Novel diapedisis method uses shear flow following siRNA knockdown in human monocytes, to improve transendothelial migration.
Keywords: migration, vascular biology, transfusion medicine, macrophages
Abstract
Monocyte recruitment to inflammatory sites and their transendothelial migration into tissues are critical to homeostasis and pathogenesis of chronic inflammatory diseases. However, even short-term suspension culture of primary human monocytes leads to phenotypic changes. In this study, we characterize the functional effects of ex vivo monocyte culture on the steps involved in monocyte transendothelial migration. Our data demonstrate that monocyte diapedesis is impaired by as little as 4 h culture, and the locomotion step is subsequently compromised. After 16 h in culture, monocyte diapedesis is irreversibly reduced by ∼90%. However, maintenance of monocytes under conditions mimicking physiological flow (5–7.5 dyn/cm2) is sufficient to reduce diapedesis impairment significantly. Thus, through the application of shear during ex vivo culture of monocytes, our study establishes a novel protocol, allowing functional analyses of monocytes not currently possible under static culture conditions. These data further suggest that monocyte-based therapeutic applications may be measurably improved by alteration of ex vivo conditions before their use in patients.
Introduction
Monocytes are critical to acute and chronic inflammation, and their phenotype can shift the balance of disease progression versus regression. The initial step in the response of monocytes to danger signals is their recruitment and transendothelial migration from the circulation into compromised tissues. Transendothelial migration is a sequential, multistep process: rolling/capture, firm adhesion, locomotion, and diapedesis [1]. These interactions with endothelium rapidly trigger expression of multiple genes [2, 3] and contribute to their differentiation within tissues to macrophages and DCs [4–6].
The importance of using normal human monocytes to define mechanisms involved in disease pathogenesis is highlighted by studies demonstrating that leukemic cell lines frequently used to study “human monocytes” have distinct responses to inflammatory stimuli compared with primary human monocytes [7, 8]. Difficulties with transduction of primary human monocytes have also been eliminated by the development of reproducible protocols to knockdown genes in monocytes using nucleofection (e.g., ref. [9]). However, another major challenge for studies using primary monocytes is their rapid phenotypic alteration during ex vivo culture, including differentiation. Macrophage-specific enzymatic activities increase, and chemotaxis decreases at Days 1 and 3, respectively [10, 11], even in optimized culture conditions, where monocytes are suspended and gently agitated in nonadherent vessels, such as Teflon bags or polypropylene tubes [12]. Thus, in vitro experiments used to determine the multiple steps in transendothelial migration [1] have relied on immediate evaluation of freshly isolated monocytes. However, temporal effects of ex vivo culture on transendothelial migration have not been examined, and the extent to which incubation following gene knockdown alters monocyte responsiveness is unknown.
Monocyte transendothelial migration is important, not only for in vitro mechanistic studies but also for investigating possible therapeutic applications of monocytes. Monocytes have been proposed as promising cellular vehicles to deliver therapeutic products to sites of arteriogenesis [13], Alzheimer's disease [14], and tumor growth and metastasis [15]. Transplantation of monocytes and their progenitors has also been considered an attractive alternative to stem cell transplantation for neural and vascular ischemia repair [16, 17]. Administration (i.v.) of monocytes may be comparable with, or even preferable to, direct transplantation to affected sites [16], and monocytes are significantly more effective than differentiated macrophages [18]. Therefore, effective monocyte transendothelial migration and locally induced differentiation appear to enhance clinical outcomes.
In this study, we demonstrate that ex vivo suspension culture rapidly impairs monocyte transendothelial migration and that this impairment can be rescued significantly by culture conditions mimicking physiological flow. Thus, our data establish a novel approach that minimizes the effects of ex vivo incubation of human monocytes and allows evaluation of the contribution of specific genes using siRNA knockdown.
MATERIALS AND METHODS
Human endothelial cell culture and monocyte isolation
HUVECs (Cascade Biologics; current supplier Life Sciences, Grand Island, NY, USA) were cultured using M199 supplemented with 20% FCS, EGM-2 SingleQuots (Lonza, Basel, Switzerland), and antibiotics and used for transendothelial migration at Passage 2.
Monocyte isolation and handling
Platelet-depleted human monocytes were prepared from freshly drawn citrate anticoagulated whole blood from healthy donors by a two-step isolation: density separation with Ficoll-Paque Plus (GE Healthcare, Pittsburgh, PA, USA) and negative selection using the Monocyte Isolation Kit II (Miltenyi Biotec, Auburn, CA, USA), plus biotinylated anti-CD42b antibodies (GeneTex, Irvine, CA, USA) [3, 19], according to the manufacturer's instructions. Isolated monocytes were kept at room temperature between the procedures (<30 min). Monocytes to be subjected to the in vitro transendothelial migration were resuspended in assay medium (phenol red-free M199 with 20% FCS, 20 mM HEPES, pH 8.0) and kept on ice until use. The University of Washington Human Subjects Review Committee has approved all protocols.
Ex vivo culture of monocytes
Fresh monocytes were suspended at 3.5–4 × 106 in 4 ml IMDM, supplemented with 20% human plasma-derived serum, and cultured without shaking in a vertical polypropylene, 50-ml conical tube in 5% CO2 incubator at 37°C. To maintain monocytes under conditions that more closely mimic those in the circulation, flow-like conditions were introduced by incubating the monocyte suspension under the same conditions, except the tubes were shaken on an orbital shaker (Unimax 1010; Heidolph, Schwabach, Germany). Shaking speeds to model physiological flow conditions were calculated using the formula for estimation of the maximal wall shear stress [τmax (dyn/cm2)] at the bottom of a round vessel using the formula τmax = a[ρη (2π f)3]1/2, where a is the radius of orbital rotation of the shaker (1 cm), ρ is the density of the culture medium (1.0 g/cm3), η is the viscosity of the medium (assumed to be 7.5×10−3 dyn×s/cm2), and f is the frequency of rotation (rotation/s) [20, 21]. Shear stresses of 2, 5, 7.5, and 10 dyn/cm2 were attained at shaking frequencies of 78, 145, 187, and 226 rpm, respectively. Although the equation above is for calculation of τmax in round- and flat-bottom vessels, 50-ml conical tubes were used, as pilot experiments using polypropylene Petri dishes showed an increase in granularity of the monocytes and lower frequency of diapedesis.
Ex vivo culture was stopped by transferring the vessels to ice, and the Annexin V-based Dead Cell Removal Kit (Miltenyi Biotec) was used for enrichment of healthy cells (94±0.9% viability comparable with fresh monocytes, as determined by trypan blue exclusion after the enrichment procedure). The Dead Cell Removal Kit process itself did not alter monocyte transendothelial migration (data not shown). Monocytes were kept on ice until addition to the migration assay. Pilot experiments also showed that monocytes incubated in vessels other than polypropylene tubes, such as tissue-culture plates and dishes coated with Hydron, a cell adhesion-resistant agent [poly(hydroxyethyl methacrylate)] [22], had even greater impairment of transendothelial migration than polypropylene tubes (data not shown).
In vitro transendothelial migration assay under static conditions
The original reported conditions [23] for static assay were modified to enable simultaneous recording of multiple conditions under identical parameters [24]. Briefly, HUVECs at Passage 2 were grown to confluence on thin collagen gels (∼0.12-mm thickness) prepared in a multiwell glass-bottom dish (9-mm diameter and ∼3-mm depth wells; 40-mm diameter dish). After treatment of the collagen gels with human plasma fibronectin, HUVECs were seeded at 6.0 × 104/well and grown to confluence (4–5 days). To avoid evaporation, all incubations were performed in a sterile, humidified chamber. The HUVEC monolayers were preactivated for 4 h with human rTNF-α (10 ng/ml; R&D Systems, Minneapolis, MN, USA). Prior to the experiment, the HUVEC monolayers and fresh monocytes or ex vivo-cultured monocytes were each equilibrated for 60 min at 37°C in assay medium. Experiments were started by addition of a monocyte suspension (50 μl droplet/well, 3–4×104 cells) to the HUVECs (100 μl/well). Multiple fields were chosen randomly so that at least 25 diapedesis events/condition could be monitored. The recording was started 10 min after addition of monocytes.
Time-lapse images were acquired with a 20× phase contrast objective lens on an inverted microscope (Axiovert 200M; Carl Zeiss, Jena, Germany), equipped with a charge-coupled device camera (AxioCam MRm; Carl Zeiss) and controlled by AxioVision software (Version 4.5; Carl Zeiss). The culture dish was maintained at 37°C in a humidified 5% CO2 chamber mounted on the stage. Image acquisition was done sequentially on the defined fields to be monitored in a single time-point with the maximal speed setting of the AxioVision software. Intervals of acquisition were dependent on the number of positions (30–60 s).
Blinded analysis of monocyte migration was performed by two investigators using ImageJ with MTrackJ plug-in software (Erik Meijering, Ph.D., Biomedical Imaging Group Rotterdam of the Erasmus MC-University Medical Center Rotterdam, Netherlands). Monocyte cell bodies were traced on the time-lapse images by moving the MTrackJ pointer when they displaced more than their radius. Individual monocytes were tracked on the apical surfaces up to the last frame or completion of diapedesis, which was defined as the frame where the monocyte cell bodies, retracting between endothelial junctions, disappeared from the apical surface of HUVEC monolayers. Reverse transendothelial migration of freshly isolated monocytes is rarely observed under our experimental conditions.
Statistic analysis
All experimental data are shown as mean ± sem with the indicated numbers of experiments or analyzed video fields. Paired t-tests were used to compare means calculated from three to four different experiments for monocytes prepared from a single donor but subjected to different conditions. Otherwise, unpaired t-tests were applied. All statistical analyses were two-sided using InStat (GraphPad Software, La Jolla, CA, USA), and P values <0.05 were considered significant.
RESULTS AND DISCUSSION
Ex vivo static culture of primary monocytes rapidly impairs their transendothelial migration capability
To assess roles of two ADAM proteases in primary monocyte transendothelial migration, effects of their siRNA knockdown were tested [24]. To optimize siRNA delivery to monocytes and monocyte survival, we used the Amaxa Nucleofector system for transfection, followed by a 16-h static suspension culture, a time period that allowed target protein turnover and maximal knockdown [24]. However, independent of siRNA transfection, monocytes incubated under static suspension culture conditions for 16 h showed impaired transendothelial migration, as monitored by time-lapse video microscopy (<10%; data not shown). Thus, we focused on characterization of approaches necessary to minimize the impairment of monocyte transendothelial migration during ex vivo suspension culture.
As shown in Fig. 1, migration of freshly isolated monocytes was compared with monocytes from the same donor following 16 h culture under static conditions. Fresh monocytes spread and locomote on the endothelium and undergo diapedesis between the endothelial junctions, thereby completing their paracellular transendothelial migration (Supplemental Video 1), as reported previously [23, 25]. In contrast, monocyte spreading on endothelium is defective following 16 h of culture (Supplemental Video 2), and locomotion and diapedesis are rare in the 2-h experiment. Approximately 80% of freshly isolated monocytes complete transendothelial migration, whereas only 10% do after 16 h culture (Fig. 1). Defective monocyte transmigration following ex vivo culture is not a result of dead and/or apoptotic cells, as Annexin V+ cells were removed prior to the migration assay (94±0.9% viability by trypan blue exclusion).
Figure 1. Primary monocytes lose their ability to complete transendothelial migration after 16 h ex vivo incubation at 37°C under static conditions.
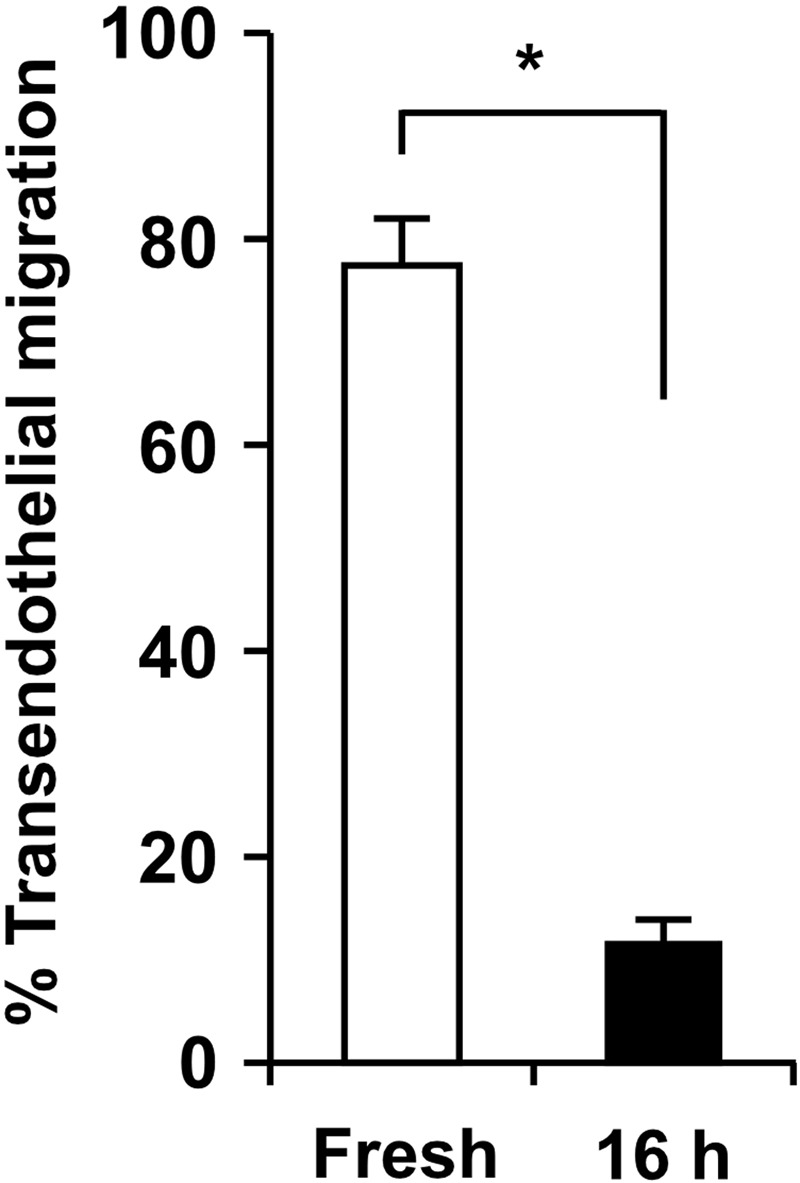
Freshly isolated human monocytes (left column; Supplemental Video 1) or monocytes incubated for 16 h at 37°C in a polypropylene tube (right column; Supplemental Video 2) were added to TNF-α-activated HUVEC monolayers, and monocyte transendothelial migration under static conditions was monitored for 2 h by time-lapse video microscopy. The ex vivo incubation time was selected to allow optimal knockdown of proteins of interest in primary monocytes using siRNA constructs [24]. Individual monocytes were tracked on the apical surfaces up to the last frame or completion of diapedesis, which was defined as the frame where the monocyte cell bodies, retracting between endothelial junctions, disappeared from the apical surface of HUVEC monolayers. A monocyte that underwent diapedesis was evaluated as one that completed transendothelial migration, and the data are expressed as percent of total monocytes in the microscopic field over a 2-h observation period. Means ± sem from independent experiments using three different monocyte donors are shown; *P < 0.01 (paired t-test).
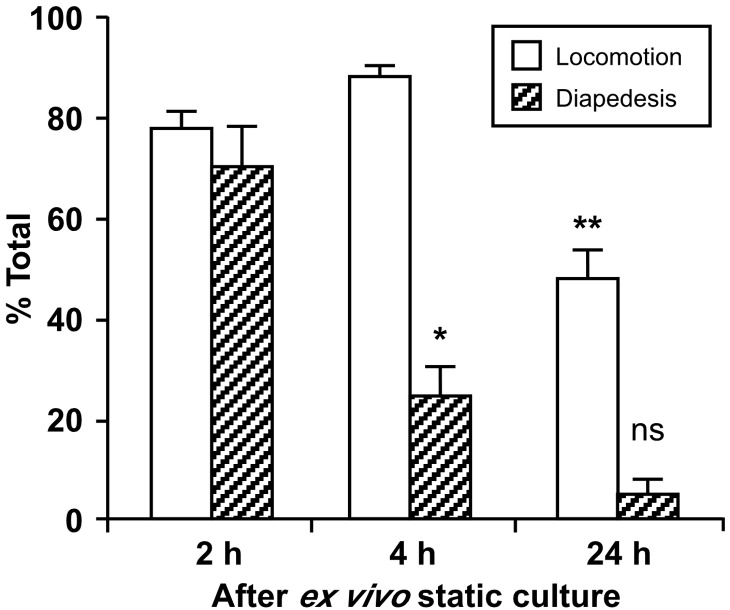
We further analyzed the effect of different incubation times (up to 24 h) on monocyte locomotion and diapedesis (Fig. 2). Ex vivo monocyte culture for 4 h is sufficient to decrease dramatically their frequency of diapedesis (Supplemental Video 4) relative to 2 h culture (Supplemental Video 3), in which transendothelial migration is comparable with freshly isolated monocytes. However, locomotion was not altered after 4 h incubation, although it was reduced after 24 h. Interestingly, defects in spreading (Supplemental Video 2) and persistence of retracting tails during locomotion (Supplemental Video 4) have also been shown for monocytes in which small GTPase signaling molecules are inhibited [26–28]. Thus, small GTPase signaling cascades may be impaired or altered as part of monocyte differentiation. Taken together, static ex vivo culture of primary monocytes first impairs their diapedesis and subsequently their locomotion.
Figure 2. Static incubation of primary monocytes first impairs monocyte diapedesis and subsequently their locomotion.
Human monocytes were incubated ex vivo for 2 h (Supplemental Video 3), 4 h (Supplemental Video 4), and 24 h and then subjected to the transendothelial migration assay, as described in Fig. 1. The numbers of monocytes that undergo locomotion and/or diapedesis are expressed as percent of total monocytes in the microscopic field (>25). Means ± sem of three fields/incubation time are shown; *P < 0.02 relative to diapedesis of monocytes after 2 h incubation; **P < 0.002 relative to locomotion of monocytes after 4 h incubation (unpaired t-test); ns, not significant for comparison with diapedesis after 4 h incubation. Representative data from two independent experiments using monocytes from two different donors are shown.
Application of shear flow during ex vivo culture preserves diapedesis more effectively than static culture conditions
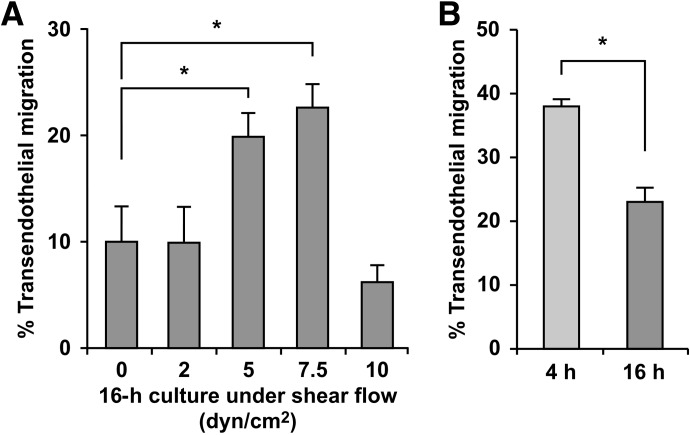
To overcome the rapid decline in transendothelial migration, gentle agitation commonly used for monocyte storage was tested. Shaking for 16 h at 2 dyn/cm2 failed to rescue impaired transendothelial migration (Fig. 3A). This is in contrast to the outcome with gentle agitation of suspended neutrophils (estimated to be 2 dyn/cm2) which retains their chemotactic potential better than static culture [29].
Figure 3. Application of shear flow during ex vivo incubation leads to less impairment of monocyte transendothelial migration than static incubation.
To maintain monocytes under conditions during ex vivo incubation that more closely mimic those in the circulation, shear flow was introduced by shaking the freshly prepared monocytes in polypropylene tubes with a rotary shaker. (A) Monocytes were shaken for 16 h at various speeds to attain fluid flow conditions that are estimated as shear stresses of 2, 5, 7.5, and 10 dyn/cm2, and their frequencies of diapedesis on TNF-activated HUVEC monolayers were evaluated by 2-h time-lapse microscopy, as described in Fig. 1. Means ± sem from experiments using four different monocyte donors for 5 dyn/cm2, and three for other conditions are shown; *P < 0.05 (unpaired t-test). (B) Monocytes were isolated from individual donors and shaken separately at 7.5 dyn/cm2 for 4 and 16 h (fourth column of Fig. 3A), and the frequencies of diapedesis were evaluated immediately after the incubation and then compared. Means ± sem from independent experiments using three different monocyte donors are shown; *P < 0.05 (paired t-test).
To model conditions in the circulation more closely, higher fluid shear was introduced during 16 h ex vivo culture (Fig. 3A). With the use of ex vivo shaking conditions that approximate 5 and 7.5 dyn/cm2, transendothelial migration frequencies are 2.3-fold higher compared with static culture and gentle shaking conditions (2 dyn/cm2). The higher fluid shear would be predicted to lead to less contact based on a theoretical model of contact dynamics between leukocyte-mimicking particles and solid surfaces under flow conditions. This model predicts that the contact force and duration under the optimal shear stress (7.5 dyn/cm2) could be twofold lower and tenfold shorter, respectively, than those under 2 dyn/cm2 [30]. Thus, decreased contact may contribute to the beneficial effects of fluid shear. A shorter culture period under shear flow conditions (4 h at 7.5 dyn/cm2) shows even better preservation of transendothelial migration capability (Fig. 3B). Our pilot experiments also showed that impaired transendothelial migration was rescued more effectively in conical tubes compared with petri dishes, suggesting that additional variables, such as fluid surface area, exposed to air and evaporation, may negatively impact the beneficial effects of fluid shear. However, together, these data demonstrate that culture under conditions of shear flow can protect monocyte transendothelial migratory capacity significantly.
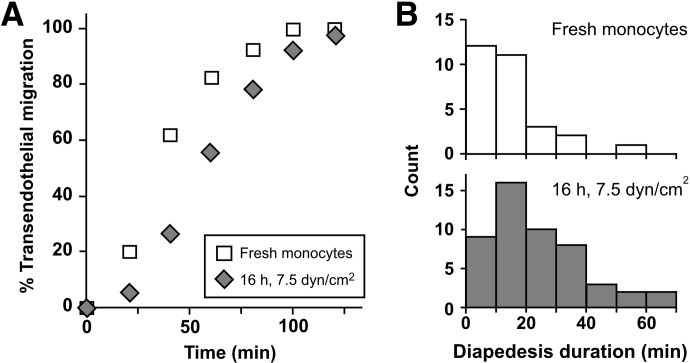
Desptie improved migratory capacity using shear flow culture conditions, completion of transendothelial migration is delayed after 16 h incubation with shear compared with freshly isolated monocytes [Fig. 4A; 32.1±2.6 vs. 81.8±10.4 min; n=4 and 5 experiments with monocytes prepared from different donors, respectively; P<0.01 (unpaired t-test with Welch correction)]. Elapsed time to complete diapedesis is also prolonged relative to freshly isolated monocytes [Fig. 4B; 13.2±1.5 vs. 43.8±8.8 min; n=4 and 5 experiments, respectively; P<0.05 (unpaired t-test with Welch correction)]. However, these analyses establish importantly that monocytes cultured for 16 h under 7.5 dyn/cm2 shear flow retain their ability to undergo diapedesis, in contrast to a loss of this capacity for monocytes maintained under static conditions. Our recent application of this approach enabled us to determine the relative contribution of the transmembrane protease ADAM17 to the different steps in monocyte transendothelial cell migration [24].
Figure 4. Analysis of diapedesis further establishes that monocytes cultured under shear flow conditions retain their ability to undergo diapedesis.
Monocytes freshly prepared or incubated under conditions of 7.5 dyn/cm2 for 16 h were analyzed by time-lapse microscopy of transendothelial migration. Individual monocytes that successfully underwent diapedesis were analyzed. Representative data from one of at least four experiments with different monocyte donors are shown (n=29 and 54 monocytes for monocytes freshly isolated and incubated with shear flow, respectively). (A) Elapsed times for individual monocytes to complete their transendothelial migration were defined as in Fig. 1. Means ± sem are 29.9 ± 3.8 and 50.7 ± 4.3 min, respectively; P < 0.005 (unpaired t-test). (B) Duration times for diapedesis were calculated as an interval between the frames where monocytes arrived at the place of diapedesis and where monocytes completed diapedesis as defined in Fig. 1. Means ± sem are 14.4 ± 2.0 and 25.0 ± 2.6 min, respectively; P < 0.01 (unpaired t-test). Note that although diapedesis is delayed following 16 h incubation under flow conditions, the ability of monocytes to undergo diapedesis is not lost, as observed under static incubation conditions.
Implications of shear flow maintenance of monocyte functionality
In the absence of shear flow, our data demonstrate that monocyte transendothelial migration is impaired even after short ex vivo incubations. Static incubation of purified human monocytes has been used for over 30 years to promote differentiation of monocytes to macrophages [10, 11]. After even 1 day of suspension culture, mean cell volume increases, and peroxidase enzyme activity decreases, whereas 5′-nucleotidase activity increases—changes that continue over the 5–7 days of culture and lead to a uniform shift to a macrophage morphology and a twofold increase in phagocytic capacity. Therefore, the impairment of transendothelial migration that we observe under static conditions may be part of the monocyte differentiation process and/or result from absence of shear flow conditions normally encountered by circulating monocytes. Our studies have revealed the importance of shear flow for maintenance of monocyte transendothelial migratory capacity, and thus, possible mechanisms responsible for the impairment can be distinguished in future investigations.
The benefits of shear flow conditions during ex vivo incubation of primary human monocytes uncovered by our analyses also have implications for monocyte therapeutic applications [13, 16, 31]. Ex vivo incubations are implicit in these procedures, including the interval between monocyte collection and transplantation [31], and monocyte transduction to use them as cellular vehicle [13–15, 32]. Our data establish that the time and conditions of ex vivo incubation determine the extent of impairment of monocyte transendothelial migration. Additionally, our studies indicate that minimizing ex vivo manipulation time could improve recruitment of monocytes to their targets. Most significantly, mimicking physiological flow to diminish impairment of monocyte diapedsis has the potential to optimize monocyte-based therapies further and enhance their promise as therapeutic strategies.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported, in part, by a research grant from the U.S. National Institutes of Health (R01 HL067267).
We thank Li-Chuan Huang, Cindy Chang, Takashi Osada, and Masato Kanazawa for excellent technical assistance. We also acknowledge Stephen D. Hauschka, Phillip W. L. Tai, and Robert E. Welikson from the University of Washington School of Medicine, Department of Biochemistry, for their assistance with the in vitro transendothelial migration assay using their microscope and monitoring system. Francis W. Luscinskas, John M. Harlan, Carole L. Wilson, and Jingjing Tang also provided helpful comments and discussion.
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
- ADAM17
- a disintegrin and metalloproteinase domain 17
- GTPase
- guanosine triphosphate hydrolase
- siRNA
- small interfering RNA
AUTHORSHIP
Y.T. performed, designed, and analyzed experiments. J.M.F. helped analyze experiments and contributed to data analysis. E.W.R. initiated the study and designed and supervised the research project. Y.T. and E.W.R. contributed to data analysis and interpretation and wrote the paper.
DISCLOSURES
The authors declare no conflicts of interest.
REFERENCES
- 1. Ley K., Laudanna C., Cybulsky M. I., Nourshargh S. (2007) Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat. Rev. Immunol. 7, 678–689 [DOI] [PubMed] [Google Scholar]
- 2. Thomas-Ecker S., Lindecke A., Hatzmann W., Kaltschmidt C., Zanker K. S., Dittmar T. (2007) Alteration in the gene expression pattern of primary monocytes after adhesion to endothelial cells. Proc. Natl. Acad. Sci. USA 104, 5539–5544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Williams M. R., Sakurai Y., Zughaier S. M., Eskin S. G., McIntire L. V. (2009) Transmigration across activated endothelium induces transcriptional changes, inhibits apoptosis, and decreases antimicrobial protein expression in human monocytes. J. Leukoc. Biol. 86, 1331–1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Randolph G. J., Beaulieu S., Lebecque S., Steinman R. M., Muller W. A. (1998) Differentiation of monocytes into dendritic cells in a model of transendothelial trafficking. Science 282, 480–483 [DOI] [PubMed] [Google Scholar]
- 5. Muller W. A., Randolph G. J. (1999) Migration of leukocytes across endothelium and beyond: molecules involved in the transmigration and fate of monocytes. J. Leukoc. Biol. 66, 698–704 [DOI] [PubMed] [Google Scholar]
- 6. Westhorpe C. L., Dufour E. M., Maisa A., Jaworowski A., Crowe S. M., Muller W. A. (2012) Endothelial cell activation promotes foam cell formation by monocytes following transendothelial migration in an in vitro model. Exp. Mol. Pathol. 93, 220–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vaddi K., Newton R. C. (1994) Regulation of monocyte integrin expression by β-family chemokines. J. Immunol. 153, 4721–4732 [PubMed] [Google Scholar]
- 8. Kohro T., Tanaka T., Murakami T., Wada Y., Aburatani H., Hamakubo T., Kodama T. (2004) A comparison of differences in the gene expression profiles of phorbol 12-myristate 13-acetate differentiated THP-1 cells and human monocyte-derived macrophage. J. Atheroscler. Thromb. 11, 88–97 [DOI] [PubMed] [Google Scholar]
- 9. Bhattacharjee A., Mishra R. S., Feldman G. M., Cathcart M. K. (2008) In vivo validation of signaling pathways regulating human monocyte chemotaxis. J. Immunol. Methods 330, 86–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stevenson H. C., Katz P., Wright D. G., Contreras T. J., Jemionek J. F., Hartwig V. M., Flor W. J., Fauci A. S. (1981) Human blood monocytes: characterization of negatively selected human monocytes and their suspension cell culture derivatives. Scand. J. Immunol. 14, 243–256 [DOI] [PubMed] [Google Scholar]
- 11. Van der Meer J. W., van de Gevel J. S., Blusse van Oud Alblas A., Kramps J. A., van Zwet T. L., Leijh P. C., van Furth R. (1982) Characteristics of human monocytes cultured in the Teflon culture bag. Immunology 47, 617–625 [PMC free article] [PubMed] [Google Scholar]
- 12. Miller P. J., Stevenson H. C. (1986) Suspension culture of human monocytes. Methods Enzymol. 132, 243–250 [DOI] [PubMed] [Google Scholar]
- 13. Herold J., Tillmanns H., Xing Z., Strasser R. H., Braun-Dullaeus R. C. (2006) Isolation and transduction of monocytes: promising vehicles for therapeutic arteriogenesis. Langenbecks Arch. Surg. 391, 72–82 [DOI] [PubMed] [Google Scholar]
- 14. Lebson L., Nash K., Kamath S., Herber D., Carty N., Lee D. C., Li Q., Szekeres K., Jinwal U., Koren J., Dickey C. A., Gottschall P. E., Morgan D., Gordon M. N. (2010) Trafficking CD11b-positive blood cells deliver therapeutic genes to the brain of amyloid-depositing transgenic mice. J. Neurosci. 30, 9651–9658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. De Palma M., Mazzieri R., Politi L. S., Pucci F., Zonari E., Sitia G., Mazzoleni S., Moi D., Venneri M. A., Indraccolo S., Falini A., Guidotti L. G., Galli R., Naldini L. (2008) Tumor-targeted interferon-α delivery by Tie2-expressing monocytes inhibits tumor growth and metastasis. Cancer Cell. 14, 299–311 [DOI] [PubMed] [Google Scholar]
- 16. Sanberg P. R., Park D. H., Kuzmin-Nichols N., Cruz E., Hossne N. A., Jr., Buffolo E., Willing A. E. (2010) Monocyte transplantation for neural and cardiovascular ischemia repair. J. Cell. Mol. Med. 14, 553–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ritter M. R., Banin E., Moreno S. K., Aguilar E., Dorrell M. I., Friedlander M. (2006) Myeloid progenitors differentiate into microglia and promote vascular repair in a model of ischemic retinopathy. J. Clin. Invest. 116, 3266–3276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Silvestre J-S., Mallat Z., Tedgui A., Lévy B. I. (2008) Post-ischaemic neovascularization and inflammation. Cardiovasc. Res. 78, 242–249 [DOI] [PubMed] [Google Scholar]
- 19. Alcaide P., Lim Y. C., Luscinskas F. W., Fresno M. (2010) Mucin AgC10 from Trypanosoma cruzi interferes with L-selectin-mediated monocyte adhesion. Infect. Immun. 78, 1260–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ley K., Lundgren E., Berger E., Arfors K. (1989) Shear-dependent inhibition of granulocyte adhesion to cultured endothelium by dextran sulfate. Blood 73, 1324–1330 [PubMed] [Google Scholar]
- 21. Kraiss L. W., Weyrich A. S., Alto N. M., Dixon D. A., Ennis T. M., Modur V., McIntyre T. M., Prescott S. M., Zimmerman G. A. (2000) Fluid flow activates a regulator of translation, p70/p85 S6 kinase, in human endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 278, H1537–H1544 [DOI] [PubMed] [Google Scholar]
- 22. Folkman J., Moscona A. (1978) Role of cell shape in growth control. Nature 273, 345–349 [DOI] [PubMed] [Google Scholar]
- 23. Muller W. A., Weigl S. A. (1992) Monocyte-selective transendothelial migration: dissection of the binding and transmigration phases by an in vitro assay. J. Exp. Med. 176, 819–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tsubota Y., Frey J. M., Tai P. W., Welikson R. E., Raines E. W. (2013) Monocyte ADAM17 promotes diapedesis during transendothelial migration: identification of steps and substrates targeted by metalloproteinases. J. Immunol. 190, 4236–4244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schenkel A. R., Mamdouh Z., Muller W. A. (2004) Locomotion of monocytes on endothelium is a critical step during extravasation. Nat. Immunol. 5, 393–400 [DOI] [PubMed] [Google Scholar]
- 26. Aepfelbacher M., Essler M., Huber E., Czech A., Weber P. C. (1996) Rho is a negative regulator of human monocyte spreading. J. Immunol. 157, 5070–5075 [PubMed] [Google Scholar]
- 27. Watson J. M., Harding T. W., Golubovskaya V., Morris J. S., Hunter D., Li X., Haskill J. S., Earp H. S. (2001) Inhibition of the calcium-dependent tyrosine kinase (CADTK) blocks monocyte spreading and motility. J. Biol. Chem. 276, 3536–3542 [DOI] [PubMed] [Google Scholar]
- 28. Worthylake R. A., Lemoine S., Watson J. M., Burridge K. (2001) RhoA is required for monocyte tail retraction during transendothelial migration. J. Cell Biol. 154, 147–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Miyamoto M., Sasakawa S. (1987) Studies on granulocyte preservation. III. Effect of agitation on granulocyte concentrates. Transfusion (Paris) 27, 165–166 [DOI] [PubMed] [Google Scholar]
- 30. Zhao Y., Chien S., Weinbaum S. (2001) Dynamic contact forces on leukocyte microvilli and their penetration of the endothelial glycocalyx. Biophys. J. 80, 1124–1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Herold J., Pipp F., Fernandez B., Xing Z., Heil M., Tillmanns H., Braun-Dullaeus R. C. (2004) Transplantation of monocytes: a novel strategy for in vivo augmentation of collateral vessel growth. Hum. Gene Ther. 15, 1–12 [DOI] [PubMed] [Google Scholar]
- 32. Mayne G. C., Borowicz R. A., Greeneklee K. V., Finlay-Jones J. J., Williams K. A., Hart P. H. (2003) Centrifugation facilitates transduction of green fluorescent protein in human monocytes and macrophages by adenovirus at low multiplicity of infection. J. Immunol. Methods 278, 45–56 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.