Abstract
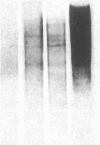
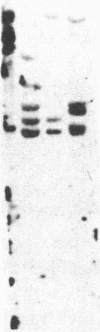
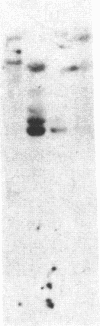
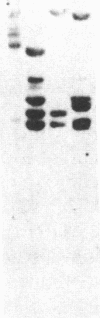
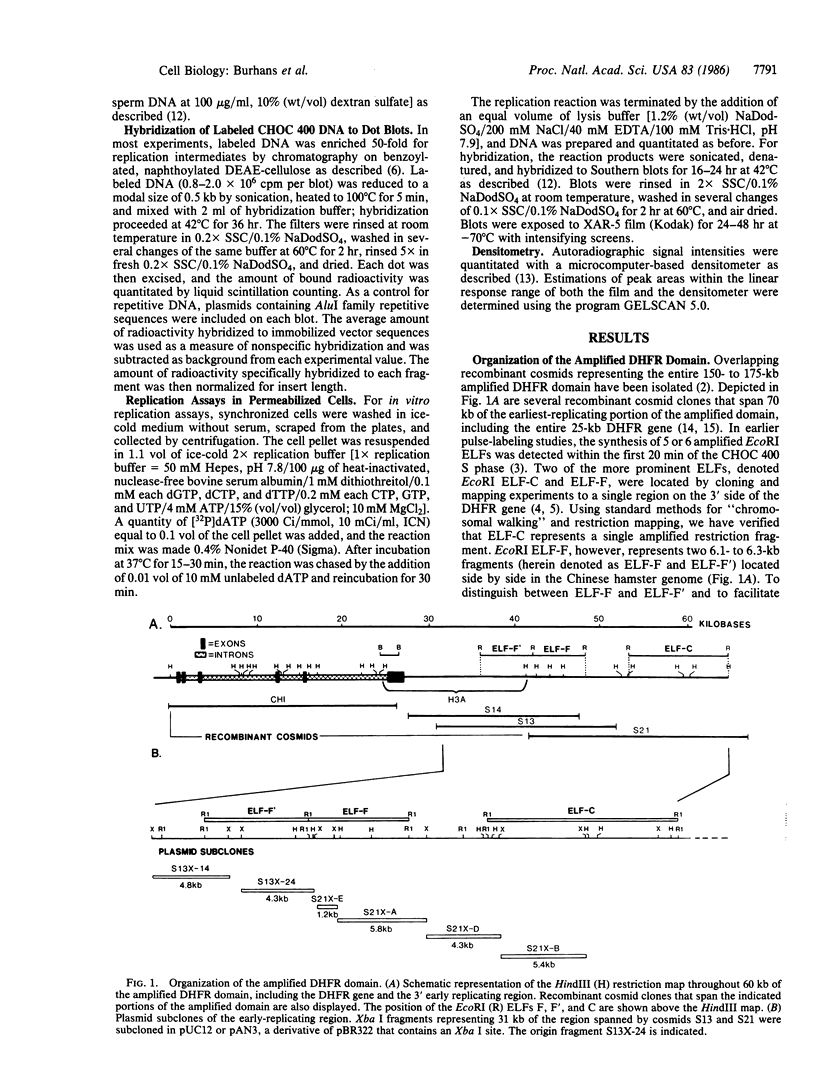
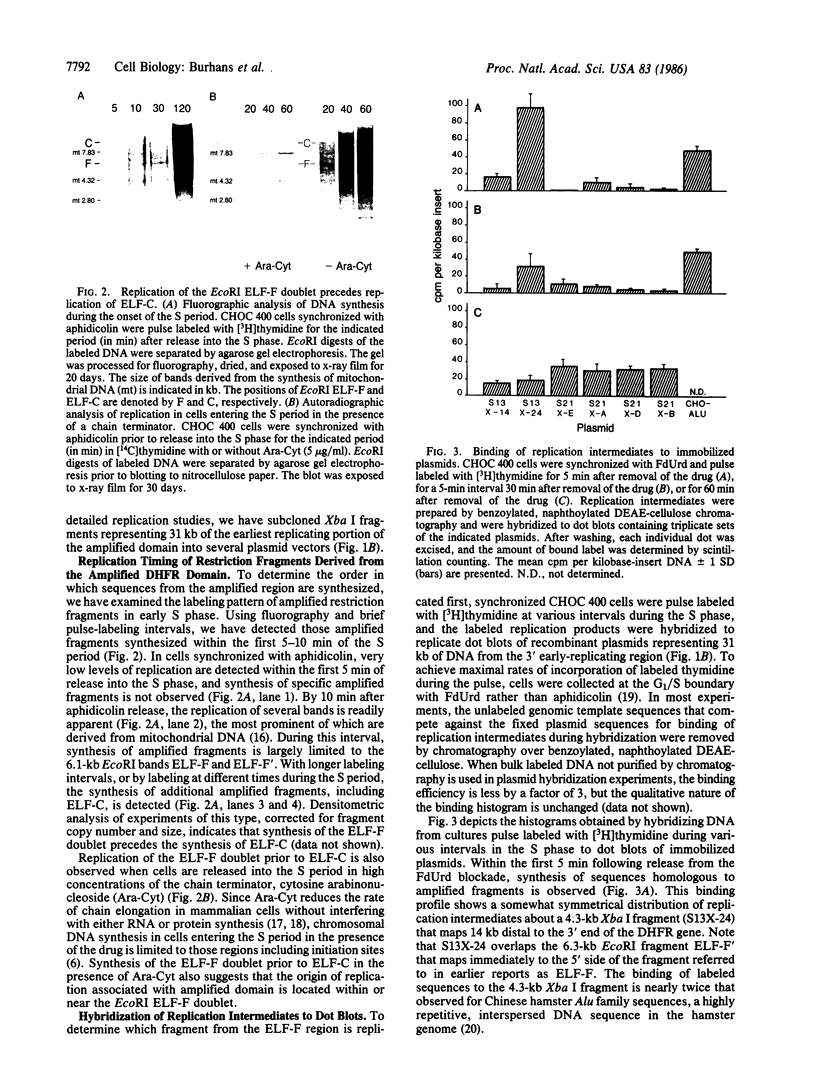
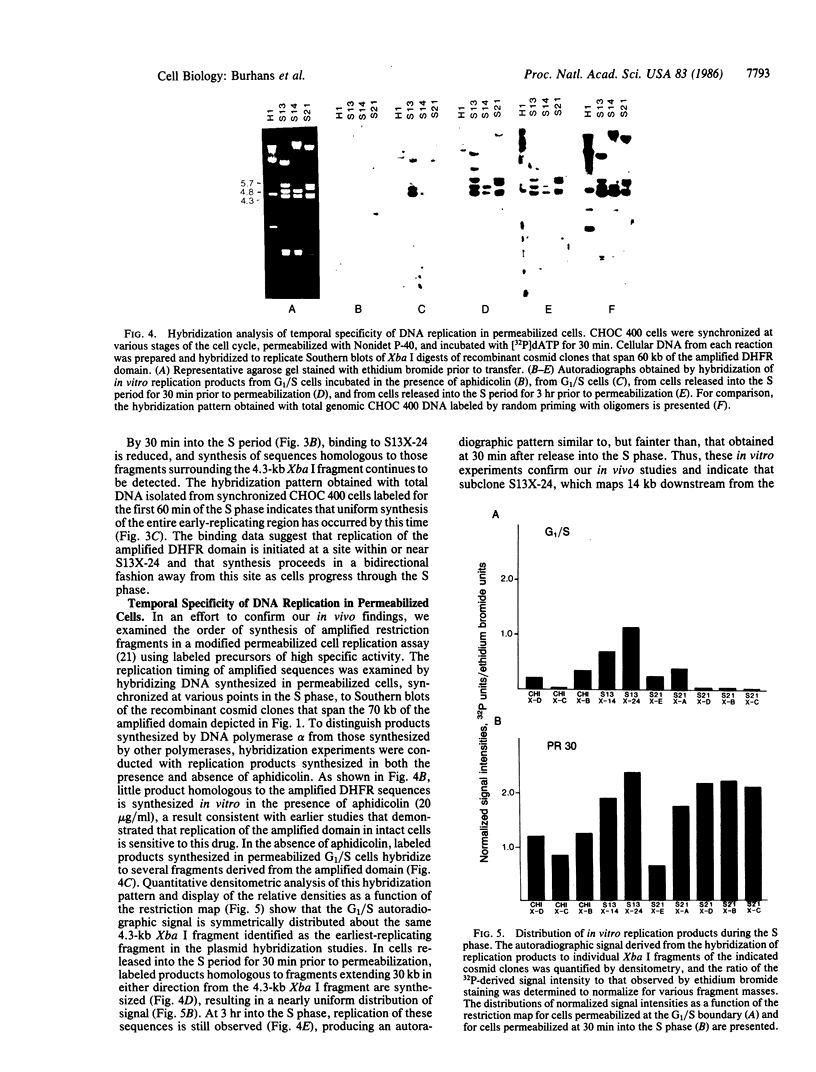
Autoradiography of restriction digests of DNA labeled in early S phase indicates that replication of the amplified dihydrofolate reductase (DHFR) domain of methotrexate-resistant CHOC 400 cells initiates within a 6.1-kilobase pair (kb) EcoRI-doublet located on the 3' side of the DHFR gene. To localize the DHFR origin fragment, synchronized CHOC 400 cells were either pulse labeled with [3H]thymidine in vivo or permeabilized and incubated with [32P]dATP under conditions that support limited chromosomal DNA replication. The temporal order of replication of amplified fragments was determined by hybridization of the in vivo or in vitro replication products to cloned fragments spanning the earliest-replicating portion of the DHFR domain. At the G1/S boundary, the labeled products derived from the replication of amplified sequences, either in whole or permeabilized cells, are distributed about an amplified 4.3-kb Xba I fragment that maps 14 kb downstream from the DHFR gene. As cells progress through the S phase, bidirectional replication away from this site is observed. These studies indicate that the 4.3-kb Xba I fragment contains the origin of replication associated with the amplified DHFR domain.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burhans W. C., Selegue J. E., Heintz N. H. Replication intermediates formed during initiation of DNA synthesis in methotrexate-resistant CHOC 400 cells are enriched for sequences derived from a specific, amplified restriction fragment. Biochemistry. 1986 Jan 28;25(2):441–449. doi: 10.1021/bi00350a025. [DOI] [PubMed] [Google Scholar]
- CHEONG L., RICH M. A., EIDINOFF M. L. Mechanism of growth inhibition of H.Ep. 1 cells by 5-fluorodeoxycytidine and 5-fluorodeoxyuridine. Cancer Res. 1960 Dec;20:1602–1607. [PubMed] [Google Scholar]
- Carothers A. M., Urlaub G., Ellis N., Chasin L. A. Structure of the dihydrofolate reductase gene in Chinese hamster ovary cells. Nucleic Acids Res. 1983 Apr 11;11(7):1997–2012. doi: 10.1093/nar/11.7.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzarelli N. R. The mechanism of action of inhibitors of DNA synthesis. Annu Rev Biochem. 1977;46:641–668. doi: 10.1146/annurev.bi.46.070177.003233. [DOI] [PubMed] [Google Scholar]
- Dinter-Gottlieb G., Kaufmann G. Aphidicolin arrest irreversibly impairs replicating simian virus 40 chromosomes. J Biol Chem. 1983 Mar 25;258(6):3809–3812. [PubMed] [Google Scholar]
- Hay R. T., DePamphilis M. L. Initiation of SV40 DNA replication in vivo: location and structure of 5' ends of DNA synthesized in the ori region. Cell. 1982 Apr;28(4):767–779. doi: 10.1016/0092-8674(82)90056-3. [DOI] [PubMed] [Google Scholar]
- Heintz N. H., Hamlin J. L. An amplified chromosomal sequence that includes the gene for dihydrofolate reductase initiates replication within specific restriction fragments. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4083–4087. doi: 10.1073/pnas.79.13.4083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heintz N. H., Hamlin J. L. In vivo effects of cytosine arabinoside on deoxyribonucleic acid replication in Chinese hamster ovary cells. 1. Resolution of differential effects on mitochondrial and nuclear deoxyribonucleic acid synthesis. Biochemistry. 1983 Jul 19;22(15):3552–3557. doi: 10.1021/bi00284a003. [DOI] [PubMed] [Google Scholar]
- Heintz N. H., Hamlin J. L. In vivo effects of cytosine arabinoside on deoxyribonucleic acid replication in Chinese hamster ovary cells. 2. Cytosine arabinoside affects the rate of synthesis but not the pattern of labeling of an amplified chromosomal sequence at the onset of the S period. Biochemistry. 1983 Jul 19;22(15):3557–3562. doi: 10.1021/bi00284a004. [DOI] [PubMed] [Google Scholar]
- Heintz N. H., Milbrandt J. D., Greisen K. S., Hamlin J. L. Cloning of the initiation region of a mammalian chromosomal replicon. 1983 Mar 31-Apr 6Nature. 302(5907):439–441. doi: 10.1038/302439a0. [DOI] [PubMed] [Google Scholar]
- Huberman J. A. New views of the biochemistry of eucaryotic DNA replication revealed by aphidicolin, an unusual inhibitor of DNA polymerase alpha. Cell. 1981 Mar;23(3):647–648. doi: 10.1016/0092-8674(81)90426-8. [DOI] [PubMed] [Google Scholar]
- Kristensen T., Prydz H. Isolated HeLa cell nuclei synthesize meaningful DNA. Nucleic Acids Res. 1985 May 24;13(10):3551–3560. doi: 10.1093/nar/13.10.3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labarca C., Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980 Mar 1;102(2):344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Milbrandt J. D., Azizkhan J. C., Greisen K. S., Hamlin J. L. Organization of a Chinese hamster ovary dihydrofolate reductase gene identified by phenotypic rescue. Mol Cell Biol. 1983 Jul;3(7):1266–1273. doi: 10.1128/mcb.3.7.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milbrandt J. D., Heintz N. H., White W. C., Rothman S. M., Hamlin J. L. Methotrexate-resistant Chinese hamster ovary cells have amplified a 135-kilobase-pair region that includes the dihydrofolate reductase gene. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6043–6047. doi: 10.1073/pnas.78.10.6043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoya-Zavala M., Hamlin J. L. Similar 150-kilobase DNA sequences are amplified in independently derived methotrexate-resistant Chinese hamster cells. Mol Cell Biol. 1985 Apr;5(4):619–627. doi: 10.1128/mcb.5.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid C. W., Jelinek W. R. The Alu family of dispersed repetitive sequences. Science. 1982 Jun 4;216(4550):1065–1070. doi: 10.1126/science.6281889. [DOI] [PubMed] [Google Scholar]
- Tracy R. P., Young D. S. A densitometer based on a microcomputer and TV camera, for use in the clinical laboratory. Clin Chem. 1984 Mar;30(3):462–465. [PubMed] [Google Scholar]
- Tsurimoto T., Matsubara K. Multiple initiation sites of DNA replication flanking the origin region of lambda dv genome. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7402–7406. doi: 10.1073/pnas.81.23.7402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vindeløv L. L., Christensen I. J., Nissen N. I. A detergent-trypsin method for the preparation of nuclei for flow cytometric DNA analysis. Cytometry. 1983 Mar;3(5):323–327. doi: 10.1002/cyto.990030503. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]