Abstract
Background:
Human papillomavirus (HPV) has been recognized as a necessary, but not sufficient, cause of cervical cancer.
Aim:
In this study, we investigated the prevalence of HPV and the genotype distribution in women from Natal, North-East Brazil, with normal cytology and with cervical lesions of different degrees.
Subjects and Methods:
Included in this study were 110 women with a normal cytology and 315 with a previous history of cervical cytological abnormalities. The patients were enrolled between January 2005 and December 2008. The cytopathological analyzes were performed by the Pap smear exam, and the pre-malignant and maligant lesions were confirmed based on the histopathological analysis. The presence of HPV was detected by polymerase chain reaction with genotyping by dot blot hybridization. All the data were included in a database, using the software SPSS, Version 10.0 (Chicago Il, USA).
Results:
Overall HPV prevalence was 65.2% (277/425), with 85.9% (238/277) single and 14.1% (39/277) multiple infection. The most prevalent HPV types were HPVs 16, 58, 18, 31, and 45. HPV 16 was the most prevalent genotype, independently of the health status of patients. HPV 58 was the second most prevalent type in women with normal cytology and in those who had mild or moderate dysplasia. HPV 58 presented equal prevalence to HPV 18 in patients with severe dysplasia. However, it was less prevalent than HPV 18 in women with cervical cancer.
Conclusions:
The results show a high prevalence of HPV 58, especially in women with mild and moderate dysplasia, revealing the high-frequency circulation of this genotype of HPV in the local population. This finding suggests the need to include this genotype in future HPV vaccines targeting women in this region.
Keywords: Human papillomavirus, Risk factors, Uterine cervical lesions
Introduction
The human papillomavirus (HPV) infection is one of the most common sexually transmissible infections in the world, especially in developing countries, where the prevalence of asymptomatic infection varies from 2% to 44%, depending on the population and studied region.[1] Some studies show that most sexually active individuals are exposed to and acquire infection from this virus at some phase in their lives.[2,3] HPV infection is most prevalent in young adults, at the beginning of their sexual activity, with a subsequent decline in the prevalence with increasing age, likely a result of the development of an immune response against the virus or due to the reduction of sexual activity as well as the number of partners.[4,5,6]
Of the more than 120 different HPV types that have been catalogued, more than 40 are known to infect the epithelium of the anogenital tract and other mucosal areas of the human body. They are classified as high or low oncogenic risk according to their involvement in the genesis of benign or malignant lesions.[7,8] Among these, at least 15 are considered high-risk HPV (HR-HPV) and are strongly associated with progression of the cervical lesions of low-grade to high-grade and invasive cancer.[9] Studies on the prevalence of genotypes indicate that HPV 16 is the most prevalent type in the different regions of the world.[1,10,11] Nevertheless, the frequency of the HR-HPV types may vary according to geographic, demographic, and clinical-pathological factors,[12,13] and may also be influenced by the methods used for detection.[14]
After many years of clinical, epidemiological, and experimental studies, it is widely accepted that epithelium cervical infection by HR-HPV is a necessary, but not sufficient, cause of the development of cervical cancer as well as a significant proportion of other anogenital and oral squamous cell carcinomas preceded by cellular abnormalities that can be identified by cytological or histopathological exams.[3,15,16,17]
In Brazil, cervical cancer is the third most prevalent malignant neoplasia among women and continues to be a serious public health problem, showing remarkable differences in the incidence among different regions of the country.[18,19] Studies have shown that HPV16 is the predominant type, but the prevalence of the other HR-HPV types varies according to the region analyzed.[18,20,21,22] This study evaluated the prevalence of infection and the distribution of HPV types in women of Rio Grande do Norte, North-East Brazil, with normal cytology and with cervical lesions of different degree, including cervical cancer.
Subjects and Methods
Population studied and sample collection
The study involved 251 women who were referred to the Luis Antonio Hospital in Natal, Rio Grande do Norte, Brazil, with a previous history of cytological abnormalities and 185 women enrolled among those who voluntarily entered the cancer screening program and were analyzed by cytological analysis. The patients were enrolled in the period between January 2005 and December 2008. All subjects participating in this study were informed about the methodology and objectives of the research. The inclusion criteria were agreeing to participate in the study and answering a standardized epidemiological questionnaire. On the other hand, the exclusion criteria were current pregnancy, having had a miscarriage or delivery less than 60 days before the collection, having undergone hysterectomy, and mental deficiency that would compromise their understanding and/or their responses when they filled out the questionnaire. All the patients included in the study signed an informed consent.
Two samples of uterine cervical exfoliated cells were collected from women participating in the screening program, using a cytobrush (Kolpalst LTDA, Brazil): One for cytological analysis by Pap smear, and the other for the molecular detection of HPV. The smears were analyzed by a trained pathologist from the Department of Pathology at the Federal University of Rio Grande do Norte, and the cytopathological reports were based on the 1991 Bethesda system for cytologic diagnosis.[23] The 64 patients who presented alterations in the cytological exam were submitted to histopathological analysis. We excluded 11 patients from analysis: Five presented scant squamous cellularity, considered unsatisfactory for evaluation, and in six specimens we did not obtain amplifiable deoxyribonucleic acid (DNA), leaving a total of 174 samples.
In patients who were referred to the hospital with a previous history of cervical alterations, the lesion was identified by colposcopy and a fragment of tissue was collected by biopsy and processed for histopathology. The detected uterine cervical lesions were classified as mild dysplasia cervical intraepithelial neoplasia (CIN 1), moderate dysplasia (CIN 2), severe dysplasia (CIN 3), and invasive cancer. In patients with the diagnosis of CIN 3 or invasive cancer, another fragment of tissue was obtained after the conization procedure in surgery and re-examined for result confirmation, and a fragment of tissue was processed for DNA extraction.
DNA extraction
For molecular analysis, the samples of cervical exfoliated cells were conditioned in a tube containing a homemade preserving solution [Phosphate Buffer Saline (PBS) + vancomycin (0.25 U/mL) + nystatin (6.25 μg/mL)] and sent to a laboratory where it was processed for DNA extraction, using rapid isolation of DNA from mammals’ protocol, with proteinase K, according to Sambrook and Russel, 2001.[24]
Small fragments of tissue obtained by colposcopy-directed biopsy or collected during the surgical procedure were conditioned as described for exfoliated cells. To DNA extraction, the samples were incubated in 200 μL of digestion buffer (0.01 M Tris-HCl; 0.02 M EDTA; 0.1 M NaCl; 0.5% SDS; pH 8.0) and 20 μL Proteinase K 10 mg/mL (© Life Technologies Corporation) at 42°C overnight, 56°C for 3 h, and further at 95°C for 5 min. After this, the DNA extraction was performed using the phenol/chloroform method, according to Sambrook and Russel, 2001.[24]
Aliquots with around 30 ng of DNA were submitted to a polymerase chain reaction (PCR) to amplify a 110 bp fragment of the human ß-globin gene, using the primers PCO3+/PCO4+[25] to analyze the quality of target DNA and the absence of PCR inhibitors.
Detection and typing HPV
All the samples that were ß-globin positive were tested for HPV DNA detection by PCR using the primers MY09/11.[26] The PCR products were submitted to genotyping for the individual HPV types by dot blot hybridization, according to Manos et al.[26] using the probes for the following HPV types: 6, 11, 16, 18, 31, 33, 35, 39, 42, 45, 51, 52, 53, 54, 55, 56, 57, 58, 59, and 68. We used the term “multiple infections” to refer to cases in which we detected more than one HPV type. Also, we classified the oncogenic risk of the detected virus according Muñoz et al.[9] HR-HPV types included 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68. HPVs 6, 11, 42 and 54 were considered low risk, and HPV 53 was considered like probable high-risk. HPV 55 and 57 were classified like undetermined.
This study was performed in compliance with relevant laws and institutional guidelines and in accordance with the ethical standards of the Declaration of Helsinki. It was approved by the Ethical Committee in Research of Federal University of Rio Grande do Norte.
Results
We included in the study just the women who agreed in participate and do not present any exclusion criteria. From these 436 women initially enrolled, 11 were excluded from analysis, because their cytological samples were considered unsatisfactory for evaluation (5 women), or we did not obtain amplifiable DNA (6 women). Thus, the studied population consisted of 425 women aged 15-65 years, with an average of 39.4 years. All patients who had their samples analyzed for HPV detection responded to a questionnaire through, which it was possible to trace the epidemiological profile of the participants in the study. The majority (52.5%) (223/425) of participants were aged between 31 and 45 years had not completed the basic education level (52.9%) (225/425), were of non-white ethnicity (54.6%) (232/425), were married or living in a stable relationship with their partner (73.2%) (311/425), had the first sexual intercourse at an age between 14 and 17 years (53.6%) (228/425), and not had more than one sexual partner throughout life (52.0%) (221/425) and at least one pregnancy (79.1%) (336/425) [Table 1].
Table 1.
Socio-demographic characteristics of the studied population

From the 174 women enrolled in the screening program and included in the study, 110 presented normal cytology and the remaining 64 who were re-analyzed by histopathology showed alterations classified as CIN 1. After the histopathological analysis, the patients were divided into five groups, based on their health status. Of the 425 samples analyzed, 110 (25.9%) did not present any cytological abnormalities in the exam and were classified in the normal cytology group, 64 (15.1%) exhibited mild dysplasia (CIN 1), 32 (7.5%) had moderate dysplasia (CIN 2), 121 (28.5%) had severe dysplasia (CIN 3), and 98 (23.1%) had invasive cervical cancer.
Among the 425 patients included in the study, 277 (65.2%) were positive for HPV, with 85.9% (238/277) of the samples containing a single HPV infection, and 14.1% (39/277) were infected by more than one HPV type. Considering both single and multiple infections, the overall HPV prevalence of infection varied in the different groups, according to health status. The overall prevalence of HPV found in this study was 65.2% (277/425), being 24.5% (27/110) in women with normal cytology, 62.5% (40/64) in those with mild dysplasia (CIN 1), 75.0% (24/32) in moderate dysplasia (CIN 2), 82.6% (110/121) in severe dysplasia (CIN 3) and 87.8% (86/98) in patients with invasive cervical cancer. We identified 14 different HPV genotypes and in 5 samples the genotypes could not be identified with the used probes.
Most patients were infected with HPV genotypes of high oncogenic risk, regardless of their health status. In women with a normal cytology, the most prevalent HPV genotypes were HPV 16 (14.5%) and HPV 58 (6.3%). In the group of women with CIN 1, the most common types were HPV 16 (20.3%), HPV 58 (14.1%), HPV 57 (10.9%), and HPV 56 (2.5%). In the lesions classified as CIN 2, the most prevalent types were HPV 16 (43.8%), HPV 58 (15.6%), followed by HPV 18, HPV 45, and HPV 59, each of them with 6.2%. In patients with severe dysplasia (CIN 3) the most prevalent types were HPV 16 (52.9%), HPV 18 (8.3%), and HPV 58, (8.3%), followed by HPV 31 (6.6%). In patients with invasive cervical cancer, the HPV genotypes with higher prevalence were HPV 16 (56.1%), HPV 18 (11.2%), HPV 58 (8.2%), and HPV 31 (5.1%) [Table 2]. Considering only the HPV positive cases, and including single and multiple infection, the distribution of HPV genotypes showed that HPV 16 was the most prevalent genotype, independently of the health status of patients, presenting overall prevalence of 58.5%, followed by HPV 58 (14.4%), HPV 18 (10.1%), HPV 31 (5.8%), and HPV 45 with (5.1%) [Table 3].
Table 2.
Distribution of HPV types with respective prevalence, including single and multiple infection, stratified according to health status of the patients
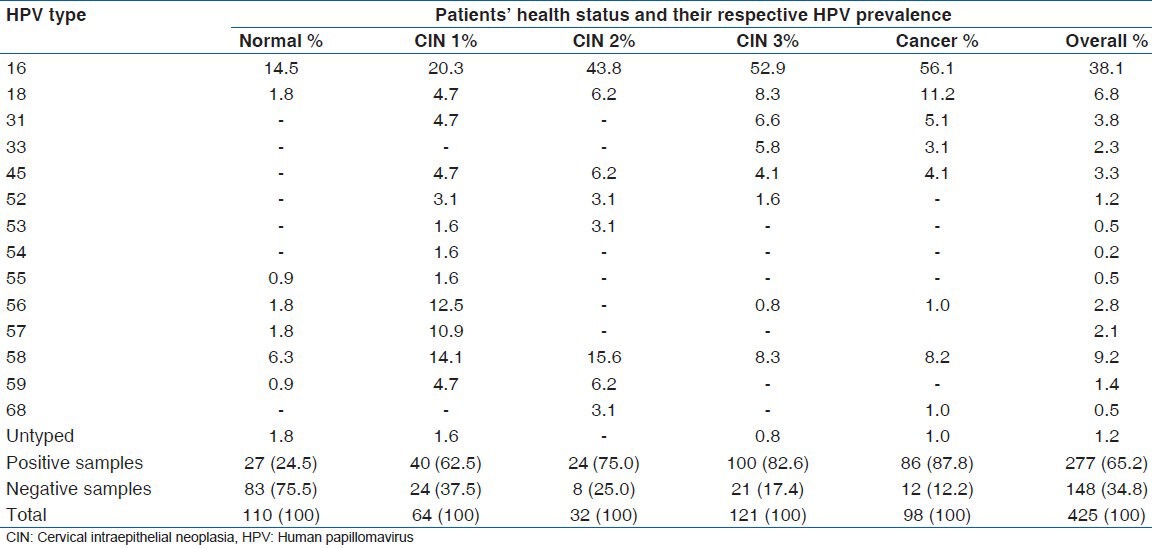
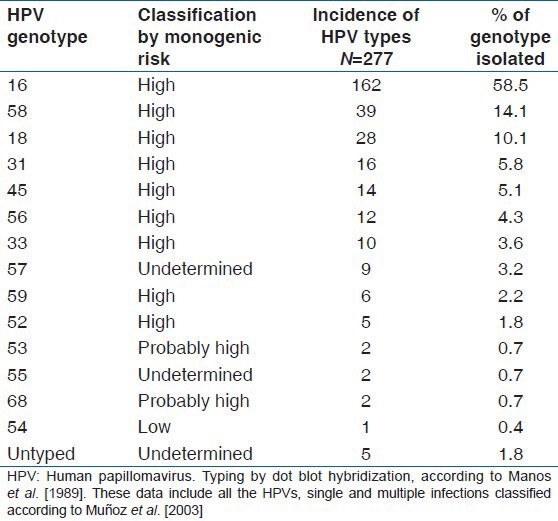
Table 3.
Prevalence of genotype infection of human papillomavirus in 277 women with normal cytology and with cervical lesions of different grade
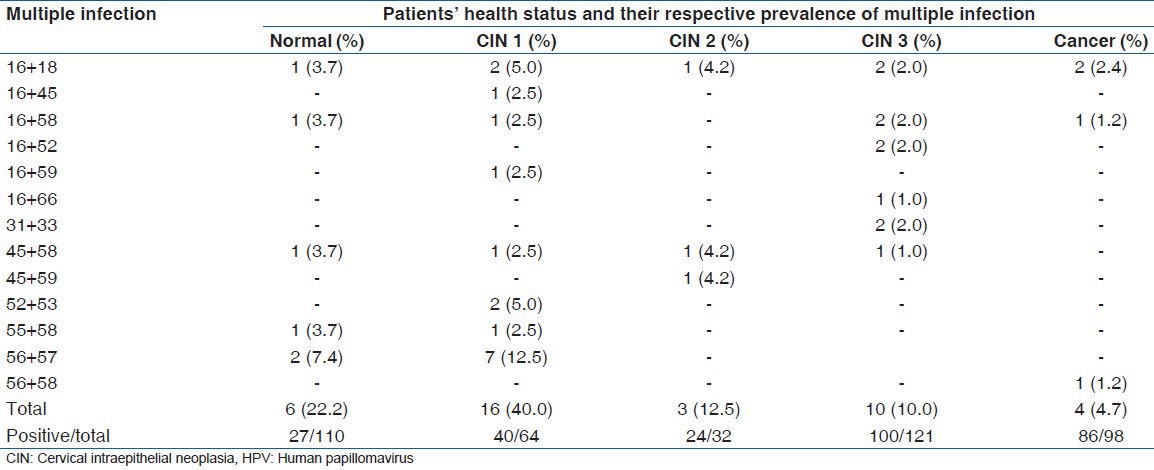
The simultaneous infection by two different types of HPV was found in 39 of the 277 patients who tested positive for HPV, representing prevalence of 14.1%. The most prevalent association occurred between HPV 56 and HPV 57, found in 12.5% of women with CIN1 and in 7.4% of those with a normal cytology, followed by the combinations HPV 52 + HPV 53 and HPV 16 + HPV 18, both found in 5.0% of the samples from patients with CIN 1 [Table 4].
Table 4.
Distribution of cases of multiple infection by HPV, according to health status of the patients
Discussion
Studies have shown that HPV 16 is the predominant genotype in all regions of Brazil, independently of the health status of the women analyzed. However, regional variations in the prevalence have been observed. In relation to the other HPV genotypes, even greater regional variations in prevalence have been found.[20,22,27] In the present study, we analyzed the distribution of HPV genotypes found in women from Rio Grande do Norte, North-East of Brazil.
HPV 58 was the second most common genotype in women with a normal cytology as well as in those with CIN 1 and CIN 2. The prevalence found in women with normal cytology was 6.3%, more than that found in women from Recife,[27] also in North-East Brazil, where HPV 58 was the third in prevalence (1.8%), and it was also higher than the prevalence reported for the countries of South America, including Brazil[1] in a meta-analysis (1.4%). In women with CIN 1, the prevalence (14.1%) was higher than that found in women of Recife,[27] where HPV 58 was only the fourth in prevalence with a percentage of 6.4%. The highest prevalence of HPV 58 (15.6%) observed in this study was found in women with cervical lesions classified as CIN 2. In this study, the prevalence of HPV 58 in women with invasive cervical cancer was 8.2%, higher than that found in women from Recife (3.4%).[27]
Considering only cases positive for HPV, the overall prevalence obtained showed that the five most common genotypes in descending order were HPV 16 (58.5%), HPV 58 (14.1%), HPV 18 (10.1%), HPV 31 (5.8%), and HPV 45 (5.1%). In a similar study involving women from South Brazil (22), the five most prevalent genotypes were HPV 16 (26.9%), HPV 58 (12.9%), HPV 11 (9.7%), HPV 33 (8.6%), and HPV 18 (7.5%). In both studies, HPV 58 was found to be the second most prevalent genotype, showing very similar prevalence. The higher prevalence of HPV 16 and HPV 18 observed in this study, compared to Paesi et al.[22] may have been caused by the inclusion of more cases of severe dysplasia and invasive cervical cancer in our study.
When we considered only women with normal cytology, the prevalence of HPV in general, and of the HPV 58 individually, found in this study was higher than the average reported for six South American countries, including Brazil.[1] In our study, we found a higher prevalence of HPV 58 infection among young women aged up 30 years, differing in this respect, from results obtained in women in Taiwan.[28]
It is important to highlight the presence of HPV 57, which was not described in other studies performed in Brazil,[20,22,27] nor in countries of South America, including Brazil,[1] and HPV 59, which had only been detected in a study performed in Northern Brazil.[29] In our study, these two types of HPV were found with the overall prevalence of 2.1% and 1.4%, respectively. Individually, the prevalence of HPV 59 in the women with moderate dysplasia (6.2%) and HPV 57 was higher among the women with mild dysplasia (10.9%). Considering that HPV 57 is not considered a HR-HPV type and that it was detected only in normal and CIN 1 lesions, and in co-infection with a HR genotype, HPV 56, there is a possibility that HPV 57 acts as only a passenger genotype.
HPV 58 is a virus cloned in 1990 that is phylogenetically related to HPV 16 and classified in the genus Alfapapillomavirus, species groups α-9, consisting almost entirely of carcinogenic types, having as the main type specie HPV 16, covering also several other type species that are HR-HPV 16-related, including HPV 58.[30] HPV58 presents a strong association with CIN of a different grade and has been isolated from specimens of condiloma, pre-malignant lesions, and invasive cancer.[31] In a study done by Chan et al.[32] it was observed that HPV 58 infection presented a positive predictive value of 68.6% for CIN among Chinese women. In the present study, we observed a high HPV 58 prevalence, principally in the women with mild and moderate dysplasia, being slightly lower in those with severe dysplasia and invasive cancer. In the study, involving women of Southern Brazil, Paesi et al.[22] reported that HPV 58 is more likely to be found in women with mild rather than moderate or severe dysplasia.
Our study found a high prevalence of HPV 58 in the studied population. However, the decrease of its prevalence in more severe lesions suggests that infection with HPV 58 is more likely to cure, compared with HPV 16 and HPV 18. It has been reported that patients infected with the HPV 58-related group presented a more favorable prognosis when compared to those infected with the HPV 18-related group. The multivariate analysis, in which the relative risk of mortality was set by the Cox hazards model, found that the risk of death for the HPV 58 and 18-related groups was 0.32 and 1.87, respectively, when compared with HPV 16.[12]
Studies show that HPV 16 is considered the most prevalent HPV type around the world, followed by HPV 18 and 45.[9,33] According to some studies conducted in different regions of Brazil, the prevalence of HPV 58 genotype in Brazilian women seems to be great.[20,22] This study indicates that HPV 58 is a common genotype in the studied population. This finding can help to define the strategies to combat the HPV-related disease.
It has been demonstrated that prophylactic vaccination with virus-like particles induces an efficient immune response against HPV 16 and 18.[34] For the other less prevalent HPV genotypes, the current vaccine appears to offer just a modest cross-protection.[35] In Brazil, the HPV vaccination has already been approved, but it has not been effectively implemented in public health units. Based on our results, the HPV vaccination would protect more than 70% of the patients against high-grade lesions and cancer. However, the relatively high prevalence of HPV 58 found in studies conducted in different regions of Brazil[20,22,27,29] as well as in other countries,[28,36] highlights the importance of future vaccines to include other HPV genotypes, particularly HPV 58, to increase the potential for prevention of cervical cancer, and other HPV-associated diseases, closer to 100%.[37]
The present study has some limitations, such as the fact that it included only women attending public health units, which generally present a lower socioeconomic status, who may not be so representative of the local female population. Besides, there are some cultural and socio-demographic differences among the populations from the different states of Northeast Brazil that can limit the extrapolation of our findings to the whole region.
Conclusion
The high prevalence of HPV 58 detected in this and in other studies conducted in Brazil suggests that this HPV genotype circulates in a high frequency in the female population and highlights the importance of inclusion of this genotype in the composition of future vaccines against HPV, especially, those directed towards Brazilian women.
Acknowledgments
We would like to thank CNPq (National Council for Scientific and Technological Development) and Coordination for the Improvement of Higher Education Personnel (CAPES) for financial support.
Footnotes
Source of Support: CNPq (National Council for Scientific and Technological Development) and Coordination for the Improvement of Higher Education Personnel (CAPES).
Conflict of Interest: None declared.
References
- 1.de Sanjosé S, Diaz M, Castellsagué X, Clifford G, Bruni L, Muñoz N, et al. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: A meta-analysis. Lancet Infect Dis. 2007;7:453–9. doi: 10.1016/S1473-3099(07)70158-5. [DOI] [PubMed] [Google Scholar]
- 2.Baseman JG, Koutsky LA. The epidemiology of human papillomavirus infections. J Clin Virol. 2005;32(Suppl 1):S16–24. doi: 10.1016/j.jcv.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 3.Trottier H, Franco EL. The epidemiology of genital human papillomavirus infection. Vaccine. 2006;24(Suppl 1):S1–15. doi: 10.1016/j.vaccine.2005.09.054. [DOI] [PubMed] [Google Scholar]
- 4.Castle PE, Schiffman M, Herrero R, Hildesheim A, Rodriguez AC, Bratti MC, et al. A prospective study of age trends in cervical human papillomavirus acquisition and persistence in Guanacaste, Costa Rica. J Infect Dis. 2005;191:1808–16. doi: 10.1086/428779. [DOI] [PubMed] [Google Scholar]
- 5.Fernandes TA, Meissner RV, Bezerra LF, Azevedo PR, Fernandes JV. Human papillomavirus infection in women attended at a cervical cancer screening service in Natal, Brazil. Braz J Microbiol. 2008;39:573–8. doi: 10.1590/S1517-838220080003000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan PK, Chang AR, Yu MY, Li WH, Chan MY, Yeung AC, et al. Age distribution of human papillomavirus infection and cervical neoplasia reflects caveats of cervical screening policies. Int J Cancer. 2010;126:297–301. doi: 10.1002/ijc.24731. [DOI] [PubMed] [Google Scholar]
- 7.de Villiers EM, Fauquet C, Broker TR, Bernard HU, zur Hausen H. Classification of papillomaviruses. Virology. 2004;324:17–27. doi: 10.1016/j.virol.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 8.Bernard HU, Burk RD, Chen Z, van Doorslaer K, Hausen Hz, de Villiers EM. Classification of papillomaviruses (PVs) based on 189 PV types and proposal of taxonomic amendments. Virology. 2010;401:70–9. doi: 10.1016/j.virol.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muñoz N, Bosch FX, de Sanjosé S, Herrero R, Castellsagué X, Shah KV, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348:518–27. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 10.Bosch FX, Burchell AN, Schiffman M, Giuliano AR, de Sanjose S, Bruni L, et al. Epidemiology and natural history of human papillomavirus infections and type-specific implications in cervical neoplasia. Vaccine. 2008;26(Suppl 10):K1–16. doi: 10.1016/j.vaccine.2008.05.064. [DOI] [PubMed] [Google Scholar]
- 11.Clifford GM, Rana RK, Franceschi S, Smith JS, Gough G, Pimenta JM. Human papillomavirus genotype distribution in low-grade cervical lesions: Comparison by geographic region and with cervical cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:1157–64. doi: 10.1158/1055-9965.EPI-04-0812. [DOI] [PubMed] [Google Scholar]
- 12.Clifford GM, Gallus S, Herrero R, Muñoz N, Snijders PJ, Vaccarella S, et al. Worldwide distribution of human papillomavirus types in cytologically normal women in the international agency for research on cancer HPV prevalence surveys: A pooled analysis. Lancet. 2005;366:991–8. doi: 10.1016/S0140-6736(05)67069-9. [DOI] [PubMed] [Google Scholar]
- 13.Li N, Franceschi S, Howell-Jones R, Snijders PJ, Clifford GM. Human papillomavirus type distribution in 30,848 invasive cervical cancers worldwide: Variation by geographical region, histological type and year of publication. Int J Cancer. 2011;128:927–35. doi: 10.1002/ijc.25396. [DOI] [PubMed] [Google Scholar]
- 14.Qu W, Jiang G, Cruz Y, Chang CJ, Ho GY, Klein RS, et al. PCR detection of human papillomavirus: Comparison between MY09/MY11 and GP5+/GP6+primer systems. J Clin Microbiol. 1997;35:1304–10. doi: 10.1128/jcm.35.6.1304-1310.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.zur Hausen H. Papillomaviruses in the causation of human cancers: A brief historical account. Virology. 2009;384:260–5. doi: 10.1016/j.virol.2008.11.046. [DOI] [PubMed] [Google Scholar]
- 16.Arbyn M, de Sanjosé S, Saraiya M, Sideri M, Palefsky J, Lacey C, et al. EUROGIN 2011 roadmap on prevention and treatment of HPV-related disease. Int J Cancer. 2012;131:1969–82. doi: 10.1002/ijc.27650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Villa LL. Vaccines against papillomavirus infections and disease. Salud Publica Mex. 2003;45(Suppl 3):S443–8. doi: 10.1590/s0036-36342003000900019. [DOI] [PubMed] [Google Scholar]
- 18.Rabelo-Santos SH, Zeferino L, Villa LL, Sobrinho JP, Amaral RG, Magalhães AV. Human papillomavirus prevalence among women with cervical intraepithelial neoplasia III and invasive cervical cancer from Goiânia, Brazil. Mem Inst Oswaldo Cruz. 2003;98:181–4. doi: 10.1590/s0074-02762003000200003. [DOI] [PubMed] [Google Scholar]
- 19.Brazil: National Institute of Câncer José Alencar Gomes da Silva-General Coordination of Strategic Actions – Coordination of Prevalence and Surveillance Estimate/2012 – Cancer Incidence; 2011. [Cited on 2013 Jan 31]. Available from: http://www.inca.gov.br/estimativa/2012 . [Google Scholar]
- 20.Camara GN, Cerqueira DM, Oliveira AP, Silva EO, Carvalho LG, Martins CR. Prevalence of human papillomavirus types in women with pre-neoplastic and neoplastic cervical lesions in the Federal District of Brazil. Mem Inst Oswaldo Cruz. 2003;98:879–83. doi: 10.1590/s0074-02762003000700003. [DOI] [PubMed] [Google Scholar]
- 21.Fernandes JV, Meissner Rde V, de Carvalho MG, Fernandes TA, de Azevedo PR, Villa LL. Prevalence of HPV infection by cervical cytologic status in Brazil. Int J Gynaecol Obstet. 2009;105:21–4. doi: 10.1016/j.ijgo.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 22.Paesi S, Serafini EP, Barea F, Madi SR, Echeverrigaray S. High prevalence of human papillomavirus type 58 in patients with cervical pre-malignant lesions in southern Brazil. J Med Virol. 2009;81:1270–5. doi: 10.1002/jmv.21410. [DOI] [PubMed] [Google Scholar]
- 23.The 1988 Bethesda System for reporting cervical/vaginal cytological diagnoses. National Cancer Institute Workshop. JAMA. 1989;262:931–4. [PubMed] [Google Scholar]
- 24.Sambrook J, Russel DW. 3rd ed. New York: Cold Spring Harbor Laboratory Press; 2001. Molecular Cloning: A Laboratory Manual. [Google Scholar]
- 25.Saiki RK, Scharf S, Faloona F, Mullis KB, Horn GT, Erlich HA, et al. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985;230:1350–4. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- 26.Manos MM, Ting T, Wright DK, Lewis AJ, Broker TR, Wolinsky SM. The use of polymerase chain reaction amplification for the detection of genital human papillomavirus. Cancer Cel Mol Diagnos Hum Cancer. 1989;7:209–14. [Google Scholar]
- 27.Lorenzato F, Ho L, Terry G, Singer A, Santos LC, De Lucena Batista R, et al. The use of human papillomavirus typing in detection of cervical neoplasia in Recife (Brazil) Int J Gynecol Cancer. 2000;10:143–150. doi: 10.1046/j.1525-1438.2000.00007.x. [DOI] [PubMed] [Google Scholar]
- 28.Lin H, Ma YY, Moh JS, Ou YC, Shen SY, ChangChien CC. High prevalence of genital human papillomavirus type 52 and 58 infection in women attending gynecologic practitioners in South Taiwan. Gynecol Oncol. 2006;101:40–5. doi: 10.1016/j.ygyno.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 29.Noronha VL, Cruz EM, Pinho CN, Mello WA, Villa LL, Russomano FB. Human papillomavirus (HPV) in women screened to cervical uterine cancer, Belém - Pará - Brazil. J Bras Doenças Sex Transm. 2011;23:5–11. [Google Scholar]
- 30.Schiffman M, Rodriguez AC, Chen Z, Wacholder S, Herrero R, Hildesheim A, et al. A population-based prospective study of carcinogenic human papillomavirus variant lineages, viral persistence, and cervical neoplasia. Cancer Res. 2010;70:3159–69. doi: 10.1158/0008-5472.CAN-09-4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guan P, Howell-Jones R, Li N, Bruni L, de Sanjosé S, Franceschi S, et al. Human papillomavirus types in 115,789 HPV-positive women: A meta-analysis from cervical infection to cancer. Int J Cancer. 2012;131:2349–59. doi: 10.1002/ijc.27485. [DOI] [PubMed] [Google Scholar]
- 32.Chan SY, Delius H, Halpern AL, Bernard HU. Analysis of genomic sequences of 95 papillomavirus types: Uniting typing, phylogeny, and taxonomy. J Virol. 1995;69:3074–83. doi: 10.1128/jvi.69.5.3074-3083.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clifford G, Franceschi S, Diaz M, Muñoz N, Villa LL. Chapter 3: HPV type-distribution in women with and without cervical neoplastic diseases. Vaccine. 2006;24(Suppl 3):S3/26–34. doi: 10.1016/j.vaccine.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 34.Stanley M. Human papillomavirus vaccines versus cervical cancer screening. Clin Oncol (R Coll Radio) 2008;20:388–94. doi: 10.1016/j.clon.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 35.Ault KA. Human papillomavirus vaccines and the potential for cross-protection between related HPV types. Gynecol Oncol. 2007;107(2 Suppl 3):S31–3. doi: 10.1016/j.ygyno.2007.08.059. [DOI] [PubMed] [Google Scholar]
- 36.Song JS, Kim EJ, Choi J, Gong G, Sung CO. Significance of HPV-58 infection in women who are HPV-Positive, Cytology-Negative and living in a country with a high prevalence of HPV-58 infection. PLoS One. 2013;8:e58678. doi: 10.1371/journal.pone.0058678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frazer IH, Leggatt GR, Mattarollo SR. Prevention and treatment of papillomavirus-related cancers through immunization. Annu Rev Immunol. 2011;29:111–38. doi: 10.1146/annurev-immunol-031210-101308. [DOI] [PubMed] [Google Scholar]


