Abstract
Background & objectives:
There has been an extensive invasion of tuberculosis at the global level by multidrug resistant as well as extensively drug resistant organisms. Attempts to recover the pathogen in pure culture have frequently failed since the specimens are often highly contaminated and also due to use of insufficient or over-active decontamination procedures. Hence in the present study different methods of decontamination were tested to evaluate their independent efficacies for culture of Mycobacterium tuberculosis.
Methods:
A total of 359 samples (241 sputum, 59 urine, 50 endometrium biopsy, 9 pus samples) from clinically suspected cases of tuberculosis were subjected to four different methods of decontamination followed by inoculation in Lowenstein-Jensen medium (LJM), and bilayered medium (BLM) and Kirchner's liquid medium (KLM) to determine the influence of differential decontamination processes. Sputum scanty and positive specimens were graded and each sample was subjected to decontamination by four different techniques.
Results:
Treatment of specimens with 4 per cent NaOH yielded minimum recovery of pure cultures, while use of 2 per cent NaOH produced higher number of contaminants compared to other methods of decontamination. Addition of N-acetyl L-cystein (NALC) coupled with 2 per cent NaOH to the samples for decontamination provided fairly reasonable recovery, but the highest number of M. tuberculosis cultures could be obtained when the specimens were treated with tri-sodium phosphate and benzalkonium (TSPB). Among the sputum positive cases recovery of growth of M. tuberculosis was higher with greater number of bacilli present in the specimens. Regarding the influence of culture media, BLM produced not only rapid growth, but reasonably higher rate of isolation of M. tuberculosis.
Interpretation & conclusions:
Although use of TSPB was found to be an efficient method of decontamination for successful isolation of M. tuberculosis from contaminated samples, both NALC+ 2 per cent NaOH and TSPB also showed significant recovery of M. tuberculosis cultures in BLM that can facilitate early diagnosis and initiation of treatment.
Keywords: Acid fast bacilli, bilayered medium, decontamination, Mycobacterium tuberculosis, trisodium phosphate benzalkonium
Tuberculosis (TB) continues to be one of the most fatal infections of the present time despite a well-planned therapeutic regimen recommended by the World Health Organization (WHO). In 2011, there were an estimated 8.7 million new cases of TB (13% co-infected with HIV), of whom 1.4 million people died. India is regarded as the country with highest number of tuberculosis according to the statistics of WHO for 2011. This has given an estimated incidence of 2.2 million new cases of tuberculosis in India. The estimated prevalence of tuberculosis in India for 2011 is given as 3.1 million1. Approximately 80 per cent of the active cases of TB in the world are seen in 22 low and middle income countires2. For isolation and cultivation of Mycobacterium tuberculosis there is an inherent, urgent and vital requirement for correct and early detection of the pathogen. Much of the effort is hampered due to the presence of different bacteria and fungi in the patients’ sputum as contaminants3. This results in delay in confirmation of the causative organism that ultimately interferes in the initiation of the treatment schedule. The contaminated sputum specimens slow down the process of confirmation of the presence of M. tuberculosis indicating a pressing need for establishing an inexpensive method for decontamination3. Decontamination with acids, alkalies, or even detergents is a common practice as mycobacteria are resistant to such agents4. As a result samples are processed by various methods that are being practiced for many years4. Certain decontaminating agents destroy a substantial number of mycobacteria along with the contaminants, while others are too weak to destroy them3,4,5. The resulting consequence is a costly delay in detecting the tubercle bacilli thereby slowing down the quintessential process of initiation of therapy. Petroff's method using 4 per cent sodium hydroxide (NaOH) is effective in removing the contaminants, but a large number of mycobacteria are also killed simultaneously thereby proving the limitation of the process5. Use of 2 per cent NaOH is less practiced due to its inability to destroy all the unwanted microbes6,7.
In another effective sample processing method for successful cultivation of M. tuberculosis a mixture of NaOH, sodium citrate and N-acetyl L-cysteine is added to digest the sample for the unbeaten recovery of causative organism8. Application of trisodium phosphate along with the mucolytic agent benzalkonium to specimens containing M. tuberculosis is also routinely practiced as the bacilli can withstand the action of these agents for as long as overnight and careful timing of exposure is not required3,9. All these processes of decontamination were followed by centrifugation to concentrate the sample, before being inoculated on to specific media.
Thus a suitable decontamination process is an absolute necessity that may provide sufficient control in removing the undesirable contaminants for the best result. The present study describes a comparative analysis of various decontamination methods for subsequent successful cultivation of M. tuberculosis in Kirchncer's liquid medium (KLM), Lowenstein Jensen medium (LJM) and the recently developed novel bilayered medium (BLM)10.
Material & Methods
The study was conducted at the Department of Microbiology, Nil Ratan Sircar Medical College, Department of Microbiology, KPC Medical College and Department of Microbiology, Herbicure Healthcare Bio-Herbal Research Foundation, Kolkata, West Bengal, India. Approval from the institutional ethics committee of the Institute of Postgraduate Medical Education and Research (IPGMER), Kolkata, was obtained prior to initiation of the study when all the three authors were working in the same Department of Microbiology, IPGMER, Kolkata.
Strains: M. tuberculosis H37Rv 102 and H37Ra 16 were used as standards to compare the growth of the new cultures from various specimens after proper decontamination.
Clinical materials: A total of 359 consecutive clinical samples were collected from suspected cases of tuberculosis attending the Department of Microbiology, Institute of Postgraduate Medical Education and Research, Kolkata, during August 2006 to December 2010. When a sample volume was less than 2 ml, it was not included in the study. All the specimens were obtained from treatment naïve patients only. The patients who were found to have started treatment were excluded from this study.
Specimens included 241 sputum samples, 59 urine samples, 50 endometrium samples and only nine pus samples. About 8 to 10 ml of different samples were collected from each patient, as every sample had to be divided into four parts for treatment with four different reagents of decontamination. Since the amount was 2 to 2.5 ml in case of all the pus samples these were subjected to homogenization with the help of vortex after addition of 0.5 to 1 ml of sterile saline to each sample. This was then divided into four equal aliquots and processed for decontamination. Each tissue from the endometrium was homogenized in a tissue homogenizer under strict sterile condition after addition of 5 ml of sterile saline. This was then centrifuged and fluid from the top was processed for decontamination.
The specimens were collected from the Directly Observed Treatment – short course (DOTs) clinic, different outpatient departments (Chest, Medicine, Paediatrics), as well as from the indoor patients (Chest, Medicine, Paediatrics) with suggestive history of tuberculosis and were transported to the laboratory of IPGMER without any further delay. The transportation time was carefully controlled and never exceeded 30 min.
Most of the samples were processed immediately in the laboratory. All sputum and pus samples were collected in sterile containers, each smear was prepared on a clean glass slide in a drop of formalin taking proper precautions, dried and heat fixed and finally stained with Ziehl-Neelsen (Z-N) method10 and graded. Grading was made from scanty to + to +++ as per Revised National Tuberculosis Control Programme (RNTCP) guidelines11. Each sample was then subjected to decontamination by four different techniques.
Samples were collected after obtaining written informed consent of individual patients. Clinical details of patients regarding age, sex, history of persistent cough or other symptoms for more than 3 wk, weight loss, pyrexia, rise of temperature, particularly in the evening, night sweat, chest pain, loss of appetite, shortness of breath, malaise, haemoptysis, family history of tuberculosis, any other complaints, and history of intake of antitubercular drugs were recorded for all the patients.
Procedures for decontamination: Each sample was divided into four equal parts. To the first part was added an equal amount of 4 per cent NaOH solution, mixture was incubated at 37°C for 15 min with occasional shaking. In highly contaminated samples incubation time was further extended for additional 10 min. The fluid was centrifuged, the deposit was suspended in 4 ml sterile distilled water, centrifuged again at 3000 g for 10 min5,7. The sediment was mixed with 1 ml of sterile distilled water and 0.1 ml of this was used as an inoculum to each medium12.
An equal amount of 2 per cent NaOH was added to the second part of the specimen, incubated at 37°C for 15 min, neutralized with 8 per cent HCl and finally centrifuged at 3000 g for 15 min6. The deposit was suspended in 1 ml sterile distilled water, 0.1 ml from which was given as an inoculum to different media.
The third part of the sample was treated with an equal volume of N-acetyl-L-cysteine (NALC) plus 2 per cent NaOH, the mixture was vortexed for 20 sec and kept at room temperature for 15 min. To this was added phosphate buffer and centrifuged at 3000 g for 15 min. The deposit was re-suspended in 1 ml of buffer, and 0.1 ml from this was used as an inoculum to different media8.
The last part of the sample was treated in the following manner: 5 g analar trisodium phosphate was dissolved in 20 ml hot sterile distilled water, to which 0.35 ml of 17 per cent benzalkonium was added. Equal parts of specimen and this solution were mixed in a mechanical shaker, allowed to stand for 30 min at room temperature; this was neutralized by phosphate buffer and centrifuged at 3000 g for 15 min3,9. The concentrated deposit was resuspended in 1 ml of sterile normal saline, 0.1 ml from which was inoculated onto various culture media.
Media: The biological components were obtained from Oxoid, (UK). Kirchner's liquid medium (KLM) and Lowenstein-Jensen medium (LJM) were prepared as per standard protocol7,13. The bilayered medium (BLM) consisted of a lower layer of LJM without malachite green, the upper layer contained Middlebrook 7H10 agar medium supplemented with antibiotics and antifungal agents to avoid contaminants plus analar tetrazolium chloride as an indicator whose colour gets changed after growth of tubercle bacilli due to reduction10.
Growth characteristics: Inoculated bottles were incubated at 37°C up to 8 wk (for LJM) and 3-4 wk (for KLM and BLM). All the bottles were checked for appearance of growth every day before these were finally discarded as failure of growth.
Inoculation of known standard strains M. tuberculosis H37Rv102 and H37Ra16 was routinely made to compare the rates of recovery of the clinical isolates on the three different types of culture media. Moreover, these cultures were grown in KLM for 10 days, vortexed, diluted and given as inoculum to different media to confirm the growth characteristics and time to positivity. This process was repeated once every month throughout the entire period of study. McFarland standard 0.5 (a turbidity standard prepared by adding 0.5 ml of a barium chloride solution to 99.5 ml of 1% H2SO4) was routinely taken for inoculation of M. tuberculosis H37Rv102 and H37Ra16. For LJM and BLM the bottles of different media inoculated with decontaminated clinical specimens or with standard control strains were kept in a slanting position for about 2 h for flow of the inocula over the surface of the slant10. Incubation of these slants and KLM was in vertical position at 37°C. The growth was confirmed for Mycobacterium spp. after performing Z-N staining and different biochemical tests, like niacin test, nitrate reduction test and catalase test14,15.
Statistical anaysis: Sign test or binomial test was followed for this analysis16.
Results
To determine the growth pattern of the isolates in different media the reference strains M. tuberculosis H37Ra 16 and H37Rv 102 were always included in all the media as standard controls. The growth appeared as turbidity in KLM within 10-12 days. In LJM typically rough, tough and buff coloured growth of mycobacteria appeared frequently after 3 wk; however, some specimens took a longer period for exhibiting growth (6-8 wk).
In BLM growth was detected earliest with the help of a hand lens against transmitted light by 48 h. There was either earlier appearance of colour change followed by appearance of translucent colonies or vice versa. After 4-5 days the medium became red due to reduction of the tetrazolium indicator demonstrating growth. Small translucent colonies appeared that were partially above the surface of the media and partially submerged in the top layer. A confluent growth was observed with further incubation.
Growth from the standard strains of M. tuberculosis H37Ra 16 and H37Rv 102 and from clinical samples was confirmed by various biochemical tests and by Z-N staining; typical mycobacterial morphology could be observed as small, straight or slightly curved, non-sporing acid fast rods throughout the entire smear. Positive reaction in niacin test was the basis of differentiation of M. tuberculosis from the M. tuberculosis complex and non-tubercular mycobacteria since apart from M. tuberculosis very rarely M. bovis, M. simiae and M. kansasii may exhibit such a reaction with this test17,18.
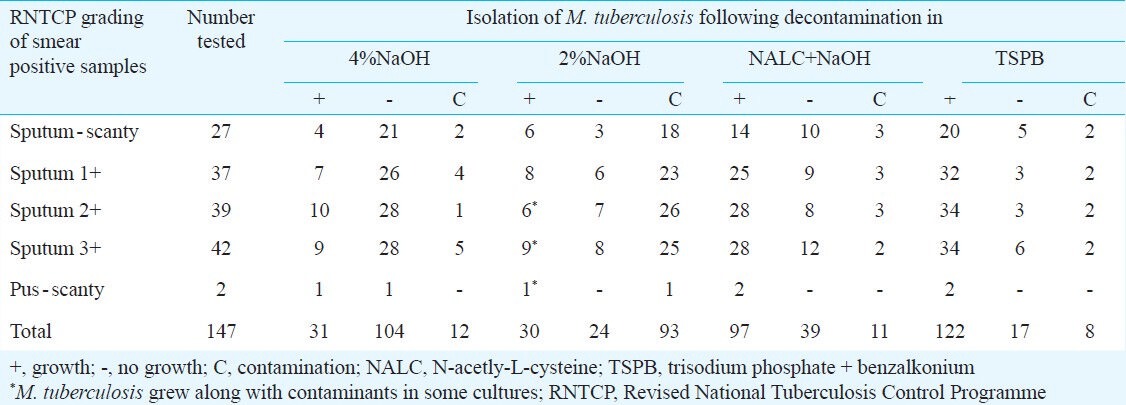
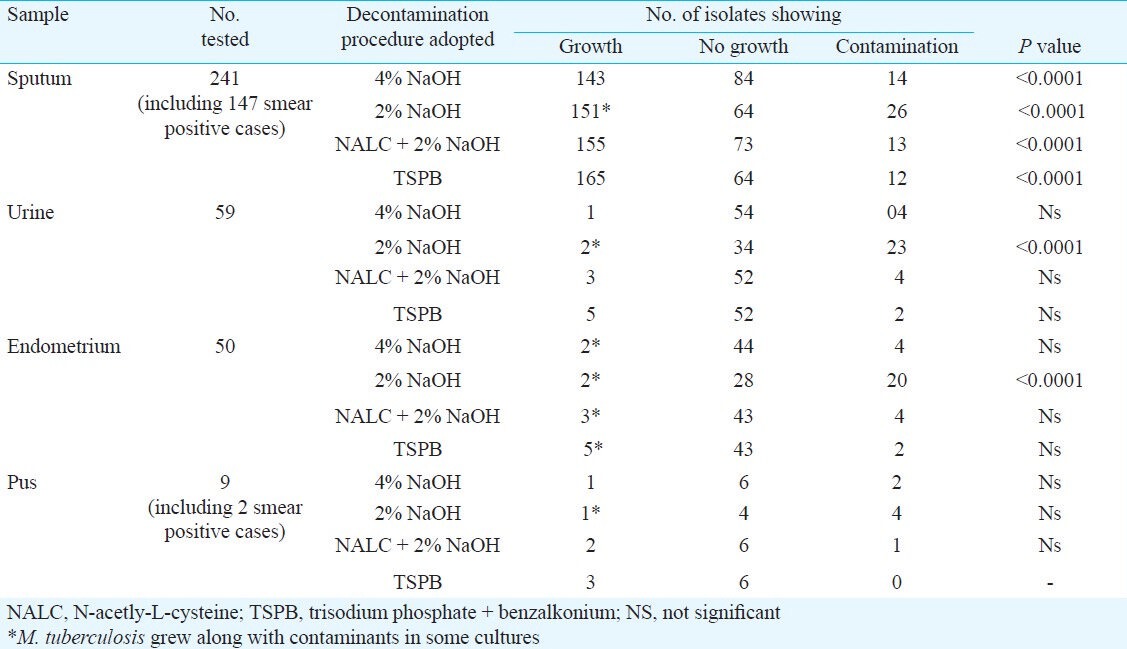
Each specimen was first examined microscopically in a direct smear prepared from the untreated specimen. Of the 241 sputum samples, 147 showed presence of acid fast bacilli by Z-N staining, and were graded following RNTCP guideline. Of the 147 smear positive cases, 104 failed to grow when the specimens were treated with 4 per cent NaOH, while only 24 samples produced no growth after treatment with 2 per cent NaOH, but a total of 93 cultures were contaminants when 2 per cent NaOH was the decontaminating agent (Table I). Decontamination with NALC + NaOH turned out to be more potent as 97 smears produced typical growth of M. tuberculosis in LJM and only 11 samples were grown as contaminants. However, TSPB was the most active decontaminating agent as 122 out of 147 gave rise to typical M. tuberculosis cultures, coupled with growth failure in 17 specimens and contamination in only eight (Table I). Of the two pus samples, one each could be grown when treated with 4 and 2 per cent NaOH, while both the samples yielded M. tuberculosis in LJM when decontamination with the other two methods was followed (Table I).
Table I.
Rate of recovery of M. tuberculosis from smear positive sputum samples and pus samples following different methods of decontamination
The recovery of pure cultures of M. tuberculosis in LJM (Table II) was satisfactory following decontamination TSPB and NALC + NaOH, while treatment with 4 and 2 per cent NaOH yielded contaminants more frequently. However, in many cases growth was found to be associated with contaminants. Treatment of these samples with NALC + 2 per cent NaOH provided 151 pure cultures while decontamination with TSPB successfully yielded 158 typical cultures of M. tuberculosis. Similarly samples from urine, endometrium and pus yielded greater number of recovery when these were treated with TSPB or NALC + 2 per cent NaOH compared to when treated with 4 or 2 per cent NaOH.
Table II.
Cultivation of M. tuberculosis in Lowenstein-Jensen medium (LJM) following various methods of decontamination
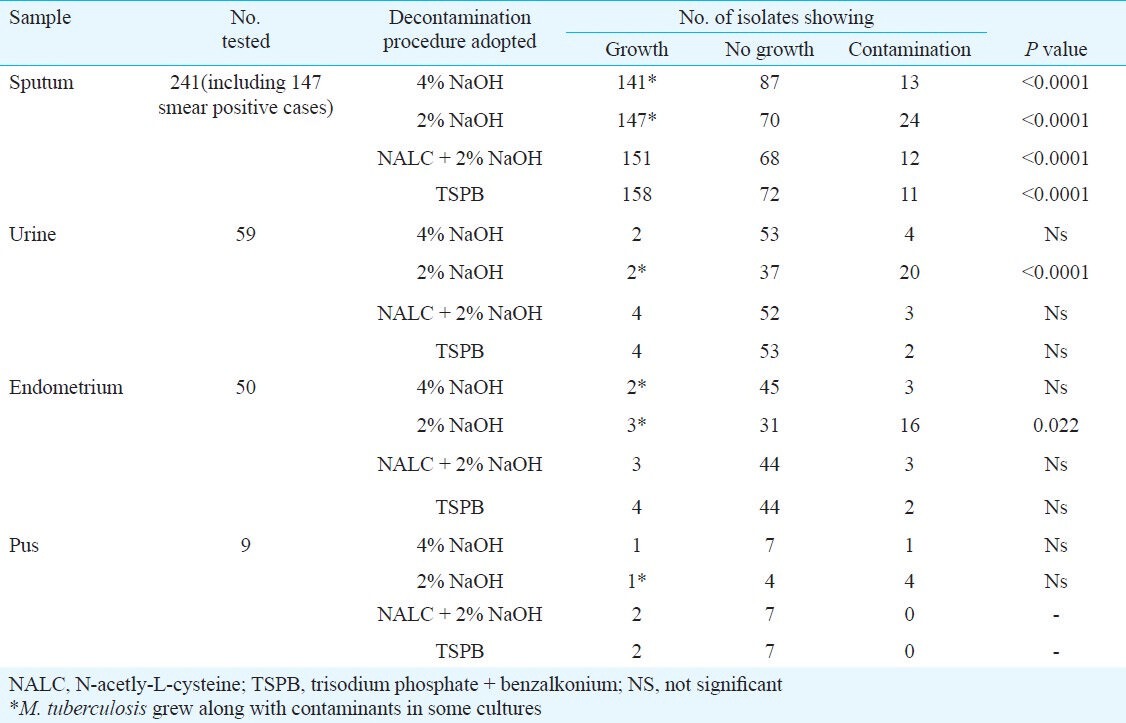
Growth of M. tuberculosis started much earlier in BLM and compared to LJM the number of cultures with growth was also distinctly higher in this medium (Table III). Following the decontamination procedure by TSPB, 168 of 241 sputum samples revealed growth of M. tuberculosis, while treatment with NALC + 2 per cent NaOH yielded 157 pure cultures of M. tuberculosis. Compared to LJM, recovery of M. tuberculosis cultures was also more in BLM after treatment of the samples with either 4 per cent or 2 per cent NaOH (Table III).
Table III.
Recovery of M. tuberculosis in bilayered medium (BLM) through differential decontamination procedures
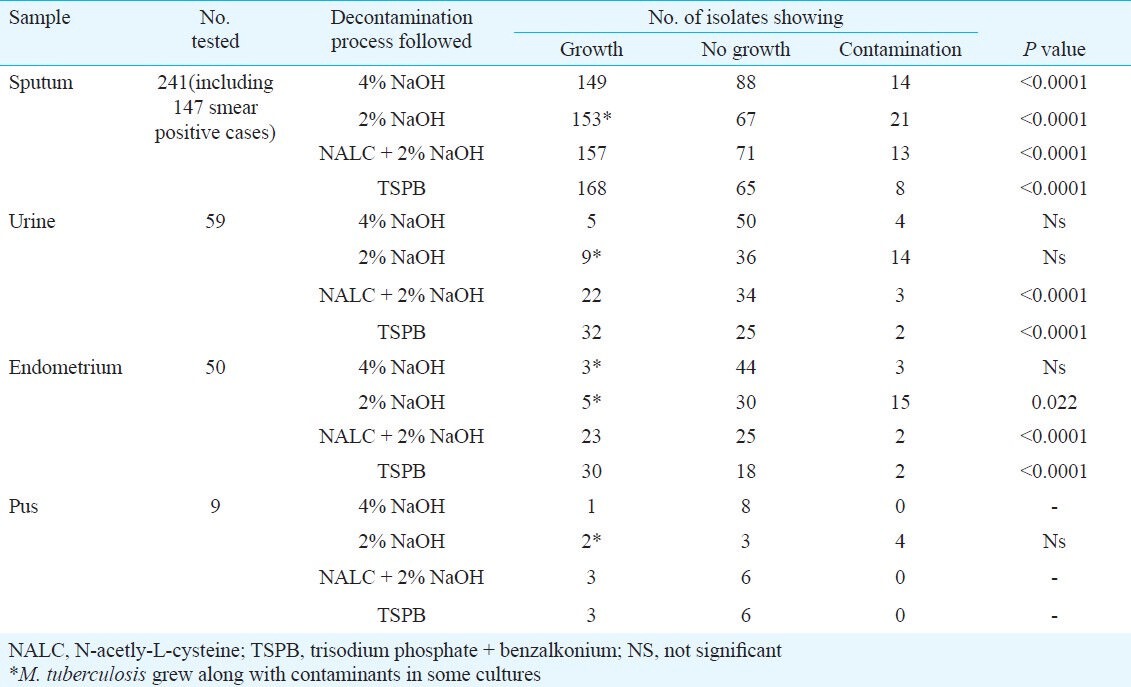
The growth of Mycobacterium from various specimens started appearing after 9 days in KLM as turbidity that became more clearly visible within 12 days (Table IV).
Table IV.
Turbid growth of M. tuberculosis in Kirchner's liquid medium (KLM) after decontamination with various agents
Discussion
Although there are definite and standard culture media for growing M. tuberculosis, but it is often difficult to obtain a pure culture due to various reasons. Specimens from suspected patients include fresh sputum, gastric washings, urine, pleural fluid, cerebrospinal fluid, joint fluid, biopsy material or other suspected materials, many of which are frequently associated with contaminating bacteria and fungi17. Hence most specimens need to be decontaminated with various agents, neutralized and concentrated by centrifugation before being inoculated into specific culture media. Specimens from sterile sites do not need decontamination and are, therefore, not subjected to decontamination procedures18.
Continuous monitoring systems for detection of M. tuberculosis have reported higher contamination rates than traditional radiometric technologies4,19. In low and middle income countries, detection of patients suffering from tuberculosis is best done by direct sputum smear microscopy which is fast, inexpensive and specific3,20, but recurrent contamination hinders the identification of causative organism thereby delaying diagnosis, treatment and destroying valuable time. Therefore, a proper and effective decontamination method is of utmost importance in saving time and life of the tuberculosis patients. In this study it was noted that sputum samples with higher number of acid fast bacilli resulted in higher rate of isolation of pure cultures of M. tuberculosis. On a very rare occasion a positive smear had failed to grow in LJM, which might possibly be due to the complex selection pressure present in the medium that did not allow small number of bacilli in the sputum to grow.
Application of 4 per cent NaOH appears to be a rather strong form of decontamination since about 60 per cent of tubercle bacilli may be killed by following such a procedure15. Decontamination with 2 per cent NaOH cannot be recommended as the number of contaminants was much higher compared to other processes. Moreover, it is often difficult to achieve an exact point of neutralization with 8 per cent HCl; mycobacteria get damaged if the mixture is acidic, while they fail to grow when it is alkaline15.
Sodium citrate added during decontamination along with NALC and 2 per cent NaOH in order to bind heavy metal ions that may be present in the specimens, often inactivate NALC resulting in reduced mucolysis and poor isolation21. The methods of application of NaOH alone and in combination with NALC are now widely used in modern laboratories for decontamination of samples from tuberculosis patients22. Addition of benzalkonium to trisodium phosphate appears to be a reasonable digestion procedure since these are fairly non-toxic to mycobacteria and a reasonably better mucolytic reagent3,4,23.
Amongst the four methods of decontamination use of 2 and 4 per cent of NaOH was least expensive but the results obtained were not satisfactory. Treatment with NALC + NaOH was costly while TSPB was intermediate in cost but proved to be the best decontaminant of the four methods used in this study.
In conclusion, the present study showed the efficacy of BLM over LJM and KLM as the rate of isolation and cultivation of M. tuberculosis was distinctly better in BLM. Although decontamination with the help of TSPB turned out to be the best of all the decontamination methods used with the highest recovery of bacteria, the findings revealed that NANC + NaOH may be considered as an equally efficient decontamination procedure.
Acknowledgment
The authors are grateful to Dr V.M. Katoch, the then Director, National JALMA Institute for Leprosy and other Mycobacterial Diseases, Agra, India, for providing standard strains of M. tuberculosis H37Rv102 and H37Ra16.
References
- 1.Geneva: WHO; 2012. World Health Organization, Global tuberculosis control; p. 9. [Google Scholar]
- 2.Lawn SD, Zumla AI. Tuberculosis. Lancet. 2011;378:57–72. doi: 10.1016/S0140-6736(10)62173-3. [DOI] [PubMed] [Google Scholar]
- 3.Steingart KR, Vivienne NG, Megan H, Hopewell PC, Ramsay A, Cunningham J, et al. Sputum processing methods to improve the sensitivity of smear microscopy for tuberculosis: A Systematic Review. Lancet Infect Dis. 2006;6:664–74. doi: 10.1016/S1473-3099(06)70602-8. [DOI] [PubMed] [Google Scholar]
- 4.Burdz TVN, Wolfe J, Kabani A. Evaluation of sputum decontamination methods for Mycobacterium tuberculosis using viable colony counts and flow cytometry. Diag Microbiol Infect Dis. 2003;47:503–9. doi: 10.1016/s0732-8893(03)00138-x. [DOI] [PubMed] [Google Scholar]
- 5.de Kantor IN, Laszlo A. Tuberculosis: Laboratory procedure for developing countries. In: Gangadharan PRJ, editor. Mycobacteria basic aspects. Vol. 1. New York: Chapman Hall ITP; 1998. pp. 351–99. [Google Scholar]
- 6.Tomita M, Takeno H, Yoshida S, Suzuki K, Sakatani M. Comparison of BBL Mycoprep and 2% NaOH decontamination procedures for MGIT. Kekkaku. 2008;83:471–3. [PubMed] [Google Scholar]
- 7.Laidlaw M. Mycobacterium: tubercle bacilli. In: Collee JG, Duguid JP, Fraser AG, Marmion BP, editors. Mackie & McCartney practical medical microbiology. Vol. 2. Edinburgh: Churchill Livingstone; 1989. pp. 399–416. [Google Scholar]
- 8.Buijtels PC, Petit PL. Comparison of NaOH-N-acetyl cysteine and sulfuric acid decontamination methods for recovery of mycobacteria from clinical specimens. J Microbiol Methods. 2005;62:83–8. doi: 10.1016/j.mimet.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 9.Kumar B, Vinay S, Abbas A. Robbins basic pathology. United Kingdom: W.B. Saunders Company; 2007. pp. 516–22. [Google Scholar]
- 10.Bhattacharya S, Ray R, Roy Chowdhury N, Dasgupta A, Dastidar SG. Comparison of a novel bilayered medium with the conventional media for cultivation of Mycobacterium tuberculosis. Indian J Med Res. 2009;130:561–6. [PubMed] [Google Scholar]
- 11.Rajpal S, Dhingra VK, Aggarwal JK. Sputum grading as predictor of treatment outcome in pulmonary tuberculosis. Indian J Tuberc. 2002;49:139–41. [Google Scholar]
- 12.Watt B, Rayner A, Harris G. Mycobacterium. In: Fraser AG, Marmion BP, Simmons A, editors. Mackie & McCartney's practical medical microbiology. II. Edinburgh: Churchill Livingstone; 1996. pp. 329–41. [Google Scholar]
- 13.Barrow GI, Feltham RKA. 3rd ed. Cambridge: Cambridge University Press; 2003. Cowan and Steel's manual for the identification of medical bacteria; p. 204. [Google Scholar]
- 14.Grange JM, Yates MD, de Kantor IN. 2nd ed. IV. Geneva, Switzerland: World Health Organization; 1996. Differentiation of M. bovis from other members of the M. tuberculosis complex. In: Guidelines for speciation within the Mycobacterium tuberculosis complex. Emerging and other communicable diseases, surveillance and control; pp. 8–11. [Google Scholar]
- 15.Kamerbeek J, Schouls L, Kolk A, Agterveld M Van, Soolingen D Van, Kuijper S, et al. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J Clin Microbiol. 1997;35:907–14. doi: 10.1128/jcm.35.4.907-914.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campbell I. Chi-squared and Fisher-Irwin tests of two-by-two tables with small sample recommendations. Stat Med. 2007;26:3661–75. doi: 10.1002/sim.2832. [DOI] [PubMed] [Google Scholar]
- 17.Nolte FS, Metchock B. Mycobacterium. In: Murray PR, Baron EJ, Tenover FC, Yolken RH, editors. Manual of clinical microbiology. Washington DC: American Society for Micobacteriology; 1995. pp. 400–33. [Google Scholar]
- 18.Tatsioni A, Zarin DA, Aronson N, et al. Challenges in systematic reviews of diagnostic technologies. Ann Intern Med. 2005;142:1048–55. doi: 10.7326/0003-4819-142-12_part_2-200506211-00004. [DOI] [PubMed] [Google Scholar]
- 19.Gebre-Selassie S. Evaluation of the concentration sputum smear technique for the laboratory diagnosis of pulmonary tuberculosis. Trop Doct. 2003;33:160–2. doi: 10.1177/004947550303300313. [DOI] [PubMed] [Google Scholar]
- 20.Angeby KA, Alvarado-Galvez C, Pineda-Garcia L, Hoffner SE. Improved sputum microscopy for a more sensitive diagnosis of pulmonary tuberculosis. Int J Tuberc Lung Dis. 2000;4:684–7. [PubMed] [Google Scholar]
- 21.Yesilkaya H, Barer MR, Andrew PW. Antibiotic resistance may affect alkali decontamination of specimen containing mycobacteria. Diagn Microbiol Infect Dis. 2004;50:153–5. doi: 10.1016/j.diagmicrobio.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 22.Iseman MD. Philadelphia: Lippincott, Williams and Wilkins; 2000. A clinician's guide to tuberculosis; pp. 29–32. [Google Scholar]
- 23.Vasanthkumari R. A single step culture technique for tubercle bacilli. Tubercle. 1990;71:267–70. doi: 10.1016/0041-3879(90)90039-b. [DOI] [PubMed] [Google Scholar]


