Abstract
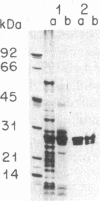
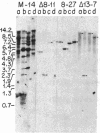
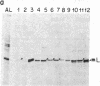
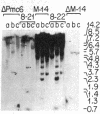
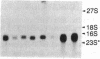
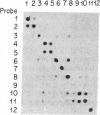
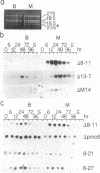
Bundle sheath chloroplasts of maize leaves contain about one-fourth as much light-harvesting chlorophyll a/b binding protein of photosystem II (LHCP-II) as do mesophyll chloroplasts. We have determined that this difference is, in part, the result of differential expression of different LHCP-II genes. We have prepared and partially characterized cDNA clones specific for six LHCP-II genes of maize. Transcripts of these six LHCP-II genes are present at vastly different levels and account for about 95% of total LHCP-II mRNAs in bundle sheath and mesophyll cells of illuminated dark-grown maize leaves. Three genes are preferentially expressed in mesophyll cells, and their mRNAs constitute about 54% of the total LHCP-II transcripts in greening (24 hr) maize leaves. Two genes are expressed equally in bundle sheath and mesophyll cells. Most interestingly, the RNA of one gene that contributes about 8% of the total LHCP-II transcripts in leaves greening for 24 hr is present at a much higher level in bundle sheath than in mesophyll cells. Moreover, immunoblot analysis of maize thylakoids reveals at least five sizes of LHCP-II; these also differ from one another in their relative abundance in bundle sheath and mesophyll cells of developing maize leaves.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apel K., Kloppstech K. The plastid membranes of barley (Hordeum vulgare). Light-induced appearance of mRNA coding for the apoprotein of the light-harvesting chlorophyll a/b protein. Eur J Biochem. 1978 Apr 17;85(2):581–588. doi: 10.1111/j.1432-1033.1978.tb12273.x. [DOI] [PubMed] [Google Scholar]
- Bassi R., dal Belin Peruffo A., Barbato R., Ghisi R. Differences in chlorophyll-protein complexes and composition of polypeptides between thylakoids from bundle sheaths and mesophyll cells in maize. Eur J Biochem. 1985 Feb 1;146(3):589–595. doi: 10.1111/j.1432-1033.1985.tb08692.x. [DOI] [PubMed] [Google Scholar]
- Bennett J. Biosynthesis of the light-harvesting chlorophyll a/b protein. Polypeptide turnover in darkness. Eur J Biochem. 1981 Aug;118(1):61–70. doi: 10.1111/j.1432-1033.1981.tb05486.x. [DOI] [PubMed] [Google Scholar]
- Coruzzi G., Broglie R., Cashmore A., Chua N. H. Nucleotide sequences of two pea cDNA clones encoding the small subunit of ribulose 1,5-bisphosphate carboxylase and the major chlorophyll a/b-binding thylakoid polypeptide. J Biol Chem. 1983 Feb 10;258(3):1399–1402. [PubMed] [Google Scholar]
- Cuming A. C., Bennett J. Biosynthesis of the light-harvesting chlorophyll a/b protein. Control of messenger RNA activity by light. Eur J Biochem. 1981 Aug;118(1):71–80. doi: 10.1111/j.1432-1033.1981.tb05487.x. [DOI] [PubMed] [Google Scholar]
- Dean C., Elzen P., Tamaki S., Dunsmuir P., Bedbrook J. Differential expression of the eight genes of the petunia ribulose bisphosphate carboxylase small subunit multi-gene family. EMBO J. 1985 Dec 1;4(12):3055–3061. doi: 10.1002/j.1460-2075.1985.tb04045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delepelaire P., Chua N. H. Electrophoretic purification of chlorophyll a/b-protein complexes from Chlamydomonas reinhardtii and spinach and analysis of their polypeptide compositions. J Biol Chem. 1981 Sep 10;256(17):9300–9307. [PubMed] [Google Scholar]
- Dunsmuir P., Smith S. M., Bedbrook J. The major chlorophyll a/b binding protein of petunia is composed of several polypeptides encoded by a number of distinct nuclear genes. J Mol Appl Genet. 1983;2(3):285–300. [PubMed] [Google Scholar]
- Dunsmuir P. The petunia chlorophyll a/b binding protein genes: a comparison of Cab genes from different gene families. Nucleic Acids Res. 1985 Apr 11;13(7):2503–2518. doi: 10.1093/nar/13.7.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franssen H., Goldbach R., Broekhuijsen M., Moerman M., van Kammen A. Expression of Middle-Component RNA of Cowpea Mosaic Virus: In Vitro Generation of a Precursor to Both Capsid Proteins by a Bottom-Component RNA-Encoded Protease from Infected Cells. J Virol. 1982 Jan;41(1):8–17. doi: 10.1128/jvi.41.1.8-17.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollmer I., Apel K. The phytochrome-controlled accumulation of mRNA sequences encoding the light-harvesting chlorophyll a/b protein of barley (Hordeum vulgare L.). Eur J Biochem. 1983 Jun 15;133(2):309–313. doi: 10.1111/j.1432-1033.1983.tb07463.x. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Kirchanski S. J., Park R. B. Comparative Studies of the Thylakoid Proteins of Mesophyll and Bundle Sheath Plastids of Zea mays. Plant Physiol. 1976 Sep;58(3):345–349. doi: 10.1104/pp.58.3.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamppa G. K., Morelli G., Chua N. H. Structure and developmental regulation of a wheat gene encoding the major chlorophyll a/b-binding polypeptide. Mol Cell Biol. 1985 Jun;5(6):1370–1378. doi: 10.1128/mcb.5.6.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas R. An improved colony hybridization method with significantly increased sensitivity for detection of single genes. Plasmid. 1983 Nov;10(3):296–298. doi: 10.1016/0147-619x(83)90045-8. [DOI] [PubMed] [Google Scholar]
- Mayfield S. P., Taylor W. C. Carotenoid-deficient maize seedlings fail to accumulate light-harvesting chlorophyll a/b binding protein (LHCP) mRNA. Eur J Biochem. 1984 Oct 1;144(1):79–84. doi: 10.1111/j.1432-1033.1984.tb08433.x. [DOI] [PubMed] [Google Scholar]
- Metz J. G., Krueger R. W., Miles D. Chlorophyll-Protein Complexes of a Photosystem II Mutant of Maize : Evidence that Chlorophyll-Protein a-2 and a Chlorophyll-Protein Complex Derived from a Photosystem I Antennae System Comigrate on Polyacrylamide Gels. Plant Physiol. 1984 May;75(1):238–241. doi: 10.1104/pp.75.1.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson T., Harpster M. H., Mayfield S. P., Taylor W. C. Light-regulated gene expression during maize leaf development. J Cell Biol. 1984 Feb;98(2):558–564. doi: 10.1083/jcb.98.2.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnes J. R., Velan B., Felsenfeld A., Ramanathan L., Ferrini U., Appella E., Seidman J. G. Mouse beta 2-microglobulin cDNA clones: a screening procedure for cDNA clones corresponding to rare mRNAs. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2253–2257. doi: 10.1073/pnas.78.4.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichersky E., Bernatzky R., Tanksley S. D., Breidenbach R. B., Kausch A. P., Cashmore A. R. Molecular characterization and genetic mapping of two clusters of genes encoding chlorophyll a/b-binding proteins in Lycopersicon esculentum (tomato). Gene. 1985;40(2-3):247–258. doi: 10.1016/0378-1119(85)90047-2. [DOI] [PubMed] [Google Scholar]
- Schuster G., Ohad I., Martineau B., Taylor W. C. Differentiation and development of bundle sheath and mesophyll thylakoids in maize. Thylakoid polypeptide composition, phosphorylation, and organization of photosystem II. J Biol Chem. 1985 Sep 25;260(21):11866–11873. [PubMed] [Google Scholar]
- Sheen J. Y., Bogorad L. Differential expression of the ribulose bisphosphate carboxylase large subunit gene in bundle sheath and mesophyll cells of developing maize leaves is influenced by light. Plant Physiol. 1985 Dec;79(4):1072–1076. doi: 10.1104/pp.79.4.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverthorne J., Tobin E. M. Demonstration of transcriptional regulation of specific genes by phytochrome action. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1112–1116. doi: 10.1073/pnas.81.4.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]