Abstract
Background:
Microalbuminuria is an independent predictor of retinopathy, so absence of microalbuminuria may tend clinician not to screen for diabetic retinopathy (DR).
Aim:
The aim of our study was to estimate prevalence of DR in patients with type 2 diabetes who have normoalbuminuria, and to study predictors for DR, which can identify these high-risk individuals.
Subjects and Methods:
In a prospective cross-sectional study that included patients with type 2 DM and normoalbuminuria. Diagnosis of DR was made by a trained ophthalmologist based on the presence of clinical features in the fundus of both eyes following the International Clinical DR guidelines. The statistical analyses were performed using Statistical Package for the Social Sciences 15.0 version software (Chicago, IL, USA). The continuous variables expressed as means (SD and Student's t-test or Mann–Whitney test were used, as appropriate, to determine differences in them. Categorical variables were presented as percentage. The Pearson's Chi-square test or Fisher's exact test, as appropriate, was used to determine the differences in them.
Results:
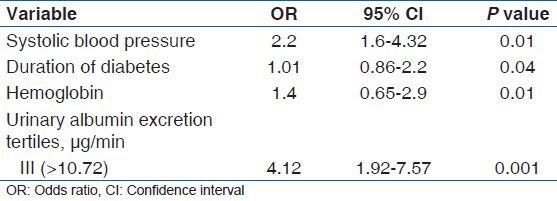
A total of 226 patients with type 2 DM and normoalbuminuria were enrolled in the study that included 110 males (48.6%), and 116 females (51.4%) Mean (SD) duration of diabetes was 8.2 (5.6) years. DR of any grade was present in 49/226 (22%) patients. Of the patients with DR of any grade, 31/49 (63%) had mild non-proliferative diabetic retinopathy (NPDR) 10/49 (22%) had moderate to severe NPDR and 8/49 (15%) had PDR. Duration of diabetes (OR 1.01, 95% CI, 0.86-2.2, P = 0.04), higher systolic blood pressure (OR 2.2, 95% CI, 1.6-4.5, P = 0.01), low hemoglobin (OR 1.4, 95% CI, 0.45-2.9, P = 0.01), and a higher tertile of urinary albumin excretion rate (OR 4.12, 95% CI, 1.92-7.57, P = 0.001) had independently significant association with DR.
Conclusion:
The risk of DR exists in patients with type 2 diabetes even in normoalbuminuric individuals. Close monitoring is particularly needed if patients have longer duration of diabetes, hypertension, anemia, or high normal albuminuria.
Keywords: Albuminuria, Diabetic retinopathy, Predictors, Type 2 diabetes mellitus
Introduction
Diabetes mellitus is one of the fastest growing diseases in our country and has become major public health concern in recent times. Micro-vascular disease is a common complication of type 2 diabetes and diabetic retinopathy (DR) and nephropathy represent one of the leading causes of visual impairment[1] and end-stage renal disease respectively in adults of both developed and developing world.[2] Numerous studies have shown various risk-factors for DR that include poor glycemic control, hypertension, dyslipidemia, age of the patient, duration of diabetes, microalbuminuria, and cigarette smoking.[3,4,5] Persistence and progression of microalbuminuria in patients with diabetes is not only a marker of nephropathy and cardiovascular risk but also of severe ocular morbidity.[6,7,8] Its presence has been well correlated with presence of DR in patients with type 1 diabetes,[9] the concordance rate between these two micro-vascular complications seems lower in type 2 diabetes.[10,11] This indicates that patients with type 2 diabetes may have DR without microalbuminuria. This has been supported by several studies that reported 10-30% prevalence of DR in patients with type 2 diabetes who have normoalbuminuria.[12,13,14,15,16]
Taking into account, this fact that microalbuminuria is an independent predictor of retinopathy, presence of DR in patients with type 2 diabetes who have normoalbuminuria may be overlooked because absence of microalbuminuria may tend clinician not to screen for DR. The aim of our study was to estimate prevalence of DR in patients with type 2 diabetes who have normoalbuminuria and to study predictors for DR, which can identify these high-risk individuals.
Subjects and Methods
Study population
This was a prospective cross-sectional study. The present study included patients with type 2 DM and normoalbuminuria, who presented to the Department of Medicine and were referred to Ophthalmology at Era's Lucknow Medical College and Hospital between July 2010 and December 2011 for screening of DR. They all underwent a dilated fundus examination and fundus photographic evaluation Type 2 DM was diagnosed if the patients had a fasting plasma glucose level ≥ 126 mg/dl or a 2 h post-glucose level after a 75 g oral glucose tolerance test ≥ 200 mg/dl[17] or random plasma glucose ≥ 200 mg/dl with osmotic symptoms. Normoalbuminuria was defined as a urinary albumin excretion rate (UAER) < 20 μg/min in 2 out of 3 consecutive tests taken within 2-3 months.[18] The exclusion criteria, included (1) an age < 18 years or > 80 years, (2) accelerated hypertension (3) pregnancy, (4) malignancies, (5) patients with end organ diseases such as hepatic failure or heart failure, (6) acute systemic infection, (7) patients with acute complications of diabetes and (8) patients with h/o non diabetic kidney disease. The study protocol was approved by the Institutional ethical committee, and this study was conducted according to the principles of the Declaration of Helsinki. A written informed consent was taken from all the participants.
The collected data included age, gender, duration of diabetes and history of medication, anthropometric parameters, (which included weight, height, body mass index (BMI), and waist/hip ratio), systolic and diastolic blood pressures, serum creatinine, hemoglobin and albumin levels, total cholesterol and high-density lipoprotein (HDL) cholesterol levels, triglyceride and serum uric acid levels, the UAER. Fasting venous blood samples were taken for the determination of the serum level of total cholesterol, HDL cholesterol, and triglyceride. The UAER was assessed via 24 h urine collection and measured by immunoturbidimetric assay using MODULAR automated clinical chemistry analyzers (Roche Diagnostic, Mannheim, Germany). The BMI was calculated as weight (kg)/height (m2). The blood pressure was measured twice, 5 min apart, using a random zero sphygmomanometer with the patient seated after 10 min of rest. Hypertension was defined as a blood pressure measurement of above 140/90 mmHg in the right upper limb supine position or when the patient was on anti-hypertensive medication. The estimated GFR (glomerular filtration rate). It was calculated using the Modification of Diet in Renal Disease four-variable equation at the time of CT scanning: EGFR = 186 × serum creatinine-1.154 × age-0.203 × 1.212 (if black) × 0.742 (if female).[19]
Each participant underwent a comprehensive ophthalmic examination that included automated refraction (RK-F1, Canon), and stereoscopic fundus examination using an indirect ophthalmoscope, after pupil dilatation with 1.0% tropicamide. Fundus photographs (45°) were taken from both eyes of each participant using a digital fundus camera (TRC-NW 100 camera; Nikon Japan). The photographs were taken in 1-field per eye, centered on the macula. Diagnosis of DR was made by a trained ophthalmologist by an indirect ophthalmoscopic examination based on the presence of clinical features in the fundus of both eyes following the International Clinical DR guidelines, and classified as (i) no apparent retinopathy, (ii) mild non-proliferative diabetic retinopathy (NPDR), (iii) moderate NPDR, (iv) severe NPDR, or (iv) proliferative diabetic retinopathy (PDR).[20]
Statistical analysis
The statistical analyses were performed using Statistical Package for the Social Sciences 15.0 version software (Chicago, IL, USA). The data for continuous variable is expressed as means (SD). The Student's t-test or Mann-Whitney test were used, as appropriate, to determine differences in continuous variables. Categorical variables are presented as percentage. The Pearson's Chi-square test or Fisher's exact test, as appropriate, was used to determine the differences in categorical variables. Both univariate and multivariate logistic regression analyses were performed to determine the various risk-factors for the presence of DR. From the univariate analysis, variables with P < 0.05 and those, which were already established as risk-factors were included in the multivariate logistic regression analysis. P values < 0.05 were considered statistically significant.
Results
A total of 226 patients were enrolled in this study. The mean age of enrolled patients was 56 (12) years. This study included 110 males (48.6%) and 116 females (51.4%) Mean (SD) duration of diabetes was 8.2 (5.6) years. The prevalence of DR was assessed according to the DR staging system presented by the International Clinical DR guidelines. DR of any grade was present in 49/226 (22%) patients. Of the patients with DR of any grade, 31/49 (63%) had mild NPDR 10/49 had moderate to severe NPDR and 8/49 (15%) had PDR. Comparisons of clinical characteristics between the patients with DR and without DR are shown in Table 1. The patients with DR were older than those without DR and DR was more prevalent in females than in males. The patients with DR had lower BMI, higher systolic blood pressure, longer duration of diabetes, lower hemoglobin levels, higher serum LDL cholesterol levels, lower estimated GFR, higher UAER, and less use of renin-angiotensin system (RAS) inhibitors compared with those without DR. In univariate logistic regression analysis [Table 2] with the presence of DR as dependent variable, age, gender, BMI, systolic blood pressure, duration of diabetes, hemoglobin levels, and higher tertile of UAER were significantly associated with the presence of DR. Multivariate logistic regression analysis [Table 3] was done to determine the predictors of DR in normoabuminuric people with type 2 DM. Data revealed that the duration of diabetes (OR 1.01, 95% CI, 0.86-2.2, P = 0.04), higher systolic blood pressure (OR 2.2, 95% CI, 1.6-4.5, P = 0.01), low hemoglobin (OR 1.4, 95% CI, 0.45-2.9, P = 0.01), and a higher tertile of UAER (OR 4.12, 95% CI, 1.92-7.57, P = 0.001) had independently significant association with DR.
Table 1.
Clinical and biochemical characteristics of patients with and without diabetic retinopathy
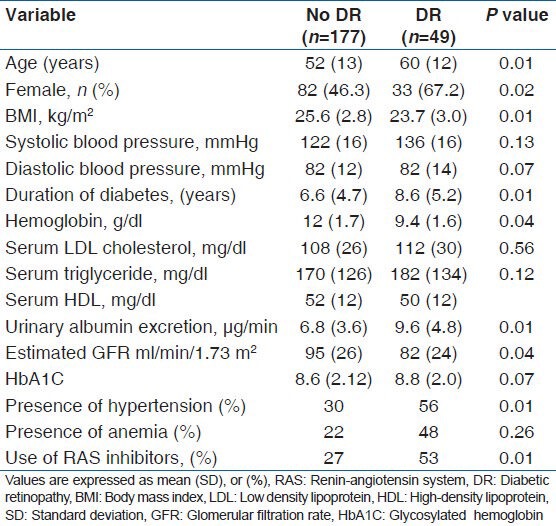
Table 2.
Univariate logistic regression analysis with diabetic retinopathy as a dependent variable in normoalbuminuric people with type 2 diabetes mellitus
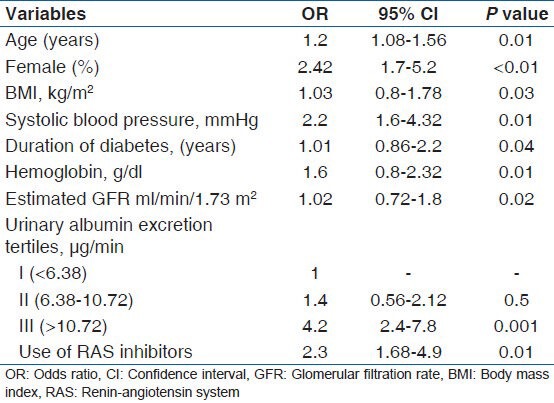
Table 3.
Multivariate logistic regression analysis to determine the predictor of diabetic retinopathy in normoalbuminuric people with type 2 diabetes mellitus
Discussion
DR is a highly specific vascular complication and a sight-threatening problem related to diabetes. DR is characterized by gradually progressive alterations in the retinal microvasculature, leading to retinal hypo perfusion, increased vascular permeability, and pathologically intraocular proliferation of retinal vessels. Both DR and nephropathy are micro-vascular complications of diabetes. With the retina and glomerulus, diabetes-specific micro-vascular disease is characterized by similar pathophysiologic features. Chronic hyperglycemia is the central initiating factor for all types of diabetic micro-vascular disease.
Previous studies have provided evidence to support the suggestion that DR and DN progress in a parallel manner; thus, the presence of one is believed to predict the development of the other.[11,21] In our study, the duration of diabetes was found to be significantly higher in patients with DR than in those without DR. The duration of diabetes is regarded as a marker for long-term exposure to hyperglycemia. Previously published studies also identified duration of diabetes to be an independent risk-factor for retinopathy.[22,23]
Higher systolic blood pressure was found to be an independent risk-factor for DR. In our study, presence of hypertension and higher systolic blood pressure was reported in patients with DR than those without DR. The UK Prospective Diabetes Study (UKPDS) also found higher relative risk for incidence of retinopathy with higher systolic blood pressure.[24] It is known that DR can be affected by the hemodynamic changes induced by hypertension, such as impaired auto regulation and hyperperfusion.[25] In addition, hypertension independent of hyperglycemia is known to up-regulate the expression of vascular endothelial growth factor in retinal endothelial cells and ocular fluids, which can promote DR.[26]
Anemia was found to be another risk-factor of DR in our study. Individuals with anemia were more likely to develop DR than individuals without anemia, perhaps because of anemia-induced retinal hypoxia. Hypoxia may alter angiogenesis, capillary permeability, vasomotor response, and cell survival,[27] These results are consistent with those reported in the ETDRS, in which low hematocrit was identified as an independent risk-factor for the development of high-risk PDR and visual impairment.[28] Another large cross-sectional study showed that the odds ratio of having any retinopathy and also risk of severe retinopathy were higher for individuals with an hemoglobin level of < 12 g/dl, as compared with those level ≥ 12 g/dl. In addition, DR patients with low hemoglobin levels had over fivefold increased risk of severe retinopathy, as compared to those with higher hemoglobin levels.[29] These results suggested that subjects with low hemoglobin levels tended to have an increased risk of retinopathy, especially that of the severe form. Other studies have corroborated this finding and have also found evidence of improvement in the DR status following correction of anemia.[30,31]
Albuminuria was significantly higher in our patients with DR than in those without DR, and estimated GFR was negatively correlated to DR. Further analysis showed that highest tertile of albuminuria was observed to be an independent risk factor for the development of DR and the severity of DR in patients with T2DM appeared to be aggravated with an increasing severity of albuminuria even if it was in the normal range.
There are studies that show risk of development of DR in patients who have normoalbuminuria.[12,13,14,15,16] Normoalbuminuria does not always imply normal renal function. In type 2 diabetes, concordance between reduced glomerular filtration rate and albuminuria is much lower than in type 1 diabetes. Of patients with type 2 diabetes from the UKPDS, 67% were normoalbuminuric at the time they developed chronic kidney disease; of these 51% remained normoalbuminuric, whereas 16% developed albuminuria thereafter.[32] Therefore, it should be remembered that many patients with type 2 diabetes may have reduced GFR, which is a risk-factor for DR without significant albuminuria primarily or secondary to the use of RAS inhibitors. The increasing use of blockers of the RAS also has been claimed to explain the weaker association of the nonalbuminuric CKD phenotype with DR. RAS blockers are more effective than other antihypertensives in slowing nephropathy progression in proteinuric diabetic patients and can decrease proteinuria in diabetes.[33] These agents were also shown to be effective in preventing DR in individuals with diabetes who have proteinuria.[34]
There were several limitations to the present study that are noteworthy, as they may affect the generalization of our findings. Being a cross-sectional study, the results do not provide definite information on a cause-and-effect relationship. In addition, because our study population was hospital based, composed solely of patients from a single center, majority from rural background, there was unavoidable bias of selection, information, and confounding variables. Prospective and larger studies are required to overcome these limitations and to draw more robust conclusions.
Conclusion
The risk of DR exists in patients with type 2 diabetes even in normoalbuminuric individuals. Absence of microalbuminuria should not be the criteria to defer for screening for DR. Close monitoring is particularly needed if patients have longer duration of diabetes, hypertension, anemia or high normal albuminuria.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Congdon NG, Friedman DS, Lietman T. Important causes of visual impairment in the world today. JAMA. 2003;290:2057–60. doi: 10.1001/jama.290.15.2057. [DOI] [PubMed] [Google Scholar]
- 2.Ritz E, Rychlík I, Locatelli F, Halimi S. End-stage renal failure in type 2 diabetes: A medical catastrophe of worldwide dimensions. Am J Kidney Dis. 1999;34:795–808. doi: 10.1016/S0272-6386(99)70035-1. [DOI] [PubMed] [Google Scholar]
- 3.Unnikrishnan RI, Rema M, Pradeepa R, Deepa M, Shanthirani CS, Deepa R, et al. Prevalence and risk factors of diabetic nephropathy in an urban South Indian population: The chennai urban rural epidemiology study (CURES 45) Diabetes Care. 2007;30:2019–24. doi: 10.2337/dc06-2554. [DOI] [PubMed] [Google Scholar]
- 4.West SK, Munoz B, Klein R, Broman AT, Sanchez R, Rodriguez J, et al. Risk factors for Type II diabetes and diabetic retinopathy in a mexican-american population: Proyecto VER. Am J Ophthalmol. 2002;134:390–8. doi: 10.1016/s0002-9394(02)01595-7. [DOI] [PubMed] [Google Scholar]
- 5.Pedro RA, Ramon SA, Marc BB, Juan FB, Isabel MM. Prevalence and relationship between diabetic retinopathy and nephropathy, and its risk factors in the North-East of Spain, a population-based study. Ophthalmic Epidemiol. 2010;17:251–65. doi: 10.3109/09286586.2010.498661. [DOI] [PubMed] [Google Scholar]
- 6.de Zeeuw D, Parving HH, Henning RH. Microalbuminuria as an early marker for cardiovascular disease. J Am Soc Nephrol. 2006;17:2100–5. doi: 10.1681/ASN.2006050517. [DOI] [PubMed] [Google Scholar]
- 7.Klausen K, Borch-Johnsen K, Feldt-Rasmussen B, Jensen G, Clausen P, Scharling H, et al. Very low levels of microalbuminuria are associated with increased risk of coronary heart disease and death independently of renal function, hypertension, and diabetes. Circulation. 2004;110:32–5. doi: 10.1161/01.CIR.0000133312.96477.48. [DOI] [PubMed] [Google Scholar]
- 8.Stratton IM, Kohner EM, Aldington SJ, Turner RC, Holman RR, Manley SE, et al. UKPDS 50: Risk factors for incidence and progression of retinopathy in Type II diabetes over 6 years from diagnosis. Diabetologia. 2001;44:156–63. doi: 10.1007/s001250051594. [DOI] [PubMed] [Google Scholar]
- 9.Lövestam-Adrian M, Agardh E, Agardh CD. The temporal development of retinopathy and nephropathy in type 1 diabetes mellitus during 15 years diabetes duration. Diabetes Res Clin Pract. 1999;45:15–23. doi: 10.1016/s0168-8227(99)00055-8. [DOI] [PubMed] [Google Scholar]
- 10.Neil A, Hawkins M, Potok M, Thorogood M, Cohen D, Mann J. A prospective population-based study of microalbuminuria as a predictor of mortality in NIDDM. Diabetes Care. 1993;16:996–1003. doi: 10.2337/diacare.16.7.996. [DOI] [PubMed] [Google Scholar]
- 11.Retnakaran R, Cull CA, Thorne KI, Adler AI, Holman RR UKPDS study group. Risk factors for renal dysfunction in type 2 diabetes: U.K. Prospective Diabetes Study 74. Diabetes. 2006;55:1832–9. doi: 10.2337/db05-1620. [DOI] [PubMed] [Google Scholar]
- 12.Rani PK, Raman R, Gupta A, Pal SS, Kulothungan V, Sharma T. Albuminuria and diabetic retinopathy in type 2 diabetes Mellitus Sankara Nethralaya diabetic retinopathy epidemiology and molecular genetic study (SN-DREAMS, report 12) Diabetol Metab Syndr. 2011;3:9. doi: 10.1186/1758-5996-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manaviat MR, Afkhami M, Shoja MR. Retinopathy and microalbuminuria in type II diabetic patients. BMC Ophthalmol. 2004;4:9. doi: 10.1186/1471-2415-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen H, Zheng Z, Huang Y, Guo K, Lu J, Zhang L, et al. A microalbuminuria threshold to predict the risk for the development of diabetic retinopathy in type 2 diabetes mellitus patients. PLoS One. 2012;7:e36718. doi: 10.1371/journal.pone.0036718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ra H, Yoo JH, Ban WH, Song HC, Lee SS, Kim SR, et al. Predictors for diabetic retinopathy in normoalbuminuric people with type 2 diabetes mellitus. Diabetol Metab Syndr. 2012;4:29. doi: 10.1186/1758-5996-4-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.An JH, Cho YM, Yu HG, Jang HC, Park KS, Kim SY, et al. The clinical characteristics of normoalbuminuric renal insufficiency in Korean type 2 diabetic patients: A possible early stage renal complication. J Korean Med Sci. 2009;24:S75–81. doi: 10.3346/jkms.2009.24.S1.S75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–53. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 18.Molitch ME, DeFronzo RA, Franz MJ, Keane WF, Mogensen CE, Parving HH, et al. Nephropathy in diabetes. Diabetes Care. 2004;27:S79–83. doi: 10.2337/diacare.27.2007.s79. [DOI] [PubMed] [Google Scholar]
- 19.Wilkinson CP, Ferris FL, 3rd, Klein RE, Lee PP, Agardh CD, Davis M, et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 2003;110:1677–82. doi: 10.1016/S0161-6420(03)00475-5. [DOI] [PubMed] [Google Scholar]
- 20.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of diet in renal disease study group. Ann Intern Med. 1999;130:461–70. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 21.Girach A, Vignati L. Diabetic micro vascular complications: Can the presence of one predict the development of another? J Diabetes Complications. 2006;20:228–37. doi: 10.1016/j.jdiacomp.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 22.Pradeepa R, Anitha B, Mohan V, Ganesan A, Rema M. Risk factors for diabetic retinopathy in a South Indian Type 2 diabetic population: The Chennai Urban Rural Epidemiology Study (CURES) Eye Study 4. Diabet Med. 2008;25:536–42. doi: 10.1111/j.1464-5491.2008.02423.x. [DOI] [PubMed] [Google Scholar]
- 23.Rani PK, Raman R, Chandrakantan A, Pal SS, Perumal GM, Sharma T. Risk factors for diabetic retinopathy in self-reported rural population with diabetes. J Postgrad Med. 2009;55:92–6. doi: 10.4103/0022-3859.48787. [DOI] [PubMed] [Google Scholar]
- 24.Adler AI, Stratton IM, Neil HA, Yudkin JS, Matthews DR, Cull CA, et al. Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): Prospective observational study. BMJ. 2000;321:412–9. doi: 10.1136/bmj.321.7258.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fuchsjäger-Mayrl G, Polak K, Luksch A, Polska E, Dorner GT, Rainer G, et al. Retinal blood flow and systemic blood pressure in healthy young subjects. Graefes Arch Clin Exp Ophthalmol. 2001;239:673–7. doi: 10.1007/s004170100333. [DOI] [PubMed] [Google Scholar]
- 26.Suzuma I, Hata Y, Clermont A, Pokras F, Rook SL, Suzuma K, et al. Cyclic stretch and hypertension induce retinal expression of vascular endothelial growth factor and vascular endothelial growth factor receptor-2: Potential mechanisms for exacerbation of diabetic retinopathy by hypertension. Diabetes. 2001;50:444–54. doi: 10.2337/diabetes.50.2.444. [DOI] [PubMed] [Google Scholar]
- 27.Irace C, Scarinci F, Scorcia V, Bruzzichessi D, Fiorentino R, Randazzo G, et al. Association among low whole blood viscosity, haematocrit, haemoglobin and diabetic retinopathy in subjects with type 2 diabetes. Br J Ophthalmol. 2011;95:94–8. doi: 10.1136/bjo.2009.172601. [DOI] [PubMed] [Google Scholar]
- 28.Davis MD, Fisher MR, Gangnon RE, Barton F, Aiello LM, Chew EY, et al. Risk factors for high-risk proliferative diabetic retinopathy and severe visual loss: Early Treatment Diabetic Retinopathy Study Report #18. Invest Ophthalmol Vis Sci. 1998;39:233–52. [PubMed] [Google Scholar]
- 29.Qiao Q, Keinänen-Kiukaanniemi S, Läärä E. The relationship between hemoglobin levels and diabetic retinopathy. J Clin Epidemiol. 1997;50:153–8. doi: 10.1016/s0895-4356(96)00335-6. [DOI] [PubMed] [Google Scholar]
- 30.Rani PK, Raman R, Rachepalli SR, Pal SS, Kulothungan V, Lakshmipathy P, et al. Anemia and diabetic retinopathy in type 2 diabetes mellitus. J Assoc Physicians India. 2010;58:91–4. [PubMed] [Google Scholar]
- 31.Ajoy Mohan VK, Nithyanandam S, Idiculla J. Microalbuminuria and low hemoglobin as risk factors for the occurrence and increasing severity of diabetic retinopathy. Indian J Ophthalmol. 2011;59:207–10. doi: 10.4103/0301-4738.81029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–53. [PubMed] [Google Scholar]
- 33.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med. 1993;329:1456–62. doi: 10.1056/NEJM199311113292004. [DOI] [PubMed] [Google Scholar]
- 34.Mauer M, Zinman B, Gardiner R, Suissa S, Sinaiko A, Strand T, et al. Renal and retinal effects of enalapril and losartan in type 1 diabetes. N Engl J Med. 2009;361:40–51. doi: 10.1056/NEJMoa0808400. [DOI] [PMC free article] [PubMed] [Google Scholar]


