Abstract
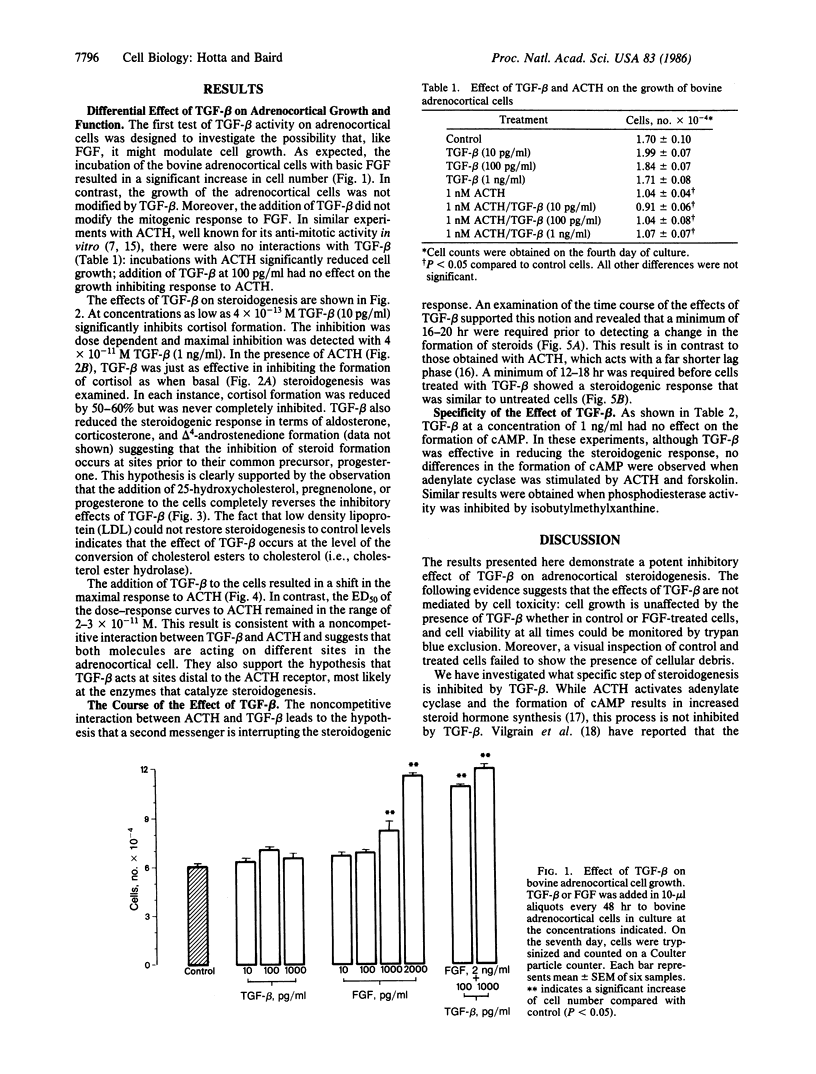
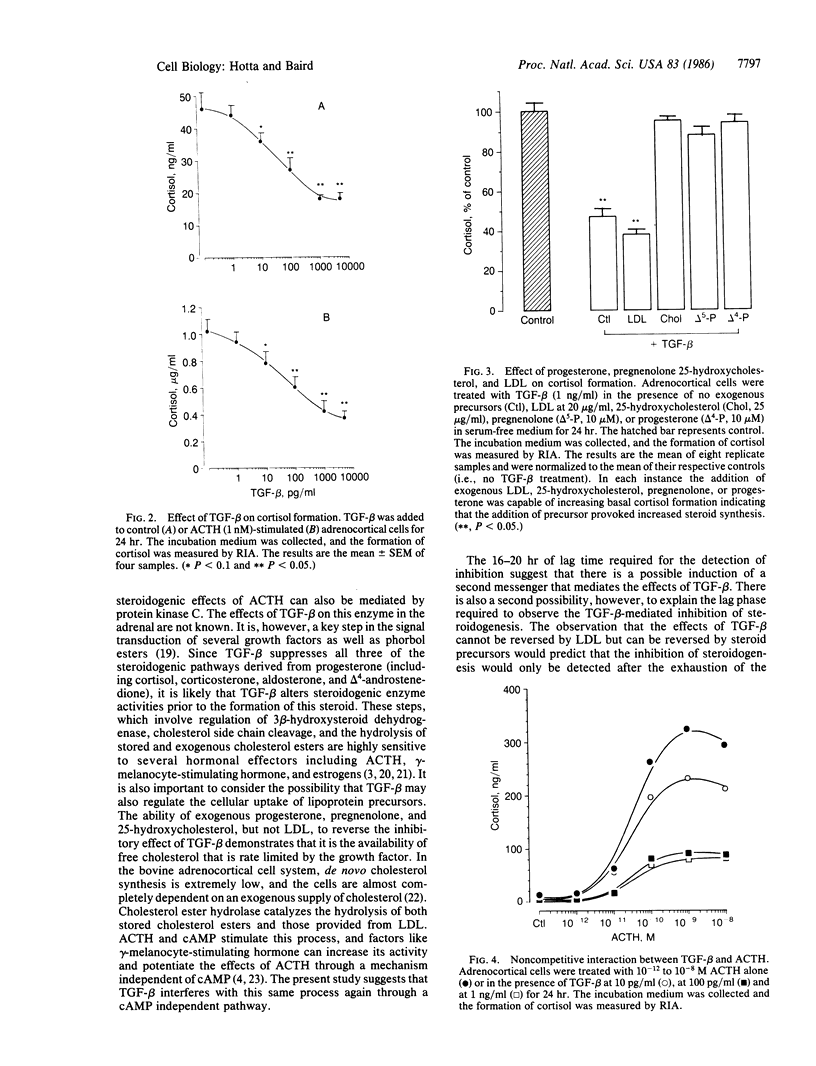
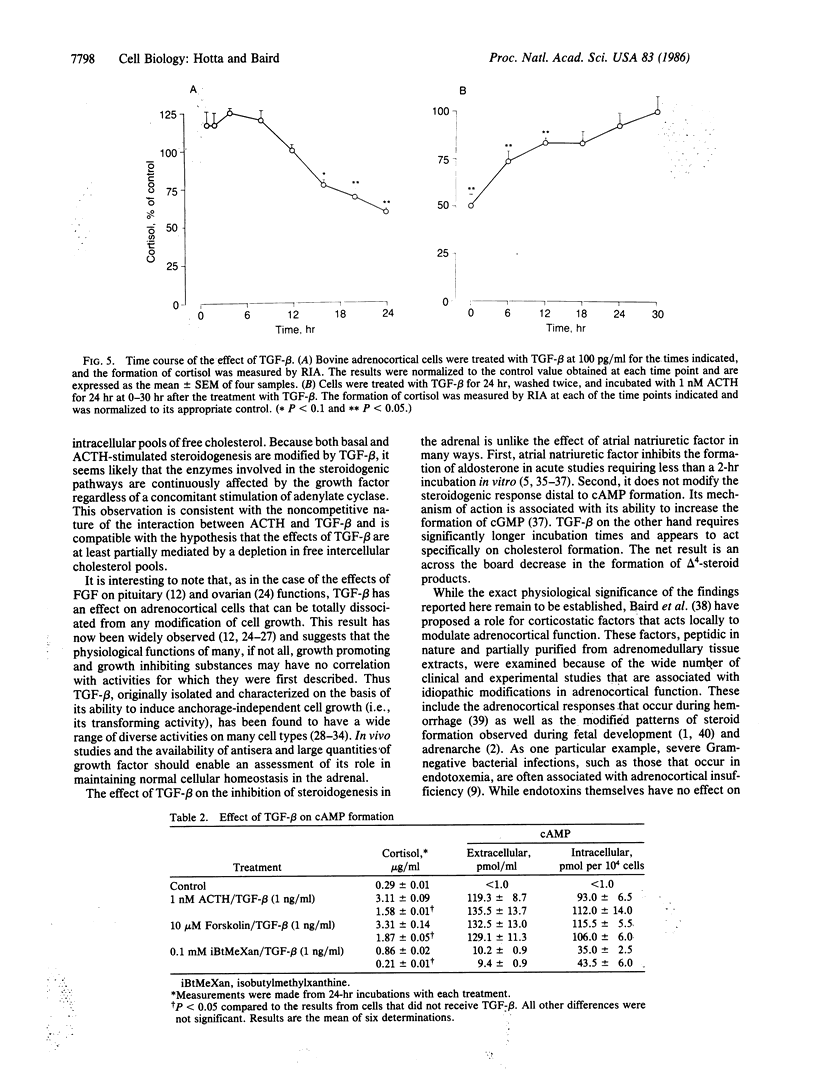
Transforming growth factor type beta (TGF-beta) suppresses basal as well as corticotropin (ACTH)-stimulated steroid formation by bovine adrenocortical cells in culture. The effect is dose dependent and is not accompanied by any change in adrenocortical cell growth. The minimum effective dose of TGF-beta is 4 X 10(-13) M (10 pg/ml), and maximal inhibition is observed at a concentration of 4 X 10(-11) M (1 ng/ml). A 16- to 20-hr incubation with TGF-beta is required to decrease steroidogenesis, and 12-18 hr are required before cells treated with TGF-beta recover complete responsiveness to corticotropin. Increases in cAMP mediated by corticotropin, forskolin, and isobutylmethylxanthine are not modified by the addition of TGF-beta; thus adenylate cyclase activity is unaffected by TGF-beta. Although TGF-beta inhibits the formation of all of the delta 4-steroids measured (including cortisol, corticosterone, aldosterone, and androstenedione), its effect can be completely reversed by the addition of 25-hydroxycholesterol, pregnenolone, or progesterone to the cells. In contrast, the addition of low density lipoprotein has no effect suggesting that TGF-beta targets the conversion of cholesterol precursors to cholesterol. The results demonstrate a highly potent effect of TGF-beta on the differentiated function of the adrenocortical cell. The inhibition of steroidogenesis can be dissociated from any effect on cell proliferation, and it occurs distal to the formation of cAMP but proximal to the formation of cholesterol. The results suggest that in the adrenal, TGF-beta or TGF-beta-like proteins may be playing an important role in modifying the differentiated state of the adrenocortical cell.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assoian R. K., Komoriya A., Meyers C. A., Miller D. M., Sporn M. B. Transforming growth factor-beta in human platelets. Identification of a major storage site, purification, and characterization. J Biol Chem. 1983 Jun 10;258(11):7155–7160. [PubMed] [Google Scholar]
- Atarashi K., Mulrow P. J., Franco-Saenz R., Snajdar R., Rapp J. Inhibition of aldosterone production by an atrial extract. Science. 1984 Jun 1;224(4652):992–994. doi: 10.1126/science.6326267. [DOI] [PubMed] [Google Scholar]
- Baird A., Esch F., Mormède P., Ueno N., Ling N., Böhlen P., Ying S. Y., Wehrenberg W. B., Guillemin R. Molecular characterization of fibroblast growth factor: distribution and biological activities in various tissues. Recent Prog Horm Res. 1986;42:143–205. doi: 10.1016/b978-0-12-571142-5.50008-2. [DOI] [PubMed] [Google Scholar]
- Baird A., Mormède P., Ying S. Y., Wehrenberg W. B., Ueno N., Ling N., Guillemin R. A nonmitogenic pituitary function of fibroblast growth factor: regulation of thyrotropin and prolactin secretion. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5545–5549. doi: 10.1073/pnas.82.16.5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartier L., Schiffrin E., Thibault G., Garcia R. Atrial natriuretic factor inhibits the stimulation of aldosterone secretion by angiotensin II, ACTH and potassium in vitro and angiotensin II-induced steroidogenesis in vivo. Endocrinology. 1984 Nov;115(5):2026–2028. doi: 10.1210/endo-115-5-2026. [DOI] [PubMed] [Google Scholar]
- Dupuis-Dauvergne M., Idelman S. Mise en évidence et analyse quantitative des phagocytes mononuclés (monocytes et macrophages tissulaires) dans les capillaires sinusoïdes du cortex surrénal du Rat. C R Acad Sci III. 1984;298(18):535–540. [PubMed] [Google Scholar]
- Esch F., Baird A., Ling N., Ueno N., Hill F., Denoroy L., Klepper R., Gospodarowicz D., Böhlen P., Guillemin R. Primary structure of bovine pituitary basic fibroblast growth factor (FGF) and comparison with the amino-terminal sequence of bovine brain acidic FGF. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6507–6511. doi: 10.1073/pnas.82.19.6507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frolik C. A., Dart L. L., Meyers C. A., Smith D. M., Sporn M. B. Purification and initial characterization of a type beta transforming growth factor from human placenta. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3676–3680. doi: 10.1073/pnas.80.12.3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodarowicz D., Baird A., Cheng J., Lui G. M., Esch F., Bohlen P. Isolation of fibroblast growth factor from bovine adrenal gland: physicochemical and biological characterization. Endocrinology. 1986 Jan;118(1):82–90. doi: 10.1210/endo-118-1-82. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Ill C. R., Hornsby P. J., Gill G. N. Control of bovine adrenal cortical cell proliferation by fibroblast growth factor. Lack of effect of epidermal growth factor. Endocrinology. 1977 Apr;100(4):1080–1089. doi: 10.1210/endo-100-4-1080. [DOI] [PubMed] [Google Scholar]
- Higuchi K., Nawata H., Kato K., Ibayashi H., Matsuo H. Alpha-human atrial natriuretic polypeptide inhibits steroidogenesis in cultured human adrenal cells. J Clin Endocrinol Metab. 1986 May;62(5):941–944. doi: 10.1210/jcem-62-5-941. [DOI] [PubMed] [Google Scholar]
- Hornsby P. J., Gill G. N. Hormonal control of adrenocortical cell proliferation. Desensitization to ACTH and interaction between ACTH and fibroblast growth factor in bovine adrenocortical cell cultures. J Clin Invest. 1977 Aug;60(2):342–352. doi: 10.1172/JCI108782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubay C. A., Weckesser E. C., Levy R. P. Occult adrenal insufficiency in surgical patients. Ann Surg. 1975 Mar;181(3):325–332. doi: 10.1097/00000658-197503000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume D. A., Halpin D., Charlton H., Gordon S. The mononuclear phagocyte system of the mouse defined by immunohistochemical localization of antigen F4/80: macrophages of endocrine organs. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4174–4177. doi: 10.1073/pnas.81.13.4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keri G., Parameswaran V., Trunkey D. D., Ramachandran J. Effects of septic shock plasma on adrenocortical cell function. Life Sci. 1981 Apr 27;28(17):1917–1923. doi: 10.1016/0024-3205(81)90299-x. [DOI] [PubMed] [Google Scholar]
- Kudo T., Baird A. Inhibition of aldosterone production in the adrenal glomerulosa by atrial natriuretic factor. Nature. 1984 Dec 20;312(5996):756–757. doi: 10.1038/312756a0. [DOI] [PubMed] [Google Scholar]
- Ling N., Esch F., Davis D., Mercado M., Regno M., Bohlen P., Brazeau P., Guillemin R. Solid phase synthesis of somatostatin-28. Biochem Biophys Res Commun. 1980 Aug 14;95(3):945–951. doi: 10.1016/0006-291x(80)91564-8. [DOI] [PubMed] [Google Scholar]
- Massagué J. Type beta transforming growth factor from feline sarcoma virus-transformed rat cells. Isolation and biological properties. J Biol Chem. 1984 Aug 10;259(15):9756–9761. [PubMed] [Google Scholar]
- Mathison J. C., Schreiber R. D., La Forest A. C., Ulevitch R. J. Suppression of ACTH-induced steroidogenesis by supernatants from LPS-treated peritoneal exudate macrophages. J Immunol. 1983 Jun;130(6):2757–2762. [PubMed] [Google Scholar]
- Moore G. P., Wilkinson M., Panaretto B. A., Delbridge L. W., Posen S. Epidermal growth factor causes hypocalcemia in sheep. Endocrinology. 1986 Apr;118(4):1525–1529. doi: 10.1210/endo-118-4-1525. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Ohashi M., Simpson E. R., Kramer R. E., Carr B. R. Regulation of low-density lipoprotein receptors in cultured bovine adrenocortical cells. Arch Biochem Biophys. 1982 Apr 15;215(1):199–205. doi: 10.1016/0003-9861(82)90295-8. [DOI] [PubMed] [Google Scholar]
- Pedersen R. C., Brownie A. C. Adrenocortical response to corticotropin is potentiated by part of the amino-terminal region of pro-corticotropin/endorphin. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2239–2243. doi: 10.1073/pnas.77.4.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen R. C., Brownie A. C., Ling N. Pro-adrenocorticotropin/endorphin-derived peptides: coordinate action on adrenal steroidogenesis. Science. 1980 May 30;208(4447):1044–1046. doi: 10.1126/science.6246578. [DOI] [PubMed] [Google Scholar]
- Rich B. H., Rosenfield R. L., Lucky A. W., Helke J. C., Otto P. Adrenarche: changing adrenal response to adrenocorticotropin. J Clin Endocrinol Metab. 1981 Jun;52(6):1129–1136. doi: 10.1210/jcem-52-6-1129. [DOI] [PubMed] [Google Scholar]
- Roberts A. B., Anzano M. A., Wakefield L. M., Roche N. S., Stern D. F., Sporn M. B. Type beta transforming growth factor: a bifunctional regulator of cellular growth. Proc Natl Acad Sci U S A. 1985 Jan;82(1):119–123. doi: 10.1073/pnas.82.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybak S. M., Ramachandran J. Mechanism of induction of delta 5-3 beta-hydroxysteroid dehydrogenase-isomerase activity in rat adrenocortical cells by corticotropin. Endocrinology. 1982 Aug;111(2):427–433. doi: 10.1210/endo-111-2-427. [DOI] [PubMed] [Google Scholar]
- SLATER J. D., BARBOUR B. H., HENDERSON H. H., CASPER A. G., BARTTER F. C. INFLUENCE OF THE PITUITARY AND THE RENIN-ANGIOTENSIN SYSTEM ON THE SECRETION OF ALDOSTERONE, CORTISOL, AND CORTICOSTERONE. J Clin Invest. 1963 Sep;42:1504–1520. doi: 10.1172/JCI104835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala G. B., Hayashi K., Catt K. J., Dufau M. L. Adrenocorticotropin action in isolated adrenal cells. The intermediate role of cyclic AMP in stimulation of corticosterone synthesis. J Biol Chem. 1979 May 25;254(10):3861–3865. [PubMed] [Google Scholar]
- Sibbald W. J., Short A., Cohen M. P., Wilson R. F. Variations in adrenocortical responsiveness during severe bacterial infections. Unrecognized adrenocortical insufficiency in severe bacterial infections. Ann Surg. 1977 Jul;186(1):29–33. doi: 10.1097/00000658-197707000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson E. R., Waterman M. R. Regulation by ACTH of steroid hormone biosynthesis in the adrenal cortex. Can J Biochem Cell Biol. 1983 Jul;61(7):692–707. doi: 10.1139/o83-088. [DOI] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B., Shull J. H., Smith J. M., Ward J. M., Sodek J. Polypeptide transforming growth factors isolated from bovine sources and used for wound healing in vivo. Science. 1983 Mar 18;219(4590):1329–1331. doi: 10.1126/science.6572416. [DOI] [PubMed] [Google Scholar]
- Veldhuis J. D., Rodgers R. J., Dee A., Simpson E. R. The insulin-like growth factor, somatomedin C, induces the synthesis of cholesterol side-chain cleavage cytochrome P-450 and adrenodoxin in ovarian cells. J Biol Chem. 1986 Feb 25;261(6):2499–2502. [PubMed] [Google Scholar]
- Vilgrain I., Cochet C., Chambaz E. M. Hormonal regulation of a calcium-activated, phospholipid-dependent protein kinase in bovine adrenal cortex. J Biol Chem. 1984 Mar 25;259(6):3403–3406. [PubMed] [Google Scholar]
- Winter J. S., Smail P. J. Effects of ACTH and estradiol on steroid production by cultured adrenal cells from an anencephalic fetus and from normal adults. Steroids. 1983 Dec;42(6):677–685. doi: 10.1016/0039-128x(83)90131-9. [DOI] [PubMed] [Google Scholar]
- Woloski B. M., Smith E. M., Meyer W. J., 3rd, Fuller G. M., Blalock J. E. Corticotropin-releasing activity of monokines. Science. 1985 Nov 29;230(4729):1035–1037. doi: 10.1126/science.2997929. [DOI] [PubMed] [Google Scholar]
- Wood C. E., Shinsako J., Keil L. C., Ramsay D. J., Dallman M. F. Apparent dissociation of adrenocorticotropin and corticosteroid responses to 15 ml/kg hemorrhage in conscious dogs. Endocrinology. 1982 Apr;110(4):1416–1421. doi: 10.1210/endo-110-4-1416. [DOI] [PubMed] [Google Scholar]
- Ying S. Y., Becker A., Baird A., Ling N., Ueno N., Esch F., Guillemin R. Type beta transforming growth factor (TGF-beta) is a potent stimulator of the basal secretion of follicle stimulating hormone (FSH) in a pituitary monolayer system. Biochem Biophys Res Commun. 1986 Mar 28;135(3):950–956. doi: 10.1016/0006-291x(86)91020-x. [DOI] [PubMed] [Google Scholar]
- Ying S. Y., Becker A., Ling N., Ueno N., Guillemin R. Inhibin and beta type transforming growth factor (TGF beta) have opposite modulating effects on the follicle stimulating hormone (FSH)-induced aromatase activity of cultured rat granulosa cells. Biochem Biophys Res Commun. 1986 May 14;136(3):969–975. doi: 10.1016/0006-291x(86)90427-4. [DOI] [PubMed] [Google Scholar]
- de Larco J. E., Todaro G. J. Growth factors from murine sarcoma virus-transformed cells. Proc Natl Acad Sci U S A. 1978 Aug;75(8):4001–4005. doi: 10.1073/pnas.75.8.4001. [DOI] [PMC free article] [PubMed] [Google Scholar]



