Abstract
Introduction
Ischemic mitral regurgitation can be defined as moderate to severe mitral leak precipitated by acute myocardial infarction. Valve repair is now the procedure of choice, but some cases can pose difficult anatomy. This review will illustrate current techniques for repairing complex ischemic mitral regurgitation.
Methods
Most patients with ischemic mitral regurgitation have predominant annular dilatation at the posterior commissure and require only ring annuloplasty. Full rigid rings are used preferentially. With leaflet tethering, adjunctive autologous pericardial patches are effective in restoring leaflet coaptation. If papillary muscle elongation or rupture occurs, Gore-Tex artificial chordal replacement performs well. With ischemic mitral regurgitation accompanying posterior ventricular aneurysms, standard trans-atrial mitral repair provides the best results, with associated aneurysms being repaired concurrently.
Results
Surgical approaches and technical outcomes of mitral repair in ischemic mitral regurgitation are illustrated in 5 patients using operative images and echocardiograms. Each method is illustrated, including ring annuloplasty, pericardial leaflet augmentation, artificial chordal replacement, and ventricular aneurysm repair. Using these techniques, virtually all ischemic mitral regurgitation can be repaired, with consequential patient benefits, even in the most complex anatomy.
Conclusions
Ischemic mitral regurgitation has been shown to have better outcomes when managed with valve repair. Using combinations of annular, leaflet, and chordal procedures, even complex ischemic mitral regurgitation can undergo autologous reconstruction with excellent long-term results.
Keywords: ischemic mitral regurgitation, mitral valve repair, survival analysis
Introduction
Several divergent concepts currently exist about the prognosis and management of ischemic mitral regurgitation (IMR). They range from “little can be done” to modify the dismal prognosis [1,2] - to mitral repair can “cure” the disorder [3]; from mitral repair should be “uniform” [4] - to prosthetic replacement achieves “more certain” results [5]. One problem is that conclusions have been based on multiple different surgical approaches between centers, and also, statistical techniques often have been suboptimal. Thus, it is likely that outcome benefits did vary between institutions, with differences in surgery and other factors, accounting for the divergent conclusions. The goal of this paper is to review repair techniques for IMR used over the past 25 years at Duke University Medical Center and to document observed outcome characteristics. Based on this experience, conclusions about future management will be made.
Pathophysiology. IMR can be defined as mitral regurgitation precipitated by myocardial infarction, with essentially normal leaflet and chordal support. IMR usually is associated with myocardial necrosis, rather than reversible ischemia, and the infarction is usually inferior [6]. IMR involves more than a “ventricular” problem.In fact, mitral valve competence requires the integrated function of all five aspects of the valve - annulus, leaflets, chordae, papillary muscles, and ventricular wall. As defined in George Burch’s classic 1966 “Papillary Muscle Dysfunction” paper [7], most cases involve predominant posterior annular dilatation, but others experience leaflet tethering due to infarct expansion. Rarely, papillary muscles can elongate or rupture, producing leaflet prolapse. The common type of IMR involves the posterior wall, usually is caused by right and/or circumflex coronary occlusion, produces moderate posterior-lateral left ventricular (LV) dysfunction, and usually will not reverse with revascularization alone. As described in the 1980’s, the decision to repair the valve should be based on the appearance of moderate or severe IMR on the intra-operative transesophageal echo (TEE), sometimes with provocative volume loading from the pump before bypass [6].
Surgical techniques. Posterior annular dilatation is an important mechanism of IMR, and has been almost uniformly corrected in our practice with a true-sized full rigid or semi-rigid ring. It has been shown that fixed reduction in anterior-posterior annular geometry is extremely important in this entity [8]. These patients often have minor leaflet abnormalities, such as fetal commissural cusps or clefts, predisposing them to opening up the valve with ischemic posterior annular dilatation [6].
Occasionally, leaflets can be tethered by infarct expansion, contributing to lack of valve closure. As Langer showed [9], a posterior leaflet autologous pericardial patch can be used to compensate for the tethering, and this approach provides a large margin of safetylong-term, especially if further geometric changes occur. For leaflet prolapse due to elongated or ruptured papillary muscles, Gore-Tex artificial chords can be placed to a viable papillary muscle, adjusting chordal lengths after ring placement, as we have described [10,11]. Echocardiograms and operative videos will be presented from 5 patients to illustrate these techniques.
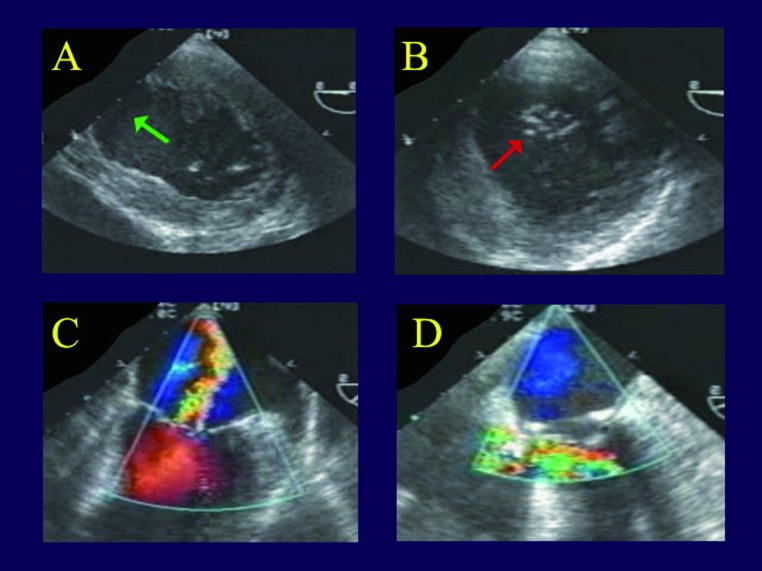
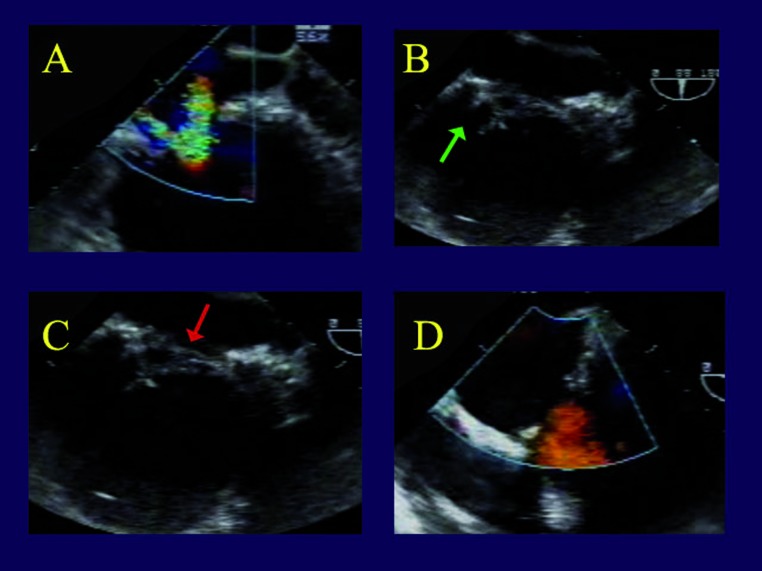
Ring annuloplasty. The first patient is a 77 year old man, experiencing an acute circumflex infarct that produced acute moderate to severe mitral regurgitation (MR) and congestive heart failure. On TEE (Figures 1 A,B), there is loss of valve coaptation at the posterior commissure (red arrow), due to annular dilatation from a large posterior lateral infarct (green arrow). This produced the characteristic loss of coaptation area and a central MR jet, but both leaflets are at the annular plane and not tethered or prolapsed (Figure 1C). After simple insertion of a full rigid ring, the valve is entirely competent (Figure 1D).
Figure 1.
Transesophageal echocardiogram in a patient undergoing valve repair for ischemic mitral regurgitation. Panel A is a short axis view of the left ventricle, showing an expanded posterior-lateral wall infarct (green arrow). Panel B shows a gap in mitral coaptation at the posterior commissure during systole (red arrow), producing a central regurgitant jet (Panel C), but the leaflets are at the annular plane. This is the most common echocardiographic appearance of ischemic mitral regurgitation. In panel D, the valve is entirely competent after full ring annuloplasty alone.
The second patient is a 55 year old obese diabetic who experienced a right coronary infarct and IMR 6 months earlier that were treated with right coronary artery (RCA) stenting. Despite revascularization, IMR and heart failure persisted at moderate to severe levels. She then developed an in-stent stenosis in the RCA and an additional left anterior descending coronary artery (LAD) lesion, and her ventricle showed residuals of the inferior infarct, with generalized dilatation. There was loss of central mitral coaptation, due to annular dilatation, a hint of tenting and leaflet tethering (Figure 2A), and moderate to severe MR with a central jet (Figure 2 B).When the valve was inspected, however, the posterior leaflet seemed fine with good closure and minimal tethering on valve testing by ventricular distension with cold saline (Figure 2C). Therefore, the valve is repaired by simple placement of a Carpentier ring, with good recovery of competence, and no residual leak (Figure 2D). So, most ischemic mitral regurgitation (MR) can be repaired with full ring annuloplasty alone, which achieves excellent results, with a documented 2% late reoperation rate, 9% late moderate MR recurrence rate [4,12], and quite satisfactory echocardiographic results [13].
Figure 2.
Clinical images in a patient with chronic ischemic mitral regurgitation. Panel A shows a hint of leaflet tethering (green arrow), with both leaflets slightly below the annular plane. Panel B demonstrates the central jet of moderate to severe ischemic mitral regurgitation. In Panel C, the posterior leaflet seems to coapt well (red arrow) with no evidence of tethering during valve testing with cold saline injection. Therefore, a simple ring annuloplasty was performed, and in Panel D, the valve is completely competent.
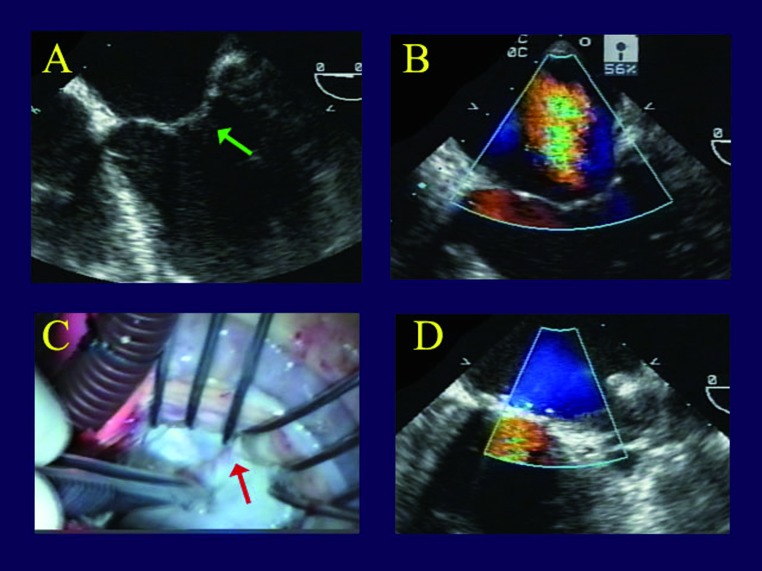
Leaflet patching for tethering. The third patient is a frail elderly female presenting with an acute circumflex infarct and severe IMR, treated initially with percutaneous coronary intervention (PCI). Unlike the first 2 patients, she had prominent posterior leaflet tethering by echocardiography (Figure 3A), and severe LV dysfunction, mainly in the lateral wall due to the infarct. On emergency angiography, she had an occluded circumflex artery, but also LAD and RCA stenoses. The circumflex was stented, but the IMR persisted, the heart failure remained severe, and the ejection fraction continued at a 0.20 level. A magnetic resonance viability study was performed. In IMR patients with low ejection fractions, we will operate if 2 of the 3 regions appear viable. In fact, this patient seemed to have good residual myocardium everywhere. Upon inspecting the valve, the right aspect of the posterior leaflet was tethered, and the posterior leaflet wouldn’t coapt, even with saline distension. In Figure 3B, one can see the posterior leaflet pulled down into the ventricle with tethering from infarct expansion. A gluteraldehyde-fixed autologous pericardial patch was inserted into the posterior leaflet to compensate for the tethering, and the valve became nicely competent. Most of what is seen in Figure 3C is patch, with the posterior leaflet moving down into the ventricle to become the entire coaptation area, and the valve is entirely competent (Figure 3D).
Figure 3.
Images of a patient with ischemic mitral regurgitation and leaflet tethering. Panel A shows both leaflets tented into the ventricle and not closing properly due to tethering (green arrow). Direct inspection in Panel B reveals taught posterior leaflet chords at the posterior commissure, inhibiting valve closure (red arrow). After insertion of an autologous pericardial patch into the posterior leaflet and placement of a Carpentier ring (Panel C), the valve is entirely competent with a large coaptation area (Panel D).
This technique produces excellent recovery of competence and surface area of coaptation. The margin of safety provided by the patch may reduce later IMR recurrence with further infarct expansion, or other problems. A transthoracic echocardiogram, obtained 6 months postoperatively, showed good valve opening, and only trivial residual leak. The patient has remained clinically stable for 3 years after mitral repair.
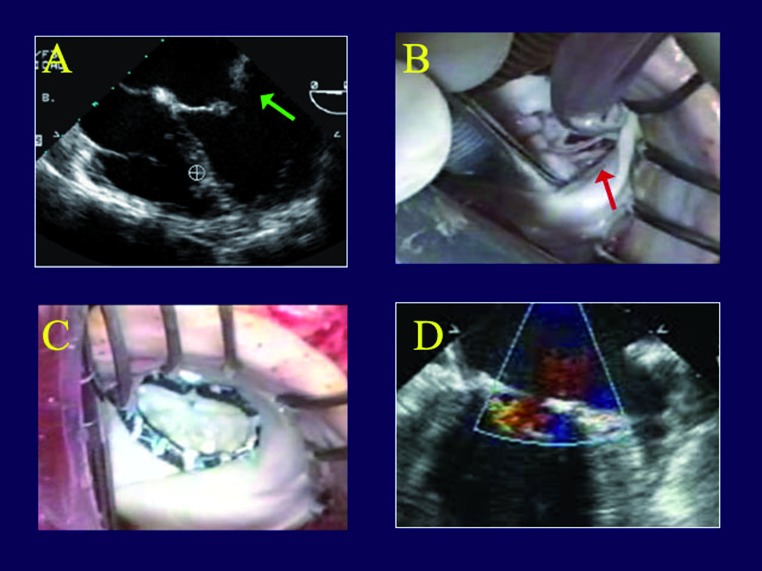
Artificial chords for leaflet prolapse. The fourth patient is a 57 year old man who had a major right coronary infarct, producing severe MR, a posterior LV aneurysm, and severe heart failure. His right coronary was occluded proximally. He also had LAD disease, and - there was a large posterior wall aneurysm (Figure 4A). The aneurysm was resected and closed primarily, approximating viable aneurysm edge to viable edge and removing all non-contractile scar. Upon inspecting the valve, he had a chronically ruptured posterior papillary muscle at the posterior commissure, with severe prolapse that was not appreciated on the echo (Figure 4B). A 2-0 Gore-Tex artificial chord was placed to a viable papillary muscle and stuffed into the ventricle for later retrieval. After ring placement, the chord was woven into the prolapsing segment and the length was adjusted. After repair, the valve was nicely competent (Figure 4C), with no residual leak (Figure 4D).
Figure 4.
Panel A is the left ventriculogram from a patient with severe ischemic mitral regurgitation and a large posterior wall aneurysm (green arrow). In Panel B is shown an associated chronically ruptured papillary muscle producing prolapse of a posterior commissural cusp (red arrow). In Panel C, an artificial chord has been placed to the commissural cusp, along with a Carpentier ring, and the valve is completely competent (Panel D).
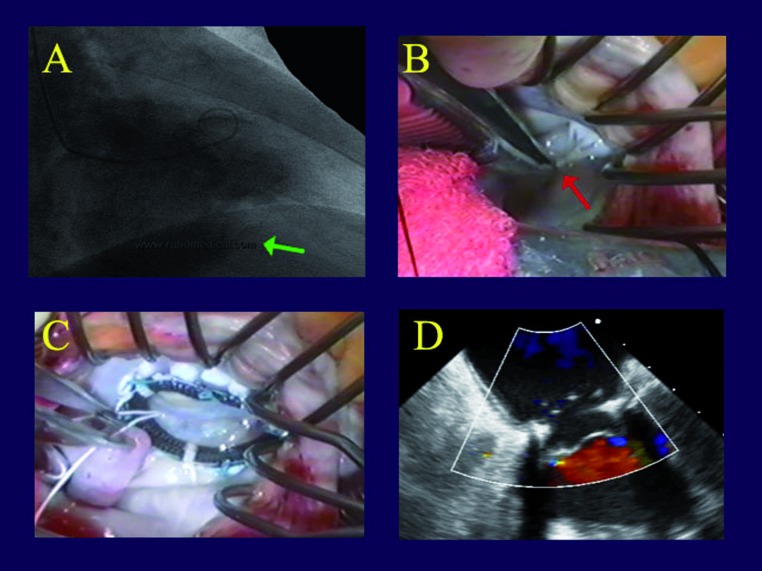
Combined chords and patches. The last patient is a 61 year old man with a remote coronary bypass for an inferior infarction, and severe persistent IMR due to simultaneous leaflet tethering and prolapse - a rare combination. In Figure 5A, one can see 2 separate jets of IMR, with the posterior leaflet tethered down into the ventricle (Figure 5B) and a segment of anterior leaflet prolapsing (Figure 5C). Thus, the patient had simultaneous posterior leaflet tethering from infarct expansion, and anterior leaflet prolapse due to papillary muscle elongation. With a patch to the posterior leaflet and an artificial chord to the anterior leaflet, the valve was completely competent with good leaflet position and no residual leak (Figure 5D). This case illustrates that some varieties of IMR can be quite complex, but with various combinations of full rings, pericardial patches, and artificial chords, the vast majority can be effectively repaired.
Figure 5.
Intraoperative transesophageal echo frames from an ischemic mitral regurgitation patient with both posterior leaflet tethering and anterior leaflet prolapse. Panel A shows 2 mitral regurgitation jets from the 2 lesions. In Panel B, the tethered posterior leaflet (green arrow) is preventing valve closure. Panel C shows a prolapsing segment of the anterior leaflet (red arrow). After repair of both lesions with a posterior leaflet pericardial patch and a chord to the anterior leaflet, the valve is fully competent (Panel D).
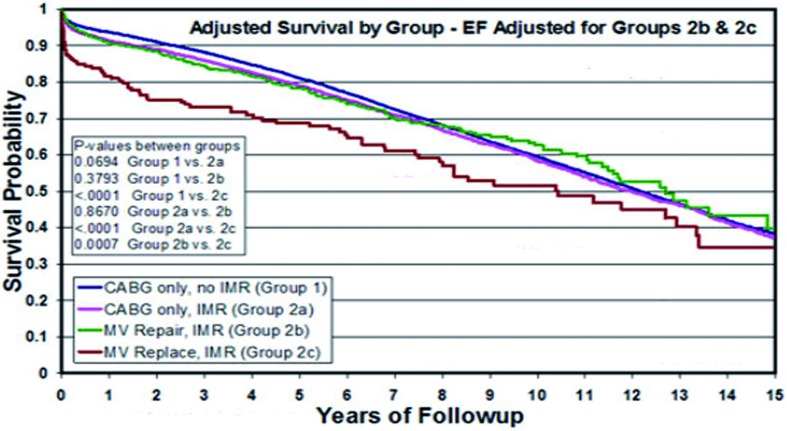
Outcome characteristics of mitral repair.From several sources, it is now clear that the generally poor prognosis after mitral valve repair for IMR (a 56% 5-year unadjusted survival in our series) is due primarily to the adverse patient profiles that are characteristic of this population, such as advanced age, numerous co-morbidities, and left ventricular dysfunction [4]. If these baseline risk factors are taken into account with a Cox model (Figure 6), valve repair seems to restore these patients to an adjusted survival that is similar to standard coronary bypass. Also of note in this analysis, is the average 14% better risk-adjusted survival with valve repair, as compared to prosthetic valve replacement [12], which is consistent with most recent analyses [14].
Figure 6.
Survival curves over 15 years of follow-up adjusted with a Cox proportional hazards model for differences in all important baseline patient characteristics. The blue curve represents adjusted survival characteristics for 16,209 patients having coronary bypass alone without intraoperative transesophageal (Group 1). The red curve represents adjusted survival for 3,181 patients with mild-to-moderate intraoperative transesophageal treated with coronary bypass alone (Group 2a). The green curve is adjusted survival for 416 patients with moderate-to-severe intraoperative transesophageal managed with mitral valve repair (Group 2b). And finally, the brown curve represents adjusted survival of 106 patients with moderate-to-severe intraoperative transesophageal receiving mitral valve replacement (Group 2c). Mitral repair for moderate-to-severe intraoperative transesophageal restored adjusted survival to levels equivalent to standard coronary bypass patients with similar risk profiles. Mitral valve replacement achieved an average 14% lower risk-adjusted survival over 15 years, as compared to valve repair. (From Milano et al. Ann Thorac Surg 2008;86:735-744).
Conclusion
In summary, the pathophysiology of ischemic MR can be complex, with aspects involving the annulus, leaflets, chords, papillary muscles, and ventricular wall. Validated repair techniques now exist for each of these problems. Based on our clinical experience, mitral valve repair should be the procedure of choice for moderate or worse IMR, as encountered in the coronary disease population. Finally, effective mitral repair restores IMR patients to a prognosis that is similar to standard coronary bypass, for a given patient’s age and co-morbidities, and repair seems superior to valve replacement in all subsets.
Footnotes
Source of Support Nil
Disclosures None declared
Presented at the 5th Expert Forum, Roland Hetzer Society, Berlin, April 20th, 2013
Cite as: Rankin JS, Daneshmand MA, Milano CA, Gaca JG, Glower DD, Smith PK. Mitral valve repair for ischemic mitral regurgitation: review of current techniques. Heart, Lung and Vessels. 2013; 5(4): 246-251.
References
- Diodato M D, Moon M R, Pasque M K. et al. Repair of ischemic mitral regurgitation does not increase mortality or improve long-term survival in patients undergoing coronary artery revascularization: A propensity analysis. Ann Thorac Surg. 2004;78:794–799. doi: 10.1016/j.athoracsur.2004.03.022. [DOI] [PubMed] [Google Scholar]
- Gorman R C, Gorman JH 3rd. Does repair of ischemic mitral regurgitation help? Ann Thorac Surg. 2003;76:1775–1776. doi: 10.1016/s0003-4975(03)00258-3. [DOI] [PubMed] [Google Scholar]
- Braun J, van de Veire N R, Klautz R J M. et al. Restrictive mitral annuloplasty cures ischemic mitral regurgitation and heart failure. Ann Thorac Surg. 2008;85:430–436. doi: 10.1016/j.athoracsur.2007.08.040. [DOI] [PubMed] [Google Scholar]
- Glower D, Tuttle R, Shaw L. et al. Patient survival characteristics after routine mitral valve repair for ischemic mitral regurgitation. J Thorac Cardiovasc Surg. 2005;129:860–868. doi: 10.1016/j.jtcvs.2004.11.023. [DOI] [PubMed] [Google Scholar]
- Gillinov A M, Wierup P N, Blackstone E H. et al. Is repair preferable to replacement for ischemic mitral regurgitation? J Thorac Cardiovasc Surg. 2001;122:1125–1141. doi: 10.1067/mtc.2001.116557. [DOI] [PubMed] [Google Scholar]
- Rankin J S, Livesey S A, Smith L R. et al. Trends in the surgical treatment of ischemic mitral regurgitation: Effects of mitral valve repair on hospital mortality. Semin Thorac Cardiovasc Surg. 1989;1:149–163. [PubMed] [Google Scholar]
- Burch G, DePasquale N, Phillips J. The syndrome of papillary muscle dysfunction. Am Heart J. 1968;75:399–415. doi: 10.1016/0002-8703(68)90097-5. [DOI] [PubMed] [Google Scholar]
- Tibayan F A, Rodriguez F, Langer F. et al. Annular remodeling in chronic ischemic mitral regurgitation: ring selection implications. Ann Thorac Surg. 2003;76:1549–1555. doi: 10.1016/s0003-4975(03)00880-4. [DOI] [PubMed] [Google Scholar]
- Langer F, Rodriguez F, Cheng A. et al. Posterior mitral leaflet extension: An adjunctive repair option for ischemic mitral regurgitation? J Thorac Cardiovasc Surg. 2006;131:868–877. doi: 10.1016/j.jtcvs.2005.11.027. [DOI] [PubMed] [Google Scholar]
- Rankin J S, Orozco R E, Addai T R. et al. Several new considerations in mitral valve repair. J Heart Valve Dis. 2004;13:399–409. [PubMed] [Google Scholar]
- Rodgers T L, Rankin J S, Orozco R E. et al. "Adjustable" artificial chordal replacement for repair of mitral valve prolapse. Ann Thorac Surg. 2006;81:1526–1528. doi: 10.1016/j.athoracsur.2005.01.042. [DOI] [PubMed] [Google Scholar]
- Milano C A, Danishmand M A, Rankin J S. et al. Survival prognosis and surgical management of ischemic mitral regurgitation. Ann Thorac Surg. 2008;86:735–744. doi: 10.1016/j.athoracsur.2008.05.017. [DOI] [PubMed] [Google Scholar]
- Williams M L, Daneshmand M A, Jollis J G. et al. Mitral gradients and frequency of recurrence of mitral regurgitation after ring annuloplasty for ischemic mitral regurgitation. Ann Thorac Surg. 2009;88:1197–1201. doi: 10.1016/j.athoracsur.2009.06.022. [DOI] [PubMed] [Google Scholar]
- Rao C, Murphy M O, Saso S. et al. Mitral valve repair or replacement for ischaemic mitral regurgitation: a systematic review. Heart Lung Circ. 2011;20:555–565. doi: 10.1016/j.hlc.2011.03.012. [DOI] [PubMed] [Google Scholar]