] [PubMed] [Google Scholar]
- 59.Magnussen CG, Koskinen J, Juonala M, Chen W, Srinivasan SR, Sabin MA, et al. A diagnosis of the metabolic syndrome in youth that resolves by adult life is associated with a normalization of high carotid intima-media thickness and type 2 diabetes mellitus risk: the Bogalusa heart and cardiovascular risk in young Finns studies. J Am Coll Cardiol. 2012;60:1631–9. doi: 10.1016/j.jacc.2012.05.056. [DOI] [PubMed] [Google Scholar]
- 60.Mente A, Yusuf S, Islam S, et al. Metabolic syndrome and risk of acute myocardial infarction a case-control study of 26,903 subjects from 52 countries. J Am Coll Cardiol. 2010;55:2390–8. doi: 10.1016/j.jacc.2009.12.053. [DOI] [PubMed] [Google Scholar]
- 61.Deedwania P, Barter P, Carmena R, Fruchart JC, Grundy SM, Haffner S, et al. Reduction of low-density lipoprotein cholesterol in patients with coronary heart disease and metabolic syndrome: analysis of the Treating to New Targets study. Lancet. 2006;368:919–28. doi: 10.1016/S0140-6736(06)69292-1. [DOI] [PubMed] [Google Scholar]
- 62.Enas EA, Mohan V, Deepa M, Farooq S, Pazhoor S, Chennikkara H. The metabolic syndrome and dyslipidemia among Asian Indians: a population with high rates of diabetes and premature coronary artery disease. J Cardiometab Syndr. 2007;2:267–75. doi: 10.1111/j.1559-4564.2007.07392.x. [DOI] [PubMed] [Google Scholar]
- 63.Enas EA, Kuruvila A, Veedu J, Mohandas A, Menon D. Diagnosis and management of metabolic syndrome: The “common soil” for diabetes and premature heart disease in India. J Prev Cardiol. 2012;2:201–18. [Google Scholar]
- 64.Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HA, Livingstone SJ, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364:685–96. doi: 10.1016/S0140-6736(04)16895-5. [DOI] [PubMed] [Google Scholar]
- 65.Conroy RM, Pyorala K, Fitzgerald AP, Sans S, Menotti A, De Backer G, et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J. 2003;24:987–1003. doi: 10.1016/s0195-668x(03)00114-3. [DOI] [PubMed] [Google Scholar]
- 66.Eichler K, Puhan MA, Steurer J, Bachmann LM. Prediction of first coronary events with the Framingham score: a systematic review. Am Heart J. 2007;153:722–31. doi: 10.1016/j.ahj.2007.02.027. [DOI] [PubMed] [Google Scholar]
- 67.Gu K, Cowie CC, Harris MI. Mortality in adults with and without diabetes in a national cohort of the U.S. population, 1971-1993. Diabetes Care. 1998;21:1138–45. doi: 10.2337/diacare.21.7.1138. [DOI] [PubMed] [Google Scholar]
- 68.Norhammar A, Tenerz A, Nilsson G, Hamsten A, Efendic S, Ryden L, et al. Glucose metabolism in patients with acute myocardial infarction and no previous diagnosis of diabetes mellitus: a prospective study. Lancet. 2002;359:2140–4. doi: 10.1016/S0140-6736(02)09089-X. [DOI] [PubMed] [Google Scholar]
- 69.Ramachandran A, Chamukuttan S, Immaneni S, Shanmugam RM, Vishnu N, Viswanathan V, et al. High incidence of glucose intolerance in Asian-Indian subjects with acute coronary syndrome. Diabetes Care. 2005;28:2492–6. doi: 10.2337/diacare.28.10.2492. [DOI] [PubMed] [Google Scholar]
- 70.Lee JW, Brancati FL, Yeh HC. Trends in the prevalence of Type 2 diabetes in Asians versus Whites: Results from the United States National Health Interview Survey, 1997-2008. Diabetes Care. 2011;34:353–7. doi: 10.2337/dc10-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Forouhi NG, Sattar N, Tillin T, McKeigue PM, Chaturvedi N. Do known risk factors explain the higher coronary heart disease mortality in South Asian compared with European men. Prospective follow-up of the Southall and Brent studies, UK? Diabetologia. 2006;49:2580–8. doi: 10.1007/s00125-006-0393-2. [DOI] [PubMed] [Google Scholar]
- 72.Chaturvedi N, Fuller JH. Ethnic differences in mortality from cardiovascular disease in the UK: do they persist in people with diabetes? J Epidemiol Community Health. 1996;50:137–9. doi: 10.1136/jech.50.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mather HM, Chaturvedi N, Fuller JH. Mortality and morbidity from diabetes in South Asians and Europeans: 11- year follow-up of the Southall Diabetes Survey, London, UK. Diabet Med. 1998;15:53–9. doi: 10.1002/(SICI)1096-9136(199801)15:1<53::AID-DIA521>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 74.Ma S, Cutter J, Tan CE, Chew SK, Tai ES. Associations of diabetes mellitus and ethnicity with mortality in a multiethnic Asian population: data from the 1992 Singapore National Health Survey. Am J Epidemiol. 2003;158:543–52. doi: 10.1093/aje/kwg199. [DOI] [PubMed] [Google Scholar]
- 75.Karter AJ, Ferrara A, Liu JY, Moffet HH, Ackerson LM, Selby JV. Ethnic disparities in diabetic complications in an insured population. JAMA. 2002;287:2519–27. doi: 10.1001/jama.287.19.2519. [DOI] [PubMed] [Google Scholar]
- 76.Kamalesh M, Subramanian U, Ariana A, Sawada S, Peterson E. Diabetes status and racial differences in post-myocardial infarction mortality. Am Heart J. 2005;150:912–9. doi: 10.1016/j.ahj.2005.02.042. [DOI] [PubMed] [Google Scholar]
- 77.Selvin E, Marinopoulos S, Berkenblit G, Rami T, Brancati FL, Powe NR, et al. Meta-analysis: glycosylated hemoglobin and cardiovascular disease in diabetes mellitus. Ann Intern Med. 2004;141:421–31. doi: 10.7326/0003-4819-141-6-200409210-00007. [DOI] [PubMed] [Google Scholar]
- 78.Ray KK, Seshasai SR, Wijesuriya S, Sivakumaran R, Nethercott S, Preiss D, et al. Effect of intensive control of glucose on cardiovascular outcomes and death in patients with diabetes mellitus: a meta-analysis of randomised controlled trials. Lancet. 2009;373:1765–72. doi: 10.1016/S0140-6736(09)60697-8. [DOI] [PubMed] [Google Scholar]
- 79.Delahoy PJ, Magliano DJ, Webb K, Grobler M, Liew D. The relationship between reduction in low-density lipoprotein cholesterol by statins and reduction in risk of cardiovascular outcomes: an updated meta-analysis. Clin Ther. 2009;31:236–44. doi: 10.1016/j.clinthera.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 80.Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ. 2009;338:b1665. doi: 10.1136/bmj.b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lewington S, Whitlock G, Clarke R, Sherliker P, Emberson J, Halsey J, et al. Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet. 2007;370:1829–39. doi: 10.1016/S0140-6736(07)61778-4. [DOI] [PubMed] [Google Scholar]
- 82.Kearney PM, Blackwell L, Collins R, Keech A, Simes J, Peto R, et al. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet. 2008;371:117–25. doi: 10.1016/S0140-6736(08)60104-X. [DOI] [PubMed] [Google Scholar]
- 83.Younis N, Williams S, Soran H. Aspirin therapy and primary prevention of cardiovascular disease in diabetes mellitus. Diabetes Obes Metab. 2009;11:997–1000. doi: 10.1111/j.1463-1326.2009.01068.x. [DOI] [PubMed] [Google Scholar]
- 84.Zhang C, Sun A, Zhang P, Wu C, Zhang S, Fu M, et al. Aspirin for primary prevention of cardiovascular events in patients with diabetes: A meta-analysis. Diabetes Res Clin Pract. 2010;87:211–8. doi: 10.1016/j.diabres.2009.09.029. [DOI] [PubMed] [Google Scholar]
- 85.Schmidt BM, Arora R. Primary prevention of cardiovascular complications in type II diabetes patients using aspirin: A complicated tale. Am J Ther. 2013;20:275–8. doi: 10.1097/MJT.0b013e3181afbf17. [DOI] [PubMed] [Google Scholar]
- 86.Strandberg TE, Pyorala K, Cook TJ, Wilhelmsen L, Faergeman O, Thorgeirsson G, et al. Mortality and incidence of cancer during 10-year follow-up of the Scandinavian Simvastatin Survival Study (4S) Lancet. 2004;364:771–7. doi: 10.1016/S0140-6736(04)16936-5. [DOI] [PubMed] [Google Scholar]
- 87.Ford I, Murray H, Packard CJ, Shepherd J, Macfarlane PW, Cobbe SM. Long-term follow-up of the West of Scotland Coronary Prevention Study. N Engl J Med. 2007;357:1477–86. doi: 10.1056/NEJMoa065994. [DOI] [PubMed] [Google Scholar]
- 88.LIPID Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. N Engl J Med. 1998;339:1349–57. doi: 10.1056/NEJM199811053391902. [DOI] [PubMed] [Google Scholar]
- 89.Cohen JC, Boerwinkle E, Mosley TH, Jr, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med. 2006;354:1264–72. doi: 10.1056/NEJMoa054013. [DOI] [PubMed] [Google Scholar]
- 90.Ference BA, Yoo W, Alesh I, Mahajan N, Mirowska KK, Mewada A, et al. Effect of long-term exposure to lower low-density lipoprotein cholesterol beginning early in life on the risk of coronary heart disease: a mendelian randomization analysis. J Am Coll Cardiol. 2012;60:2631–9. doi: 10.1016/j.jacc.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 91.Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110:227–39. doi: 10.1161/01.CIR.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
- 92.Shepherd J, Cobbe SM, Ford I, Isles CG, Lorimer AR, MacFarlane PW, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. Atheroscler. 2004;5(Suppl):91–7. doi: 10.1016/j.atherosclerosissup.2004.08.029. [DOI] [PubMed] [Google Scholar]
- 93.Downs JR, Clearfield M, Weiss S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels. JAMA. 1998;279:1615–22. doi: 10.1001/jama.279.20.1615. [DOI] [PubMed] [Google Scholar]
- 94.Kathiresan S. A PCSK9 missense variant associated with a reduced risk of early-onset myocardial infarction. N Engl J Med. 2008;358:2299–300. doi: 10.1056/NEJMc0707445. [DOI] [PubMed] [Google Scholar]
- 95.Thompson PD, Moyna NM, White CM, Weber KM, Giri S, Waters DD. The effects of hydroxy-methyl-glutaryl co-enzyme A reductase inhibitors on platelet thrombus formation. Atherosclerosis. 2002;161:301–6. doi: 10.1016/s0021-9150(01)00645-1. [DOI] [PubMed] [Google Scholar]
- 96.Dangas G, Badimon JJ, Smith DA, Unger AH, Levine D, Shao JH, et al. Pravastatin therapy in hyperlipidemia: effects on thrombus formation and the systemic hemostatic profile. J Am Coll Cardiol. 1999;33:1294–304. doi: 10.1016/s0735-1097(99)00018-2. [DOI] [PubMed] [Google Scholar]
- 97.Bonnet J, McPherson R, Tedgui A, Simoneau D, Nozza A, Martineau P, et al. Comparative effects of 10-mg versus 80-mg Atorvastatin on high-sensitivity C-reactive protein in patients with stable coronary artery disease: results of the CAP (Comparative Atorvastatin Pleiotropic effects) study. Clin Ther. 2008;30:2298–313. doi: 10.1016/j.clinthera.2008.12.023. [DOI] [PubMed] [Google Scholar]
- 98.Squizzato A, Galli M, Romualdi E, Dentali F, Kamphuisen PW, Guasti L, et al. Statins, fibrates, and venous thromboembolism: a meta-analysis. Eur Heart J. 2010;31:1248–56. doi: 10.1093/eurheartj/ehp556. [DOI] [PubMed] [Google Scholar]
- 99.Abuissa H, O’Keefe JH, Bybee KA. Statins as antiarrhythmics: A systematic review Part I: Effects on risk of atrial fibrillation. Clin Cardiol. 2009;32:544–8. doi: 10.1002/clc.20669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Abuissa H, O’Keefe JH, Bybee KA. Statins as Anti-Arrhythmics: A Systematic Review Part II: Effects on Risk of Ventricular Arrhythmias. Clin Cardiol. 2009;32:549–52. doi: 10.1002/clc.20435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fauchier L, Pierre B, de Labriolle A, Grimard C, Zannad N, Babuty D. Antiarrhythmic effect of statin therapy and atrial fibrillation a meta-analysis of randomized controlled trials. J Am Coll Cardiol. 2008;51:828–35. doi: 10.1016/j.jacc.2007.09.063. [DOI] [PubMed] [Google Scholar]
- 102.Khemasuwan D, Divietro ML, Tangdhanakanond K, Pomerantz SC, Eiger G. Statins decrease the occurrence of venous thromboembolism in patients with cancer. Am J Med. 2010;123:60–5. doi: 10.1016/j.amjmed.2009.05.025. [DOI] [PubMed] [Google Scholar]
- 103.Caroli-Bosc FX, Le Gall P, Pugliese P, Delabre B, Caroli-Bosc C, Demarquay JF, et al. Role of fibrates and HMG-CoA reductase inhibitors in gallstone formation: epidemiological study in an unselected population. Dig Dis Sci. 2001;46:540–4. doi: 10.1023/a:1005643014395. [DOI] [PubMed] [Google Scholar]
- 104.Bodmer M, Brauchli YB, Krahenbuhl S, Jick SS, Meier CR. Statin use and risk of gallstone disease followed by cholecystectomy. JAMA. 2009;302:2001–7. doi: 10.1001/jama.2009.1601. [DOI] [PubMed] [Google Scholar]
- 105.Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937–52. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 106.Reiner Z, Catapano AL, De Backer G, Graham I, Taskinen MR, Wiklund O, et al. ESC/EAS Guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS) Eur Heart J. 2011;32:1769–818. doi: 10.1093/eurheartj/ehr158. [DOI] [PubMed] [Google Scholar]
- 107.Lazar LD, Pletcher MJ, Coxson PG, Bibbins-Domingo K, Goldman L. Cost-effectiveness of statin therapy for primary prevention in a low-cost statin era. Circulation. 2011;124:146–53. doi: 10.1161/CIRCULATIONAHA.110.986349. [DOI] [PubMed] [Google Scholar]
- 108.Genest J, McPherson R, Frohlich J, Anderson T, Campbell N, Carpentier A, et al. Canadian Cardiovascular Society/Canadian guidelines for the diagnosis and treatment of dyslipidemia and prevention of cardiovascular disease in the adult - 2009 recommendations. Cana J Cardiol. 2009;25:567–79. doi: 10.1016/s0828-282x(09)70715-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lipid Modification. Cardiovadcular risk Assessment and of Blood lipids for the Primary and Secondary prevention of Cardiovadculr Disease. [accessed on May 31, 2013]. Available from: http://www.niceorg.uk/guidanceCG 67 .
- 110.Pletcher MJ, Lazar L, Bibbins-Domingo K, Moran A, Rodondi N, Coxson P, et al. Comparing impact and cost-effectiveness of primary prevention strategies for lipid-lowering. Ann Intern Med. 2009;150:243–54. doi: 10.7326/0003-4819-150-4-200902170-00005. [DOI] [PubMed] [Google Scholar]
- 111.Law MR, Wald NJ, Thompson SG. By how much and how quickly does reduction in serum cholesterol concentration lower risk of ischaemic heart disease? BMJ. 1994;308:367–72. doi: 10.1136/bmj.308.6925.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wiegman A, Hutten BA, de Groot E, Rodenburg J, Bakker HD, Buller HR, et al. Efficacy and safety of statin therapy in children with familial hypercholesterolemia: a randomized controlled trial. JAMA. 2004;292:331–7. doi: 10.1001/jama.292.3.331. [DOI] [PubMed] [Google Scholar]
- 113.Hartiala O, Magnussen CG, Kajander S, Knuuti J, Ukkonen H, Saraste A, et al. Adolescence risk factors are predictive of coronary artery calcification at middle age: the cardiovascular risk in young Finns study. J Am Coll Cardiol. 2012;60:1364–70. doi: 10.1016/j.jacc.2012.05.045. [DOI] [PubMed] [Google Scholar]
- 114.McCrindle BW, Ose L, Marais AD. Efficacy and safety of atorvastatin in children and adolescents with familial hypercholesterolemia or severe hyperlipidemia: a multicenter, randomized, placebo-controlled trial. J Pediatr. 2003;143:74–80. doi: 10.1016/S0022-3476(03)00186-0. [DOI] [PubMed] [Google Scholar]
- 115.Rader DJ, Cohen J, Hobbs HH. Monogenic hypercholesterolemia: new insights in pathogenesis and treatment. J Clin Invest. 2003;111:1795–803. doi: 10.1172/JCI18925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Macchiaiolo M, Gagliardi MG, Toscano A, Guccione P, Bartuli A. Homozygous familial hypercholesterolaemia. Lancet. 2012;379:1330. doi: 10.1016/S0140-6736(11)61476-1. [DOI] [PubMed] [Google Scholar]
- 117.DeWeerdt SE, Culliton BJ. Bethesda: Medical Communications Resources Inc; 1998. Vital signs: Discoveries in diseases of heart, lungs, and blood. [Google Scholar]
- 118.Daniels SR. Bethesda: Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents: Report from National Heart, Lung and Blood Institute; [accessed on March 20, 2013]. http://www.nhlbi.nih.gov/guidelines/cvd_ped/index.htm2011.2011 . [Google Scholar]
- 119.Enas EA, Singh V, Munjal YP, Gupta R, Patel KC, Bhandari S, et al. Recommendations of the second Indo-U.S. health summit on prevention and control of cardiovascular disease among Asian Indians. Indian Heart J. 2009;61:265–74. [PubMed] [Google Scholar]
- 120.Gajalakshmi V, Peto R, Kanaka S, Balasubramanian S. Verbal autopsy of 48 000 adult deaths attributable to medical causes in Chennai (formerly Madras), India. BMC Public Health. 2002;2:7. doi: 10.1186/1471-2458-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Roger VL, Go AS, DM, Adams RJ, Jarett D. Heart Disease and Stroke Statistics-2011 Update. A Report From the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Palaniappan L, Mukherjea A, Holland A, Ivey SL. Leading causes of mortality of Asian Indians in California. Ethn Dis. 2010;20:53–7. [PubMed] [Google Scholar]
- 123.Miller GJ, Beckles GL, Maude GH, Carson DC, Alexis SD, Price SG, et al. Ethnicity and other characteristics predictive of coronary heart disease in a developing community: principal results of the St James Survey, Trinidad. Int J Epidemiol. 1989;18:808–17. doi: 10.1093/ije/18.4.808. [DOI] [PubMed] [Google Scholar]
- 124.Aarabi M, Jackson PR. Predicting coronary risk in UK South Asians: an adjustment method for Framingham-based tools. Eur J Cardiovasc Prev Rehabil. 2005;12:46–51. [PubMed] [Google Scholar]
- 125.Enas EA. Why is there an epidemic of malignant CAD in young Indians? Asian J Clin Cardiol. 1998;1:43–59. [Google Scholar]
- 126.Enas EA. Downers Grove: Advanced Heart Lipid Clinic USA; 2013. How to beat the heart disease epidemic among South Asians: A prevention and management guide for Asian Indians and their Doctors. [Google Scholar]
- 127.Smith J, Cianflone K, Al-Amri M, Sniderman A. Body composition and the apoB/apoA-I ratio in migrant Asian Indians and white Caucasians in Canada. Clin Sci (Lond) 2006;111:201–7. doi: 10.1042/CS20060045. [DOI] [PubMed] [Google Scholar]
- 128.Sierra-Johnson J, Somers VK, Kuniyoshi FH, Garza CA, Isley WL, Gami AS, et al. Comparison of apolipoprotein-B/apolipoprotein-AI in subjects with versus without the metabolic syndrome. Am J Cardiol. 2006;98:1369–73. doi: 10.1016/j.amjcard.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 129.Joshi P, Islam S, Pais P, Reddy S, Dorairaj P, Kazmi K, et al. Risk factors for early myocardial infarction in South Asians compared with individuals in other countries. JAMA. 2007;297:286–94. doi: 10.1001/jama.297.3.286. [DOI] [PubMed] [Google Scholar]
- 130.Karthikeyan G, Teo KK, Islam S, McQueen MJ, Pais P, Wang X, et al. Lipid profile, plasma apolipoproteins, and risk of a first myocardial infarction among Asians: an analysis from the INTERHEART Study. J Am Coll Cardiol. 2009;53:244–53. doi: 10.1016/j.jacc.2008.09.041. [DOI] [PubMed] [Google Scholar]
- 131.Enas EA, Dhawan J, Petkar S. Coronary artery disease in Asian Indians: lessons learnt and the role of lipoprotein(a) Indian Heart J. 1997;49:25–34. [PubMed] [Google Scholar]
- 132.Boon MR, Karamali NS, de Groot CJ, van Steijn L, Kanhai HH, van der Bent C, et al. E-selectin is elevated in cord blood of South Asian neonates compared with Caucasian neonates. J Pediatr. 2012;160:844–8.e841. doi: 10.1016/j.jpeds.2011.11.025. [DOI] [PubMed] [Google Scholar]
- 133.Low PS, Heng CK, Saha N, Tay JS. Racial variation of cord plasma lipoprotein(a) levels in relation to coronary risk level: a study in three ethnic groups in Singapore. Pediatr Res. 1996;40:718–22. doi: 10.1203/00006450-199611000-00011. [DOI] [PubMed] [Google Scholar]
- 134.Enas EA. Coronary artery disease epidemic in Indians: a cause for alarm and call for action. J Indian Med Assoc. 2000;98:694. [PubMed] [Google Scholar]
- 135.Enas EA, Singh V, Munjal YP, Bhandari S, Yadave RD, Manchanda SC. Reducing the burden of coronary artery disease in India: challenges and opportunities. Indian Heart J. 2008;60:161–75. [PubMed] [Google Scholar]
- 136.Sachdeva A, Cannon CP, Deedwania PC, Labresh KA, Smith SC, Jr, Dai D, et al. Lipid levels in patients hospitalized with coronary artery disease: an analysis of 136,905 hospitalizations in Get With The Guidelines. Am Heart J. 2009;157:111–7 e112. doi: 10.1016/j.ahj.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 137.Enas EA. High rates of CAD in Asian Indians in the United States despite intense modification of lifestyle: What next? Current Science. 1998;74:1081–6. [Google Scholar]
- 138.Howard G, Lackland DT, Kleindorfer DO, Kissela BM, Moy CS, Judd SE, et al. Racial differences in the impact of elevated systolic blood pressure on stroke risk. JAMA Intern Med Jan. 2013;173:46–51. doi: 10.1001/2013.jamainternmed.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Flack JM, Sica DA, Bakris G, Brown AL, Ferdinand KC, Grimm RH, Jr, et al. Management of high blood pressure in Blacks: an update of the International Society on Hypertension in Blacks consensus statement. Hypertension. 2010;56:780–800. doi: 10.1161/HYPERTENSIONAHA.110.152892. [DOI] [PubMed] [Google Scholar]
- 140.Enas EA, Mehta J. Malignant coronary artery disease in young Asian Indians: thoughts on pathogenesis, prevention, and therapy. Coronary Artery Disease in Asian Indians (CADI) Study. Clin Cardiol. 1995;18:131–5. doi: 10.1002/clc.4960180305. [DOI] [PubMed] [Google Scholar]
- 141.Refsum H, Yajnik CS, Gadkari M, Schneede J, Vollset SE, Orning L, et al. Hyperhomocysteinemia and elevated methylmalonic acid indicate a high prevalence of cobalamin deficiency in Asian Indians. Am J Clin Nutr. 2001;74:233–41. doi: 10.1093/ajcn/74.2.233. [DOI] [PubMed] [Google Scholar]
- 142.Superko HR, Enas EA, Kotha P, Bhat NK, Garrett B. High-density lipoprotein subclass distribution in individuals of asian Indian descent: the National Asian Indian Heart Disease Project. Prev Cardiol Spring. 2005;8:81–6. doi: 10.1111/j.1520-037x.2005.3766.x. [DOI] [PubMed] [Google Scholar]
- 143.Anand SS, Enas EA, Pogue J, Haffner S, Pearson T, Yusuf S. Elevated lipoprotein(a) levels in South Asians in North America. Metabolism. 1998;47:182–4. doi: 10.1016/s0026-0495(98)90217-7. [DOI] [PubMed] [Google Scholar]
- 144.Hopkins PN, Hunt SC, Schreiner PJ, et al. Lipoprotein(a) interactions with lipid and non-lipid risk factors in patients with early onset coronary artery disease: results from the NHLBI Family Heart Study. Atherosclerosis. 1998;141:333–45. doi: 10.1016/s0021-9150(98)00174-9. [DOI] [PubMed] [Google Scholar]
- 145.Refsum H, Yajnik CS, Gadkari M, Schneede J, Vollset SE, Orning L, et al. Hyperhomocysteinemia and elevated methylmalonic acid indicate a high prevalence of cobalamin deficiency in Asian Indians. Am J Clin Nutr. 2001;74:233–41. doi: 10.1093/ajcn/74.2.233. [DOI] [PubMed] [Google Scholar]
- 146.McKenney JM, Davidson MH, Saponaro J, Thompson PD, Bays HE. Use of a treatment algorithm to achieve NCEP ATP III goals with atorvastatin. J Cardiovasc Pharmacol. 2005;46:594–9. doi: 10.1097/01.fjc.0000180901.70607.72. [DOI] [PubMed] [Google Scholar]
- 147.Nakamura H, Arakawa K, Itakura H, Kitabatake A, Goto Y, Toyota T, et al. Primary prevention of cardiovascular disease with pravastatin in Japan (MEGA Study): a prospective randomised controlled trial. Lancet. 2006;368:1155–63. doi: 10.1016/S0140-6736(06)69472-5. [DOI] [PubMed] [Google Scholar]
- 148.Sattar N, Preiss D, Murray HM, Welsh P, Buckley BM, de Craen AJ, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet. 2010;375:735–42. doi: 10.1016/S0140-6736(09)61965-6. [DOI] [PubMed] [Google Scholar]
- 149.Mills EJ, Wu P, Chong G, Ghement I, Singh S, Akl EA, et al. Efficacy and safety of statin treatment for cardiovascular disease: a network meta-analysis of 170,255 patients from 76 randomized trials. QJM. 2011;104:109–24. doi: 10.1093/qjmed/hcq165. [DOI] [PubMed] [Google Scholar]
- 150.Preiss D, Seshasai SR, Welsh P, Murphy SA, Ho JE, Waters DD, et al. Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy: a meta-analysis. JAMA. 2011;305:2556–64. doi: 10.1001/jama.2011.860. [DOI] [PubMed] [Google Scholar]
- 151.Waters DD, Ho JE, DeMicco DA, Breazna A, Arsenault BJ, Wun CC, et al. Predictors of new-onset diabetes in patients treated with atorvastatin: results from 3 large randomized clinical trials. J Am Coll Cardiol. 2011;57:1535–45. doi: 10.1016/j.jacc.2010.10.047. [DOI] [PubMed] [Google Scholar]
- 152.Wilson PW, Meigs JB, Sullivan L, Fox CS, Nathan DM, D’Agostino RB., Sr Prediction of incident diabetes mellitus in middle-aged adults: the Framingham Offspring Study. Arch Intern Med. 2007;167:1068–74. doi: 10.1001/archinte.167.10.1068. [DOI] [PubMed] [Google Scholar]
- 153.Rahman M, Simmons RK, Harding AH, Wareham NJ, Griffin SJ. A simple risk score identifies individuals at high risk of developing Type 2 diabetes: a prospective cohort study. Fam Pract. 2008;25:191–6. doi: 10.1093/fampra/cmn024. [DOI] [PubMed] [Google Scholar]
- 154.Kanaya AM, Wassel Fyr CL, de Rekeneire N, Shorr RI, Schwartz AV, Goodpaster BH, et al. Predicting the development of diabetes in older adults: the derivation and validation of a prediction rule. Diabetes Care. 2005;28:404–8. doi: 10.2337/diacare.28.2.404. [DOI] [PubMed] [Google Scholar]
- 155.Waters DD, LaRosa JC, Barter P, et al. Effects of high-dose atorvastatin on cerebrovascular events in patients with stable coronary disease in the TNT (treating to new targets) study. J Am Coll Cardiol. 2006;48:1793–99. doi: 10.1016/j.jacc.2006.07.041. [DOI] [PubMed] [Google Scholar]
- 156.Pedersen TR, Faergeman O, Kastelein JJ, et al. High-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial. JAMA. 2005;294:2437–45. doi: 10.1001/jama.294.19.2437. [DOI] [PubMed] [Google Scholar]
- 157.Waters DD, Ho JE, Boekholdt SM, DeMicco DA, Kastelein JJ, Messig M, et al. Cardiovascular event reduction versus new-onset diabetes during atorvastatin therapy: effect of baseline risk factors for diabetes. J Am Coll Cardiol. 2013;61:148–52. doi: 10.1016/j.jacc.2012.09.042. [DOI] [PubMed] [Google Scholar]
- 158.Shepherd J, Barter P, Carmena R, Deedwania P, Fruchart JC, Haffner S, et al. Effect of lowering LDL cholesterol substantially below currently recommended levels in patients with coronary heart disease and diabetes: the Treating to New Targets (TNT) study. Diabetes Care. 2006;29:1220–6. doi: 10.2337/dc05-2465. [DOI] [PubMed] [Google Scholar]
- 159.Ridker PM, Pradhan A, MacFadyen JG, Libby P, Glynn RJ. Cardiovascular benefits and diabetes risks of statin therapy in primary prevention: an analysis from the JUPITER trial. Lancet. 2012;380:565–71. doi: 10.1016/S0140-6736(12)61190-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wang KL, Liu CJ, Chao TF, Huang CM, Wu CH, Chen SJ, et al. Statins, risk of diabetes, and implications on outcomes in the general population. J Am Coll Cardiol. 2012;60:1231–8. doi: 10.1016/j.jacc.2012.05.019. [DOI] [PubMed] [Google Scholar]
- 161.Waters DD, Ho JE, Boekholdt SM, et al. Cardiovascular event reduction versus new-onset diabetes during atorvastatin therapy: effect of baseline risk factors for diabetes. J Am Coll Cardiol. 2013;61:148–52. doi: 10.1016/j.jacc.2012.09.042. [DOI] [PubMed] [Google Scholar]
- 162.Taylor E N, Hu FB, Curhan GC. Antihypertensive medications and the risk of incident type 2 diabetes. Diabetes Care. 2006;29:1065–70. doi: 10.2337/diacare.2951065. [DOI] [PubMed] [Google Scholar]
- 163.Mancia G, Grassi G, Zanchetti A. New-onset diabetes and antihypertensive drugs. J Hypertens. 2006;24:3–10. doi: 10.1097/01.hjh.0000194119.42722.21. [DOI] [PubMed] [Google Scholar]
- 164.Brunner NW, Ramanathan K, Wang H, Quan H, Khan NA. Effectiveness of statin prescribing on reducing mortality in South asian, chinese, and white patients with diabetes. Cana J Cardiol. 2013;29:920–6. doi: 10.1016/j.cjca.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 165.Ma Y, Culver A, Rossouw J, Olendzki B, Merriam P, Lian B, et al. Statin therapy and the risk for diabetes among adult women: do the benefits outweigh the risk? Ther Adv Cardiovasc Dis. 2013;7:41–4. doi: 10.1177/1753944712468499. [DOI] [PubMed] [Google Scholar]
- 166.Kengne AP, Patel A, Marre M, Travert F, Lievre M, Zoungas S, et al. Contemporary model for cardiovascular risk prediction in people with type 2 diabetes. Eur J Cardiovasc Prev Rehabil. 2011;18:393–8. doi: 10.1177/1741826710394270. [DOI] [PubMed] [Google Scholar]
- 167.Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215–22. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Amarenco P, Goldstein LB, Szarek M, Sillesen H, Rudolph AE, Callahan A, 3rd, et al. Effects of intense low-density lipoprotein cholesterol reduction in patients with stroke or transient ischemic attack: the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial. Stroke. 2007;38:3198–204. doi: 10.1161/STROKEAHA.107.493106. [DOI] [PubMed] [Google Scholar]
- 169.Goldstein LB, Amarenco P, Szarek M, Callahan A, 3rd, Hennerici M, Sillesen H, et al. Hemorrhagic stroke in the Stroke Prevention by Aggressive Reduction in Cholesterol Levels study. Neurology. 2008;70:2364–70. doi: 10.1212/01.wnl.0000296277.63350.77. [DOI] [PubMed] [Google Scholar]
- 170.Ebrahim S, Sung J, Song YM, Ferrer RL, Lawlor DA, Davey Smith G. Serum cholesterol, haemorrhagic stroke, ischaemic stroke, and myocardial infarction: Korean national health system prospective cohort study. BMJ. 2006;333:22. doi: 10.1136/bmj.38855.610324.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Glasser SP, Wadley V, Judd S, Kana B, Prince V, Jenny N, et al. The association of statin use and statin type and cognitive performance: analysis of the reasons for geographic and racial differences in stroke (REGARDS) study. Clin Cardiol. 2010;33:280–8. doi: 10.1002/clc.20758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Rojas-Fernandez CH, Cameron JC. Is statin-associated cognitive impairment clinically relevant? A narrative review and clinical recommendations. Ann Pharmacother. 2012;46:549–57. doi: 10.1345/aph.1Q620. [DOI] [PubMed] [Google Scholar]
- 173.Tierney EF, Thurman DJ, Beckles GL, Cadwell BL. Association of statin use with peripheral neuropathy in the U.S. population years of age or older. J Diabetes. 2013;5:207–15. doi: 10.1111/1753-0407.12013. [DOI] [PubMed] [Google Scholar]
- 174.Chong PH, Boskovich A, Stevkovic N, Bartt RE. Statin-associated peripheral neuropathy: review of the literature. Pharmacotherapy. 2004;24:1194–203. doi: 10.1592/phco.24.13.1194.38084. [DOI] [PubMed] [Google Scholar]
- 175.Golomb BA, Evans MA, Dimsdale JE, White HL. Effects of statins on energy and fatigue with exertion: results from a randomized controlled trial. Arch Intern Med. 2012;172:1180–2. doi: 10.1001/archinternmed.2012.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wardle J, Armitage J, Collins R, Wallendszus K, Keech A, Lawson A. Randomised placebo controlled trial of effect on mood of lowering cholesterol concentration. Oxford Cholesterol Study Group. BMJ. 1996;313:75–8. doi: 10.1136/bmj.313.7049.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Bays H. Statin safety: an overview and assessment of the data--2005. Am J Cardiol. 2006;97:6C–26C. doi: 10.1016/j.amjcard.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 178.Pasternak RC, Smith SC, Jr, Bairey-Merz CN, Grundy SM, Cleeman JI, Lenfant C. ACC/AHA/NHLBI clinical advisory on the use and safety of statins. J Am Coll Cardiol. 2002;40:567–72. doi: 10.1016/s0735-1097(02)02030-2. [DOI] [PubMed] [Google Scholar]
- 179.Gotto AM., Jr The case for over-the-counter statins. Am J Cardiol. 2004;94:753–6. doi: 10.1016/j.amjcard.2004.05.058. [DOI] [PubMed] [Google Scholar]
- 180.Downs JR, Clearfield M, Weiss S, Whitney E, Shapiro D, Beere P, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels. JAMA. 1998;279:1615–22. doi: 10.1001/jama.279.20.1615. [DOI] [PubMed] [Google Scholar]
- 181.Dujovne CA. Side effects of statins: hepatitis versus “transaminitis”-myositis versus “CPKitis”. Am J Cardiol. 2002;89:1411–3. doi: 10.1016/s0002-9149(02)02356-1. [DOI] [PubMed] [Google Scholar]
- 182.LaRosa JC, Grundy SM, Waters DD, Shear C, Barter P, Fruchart JC, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005;352:1425–35. doi: 10.1056/NEJMoa050461. [DOI] [PubMed] [Google Scholar]
- 183.Fenofibrate patient information and physician prescribing information. [accessed on Ocober 1, 2013]. Available from: www:Abbott/Abbvie .
- 184.Waters DD, Ku I. Early statin therapy in acute coronary syndromes: the successful cycle of evidence, guidelines, and implementation. J Am Coll Cardiol. 2009;54:1434–7. doi: 10.1016/j.jacc.2009.05.062. [DOI] [PubMed] [Google Scholar]
- 185.Yoo J, Lee S, Kim K, Yoo S, Sung E, Yim J. Relationship between insulin resistance and serum alanine aminotransferase as a surrogate of NAFLD (nonalcoholic fatty liver disease) in obese Korean children. Diabetes Res Clin Pract. 2008;81:321–6. doi: 10.1016/j.diabres.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 186.Kunde SS, Lazenby AJ, Clements RH, Abrams GA. Spectrum of NAFLD and diagnostic implications of the proposed new normal range for serum ALT in obese women. Hepatology. 2005;42:650–6. doi: 10.1002/hep.20818. [DOI] [PubMed] [Google Scholar]
- 187.Cohen DE, Anania FA, Chalasani N. An assessment of statin safety by hepatologists. Am J Cardiol. 2006;97:77C–81C. doi: 10.1016/j.amjcard.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 188.Vuppalanchi R, Teal E, Chalasani N. Patients with elevated baseline liver enzymes do not have higher frequency of hepatotoxicity from lovastatin than those with normal baseline liver enzymes. Am J Med Sci. 2005;329:62–5. doi: 10.1097/00000441-200502000-00002. [DOI] [PubMed] [Google Scholar]
- 189.Chalasani N, Aljadhey H, Kesterson J, Murray MD, Hall SD. Patients with elevated liver enzymes are not at higher risk for statin hepatotoxicity. Gastroenterology. 2004;126:1287–92. doi: 10.1053/j.gastro.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 190.Kiyici M, Gulten M, Gurel S, Nak SG, Dolar E, Savci G, et al. Ursodeoxycholic acid and atorvastatin in the treatment of nonalcoholic steatohepatitis. Can J Gastroenterol. 2003;17:713–8. doi: 10.1155/2003/857869. [DOI] [PubMed] [Google Scholar]
- 191.Bjornsson E, Olsson R. Outcome and prognostic markers in severe drug-induced liver disease. Hepatology. 2005;42:481–9. doi: 10.1002/hep.20800. [DOI] [PubMed] [Google Scholar]
- 192.Athyros VG, Tziomalos K, Gossios TD, Griva T, Anagnostis P, Kargiotis K, et al. Safety and efficacy of long-term statin treatment for cardiovascular events in patients with coronary heart disease and abnormal liver tests in the Greek Atorvastatin and Coronary Heart Disease Evaluation (GREACE) Study: a post-hoc analysis. Lancet. 2010;376:1916–22. doi: 10.1016/S0140-6736(10)61272-X. [DOI] [PubMed] [Google Scholar]
- 193.Chalasani N. Statins and hepatotoxicity: focus on patients with fatty liver. Hepatology. 2005;41:690–5. doi: 10.1002/hep.20671. [DOI] [PubMed] [Google Scholar]
- 194.Bays H. Statin safety: an overview and assessment of the data 2005. Am J Cardiol. 2006;97:6C–26C. doi: 10.1016/j.amjcard.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 195.Law M, Rudnicka AR. Statin safety: a systematic review. Am J Cardiol. 2006;97:52C–60C. doi: 10.1016/j.amjcard.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 196.Drug Safety Communication: Important safety label changes to cholesterol-lowering statin drugs. Posted on February 28, 2012 on Official website of US Food and Drug Administration (FDA) [accessed on September 17, 2013]; [Google Scholar]
- 197.Savarese G, Musella F, Volpe M, Paneni F, Perrone-Filardi P. Effects of atorvastatin and rosuvastatin on renal function: A meta-analysis. Int J Cardiol. 2013;167:2482–9. doi: 10.1016/j.ijcard.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 198.Wu Y, Wang Y, An C, Dong Z, Liu H, Zhang Y, et al. Effects of rosuvastatin and atorvastatin on renal function: meta-analysis. Circ J. 2012;76:1259–66. doi: 10.1253/circj.cj-11-1385. [DOI] [PubMed] [Google Scholar]
- 199.Thompson PD, Clarkson PM, Rosenson RS. An assessment of statin safety by muscle experts. Am J Cardiol. 2006;97:69C–76C. doi: 10.1016/j.amjcard.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 200.Cham S, Evans MA, Denenberg JO, Golomb BA. Statin-associated muscle-related adverse effects: a case series of 354 patients. Pharmacotherapy. 2010;30:541–53. doi: 10.1592/phco.30.6.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Hoffman KB, Kraus C, Dimbil M, Golomb BA. A survey of the FDA's AERS database regarding muscle and tendon adverse events linked to the statin drug class. PLoS One. 2012;7:e42866. doi: 10.1371/journal.pone.0042866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Nichols GA, Koro CE. Does statin therapy initiation increase the risk for myopathy? An observational study of 32,225 diabetic and nondiabetic patients. Clin Ther. 2007;29:1761–70. doi: 10.1016/j.clinthera.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 203.Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–78. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 204.Thompson PD, Clarkson P, Karas RH. Statin-associated myopathy. JAMA. 2003;289:1681–90. doi: 10.1001/jama.289.13.1681. [DOI] [PubMed] [Google Scholar]
- 205.Bottorff MB. Statin safety and drug interactions: clinical implications. Am J Cardiol. 2006;97:27C–31C. doi: 10.1016/j.amjcard.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 206.Smith CC, Bernstein LI, Davis RB, Rind DM, Shmerling RH. Screening for statin-related toxicity: the yield of transaminase and creatine kinase measurements in a primary care setting. Arch Intern Med. 2003;163:688–92. doi: 10.1001/archinte.163.6.688. [DOI] [PubMed] [Google Scholar]
- 207.McKenney JM, Davidson MH, Jacobson TA, Guyton JR. Final conclusions and recommendations of the National Lipid Association Statin Safety Assessment Task Force. Am J Cardiol. 2006;97:89C–94C. doi: 10.1016/j.amjcard.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 208.Graham DJ, Staffa JA, Shatin D, Andrade SE, Schech SD, La Grenade L, et al. Incidence of hospitalized rhabdomyolysis in patients treated with lipid-lowering drugs. JAMA. 2004;292:2585–90. doi: 10.1001/jama.292.21.2585. [DOI] [PubMed] [Google Scholar]
- 209.Rosenson RS, Bays HE. Results of two clinical trials on the safety and efficacy of pravastatin 80 and 160 mg per day. Am J Cardiol. 2003;91:878–81. doi: 10.1016/s0002-9149(03)00026-2. [DOI] [PubMed] [Google Scholar]
- 210.Davidson MH, Stein EA, Dujovne CA, Hunninghake DB, Weiss SR, Knopp RH, et al. The efficacy and six-week tolerability of simvastatin 80 and 160 mg/day. Am J Cardiol. 1997;79:38–42. doi: 10.1016/s0002-9149(96)00742-4. [DOI] [PubMed] [Google Scholar]
- 211.The statin wars: why AstraZeneca must retreat. Lancet. 2003;362:1341. [PubMed] [Google Scholar]
- 212.Williams D, Feely J. Pharmacokinetic-pharmacodynamic drug interactions with HMG-CoA reductase inhibitors. Clin Pharmacokinet. 2002;41:343–70. doi: 10.2165/00003088-200241050-00003. [DOI] [PubMed] [Google Scholar]
- 213.Omar MA, Wilson JP. FDA adverse event reports on statin-associated rhabdomyolysis. Ann Pharmacother. 2002;36:288–95. doi: 10.1345/aph.1A289. [DOI] [PubMed] [Google Scholar]
- 214.Stang P, Morris L, Kempf J, Henderson S, Yood MU, Oliveria S. The coprescription of contraindicated drugs with statins: continuing potential for increased risk of adverse events. Am J Ther. 2007;14:30–40. doi: 10.1097/01.mjt.0000208875.61480.c8. [DOI] [PubMed] [Google Scholar]
- 215.Link E, Parish S, Armitage J, Bowman L, Heath S, Matsuda F, et al. SLCO1B1 variants and statin-induced myopathy-a genomewide study. N Engl J Med. 2008;359:789–99. doi: 10.1056/NEJMoa0801936. [DOI] [PubMed] [Google Scholar]
- 216.Cziraky MJ, Willey VJ, McKenney JM, Kamat SA, Fisher MD, Guyton JR, et al. Statin safety: an assessment using an administrative claims database. Am J Cardiol. 2006;97:61C–68C. doi: 10.1016/j.amjcard.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 217.Neuvonen PJ, Backman JT, Niemi M. Pharmacokinetic comparison of the potential over-the-counter statins simvastatin, lovastatin, fluvastatin and pravastatin. Clin Pharmacokinet. 2008;47:463–74. doi: 10.2165/00003088-200847070-00003. [DOI] [PubMed] [Google Scholar]
- 218.Brener ZZ, Bilik I, Khorets B, Winchester JF, Bergman M. Rhabdomyolysis following clarithromycin monotherapy. Am J Med Sci. 2009;338:78. doi: 10.1097/MAJ.0b013e31819e221f. [DOI] [PubMed] [Google Scholar]
- 219.Davidson MH, Armani A, McKenney JM, Jacobson TA. Safety considerations with fibrate therapy. Am J Cardiol. 2007;99:3C–18C. doi: 10.1016/j.amjcard.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 220.Crestor (rosuvasatin) patient information and physician prescribing information. [accessed on October 1, 2013]. Available from: www: Aztra Zeneca .
- 221.Neuvonen PJ, Niemi M, Backman JT. Drug interactions with lipid-lowering drugs: mechanisms and clinical relevance. Clin Pharmacol Ther. 2006;80:565–81. doi: 10.1016/j.clpt.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 222.Schmidt GA, Hoehns JD, Purcell JL, Friedman RL, Elhawi Y. Severe rhabdomyolysis and acute renal failure secondary to concomitant use of simvastatin, amiodarone, and atazanavir. J Am Board Fam Med. 2007;20:411–6. doi: 10.3122/jabfm.2007.04.060187. [DOI] [PubMed] [Google Scholar]
- 223.Rowan C, Brinker AD, Nourjah P, Chang J, Mosholder A, Barrett JS, et al. Rhabdomyolysis reports show interaction between simvastatin and CYP3A4 inhibitors. Pharmacoepidemiol Drug Saf. 2009;18:301–9. doi: 10.1002/pds.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Calza L, Manfredi R, Colangeli V, Pocaterra D, Pavoni M, Chiodo F. Rosuvastatin, pravastatin, and atorvastatin for the treatment of hypercholesterolaemia in HIV-infected patients receiving protease inhibitors. Curr HIV Res. 2008;6:572–8. doi: 10.2174/157016208786501481. [DOI] [PubMed] [Google Scholar]
- 225.Nowack R. Review article: cytochrome P450 enzyme, and transport protein mediated herb-drug interactions in renal transplant patients: grapefruit juice, St John's Wort - and beyond! Nephrology (Carlton) 2008;13:337–47. doi: 10.1111/j.1440-1797.2008.00940.x. [DOI] [PubMed] [Google Scholar]
- 226.Pittler MH, Ernst E. Systematic review: hepatotoxic events associated with herbal medicinal products. Aliment Pharmacol Ther. 2003;18:451–71. doi: 10.1046/j.1365-2036.2003.01689.x. [DOI] [PubMed] [Google Scholar]
- 227.Estes JD, Stolpman D, Olyaei A, Corless CL, Ham JM, Schwartz JM, et al. High prevalence of potentially hepatotoxic herbal supplement use in patients with fulminant hepatic failure. Arch Surg. 2003;138:852–8. doi: 10.1001/archsurg.138.8.852. [DOI] [PubMed] [Google Scholar]
- 228.Whiting PW, Clouston A, Kerlin P. Black cohosh and other herbal remedies associated with acute hepatitis. Med J Aust. 2002;177:440–43. doi: 10.5694/j.1326-5377.2002.tb04886.x. [DOI] [PubMed] [Google Scholar]
- 229.Ahmed W, Khan N, Glueck CJ, Pandey S, Wang P, Goldenberg N, et al. Low serum 25 (OH) vitamin D levels (<32 ng/mL) are associated with reversible myositis-myalgia in statin-treated patients. Transl Res. 2009;153:11–6. doi: 10.1016/j.trsl.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 230.Lee JH, O’Keefe JH, Bell D, Hensrud DD, Holick MF. Vitamin D deficiency an important, common, and easily treatable cardiovascular risk factor? J Am Coll Cardiol. 2008;52:1949–56. doi: 10.1016/j.jacc.2008.08.050. [DOI] [PubMed] [Google Scholar]
- 231.Wang TJ, Pencina MJ, Booth SL, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117:503–11. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. [accessed on August 24, 2012]. Available from: http://www.epocrates.com .
- 233.Ghirlanda G, Oradei A, Manto A, Lippa S, Uccioli L, Caputo S, et al. Evidence of plasma CoQ10-lowering effect by HMG-CoA reductase inhibitors: a double-blind, placebo-controlled study. J Clin Pharmacol. 1993;33:226–9. doi: 10.1002/j.1552-4604.1993.tb03948.x. [DOI] [PubMed] [Google Scholar]
- 234.Rundek T, Naini A, Sacco R, Coates K, DiMauro S. Atorvastatin decreases the coenzyme Q10 level in the blood of patients at risk for cardiovascular disease and stroke. Arch Neurol. 2004;61:889–92. doi: 10.1001/archneur.61.6.889. [DOI] [PubMed] [Google Scholar]
- 235.Hughes K, Lee BL, Feng X, Lee J, Ong CN. Coenzyme Q10 and differences in coronary heart disease risk in Asian Indians and Chinese. Free Radic Biol Med. 2002;32:132–8. doi: 10.1016/s0891-5849(01)00783-3. [DOI] [PubMed] [Google Scholar]
- 236.Duncan AJ, Hargreaves IP, Damian MS, Land JM, Heales SJ. Decreased ubiquinone availability and impaired mitochondrial cytochrome oxidase activity associated with statin treatment. Toxicol Mech Methods. 2009;19:44–50. doi: 10.1080/15376510802305047. [DOI] [PubMed] [Google Scholar]
- 237.Marcoff L, Thompson PD. The role of coenzyme Q10 in statin-associated myopathy: a systematic review. J Am Coll Cardiol. 2007;49:2231–37. doi: 10.1016/j.jacc.2007.02.049. [DOI] [PubMed] [Google Scholar]
- 238.Laaksonen R, Riihimaki A, Laitila J, Martensson K, Tikkanen MJ, Himberg JJ. Serum and muscle tissue ubiquinone levels in healthy subjects. J Lab Clin Med. 1995;125:517–21. [PubMed] [Google Scholar]
- 239.Young JM, Florkowski CM, Molyneux SL, McEwan RG, Frampton CM, George PM, et al. Effect of coenzyme Q(10) supplementation on simvastatin-induced myalgia. Am J Cardiol. 2007;100:1400–3. doi: 10.1016/j.amjcard.2007.06.030. [DOI] [PubMed] [Google Scholar]
- 240.Langsjoen PH, Langsjoen JO, Langsjoen AM, Lucas LA. Treatment of statin adverse effects with supplemental Coenzyme Q10 and statin drug discontinuation. Biofactors. 2005;25:147–52. doi: 10.1002/biof.5520250116. [DOI] [PubMed] [Google Scholar]
- 241.Caso G, Kelly P, McNurlan MA, Lawson WE. Effect of coenzyme q10 on myopathic symptoms in patients treated with statins. Am J Cardiol. 2007;99:1409–12. doi: 10.1016/j.amjcard.2006.12.063. [DOI] [PubMed] [Google Scholar]
- 242.Emberson JR, Kearney PM, Blackwell L, Newman C, Reith C, Bhala N, et al. Lack of effect of lowering LDL cholesterol on cancer: meta-analysis of individual data from 175,000 people in 27 randomised trials of statin therapy. PLoS One. 2012;7:e29849. doi: 10.1371/journal.pone.0029849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Iribarren C, Jacobs DR, Jr, Sidney S, Claxton AJ, Gross MD, Sadler M, et al. Serum total cholesterol and risk of hospitalization, and death from respiratory disease. Int J Epidemiol. 1997;26:1191–202. doi: 10.1093/ije/26.6.1191. [DOI] [PubMed] [Google Scholar]
- 244.Jacobs DR, Jr, Hebert B, Schreiner PJ, Sidney S, Iribarren C, Hulley S. Reduced cholesterol is associated with recent minor illness: the CARDIA Study. Coronary Artery Risk Development in Young Adults. Am J Epidemiol. 1997;146:558–64. doi: 10.1093/oxfordjournals.aje.a009314. [DOI] [PubMed] [Google Scholar]
- 245.American Heart Association. Heart and Stroke Statistical Update. Circulation. 2012;125:188–97. doi: 10.1161/CIR.0b013e3182456d46. [DOI] [PubMed] [Google Scholar]
- 246.Young F, Capewell S, Ford ES, Critchley JA. Coronary mortality declines in the U.S. between and quantifying the contributions from primary and secondary prevention. Am J Prev Med. 2010;39:228–34. doi: 10.1016/j.amepre.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 247.Ford ES, Ajani UA, Croft JB, Critchley JA, Labarthe DR, Kottke TE, et al. Explaining the decrease in U.S. deaths from coronary disease, 1980-2000. N Engl J Med. 2007;356:2388–98. doi: 10.1056/NEJMsa053935. [DOI] [PubMed] [Google Scholar]
- 248.Baigent C, Blackwell L, Collins R, Emberson J, Godwin J, Peto R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373:1849–60. doi: 10.1016/S0140-6736(09)60503-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Gorelick PB, Weisman SM. Risk of hemorrhagic stroke with aspirin use: an update. Stroke. 2005;36:1801–7. doi: 10.1161/01.STR.0000174189.81153.85. [DOI] [PubMed] [Google Scholar]
- 250.Pignone M, Earnshaw S, Tice JA, Pletcher MJ. Aspirin, statins, or both drugs for the primary prevention of coronary heart disease events in men: a cost-utility analysis. Ann Intern Med. 2006;144:326–36. doi: 10.7326/0003-4819-144-5-200603070-00007. [DOI] [PubMed] [Google Scholar]
- 251.Seshasai SR, Wijesuriya S, Sivakumaran R, Nethercott S, Erqou S, Sattar N, et al. Effect of Aspirin on Vascular and Nonvascular Outcomes: Meta-analysis of Randomized Controlled Trials. Arch Intern Med. 2012;172:209–16. doi: 10.1001/archinternmed.2011.628. [DOI] [PubMed] [Google Scholar]
- 252.De Berardis G, Sacco M, Strippoli GF, Pellegrini F, Graziano G, Tognoni G, et al. Aspirin for primary prevention of cardiovascular events in people with diabetes: meta-analysis of randomised controlled trials. Bmj. 2009;339:b4531. doi: 10.1136/bmj.b4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Keech A, Simes RJ, Barter P, Best J, Scott R, Taskinen MR, et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet. 2005;366:1849–61. doi: 10.1016/S0140-6736(05)67667-2. [DOI] [PubMed] [Google Scholar]
- 254.Gaist D, Rodriguez LA, Huerta C, Hallas J, Sindrup SH. Lipid-lowering drugs and risk of myopathy: a population-based follow-up study. Epidemiology. 2001;12:565–9. doi: 10.1097/00001648-200109000-00017. [DOI] [PubMed] [Google Scholar]
- 255.Graham DJ, Staffa JA, Shatin D, et al. Incidence of hospitalized rhabdomyolysis in patients treated with lipid-lowering drugs. JAMA. 2004;292:2585–90. doi: 10.1001/jama.292.21.2585. [DOI] [PubMed] [Google Scholar]
- 256.Wald NJ, Law MR. A strategy to reduce cardiovascular disease by more than 80% BMJ. 2003;326:1419. doi: 10.1136/bmj.326.7404.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Naderi SH, Bestwick JP, Wald DS. Adherence to drugs that prevent cardiovascular disease: meta-analysis on 376,162 patients. Am J Med. 2012;125:882–7.e881. doi: 10.1016/j.amjmed.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 258.Abramson J, Wright JM. Are lipid-lowering guidelines evidence-based? Lancet. 2007;369:168–9. doi: 10.1016/S0140-6736(07)60084-1. [DOI] [PubMed] [Google Scholar]


