Abstract
Background & objectives:
The reported low relapse rates after 24 months multidrug therapy (MDT) for multibacillary leprosy (MB) led to the recommendation of reducing duration of therapy to 12 months. However, only a few reports exist on long term follow up data after 12 months fixed duration therapy (FDT). The present study was done to assess the incidence of relapse in MB leprosy patients after 12 months treatment.
Methods:
The leprosy patients detected in field surveys during 2001-2006 in Agra district, Uttar Pradesh, India, were put on WHO-MDT and followed up for treatment completion, relapse, reactions and development of disability. The assessment was done clinically by following up the patients until January 2011. Data collected were analyzed for risk and survival analysis.
Results:
The incidence of relapse was found to be 1.97/100 person years of follow up. The incidence of relapse by age (34 yr vs >34 yr), sex (male vs female), delay in detection (<36 months vs >36 months) and smear status (smear +ve vs -ve) was not found to be significantly different but patients with no nerve involvement were observed to have significantly higher relapses than those with three or more nerve involvement (P<0.05). Similarly, borderline-borderline and BB with reaction (BB/BBR) patients were observed to have significantly high relapses than among those with borderline tuberculoid or BT with reaction (BT/BTR) or borderline lipromatous/lepromatous/neuritic (BL/LL/N) type of leprosy (P<0.01).
Interpretation & conclusion:
From the observations in the study, it can be suggested that relapses occur in 12 months FDT and almost as much as reported in 24 months FDT for MB leprosy. Although, early relapses may be due to insufficient treatment, late relapses may be due to persistent dormant mycobacteria. However, a study relating to immunological response of treatment and change in immunological profile relating to the occurrence of relapses and its clinical correlates may suggest better information on causes of relapses.
Keywords: Fixed duration, incidence, leprosy, multibacillary, multidrug therapy, relapse
The relapse rates in multibacillary (MB) leprosy patients after 24 months fixed duration therapy (FDT) were observed to be very low1. This led to the recommendation to reduce duration of therapy to 12 months for MB leprosy2. However, questions were raised about the quality diagnosis of relapse as some could be late reversal reactions3,4. Histological examination was often unable to distinguish between a reaction and relapse5. A therapeutic trial with steroids was advocated for making clear diagnosis6.
One of the reasons for low relapse rate was that follow up was done usually for shorter intervals after therapy and diagnostic criteria were not clear. However, a few studies based on long-term follow up had reported crude relapse rate of about 20 per cent5 and another as 2.04/100 person years7 after release from 24 months fixed duration treatment with multi-drug therapy (MDT). Although there were many reports on relapse after 24 months fixed duration therapy but hardly a few on 12 months fixed duration therapy. Therefore, studies on medium to long term follow up were required to assess the relapse rates in leprosy patients treated with 12 months fixed duration therapy. Therefore, the present study was undertaken in a cohort of MB leprosy patients from field studies undertaken in Agra district, Uttar Pradesh, India, to find out the incidence of relapse.
Material & Methods
This study was initially planned in year 2001 as a randomized field trial aimed at comparing cure and relapse in standard 12 monthly fixed multidrug therapy with that of 12 monthly single dose of rifampicin, ofloxacin and minocycline (ROM) among MB leprosy patients detected in the field. Since ROM was suddenly discontinued by WHO from the programme and only 22 cases were randomly allocated to ROM arm by then, thereafter only MDT was given to all detected cases. The skin smear was done in all those who cooperated for it resulting in 108 patients out of 157 who completed the follow up.
Inclusion/exclusion criterion of patients for the study: The study was conducted in patients detected in the field survey in Agra district in north India, during 2001-2006. All newly detected leprosy patients diagnosed clinically as multibacillary leprosy were taken for this study. Patients included were with >5 skin lesions, either erythmatous or hypo-pigmented with definite impairment or loss of sensations and/or >2 thickened nerves. None of the patients had taken leprosy treatment earlier.
Cohort size and treatment allocation: During 2001-2006 in Agra district, several field surveys were undertaken to detect leprosy cases. In these surveys, a total of 267 cases were put on MB-MDT for fixed duration of 12 months. Of the 267 cases, 92 (34.5%) discontinued treatment at various duration, 13 patients were lost to follow up (LFU) or died during follow up period. Therefore, a total of 162 MB patients could be followed up till the end of 2010 for a mean duration of 4.08 years after completion of MDT treatment. Of the 162 patients, the follow up could be done for up to 1 yr in 8.6 per cent (14), 1-3 yr in 16.1 per cent (26) cases, 50 per cent (81) for 3-5 yr and 25.3 per cent (41) for 5-8 yr.
At the time of starting treatment, all the patients were informed about disease, its implications, known effects of treatment and benefits, possible side effects and remedies. Written informed consent from patients was taken. These patients were then put on respective treatment as per WHO guidelines7. In case of children, consent of their parents was taken. The study protocol was approved by ethics committee of the institute.
Treatment: WHO supplied MDT blister packs were used for the study. WHO recommended doses were given to children (aged ≤ 14 yr) and adults (aged >14 yr). Monthly MB-MDT was given, with supervisory dose under supervision and for rest of days patients were guided to take daily treatment doses of dapsone and clofazimine.
Follow up and assessment: Patients were visited every month till the completion of treatment for drug intake and compliance, clinical conditions and side effects. However, formal assessment of each available patient was made every six months after treatment completion for five years and annually thereafter. Lesion activity- erythema, infiltration and size, any new lesion and/or new nerve thickening or any deformity was recorded. Cure of the disease was defined as complete healing of the lesion or patch becoming flat hypopigmented with decrease in size of the lesion and/or regain of sensations. For lepromatous cases, regression of infiltration on skin and/or skin smear negativity were taken as the criteria of cure8.
Relapse and reaction: World Health Organization1 defines relapse as “A patient who successfully completes an adequate course of MDT, but subsequently develops new signs and symptoms of leprosy either during surveillance period or thereafter”. Another definition is given by Becx-Bleumink4: Appearance of new skin lesions, new activity in previously existing lesions or bacteriological index of 2+ or more or new nerve function loss or histological evidence of relapse in skin or nerve. Gradual or insidious appearance of new lesion(s) or definite increase in size of the lesion and/or appearance of new nerve thickening were taken as relapse. Any sudden redness (showing activity in lesion), swelling of the lesion with or without new lesion especially in the first 6 to 12 months of follow up, was first considered as late reaction. All such patients were put on corticosteroids (20 mg prednisolone equivalent per day). If there was no obvious change in morphology of lesion (inflammation) in 4 wk on steroids, the patients were considered as to have relapsed.
Statistical analysis: Pearson χ2 and Mantel-Hasnel test were used to compare the proportion and relapse rate8 by person years.
Results
The age distribution of patients who completed treatment and defaulted suggested that the mean age was slightly high among defaulters (46.1 vs 41.7 yr). The sex ratio of patients was also in favour of males being more. The occupation statuses although not recorded in this data file, but most males were labours, or agriculturists and most female were involved in household works.
Incidence of relapse: Of the 162 patients, during the follow up period (mean = 4.08 yr, SD=1.7 & median=4.2), 13 relapses occurred among patients treated with 12 months fixed duration WHO-MDT. These patients were followed up for 660.4 person years (PY) and the incidence of relapse was observed to be 1.97/100 PY (Figure).
Fig.
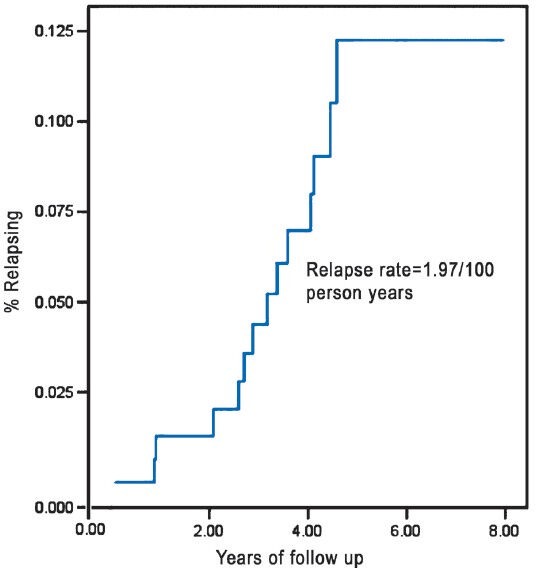
Incidence of replace in MB leprosy.
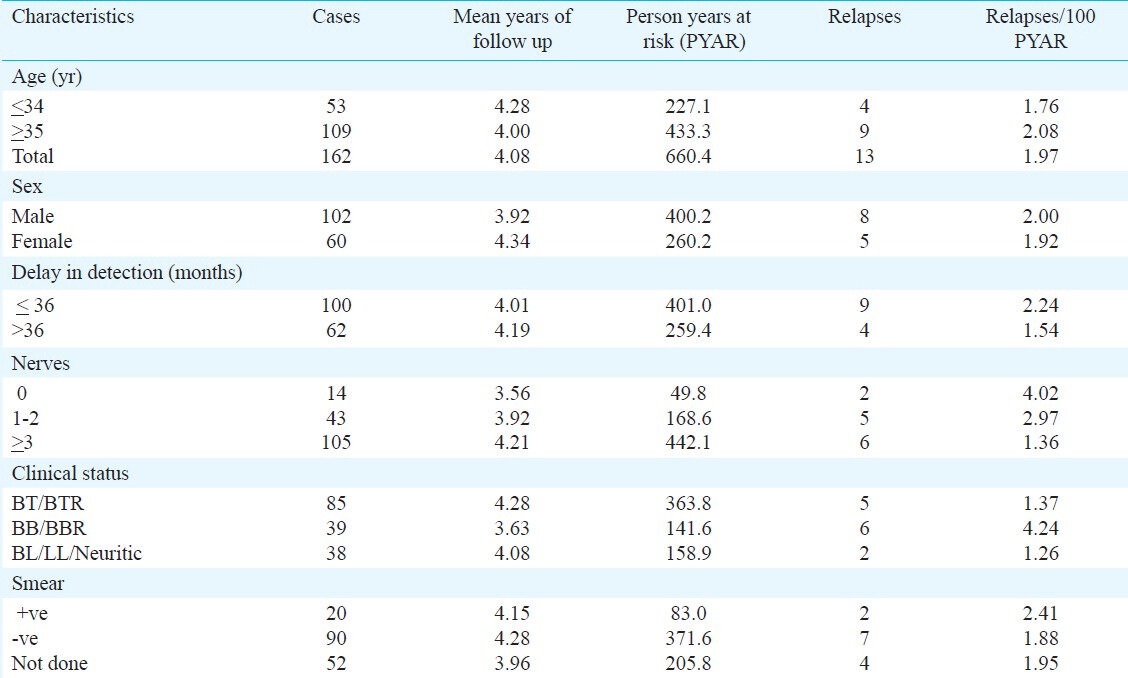
The characteristics of patients are given in the Table. Of the 162 patients, 53 were ≤34 yr. The incidence of relapse per 100 PY was found to be slightly higher among patients aged above 35 yr than in the younger patients below 35 yr (2.08 vs. 1.76), among males (2.0 vs. 1.92) than in females. The patients whose reported delay in detection was upto 36 months had high relapse rate (2.24 vs. 1.54) then those detected later than 36 months of having disease. The incidence of relapse is found highest (4.02) among cases without any nerve thickening (0 nerve) and significantly higher than in cases with 3 or more nerve thickening (4.02 vs. 1.36, P<0.05) but did not differ significantly with relapse rate in patients with 1-2 nerve thickening.
Similarly, the highest incidence of relapses was observed in borderline-borderline and BB with reaction (BB/BBR) which was significantly higher than in borderline-tuberculoid (BT) leprosy patients and BT with reaction (BTR) (4.24 vs. 1.37, P<0.01) and (4.24 vs. 1.26, P=0.02) than in borderline lepromatous / lepromatous/neuritic (BL/LL/N). Although relapse rate in smear positive patients (2.41) was slightly higher than in smear negative patients (1.88) and 1.95 in others but none of these differences was found to be significant (Table).
Table.
Incidence of relapses/1000 person years at risk (PYAR) in MB leprosy
The late reversal reaction was observed in only one patient with borderline leprosy(BB) – at 18 months after released from treatment. Beside this, four patients had early reaction during treatment and discontinued treatment.
Discussion
Measuring efficacy of a treatment is based on its long term sustainable effects on a particular disease and the re-occurrence of disease (relapse) weakens its efficacy. In the present case of MB leprosy, the relapse rates have been reported after 12 months standard MDT in field based MB cohort. A large variation was observed in relapse rate globally; low in programme based data and high in closely monitored studies. Although, some studies had reported relapse rate below 1 per cent in MB leprosy10,11,12,13,14. One study15 in which patients were treated for variable duration (of 163, 41 for 1 yr, 80 for 2 yr and the remaining till smear negativity), following up patients for long duration, observed very low relapse rate of 0.26/100 person year. However, there are reports, indicating high (gross cumulative) relapse rate of 18.6 per cent16 (16 out of 86) without converting this rate in person year terms. The variation observed also had generated discussion on the need to develop criteria of relapse17 but its sensitivity is low. Thus until then, dependence would mainly be on clinical basis of relapse.
In the present field based study, overall relapse rate was observed as 1.97/100 persons years in the MB cohort treated with 12 months MDT. Most relapses (6/13) were observed during 2 to 4 years of follow up and almost 31 per cent (4/13) beyond 4 years of follow up. High relapse rate was found in BB/BBR leprosy than in BT/BTR or BL/LL/N cases. In BB cases, two relapses occurred in 2+ smear cases after 2.7 and 4 years of completing treatment. If treatment till smear negativity is taken as index for better response to treatment, it takes longer about 45 months for bacillary clearance18 and thus treatment may need to continue for such durations.
Although it is difficult to qualify for high and low relapse rates but relapses do occur. More relapses may occur if these patients are followed up for further longer period but extent of relapse is not easy to project. One of the main reasons of relapse is ‘persisting organisms in immunologically favourable sites like dermal nerves, lymph nodes and alike19. Secondly, BB disease in leprosy spectrum is also immunological unstable and many times it may be advancing to early BB from BT. It would, therefore, be interesting to investigate the reason of relapses - is it due to insufficient treatment in some patients causing early relapse or persistent dormant mycobacteria leading to late relapse or immunological variations across populations giving mix of the above two. In the present study with 12 months fixed duration therapy, observed relapse rates were almost similar as were observed in 24 months therapy and not significantly different from other reports8. Similarly, research studies are required to investigate as to why patients with less nerve involvement relapse more than those having more nerves involvement. Is it due to the fact that clinical presentations are changing, resulting in weak correlation of histopathology and clinical classifications? A recent study suggested need for revalidation of WHO classification of MB leprosy based on results of lowering sensitivity and increasing specificity20.
Some possibilities of relatively more relapses in BB leprosy could be due to (i) a few patients might have had reversal reaction which was slow and insidious to appear; (ii) Contrary to expectations, the response to 4 wk steroids therapy was not adequate to delineate it as reaction; (iii) Too sensitive and rigid criteria having been applied for labelling reactivation as relapse. This includes appearance of new lesions / increase in size/thickening of earlier unaffected nerves and/or new nerve function Impairment (NFI); and (iv) Frequent and long-term follow up and contact with patients help to detect relapse which is likely to miss if longer follow up is done.
One limitation of the study was that many patients under field conditions did not cooperate for skin smear, resulting unknown smear status. Although histological examination of relapses has problems of definite confirmation and difficulties in field set up but should be attempted wherever feasible as this adds to information on diagnosis of relapse.
In conclusion, this study provided information on relapse rates in MB leprosy patients on 12 months fixed duration therapy. This information would be useful for programme managers.
Acknowledgment
Authors acknowledge the help extended by the institute by providing internal grant for the study and thank to all patients who cooperated in the study and the paramedical workers and District leprosy officer for the support.
References
- 1.Geneva: 1994. WHO leprosy Unit. Risk of relapse in leprosy. WHO document. WHO/CTD/LEP/94.1. [Google Scholar]
- 2.Ji B. Why multidrug therapy for multibacillary leprosy can be shortened to 12 months. Lepr Rev. 1998;69:106–9. doi: 10.5935/0305-7518.19980009. [DOI] [PubMed] [Google Scholar]
- 3.Lobo D. Treatment failures with multidrug therapy. Lepr Rev. 1992;63:93S–8S. doi: 10.5935/0305-7518.19920060. [DOI] [PubMed] [Google Scholar]
- 4.Beck-Bleumink M. Relapses among leprosy patients treated with multidrug therapy: experience in the leprosy control program of All Africa Leprosy and Rehabilitation Training Centre (ALERT) in Ethiopia; practical difficulties with diagnosing relapse, operational procedures and criteria for diagnosing relapses. Int J Lepr. 1992;60:421–35. [PubMed] [Google Scholar]
- 5.Jamet P, Ji B Mercoux Chemotherapy Group. Relapse after long term follow up of multibacillary patients treated by WHO multidrug regimen. Int J Lepr. 1995;63:195–201. [PubMed] [Google Scholar]
- 6.Mercoux Chemotherapy Group. Relapse in multibacillary patients after stopping treatment with rifampicin containing combined regimens. Int J Lepr. 1992;60:525–35. [PubMed] [Google Scholar]
- 7.Geneva: WHO; 1998. World Health Organization (WHO). Expert committee on leprosy, Technical Report Series 874, 7th report. [PubMed] [Google Scholar]
- 8.Girdhar BK, Girdhar A, Kumar A. Relapse in MB leprosy patients - Effect of length of therapy. Lepr Rev. 2000;71:144–53. doi: 10.5935/0305-7518.20000017. [DOI] [PubMed] [Google Scholar]
- 9.Le chap T. New York, USA: John Wiley & Sons; 1988. Applied catagorical data analysis. [Google Scholar]
- 10.Waters MF. Is it safe to shorten multidrug therapy for lepromatous (LL or BL) leprosy to 12 months? Lepr Rev. 1998;69:110–1. doi: 10.5935/0305-7518.19980010. [DOI] [PubMed] [Google Scholar]
- 11.The Leprosy Unit-WHO. Risk of relapse in leprosy. Indian J Lepr. 1995;67:13–26. [PubMed] [Google Scholar]
- 12.Suite M. Relapse rates following leprosy multidrug therapy. West Indian Med J. 2000;49:210–1. [PubMed] [Google Scholar]
- 13.Shaw IN, Natarajan MM, Rao GS, Jesudasan K, Christian M, Kavitha M. Log term follow up of multibacillary leprosy patients with high BI treated with WHO/MDT regimen for a fixed duration of two years. Int J Lepr Other Mycobact Dis. 2000;68:405–9. [PubMed] [Google Scholar]
- 14.Ali MK, Thorat DM, Subramaniam M, Parthasarathy G, Selveraj U, Prabhakar V. A study on trend of relapse in leprosy and factors influencing relapse. Indian J Lepr. 2005;77:105–15. [PubMed] [Google Scholar]
- 15.Poojabylaiah M, Marne RB, Varikkodan R, Bala N, Dandekeri S, Martis J. Relapses in multibacillary leprosy patients after multidrug therapy (case report) Lepr Rev. 2008;79:320–4. [PubMed] [Google Scholar]
- 16.Vara N, Agarwal M, Marfatia Y. Leprosy beyond MDT: study of follow up of 100 released from treatment cases. Indian J Lepr. 2010;82:189–94. [PubMed] [Google Scholar]
- 17.Linder K, Zia M, Kern WV, Pfau RK, Wagner D. Relapses vs reaction in multibacillary leprosy: proposal of bew criterioa. Trop Med Int Health. 2008;13:295–309. doi: 10.1111/j.1365-3156.2008.02003.x. [DOI] [PubMed] [Google Scholar]
- 18.Kumar A, Girdhar A, Girdhar BK. Pattern of bacillary clearance in MB leprosy. Acta Lepralogica. 2003;2:123–8. [PubMed] [Google Scholar]
- 19.Kaimal S, Thapa DM. Relapse in leprosy. Indian J Derm Ven Lep. 2009;75:126–35. doi: 10.4103/0378-6323.48656. [DOI] [PubMed] [Google Scholar]
- 20.Kar HK. Mumbai: Presented at 28th Beinnial Conference of IAL; 2012. Jan 27-29, W.H.O. Classification of leprosy: Need for revalidation. abstract. [Google Scholar]


