Abstract
Background and Aim:
Synthetic nerve conduits have been sought for repair of nerve defects as the autologous nerve grafts causes donor site morbidity and possess other drawbacks. Many strategies have been investigated to improve nerve regeneration through synthetic nerve guided conduits. Olfactory ensheathing cells (OECs) that share both Schwann cell and astrocytic characteristics have been shown to promote axonal regeneration after transplantation. The present study was driven by the hypothesis that tissue-engineered poly(lactic-co-glycolic acid) (PLGA) seeded with OECs would improve peripheral nerve regeneration in a long sciatic nerve defect.
Materials and Methods:
Sciatic nerve gap of 15 mm was created in six adult female Sprague-Dawley rats and implanted with PLGA seeded with OECs. The nerve regeneration was assessed electrophysiologically at 2, 4 and 6 weeks following implantation. Histopathological examination, scanning electron microscopic (SEM) examination and immunohistochemical analysis were performed at the end of the study.
Results:
Nerve conduction studies revealed a significant improvement of nerve conduction velocities whereby the mean nerve conduction velocity increases from 4.2 0.4 m/s at week 2 to 27.3 5.7 m/s at week 6 post-implantation (P < 0.0001). Histological analysis revealed presence of spindle-shaped cells. Immunohistochemical analysis further demonstrated the expression of S100 protein in both cell nucleus and the cytoplasm in these cells, hence confirming their Schwann-cell-like property. Under SEM, these cells were found to be actively secreting extracellular matrix.
Conclusion:
Tissue-engineered PLGA conduit seeded with OECs provided a permissive environment to facilitate nerve regeneration in a small animal model.
Keywords: Olfactory ensheathing cells, poly(lactic-co-glycolic acid), sciatic nerve defect, tissue engineering
INTRODUCTION
Peripheral nerve injury is a prevalent devastating event that is often caused by accidents, surgery and diseases. Nerve injury can result in permanent impairment or complete functional loss of the affected body part. Although extensive research has been going on for decades, optimal clinical outcome is yet to be achieved and more studies are necessary to optimize functional recovery of injured nerves.
Autologous nerve graft has been used for injured peripheral nerve repair as a first line therapy. However, limitations include the need for a second surgical procedure to extract the donor nerve, permanent loss of the donor nerve function, limited supply of available grafts and mismatch in size between that of the defect and the graft.[1] Allogeneic nerve grafts have also been used in nerve reconstructions, but this requires systemic immunosuppression.[2]
Thus, there is a need for an alternative nerve conduit with ideal characteristics such as biocompatibility, ability to regenerate the nerve along the whole length of the conduit, semi flexibility and easy handling during the surgery.[1] Various synthetic biomaterials have been studied in the evolution and development of artificial nerve conduits. Poly lactic-co-glygolic acid (PLGA) is approved by United States Food and Drug Administration (USFDA) for therapeutic use and for use in medical devices owing to its biodegradability and biocompatibility and is one of the most used biodegradable synthetic polymers in tissue engineering. It's use is mainly due to ease of controlling its mechanical properties and biodegradation rate.[3,4,5] PLGA hollow tubes have been long studied for use in nerve graft and showed potentially encouraging results.[3,4,5] Neurotrophic factors within the artificial nerve conduit improved the adhesion, survival and movement of the neuronal cells and assisted alignment of the nerve axons during the regeneration comparable to the nerve regeneration in autografts.[6,7] Previously, artificial nerve conduits filled with neural-differentiated human mesenchymal stem cells (hMSCs) and Schwann cells have been studied for use in nerve graft.[1,4,5] Besides hMSCs and Schwann cells, olfactory ensheathing cells (OECs) are also potential cells for nerve regeneration as they exhibit properties of both Schwann cells and astrocytes and are known to improve axonal regeneration and produce myelin after transplantation.[8,9] A previous in vivo study involving transplantation of OECs on transected nerve showed that the OECs integrated into the repaired nerves and the OEC transplanted group had increased conduction velocity and better functional recovery.[10] It is widely accepted that a 10 mm nerve gap for rat sciatic nerves can regenerate spontaneously. Therefore, a nerve gap longer than 10 mm is considered critical and would necessitate the use of a nerve conduit to bridge the gap.[11]
The present study was driven by the hypothesis that PLGA nerve conduit seeded with OECs would facilitate nerve regeneration in transected nerve injuries involving longer nerve gaps (>10 mm). We evaluated the efficacy of tissue-engineered PLGA nerve conduit seeded with OECs in bridging nerve gaps of 15 mm following sciatic nerve injury in the rat model. The nerve regeneration process was analyzed histologically and the functional recovery of the nerve was assessed electrophysiologically.
MATERIALS AND METHODS
Fabrication of PLGA nerve guide conduit
Porous PLGA nerve conduit was fabricated by immersion precipitation technique.[12] The PLGA with a lactide-glycolide ratio of 50:50 was used in the present study. One gram of PLGA was dissolved in 15 ml of methylene chloride. To form the conduit tubes, 18-gauge needles were utilized. The dip-molding process was performed within a fume hood to prevent toxic effects of methylene chloride fumes. Needles were dipped into the solution for 1 min, ensuring at least three quarters or more of the needle surface was beneath the solution line. They were then removed and hung within the fume hood for 5 min. This process was repeated for 50 cycles before the needles were left to dry for 24 h. This whole cycle was repeated twice within 4 days. After the 4th day, a clear structure was seen forming on the outer layer of the needle. The conduits were then removed from the needle scaffold by pushing the conduits off manually and sterilized using 70% ethanol. PLGA conduits of 20 mm length and about 0.5 mm diameter were obtained. There was no difficulty in handling the conduit during implantation and suturing.
Cell culture and seeding
OECs were harvested from adult female Sprague-Dawley (SD) rats. The OEC layer was stripped away from the rest of the bulb through the septum portion.[13] The tissue was minced and then digested with collagenase type I 0.3% solution. Subsequently, the cell suspension was centrifuged and cultured in Minimum Essential Medium Alpha (α-MEM) medium until passage 2. Characterization by immunostaining showed that OECs were positive for glial fibrillary acidic protein (GFAP), S100b and cytokeratin 18.[13] A total of 3 Χ 10[6] cells were seeded on each nerve conduit. The PLGA conduit was immersed in the cell suspension for 2 h to facilitate equal cell distribution throughout the conduit.
Animals
All animal work was conducted according to the Universiti Kebangsaan Malaysia (UKM) Animal Ethics Committee guidelines and prior ethical approval was obtained for the study. Six adult female SD rats weighing 250-350 g were used. The animals were given rat pellet and water and were allowed normal cage activities. Saw dust was used as bedding and was changed twice a week.
Operative procedure for implantation
Rats were placed prone under sterile conditions and skin from the clipped lateral right thigh scrubbed in a routine fashion with povidone solution. Surgeries were performed under the operating microscope (Carl Zeiss, Germany). Under deep anesthesia (ketamine 80 mg/kg, xylazine 10 mg/kg; intravenous), the right sciatic nerve was exposed through a skin incision extending from the greater trochanter to the midway distally followed by deep dissection. After nerve mobilization, a transection injury of 15 mm was performed (neurotmesis) using straight microsurgical scissors. The nerve was injured at a level as low as possible, in general, immediately above the terminal nerve ramification. The injured nerve was repaired using PLGA incorporated with OECs. Fifteen mm conduits were interposed between the proximal and distal stumps with three sutures (10/0 Ethilon) at each junction [Figure 1]. Following the implantation, the intermuscular plane was closed using a 5/0 chromic catgut suture (Ethicon) and the skin was closed using a 5/0 nylon suture (Ethicon). Chloramphenicol cream was applied to sutured skin area to protect it from infection. The contralateral left sciatic nerves were left intact in all groups and these served as controls. After the surgery, all animals were nursed in an individual ventilated cage, housed in a temperature-controlled and humidity-controlled room and were closely monitored for 3 days; there was no postoperative mortality. Incontinent sheet was used as the bedding for 3-4 days to maintain hygiene and prevent wound infection. Intramuscular ceftriaxone (Rocephin) 100 mg/kg daily was given for 3 doses and syrup Paracetamol 200 mg/kg/day was given to relieve pain. Feeding was also monitored in terms of pellets eaten and amount of fluids taken. All the animals were monitored for 6 weeks before being euthanized for further analysis.
Figure 1.
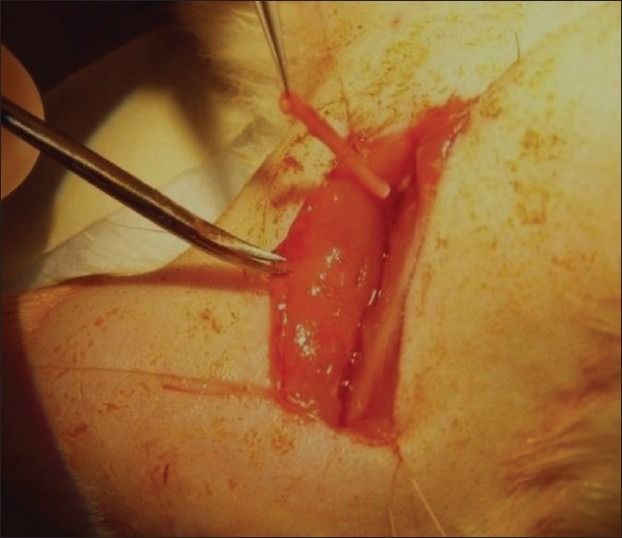
Implantation of poly(lactic-co-glygolic acid) nerve conduit seeded with olfactory ensheathing cells at transected sciatic nerve injury
Electrophysiological evaluation
For the experimental rats, electrophysiological evaluation was performed at an interval of 2nd, 4th and 6th weeks after tissue-engineered PLGA implantation. Under anesthesia (ketamine 80 mg/kg, xylazine 10 mg/kg; intravenous), a recording needle electrode was placed at the sciatic nerve and stimulated at 98 mA by stainless wire electrode connected to a DC electrical stimulator. The stimulus duration was 0.1 ms. The ground electrode was also placed in surrounding muscle tissues. The corresponding nerve conduction velocities were determined for all the six rats.
Histological evaluation
For the histological analysis (6 weeks following implantation), the animals were euthanized with pentobarbital administration. The animals were carefully dissected and the implanted constructs were removed. The regenerated nerve specimens were fixed and dehydrated. The mid-portions of the specimens were embedded in paraffin and cut into 4 μm thickness sections with an ultramicrotome and stained with hematoxylin and eosin (H and E) for observation by a light microscopy. At the same time, the specimens were observed by scanning electron microscopy.
Immunohistochemical analysis
For the immunostaining with S100, the paraffin-embedded sections were deparaffinized, rehydrated and subsequently immersed into preheated target retrieval solution in a water bath (95-98°C) for 30 min. The slides were placed under room temperature for 20 min before they were rinsed 2 or 3 times with tris buffered saline (TBS) and followed by 1 h blocking with 10% goat serum in TBS. Polyclonal anti-S100b (mouse; 1:4000; BD) was then applied overnight at 4°C. Primary antibody was detected by fluorescein isothiocyanate (FITC) anti-mouse IgG (goat; 1:50; Chemicon) and counter-staining was done with 4’,6-diamidino-2-phenylindole (DAPI) (nuclear staining). The sections were examined using a fluorescence microscope (eclipse Ti, Nikon).
Statistical analysis
Data is expressed as mean ± standard error of mean. Electrophysiological data with intergroup (3 groups) differences were tested by one-way analysis of variance (ANOVA) using SPPS 14.0 software. Before each ANOVA, a Levene's test was used to determine if the variances across the groups are homogenous. If ANOVA analysis identified a significant effect of the experimental group (between subjects factor), the post hoc HSD Tukey's test was applied for paired comparisons. Descriptive analysis was used for both immunohistochemical and histological results.
RESULTS
in vivo regeneration
All animals survived the surgery. The initial three rats developed ulcers on the 3rd day post-surgery. Eventually, these rats nibbled at the extremity of their toes. This may be due to post-surgical pain. Hence, for the subsequent rats, Paracetamol was administered for 7 days immediately post-surgery and the problem resolved.
All conduits were intact after 6 weeks of implantation, without any anastomotic disruptions in the conduits.
Electrophysiological evaluation
Nerve conduction study was used to assess neurophysiological recovery. The nerve conduction velocities at 2, 4 and 6 weeks postimplantation were compared to each other. One-way ANOVA of the three groups of data revealed a P < 0.0001. Two weeks postoperative and 4 weeks postoperative data showed no statistical difference in nerve conduction velocities. However, 6 weeks postoperative data showed a significant difference and consistently higher values compared to those at 2 and 4 weeks postimplantation [Figure 2].
Figure 2.
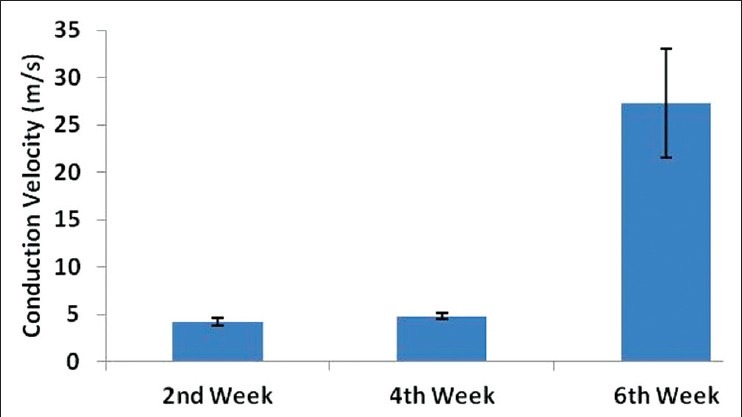
A bar diagram showing nerve conduction velocities at 2, 4 and 6 weeks following implantation of olfactory ensheathing cells seeded poly(lactic-co-glygolic acid) conduit into transected sciatic nerve. *One-way ANOVA of the three groups of data revealed P < 0.0001. The 6th weeks post implantation mean conduction velocity was significantly higher compared to the 2nd and 4th week post-implantation mean conduction velocity
Histological evaluation
Six weeks following implantation, the tissue-engineered PLGA showed the presence of spindle-shaped Schwann cell-like cells and red blood cells [Figure 3]. No marked presence of inflammatory cells was noted in the histological sections of the post-implanted conduits. Histological section from normal sciatic nerve was used as a reference.
Figure 3.
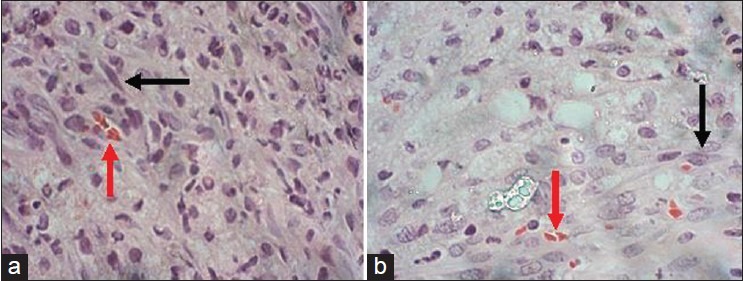
H and E staining of cross-sectional nerve (×100). Mid-portion of: (a) the implanted olfactory ensheathing cell seeded poly(lactic-co-glygolic acid) conduit (6 weeks), (b) normal sciatic nerve. The black arrow shows Schwann cell-like cells and the red arrow show red blood cells
Scanning electron microscopy
Scanning electron microscopy following 6 weeks implantation of the OECs-seeded PLGA conduit showed presence of Schwann cell-like cells that were actively secreting extracellular matrix [Figure 4]. Normal sciatic nerve was used as a reference.
Figure 4.
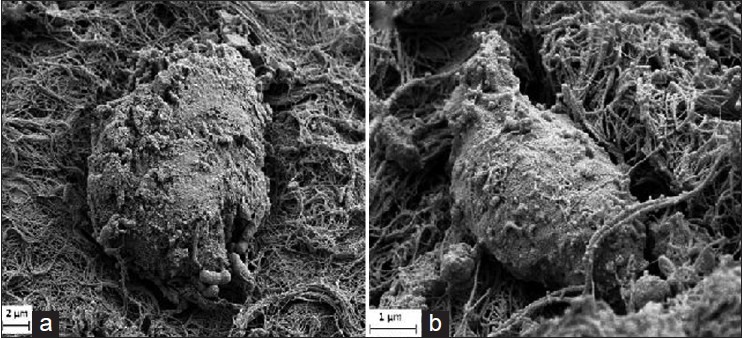
Scanning electron micrograph of: (a) olfactory ensheathing cells seeded poly(lactic-co-glygolic acid) nerve conduit at 6 weeks postimplantation at transected sciatic nerve (×3000), (b) normal Schwann cell (×10000)
Immunohistochemical evaluation
Six weeks following implantation of tissue-engineered PLGA, immunohistochemical analysis revealed the distribution of S100 protein in both the nucleus and the cytoplasm of the Schwann cell-like cells [Figure 5]. Normal sciatic nerve was used as a reference.
Figure 5.

Immunofluorescence staining of the normal nerve and implanted conduits’ cross section (×100). Cells with DAPI-counterstained nucleus (blue) are expressing S-100 (green fluorescence). (a) olfactory ensheathing cells seeded poly(lactic-co-glygolic acid) nerve conduit 6 weeks post implantation at transected sciatic nerve (6 weeks), (b) normal sciatic nerve
DISCUSSION
After an event of peripheral nerve injury, restoration of function with sufficient recovery continues to be a clinical challenge.[14] The use of artificial nerve guide conduits to bridge the gap between severed peripheral nerve stumps is widely accepted as a useful alternative.[1] Previous animal studies used PLGA conduits to bridge the gap between the transected nerve ends to enable axonal growth across the gap and histological analysis has revealed the presence of Schwann cells along these conduit.[15,16] Use of PLGA has several advantages. Being biodegradable, it does not leave any residual foreign material. Furthermore, PLGA can be tailored to release substances over a protracted period as demonstrated by its use as a drug delivery vehicle.[17] The PLGA with a lactide-glycolide ratio of 50:50 was used in the present study as our previous studies had shown that PLGA (50:50) can form scaffolds of good mechanical property and hence suitable for tissue engineering.[5,18,19]
The immersion precipitation technique was chosen for fabrication of PLGA nerve guide as this method is simple and can be used to form hollow conduits of various thicknesses.[12,20,21] Our previous physicomechanical and ultrastructural study on the PLGA conduits have shown that they have good flexibility, ability to stretch and have a porous outer layer.[22] In this study, porous PLGA nerve conduit with OECs was used to facilitate nerve regeneration. Porous conduits were utilized to encourage vascular ingrowth and nutrient permeation into the tube wall as also concurred with other studies.[12,23] In our study, the blood cells that were observed in the regenerated nerve tissues could be an early sign of angiogenesis. The postimplanted conduits showed the presence of S100-positive cells. As S100 is a specific marker for Schwann cells, we used the term Schwann-cell-like cells to refer to these S100 positive cells throughout the paper. In this study, we did not perform cell tracking on the implanted cells, hence, we could not determine whether the Schwann cell-like cells were derived from the implanted OECs or migrated into the conduit from the surrounding tissues. We can only confirm that our PLGA conduit supported the growth of Schwann cell-like cells. The presence of Schwann cell-like cells in the tissue engineered nerve conduit at 6 weeks postimplantation strengthens our belief that physiological regeneration was taking place as previous studies have shown that Schwann cells promote and facilitate axonal regrowth.[24,25]
A previous study had demonstrated that the growth rate of proximal and distal nerve stumps was 0.32 and 0.18 mm/day, respectively, in rat sciatic nerves.[26] Thus, for the nerve to regenerate across a 15 mm gap, it would have taken at least 30 days (~4 weeks). Hence, nerve conduction velocities at 2nd and 4th week postoperatively showed no statistically significant difference. However, after 6 weeks of implantation, the mean nerve conduction velocity of the PLGA tube (27.3 ± 5.7 m/s) was about 73% of that reported by Meng et al.[27] on a normal rat sciatic nerve (37.28 m/s). Another study by Kim et al.[28] reported that the nerve conduction velocity of regenerated nerve through acellular nerve graft incorporated with growth factor (nerve gap 8 mm; 6 months implantation; SD rat) was only about 60%.
Neurotrophic factors promote a variety of neural responses, like the survival and outgrowth of the motor and sensory nerve fibers and are implicated in peripheral nerve regeneration.[1,29] OEC is a useful cellular system to produce and deliver these neurotrophic factors.[30] OECs can be regarded as peripheral nerve progenitor cells since they develop from a peripheral origin, the olfactory placode. OECs are able to self-renew and differentiate to promote axonal regrowth and remyelination in the peripheral nervous system under experimental conditions.[8] This was the basis for incorporating OECs in the biodegradable nerve conduit in our study.
To conclude, the results of our preliminary study support the use of OECs-seeded porous PLGA conduit to facilitate nerve regeneration. Proof of efficacy in a study with larger sample number and in large animal model needs to be conducted before this tissue engineered nerve conduit can be considered for use in patients for peripheral nerve reconstruction. It will be an alternative to autograft nerve transplant in future clinical settings.
Footnotes
Source of Support: This work was financially supported by the grant received from Ministry of Science, Technology and Innovations, Malaysia (e-Science Fund No. 02-01-02 SF0446). The authors are grateful to Dr. Jamari S and Mr. Yazid AG from Universiti Kebangsaan Malaysia Medical Centre for technical assistance.
Conflict of Interest: None.
REFERENCES
- 1.Daly W, Yao L, Zeugolis D, Windebank A, Pandit A. A biomaterials approach to peripheral nerve regeneration: Bridging the peripheral nerve gap and enhancing functional recovery. J R Soc Interface. 2012;9:202–21. doi: 10.1098/rsif.2011.0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mackinnon SE, Doolabh VB, Novak CB, Trulock EP. Clinical outcome following nerve allograft transplantation. Plast Reconstr Surg. 2001;107:1419–29. doi: 10.1097/00006534-200105000-00016. [DOI] [PubMed] [Google Scholar]
- 3.Burg K, Thomas C. Tissue engineering. In: Akay M, editor. Wiley Encyclopedia of Biomedical Engineering. New Jersey: John Wiley and Sons; 2006. pp. 3497–512. [Google Scholar]
- 4.Bryan DJ, Holway AH, Wang KK, Silva AE, Trantolo DJ, Wise D, et al. Influence of glial growth factor and Schwann cells in a bioresorbable guidance channel on peripheral nerve regeneration. Tissue Eng. 2000;6:129–38. doi: 10.1089/107632700320757. [DOI] [PubMed] [Google Scholar]
- 5.Hidayah N, Fadzli A, Ng M, Ruszymah B, Naicker A, Shalimar A, et al. Porous PLGA sheet and acellularized muscle stuffed vein seeded with neural-differentiated MSCs are potential scaffolds for nerve regeneration. Regen Res. 2012;1:1–7. [Google Scholar]
- 6.Jiang X, Lim SH, Mao HQ, Chew SY. Current applications and future perspectives of artificial nerve conduits. Exp Neurol. 2010;223:86–101. doi: 10.1016/j.expneurol.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 7.Midha R, Munro CA, Dalton PD, Tator CH, Shoichet MS. Growth factor enhancement of peripheral nerve regeneration through a novel synthetic hydrogel tube. J Neurosurg. 2003;99:555–65. doi: 10.3171/jns.2003.99.3.0555. [DOI] [PubMed] [Google Scholar]
- 8.Imaizumi T, Lankford KL, Waxman SG, Greer CA, Kocsis JD. Transplanted olfactory ensheathing cells remyelinate and enhance axonal conduction in the demyelinated dorsal columns of the rat spinal cord. J Neurosci. 1998;18:6176–85. doi: 10.1523/JNEUROSCI.18-16-06176.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramón-Cueto A, Valverde F. Olfactory bulb ensheathing glia: A unique cell type with axonal growth-promoting properties. Glia. 1995;14:163–73. doi: 10.1002/glia.440140302. [DOI] [PubMed] [Google Scholar]
- 10.Radtke C, Wewetzer K, Reimers K, Vogt PM. Transplantation of olfactory ensheathing cells as adjunct cell therapy for peripheral nerve injury. Cell Transplant. 2011;20:145–52. doi: 10.3727/096368910X522081. [DOI] [PubMed] [Google Scholar]
- 11.Chen YS, Liu CJ, Cheng CY, Yao CH. Effect of bilobalide on peripheral nerve regeneration. Biomaterials. 2004;25:509–14. doi: 10.1016/s0142-9612(03)00548-9. [DOI] [PubMed] [Google Scholar]
- 12.Oh SH, Lee JH. Fabrication and characterization of hydrophilized porous PLGA nerve guide conduits by a modified immersion precipitation method. J Biomed Mater Res A. 2007;80:530–8. doi: 10.1002/jbm.a.30937. [DOI] [PubMed] [Google Scholar]
- 13.Yazid AG, Anuar A, Onhmar HT, Ng AM, Ruszymah BH, Amaramalar SN. Sourcing different neuro-progenitor cell for the use of nerve construct. Med J Malaysia. 2008;63:113–4. [PubMed] [Google Scholar]
- 14.Schlosshauer B, Müller E, Schröder B, Planck H, Müller HW. Rat Schwann cells in bioresorbable nerve guides to promote and accelerate axonal regeneration. Brain Res. 2003;963:321–6. doi: 10.1016/s0006-8993(02)03930-6. [DOI] [PubMed] [Google Scholar]
- 15.Hadlock T, Sundback C, Hunter D, Cheney M, Vacanti JP. A polymer foam conduit seeded with Schwann cells promotes guided peripheral nerve regeneration. Tissue Eng. 2000;6:119–27. doi: 10.1089/107632700320748. [DOI] [PubMed] [Google Scholar]
- 16.Bini TB, Gao S, Xu X, Wang S, Ramakrishna S, Leong KW. Peripheral nerve regeneration by microbraided poly(L-lactide-co-glycolide) biodegradable polymer fibers. J Biomed Mater Res A. 2004;68:286–95. doi: 10.1002/jbm.a.20050. [DOI] [PubMed] [Google Scholar]
- 17.Langer R. 1994 Whitaker Lecture: Polymers for drug delivery and tissue engineering. Ann Biomed Eng. 1995;23:101–11. doi: 10.1007/BF02368317. [DOI] [PubMed] [Google Scholar]
- 18.Salem SA, Hwei NM, Saim AB, Ho CC, Sagap I, Singh R, et al. Polylactic-co-glycolic acid mesh coated with fibrin or collagen and biological adhesive substance as a prefabricated, degradable, biocompatible and functional scaffold for regeneration of the urinary bladder wall. J Biomed Mater Res A. 2013;101:2237–47. doi: 10.1002/jbm.a.34518. [DOI] [PubMed] [Google Scholar]
- 19.Sha’ban M, Kim SH, Idrus RB, Khang G. Fibrin and poly(lactic-co-glycolic acid) hybrid scaffold promotes early chondrogenesis of articular chondrocytes: An in vitro study. J Orthop Surg Res. 2008;3:17. doi: 10.1186/1749-799X-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wan AC, Mao HQ, Wang S, Leong KW, Ong LK, Yu H. Fabrication of poly (phosphoester) nerve guides by immersion precipitation and control of porosity. Biomaterials. 2001;22:1147–56. doi: 10.1016/s0142-9612(00)00355-0. [DOI] [PubMed] [Google Scholar]
- 21.Wang S, Cai L. Polymers for fabricating nerve conduits. Int J Polym Sci. 2010;2010:20. [Google Scholar]
- 22.Ahmad-Fadzli S, Nur-Hidayah H, Naicker AS, Shalimar A, Angela NM, Mohd-Reusmaazran, et al. Developing and testing of a collagen-coated polylactic-glycolic acid (PLGA) Neural Conduit 5th Asean Arthroplasty Association Meeting; Kuala Lumpur, Malaysia. 2011. [Google Scholar]
- 23.Navarro X, Rodríguez FJ, Labrador RO, Butí M, Ceballos D, Gómez N, et al. Peripheral nerve regeneration through bioresorbable and durable nerve guides. J Peripher Nerv Syst. 1996;1:53–64. [PubMed] [Google Scholar]
- 24.Chen YY, McDonald D, Cheng C, Magnowski B, Durand J, Zochodne DW. Axon and Schwann cell partnership during nerve regrowth. J Neuropathol Exp Neurol. 2005;64:613–22. doi: 10.1097/01.jnen.0000171650.94341.46. [DOI] [PubMed] [Google Scholar]
- 25.Guénard V, Kleitman N, Morrissey TK, Bunge RP, Aebischer P. Syngeneic Schwann cells derived from adult nerves seeded in semipermeable guidance channels enhance peripheral nerve regeneration. J Neurosci. 1992;12:3310–20. doi: 10.1523/JNEUROSCI.12-09-03310.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Humphrey MF, Levy WJ, Dietrich WD, Rumpf R, Mora J. Peripheral nerve repair across a gap studied by repeated observation in a new window implant chamber. Brain Res. 1989;497:132–7. doi: 10.1016/0006-8993(89)90978-5. [DOI] [PubMed] [Google Scholar]
- 27.Meng H, Chen R, Xu L, Li W, Chen L, Zhao X. Peripferal nerve regeneration in response to synthesized nanofiber scaffold hydrogel. Life Sci J. 2012;9:42–6. [Google Scholar]
- 28.Kim BS, Yoo JJ, Atala A. Peripheral nerve regeneration using acellular nerve grafts. J Biomed Mater Res A. 2004;68:201–9. doi: 10.1002/jbm.a.10045. [DOI] [PubMed] [Google Scholar]
- 29.Schmidt CE, Leach JB. Neural tissue engineering: Strategies for repair and regeneration. Annu Rev Biomed Eng. 2003;5:293–347. doi: 10.1146/annurev.bioeng.5.011303.120731. [DOI] [PubMed] [Google Scholar]
- 30.Chiu SC, Hung HS, Lin SZ, Chiang E, Liu DD. Therapeutic potential of olfactory ensheathing cells in neurodegenerative diseases. J Mol Med (Berl) 2009;87:1179–89. doi: 10.1007/s00109-009-0528-2. [DOI] [PubMed] [Google Scholar]


