MMP28 promotes alternatively activated (M2) macrophage function, and regulates the fibrotic response in the lung.
Keywords: M1, inflammation, monocytes, epilysin
Abstract
Members of the MMP family function in various processes of innate immunity, particularly in controlling important steps in leukocyte trafficking and activation. MMP28 (epilysin) is a member of this family of proteinases, and we have found that MMP28 is expressed by macrophages and regulates their recruitment to the lung. We hypothesized that MMP28 regulates other key macrophage responses, such as macrophage polarization. Furthermore, we hypothesized that these MMP28-dependent changes in macrophage polarization would alter fibrotic responses in the lung. We examined the gene expression changes in WT and Mmp28−/− BMDMs, stimulated with LPS or IL-4/IL-13 to promote M1 and M2 cells, respectively. We also collected macrophages from the lungs of Pseudomonas aeruginosa-exposed WT and Mmp28−/− mice to evaluate changes in macrophage polarization. Lastly, we evaluated the macrophage polarization phenotypes during bleomycin-induced pulmonary fibrosis in WT and Mmp28−/− mice and assessed mice for differences in weight loss and total collagen levels. We found that MMP28 dampens proinflammatory macrophage function and promots M2 programming. In both in vivo models, we found deficits in M2 polarization in Mmp28−/− mice. In bleomycin-induced lung injury, these changes were associated with reduced fibrosis. MMP28 is an important regulator of macrophage polarization, promoting M2 function. Loss of MMP28 results in reduced M2 polarization and protection from bleomycin-induced fibrosis. These findings highlight a novel role for MMP28 in macrophage biology and pulmonary disease.
Introduction
Emerging evidence indicates that several members of the MMP family function in various processes of innate immunity, particularly in controlling important steps in leukocyte trafficking and activation [1–5]. Some mechanisms whereby MMPs affect leukocyte behavior include proteolytic processing of chemokines and release of chemotactic fragments or accessory proteins [1]. For example, matrilysin (MMP7) controls neutrophil influx into the alveolar space and activation by shedding syndecan-1, a transmembrane proteoglycan, complexed with the CXC chemokine keratinocyte-derived chemokine [2].
MMP28 is the last member of the mammalian MMP family, and it contains the prototypic domains of a MMP. It also contains a furin activation sequence; hence, it is activated within the secretion pathway [6–8]. In human and mouse tissues, (mRNA) Mmp28 is expressed at high levels in lung, heart, gastrointestinal tract, and epidermis, and the constitutive expression of this Mmp suggests a role in homeostasis [8–10]. Despite the broad, constitutive expression of Mmp28, Mmp28−/− mice have no overt phenotype when unchallenged, with the exception of an increase in circulating inflammatory cytokines associated with aging [11, 12].
As with other Mmp−/− mice, phenotypes related to immune regulation emerge with challenge. We reported that exposure to P. aeruginosa and LPS induces expression of Mmp28 by cultured BMDMs and that macrophage recruitment is accelerated into the lungs of Mmp28−/− mice in response to bacterial infection [12]. In vitro chemotaxis assays demonstrated that Mmp28−/− macrophages have an intrinsically faster ability to migrate than WT cells [12], indicating that Mmp28 functions in a cell-autonomous mechanism to moderate macrophage chemotaxis. Together, our previous findings suggest that Mmp28 functions to dampen the proinflammatory activity of infiltrating macrophages.
Macrophages have been classified into two groups: M1 and M2, although there is a continuum of macrophage polarization beyond these simplified, discrete, in vitro-based classifications. The M1 phenotype, induced by LPS, is characterized by production of high levels of proinflammatory factors, including IL-1β, IL-12, TNF-α, and iNOS [13, 14]. The M2 phenotype can be induced by TH2 cytokines, IL-4 and IL-13, and is characterized by production of IL-10, ARG-1, FIZZ-1, and CCL17 [15, 16]. In pneumonia, effective response to infection requires a balance of both polarized responses, with M1 induction occurring early (to help clear infection) and M2 responses occurring during ALI resolution [17]. In other models of muscle and liver injury, M2 cells are also important for resolution [18, 19]. Furthermore, M2 cells are also thought to be involved in regulating fibrotic responses, both within the lung and other tissues [20–23]. Hence, factors that regulate macrophage recruitment and polarization affect a broad spectrum of diseases and tissues.
In this study, we identified specific monocyte and macrophage subpopulations that express Mmp28 and factors that regulate Mmp28 expression. In addition, we evaluated the consequences of Mmp28−/− on macrophage inflammatory responses in vitro and in vivo. Our findings highlight a novel regulatory role of Mmp28 in promoting M2 function and dampening macrophage proinflammatory (M1) function. We also describe in vivo changes associated with Mmp28-dependent regulation of macrophage polarization in regulating fibrotic responses in the lung.
MATERIALS AND METHODS
Animals
WT C57BL/6 and Mmp28−/− mice [12] were used for these experiments. Age- and gender-matched mice between the ages of 8 and 12 weeks were used for all experiments. All animal protocols were approved by the University of Washington Office of Animal Welfare.
Exposure models
P. aeruginosa strain PAK, a nonmucoid, flagellated strain, obtained originally from Dr. Stephen Lory (Harvard University, Cambridge, MA, USA), was grown in LB broth at 37°C, collected, counted during stationary phase, and suspended in 20 ml PBS. Mice were received 1 × 107 bacteria in 50 μl PBS via oropharyngeal aspiration. To isolate pulmonary macrophages, the lungs were perfused with 10 ml cold PBS, serially lavaged (3×) with 1 ml PBS containing 2 mM EDTA, and homogenized as described [12]. The lavage and lung homogenates were processed for cell sorting, as described [17].
For bleomycin experiments, age- and gender-matched mice received between 0.0017 and 0.0025 U/g bleomycin (Hospira, Lake Forest, IL, USA) in a total volume of 50 μl sterile PBS. Lung tissue was harvested at various times (Days 3, 7, 14, 21, and 28). For bleomycin instillations, mice were sedated using isoflurane and intubated using an angiocatheter. Respiratory variations of fluid in a 1-ml syringe confirmed position in the airway prior to instillation of bleomycin.
Collagen was quantified using the Picrosirius Red Sircol assay (Biocolor, Carrickfergus, County Antrim, UK), per the manufacturer's protocol. Briefly, the left lung was removed and homogenized in 0.5 M acetic acid solution. A total of 200 μl of the acid homogenate was digested by adding 1 ml pepsin solution (2 mg/ml in 0.5 M acetic acid) with continuous shaking. After digestion, samples were centrifuged, and 100 μl of the supernatant containing soluble collagen was incubated with 1 ml Sircol dye reagent for 30 min at room temperature. Samples were centrifuged, and the precipitated pellet was resuspended in 1 ml Sircol alkali reagent. Collagen concentration was then determined by spectrophotometric absorbance at 540 nm as compared with a standard curve. In a separate experiment, formalin-fixed, paraffin-embedded lung tissue sections were stained with Sirius Red solution (Sigma-Aldrich, St. Louis, MO, USA), dissolved in picric acid (Sigma-Aldrich), and counterstained using Fast Green (Sigma-Aldrich). Collagen fibers were visualized using a polarizing microscope.
Macrophage cultures
BMDMs were derived from WT and Mmp28−/− mice, as described [12]. After 6–7 days of differentiation in M-CSF-containing medium, the macrophages are referred to as M0 cells.
Isolation of monocytes and pulmonary macrophages
Monocytes were collected from pooled blood obtained via cardiac puncture and anticoagulated with EDTA. Blood was diluted 1:5 in PBS and layered on top of Ficoll-Paque (GE Healthcare, Piscataway, NJ, USA) and centrifuged per the manufacturer's protocol. The mononuclear layer was collected, washed with PBS, and labeled with anti-mouse CD11b-PE Cy7 and Ly6c-Pacific Blue antibodies (BioLegend, San Diego, CA, USA). Monocytes were identified by low forward- and side-scatter and CD11b expression. Ly6clow and Ly6chigh cells were sorted by the BD FACSAria II flow cytometer. Identification and sorting of pulmonary macrophage subpopulations were performed as described using whole lung from three pooled mice/genotype [24].
BMDM activation
M0 BMDMs were transferred to 12-well plates at a density of 1 × 106 cells/well. The cells were stimulated with 100 ng/ml of Escherichia coli LPS strain O111:B4 for 24 h, 10 ng/ml each IL-4 and IL-13 for 48 h, Chlamydia pneumoniae ×24 h, 100 ng/ml IFN-γ for 24 h, or 5 μg/ml Poly(I:C) for 24 h in M-CSF-containing medium. Unstimulated macrophages served as a control.
qRT-PCR
Total RNA from cells was isolated using the RNeasy Mini kit (Qiagen, Germantown, MD, USA). The quantity and quality of RNA were determined using a NanoDrop spectrophotometer (NanoDrop, Wilmington, DE, USA). Primers and TaqMan probes (FAM dye-labeled) for Mmp28, Il6, Tnfα, Arg1, Il10, and hypoxanthine-guanine phosphoribosyltransferase were added to cDNA, synthesized from total RNA with a high-capacity cDNA archive kit (Applied Biosystems, Foster City, CA, USA). Product amplification was measured with an ABI HT7900 Fast real-time PCR system. The Ct was obtained from duplicate samples and averaged. The ΔCt was the difference between the average Ct for the specific cDNAs. The ΔΔCt was the average ΔCt at a given time-point minus the average ΔCt of Day 0 (uninfected) samples (for sorted lung macrophages) or BMDMs at Day 7 of culture (for all other macrophage/monocyte samples). The data are expressed as relative quantification calculated as 2−ΔΔCt.
Caspase 3/7 assay
BMDMs from WT and Mmp28−/− mice were plated in 96-well plates (3×104 cells/well). At Time 0, cells were treated with LPS, as described above, to polarize the cells toward M1. Control cells received media + PBS (M0). At 48 h, an equal volume of lysis buffer plus caspase 3/7 substrate, (benzyloxycarbonyl-aspartyl-glutamyl-valyl-aspartyl)2-R110, was added to each well per the manufacturer's instructions (Cell Technology, Mountain View, CA, USA). The caspase 3/7 activity was determined using excitation at 488 nm and emission at 530 nm using a Synergy 4 Hybrid Multi-Mode Microplate Reader (BioTek, Winooski, VT, USA). Results are reported as relative fluorescence units.
Microarray experiments
The integrity of total RNA samples was confirmed using an Agilent 2100 bioanalyzer (Agilent Technologies, Santa Clara, CA, USA). RNA was reverse-transcribed to cDNA, labeled, and hybridized to the MouseRef-6 v2.0 Expression BeadChip microarrays (Illumina, San Diego, CA, USA), according to the manufacturer's recommended protocols. Each BeadChip platform enables the interrogation of six samples in parallel and is comprised of 25,600 well-annotated RefSeq transcripts and over 19,100 unique genes. Hybridization, scanning and data acquisition were performed at the Genomics Shared Resource at the Fred Hutchinson Cancer Research Center. Background adjustment and quantile normalization across all experiments were performed using BeadStudio software (Illumina).
Microarray data analysis
Differential gene expression and functional analysis.
Normalized microarray data, meeting detection threshold, were used to identify differentially expressed genes using a Bayesian implementation of the parametric t-test [25], coupled with FDR analysis, based on Benjamini-Hochberg-adjusted P <0.01. We applied pair-wise comparisons using these tests to identify significant gene-expression changes between M0 and M1 genotypes, i.e., Mmp28−/− M0 versus WT M0 and Mmp28−/− M1 versus WT M1. For each comparison, differentially expressed genes (adjusted P<0.01) underwent functional analysis using WebGestalt [26]. Enrichment of GO categories was determined based on the hypergeometric test and corrected for multiple hypothesis testing using Benjamini-Hochberg's method (adjusted P<0.01 for significance cutoff). The GO database was used to depict the hierarchical relationships among over-represented, functional categories [27].
Network analysis.
Differentially expressed genes in M1 BMDMs (Mmp28−/− M1 versus WT M1) were used as seeds to create a gene product network derived from curated interaction databases, including Ingenuity and STRING [28, 29].
Statistics
Results are expressed as means ± sem. Statistical significance was determined using Student's t-test. Differences were considered significant if P < 0.05.
RESULTS
Mmp28 is highly expressed by BM cells and remains expressed during macrophage differentiation
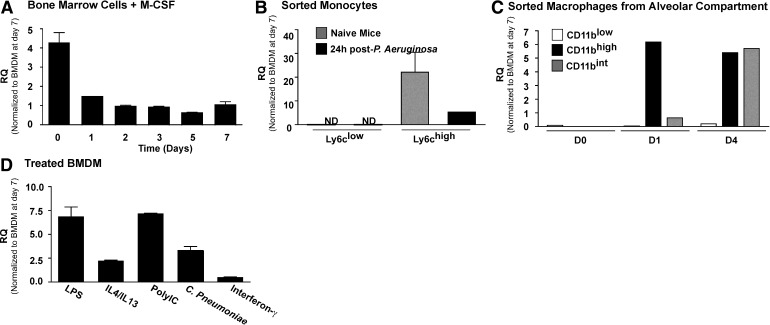
We sought to identify the temporal expression of Mmp28 during differentiation of BM cells into macrophages. We found that freshly isolated BM cells (Day 0) had the highest expression level of Mmp28 and that the expression of Mmp28 decreased approximately fourfold as the cells differentiated into macrophages (Fig. 1A). The drop in expression levels may represent a contribution from other cell types in the Day 0 cultures that do not survive the differentiation protocol or changes associated with adhesion/differentiation. Regardless of this early differential expression, we found that at all time-points, Mmp28 remained expressed (Ct=29.8±0.1), suggesting a role for this metalloproteinase in macrophage biology.
Figure 1. Expression of Mmp28 in myeloid cells.
(A) Expression of Mmp28 in BM cells at time of harvest and during differentiation to macrophages in the presence of M-CSF relative to expression at Day 7 (set to one). Mmp28 expression decreased during culture, with a slight increase at Day 7. Overall, expression remained significant at all times (n=3/genotype/time-point). RQ (Fold increase). (B) Expression of Mmp28 by monocyte populations in naive (gray bars) or infected (black bars) mice reveals selective expression of Mmp28 by Ly6chigh monocytes, with reduced expression after infection (n=3 mice/genotype). (C) Expression of Mmp28 in macrophage subpopulations isolated from the alveolar space reveals greater expression of Mmp28 by recruited macrophages (CD11bint, CD11bhigh) compared with resident cells (CD11blow; n=3 mice/genotype). (D) Expression of Mmp28 in stimulated BMDM (relative to Day 7, untreated BMDM). LPS, IL-4/13, Poly(I:C), and C. pneumoniae all increased Mmp28 gene expression, with the exception of IFN-γ, which did not alter Mmp28 expression significantly (n=3–6/genotype/condition).
Mmp28 is expressed by monocyte and macrophage subsets
We sought to determine if Mmp28 is expressed selectively by distinct monocyte and macrophage subsets isolated from mice. As in humans, two populations of monocytes have been described in the murine circulation, and each population has a specific repertoire of chemokine receptors [25]. A population of CD11b+Gr1−CCR2−CX3CR1high (also known as Ly6clow) cells enters tissues constitutively via the activity of the chemokine receptor CX3CR1. Another population of inflammatory monocytes, characterized by expression of CD11b+Gr-1highCCR2+CX3CR1int (also known as Ly6chigh), enters inflamed peripheral and lymphoid tissues via the activity of CCR2 [25].
We identified these populations in blood from WT mice and sorted them for RNA collection. We found that Mmp28 expression was restricted to the Ly6chigh monocyte population and was not detected in Ly6clow cells (Fig. 1B). Furthermore, we found that in mice with P. aeruginosa pneumonia, Mmp28 expression in circulating Ly6chigh monocytes was reduced (Fig. 1B).
Our finding that Mmp28 expression is restricted to Ly6chigh monocytes was confirmed with expression data from macrophage subsets in infected lungs. During bacterial pneumonia, we identified three alveolar and interstitial macrophage populations using cell-surface markers for CD11b, F4/80, MHCII, CD11c, and CD45, identified by flow cytometry, as published [24]. We sorted these alveolar and interstitial macrophage populations from infected WT and Mmp28−/− mice for analysis of Mmp28 expression and found that alveolar macrophages (CD11blowCD45high) do not express Mmp28. Rather, Mmp28 was expressed by the recruited populations of macrophages (CD11bintCD45int and CD11bhighCD45high cells; Fig. 1C). These findings indicate that Mmp28 is expressed selectively by, and hence, functions in a subset of, recruited monocytes/macrophages. Although our results suggest that monocytes express less Mmp28 during infection, recruited cells in the lung continue to express Mmp28, likely reflecting the complexity of its regulation and function on recruitment of myeloid cells into tissue and their inflammatory responses within these tissues.
Mmp28 is differentially regulated by cytokines and TLR agonists
We hypothesized that Mmp28 may regulate the inflammatory response of macrophages and first sought to determine the effects of TLR (toll-like receptor) agonists and cytokines to regulate Mmp28 gene expression. We established previously that E. coli LPS and P. aeruginosa up-regulate Mmp28 expression by BMDMs [12]. In addition to these stimuli, we examined the response to proinflammatory stimuli, such as the TLR3 agonist, Poly(I:C), C. pneumonia, and IFN-γ. We also examined its expression in M2 cells stimulated with IL-4 and IL-13. We found that all stimuli, except for IFN-γ, up-regulated Mmp28, suggesting that MMP28 may be broadly involved in the inflammatory function of macrophages (Fig. 1D). However, given its greater expression in M1 versus M2 cells, we hypothesized that MMP28 may have important regulatory roles in controlling M1 polarization.
Transcriptional profiling of Mmp28−/− and WT M1 BMDMs reveals enrichment of inflammatory, stress, and apoptotic pathways
Given our results that Mmp28−/− is up-regulated in M1 cells, we examined the effect of Mmp28−/− on global gene transcription in unstimulated BMDMs at Day 7 in culture (M0) and after 24 h of E. coli LPS exposure (M1), using Illumina BeadChip microarrays. We applied pair-wise comparisons to identify significant changes in gene expression between the two genotypes under M0 and M1 conditions (i.e., Mmp28−/− M0 vs. WT M0 and Mmp28−/− M1 vs. WT M1). For each comparison, differentially expressed genes (FDR<0.01) underwent functional enrichment and network analysis.
Gene-expression changes between WT and Mmp28−/− unstimulated (M0) cells showed differences in genes mapping to processes involved in catalytic activity, ribonucleoprotein, and proteasome complexes, but there was no enrichment of inflammatory or immune-related pathways (Supplemental Table 1). Importantly, these results suggest that Mmp28 does not play a significant role in macrophage differentiation. Despite a scarcity of over-represented processes, there were significant differences in gene expression between M0 WT and Mmp28−/− cells. For example, CCR5 was increased approximately twofold in Mmp28−/− M0 cells, which may be relevant to the enhanced chemotactic responses of these cells to BAL fluid, recovered from infected mice, as reported previously [12].
In contrast to the relative lack of functional enrichment observed in M0 cells, LPS-treated WT and Mmp28−/− cells (M1) showed profound differences in the expression of genes involved in immunity, inflammation, and cytokine production, showing greater enrichment of proinflammatory genes in the Mmp28−/− cells (Fig. 2A, Supplemental Table 2, and Supplemental Fig. 1). We explored putative relationships among differentially expressed genes in Mmp28−/− M1 cells using network analysis (Fig. 2B). This gene product interactome revealed a complex network orchestrated by a number of densely-connected hubs that included proinflammatory mediators (Il6, Tnfa, Il1b), signaling molecules (Traf1, Nfkb1a), transcription factors (Egr1, Junb), and regulators of apoptosis (Gadd45a, Gadd45b). This analysis captures the widespread molecular consequences of Mmp28 deletion in M1-polarized macrophages and recapitulates many of the phenotypic and functional characteristics observed in these cells. Differentially up-regulated genes in Mmp28−/− M1 cells versus WT M1 cells included many proinflammatory mediators, such as Il6, Tnfa, Il1b. The mRNA and/or protein levels of several of these genes, including Il6, Cxcl1, Cxcl2, Tnfa, Il1b, and Mcp1, were confirmed using qRT-PCR, ELISA, and/or Luminex (Fig. 3A and B).
Figure 2. Functional analysis and gene network of Mmp28-dependent genes.
(A) A functional analysis of Mmp28-dependent genes in LPS-treated (M1) macrophages. Processes are color-coded, based on their enrichment significance. Deletion of Mmp28 enhances the inflammatory, stress, and apoptotic responses of M1. (B) Increased inflammatory gene network in LPS-stimulated Mmp28−/− macrophages compared with WT cells (n=3/genotype/condition). Several densely connected, proinflammatory network nodes that differ between LPS-stimulated Mmp28−/− and WT BMDMs have been labeled (red=increased expression in Mmp28−/− cells; green=decreased expression in Mmp28−/− cells). Genes are organized by known localization of the protein: black background (secreted); yellow (membrane); light gray (cytoplasmic); dark gray (nuclear). INHBA, Inhibin-βA; IER3, immediate early response 3; MAP3K4, mitogen-activated protein kinase kinase kinase 4, MEK4, mitogen-activated protein kinase kinase 4; IFN-β1; OSM, oncostatin M.
Figure 3. Validation of gene expression arrays demonstrates a role for Mmp28 in limiting M1 and promoting M2 phenotypes.
(A) Fold increase in inflammatory gene expression for Il6, Cxcl1, and Cxcl2 in WT (black bars) and Mmp28−/− (white bars) BMDMs at 24 h post-LPS stimulation compared with untreated, M0 controls (n=3–6/genotype/condition). (B) Protein levels of secreted inflammatory mediators, TNF-α, IL-1β, CXCL2, and MCP-1 in WT and Mmp28−/− M1 cells (n=6/genotype). (C) Caspase 3/7 activity in WT and Mmp28−/− M0 and M1 cells, demonstrating increased activity in Mmp28−/− M1 cells at 48 h post-LPS (n=5–10/genotype). (D) Fold increase in gene expression of M2 markers, Fizz1, Arg1, and Il10 in WT and Mmp28−/− at 48 h post-treatment with IL-4 and IL-13 (n=6/genotype). *P < 0.05.
Other highly significant, over-represented, functional categories between M1 Mmp28−/− and WT cells included “response to stress” and “apoptosis” (Fig. 2A). Gadd45a and Gadd45b were representative genes mapping to “programmed cell death” (Fig. 2B). We further assessed the apoptosis module by measuring caspase 3/7 activity in M0 and M1 cells. This analysis showed a 35.6% increase in activity in Mmp28−/− M1 cells compared with WT M1 cells, confirming that Mmp28 inhibits macrophage apoptosis, a role we have reported previously in epithelial cells (Fig. 3C) [30]. These results imply that Mmp28 orchestrates transcriptional programs that dampen the proinflammatory state of macrophages, as well as inhibiting stress responses and apoptosis.
Mmp28 promotes M2 function
As our results above indicate that Mmp28 dampens M1 polarization, we hypothesized that Mmp28 would, in turn, promote differentiation of the M2 phenotype. We treated BMDMs of each genotype with IL-4 and IL-13 and assessed gene expression of M2 markers at 48 h using qRT-PCR. Compared with WT cells, we found that Mmp28−/− BMDMs had diminished M2-polarized responses, as determined by reduced expression of Arg1, Il10, and Fizz1 (Fig. 3D).
We also demonstrated significantly less Arg1 and Ym1 and greater Il6 expression in Mmp28−/− CD11bhigh pulmonary macrophages isolated during host responses to P. aeruginosa pneumonia. For these experiments, we sorted and collected RNA from distinct macrophage subsets at Day 1 postinfection from WT and Mmp28−/− mice. Within the recruited CD11bhigh population, Mmp28−/− cells had a 4.1 ± 1.0-fold decrease in Arg1 expression, a 3.2 ± 1.3-fold decrease in Ym1 expression, and a 1.7 ± 0.01-fold increase in Il6 expression compared with WT macrophages. Hence, lack of Mmp28 resulted in skewing of their polarization, favoring reduced M2 polarization. In concurrence with the lack of expression of Mmp28 by resident alveolar macrophages (Fig. 1C), we found no alteration in their gene expression of M2 markers in the absence of Mmp28 (data not shown).
Mmp28 promotes pulmonary fibrosis
As M2 polarization is implicated in promoting tissue fibrosis [20–23], we compared the fibrotic response to bleomycin-induced lung injury between WT and Mmp28−/− mice. With low-dose bleomycin, we observed that Mmp28−/− mice had improved recovery of total body weights starting at Days 7–9 (Fig. 4A). With high-dose bleomycin, we also observed greater recovery of body weights in Mmp28−/− mice, and by Day 14, WT mice were less active and had increased respiratory rates compared with Mmp28−/− mice (data not shown). In this high-dose group, there was a trend for greater mortality among the WT group (four deaths/n=11) compared with Mmp28−/− mice (one death/n=11; Fig. 4B).
Figure 4. MMP28 contributes to pulmonary fibrosis and M2 polarization in the lung.
(A) Mice received bleomycin and were followed for weight change as a marker of severity of illness (n=8–10 mice/genotype/condition). Mmp28−/− mice had earlier recovery of weights between Days 7 and 9. (B) In a separate experiment using high-dose bleomycin (n=11/genotype), WT mice trended toward greater mortality (P=0.12), and (C and D) surviving WT mice had greater area of lung involvement compared with Mmp28−/− mice, as determined using H&E-stained lung tissue and Visiopharm software. (E and F) Lung collagen was localized and quantified using Picrosirius Red Sircol assay on lung tissue and homogenates. (E) Lung histology at Day 14 demonstrates collagen deposition (red fibers) in WT and Mmp28−/− lungs but with greater involvement in WT lungs. (F) Homogenized lungs analyzed for collagen content demonstrate collagen accumulation in both genotypes at Days 14 and 21 but with a blunted response in Mmp28−/− mice. (G–I) Quantification of BAL total, macrophage, and neutrophil cell counts at Days 3, 7, and 14. Early after bleomycin (Day 3), WT and Mmp28−/− mice had a similar inflammatory response. At Day 7, the Mmp28−/− mice had increased macrophage recruitment and fewer neutrophils. At Day 14, greater macrophage influx in Mmp28−/− mice persisted. *P < 0.05.
As bleomycin induces patchy inflammatory and fibrotic lung disease, we determined the area of lung involvement in five cross-sectioned lobes using digitized lung slides and Visiopharm software (Hoersholm, Denmark). At Day 14, the WT mice had the greatest area of lung involvement compared with Mmp28−/− mice (52.3±3.0% vs. 33.7±3.5%; P=0.02; (Fig. 4C and D). As the most severely affected WT mice (n=4) died before assessment, these differences may be less pronounced.
In addition, total collagen in the lung was visualized and quantified using Picrosirius Red Sircol assay (Fig. 4E and F). At Days 7, 14, and 21, all bleomycin-treated mice had increased collagen from baseline (Fig. 4F), and Mmp28−/− mice had a modest reduction in collagen accumulation at Days 14 and 21 when compared with WT mice (Fig. 4F).
To assess for differences in inflammatory cell influx into the lung, mice were harvested for BAL at Days 3, 7, and 14 (Fig. 4G–I). The initial inflammatory response at Day 3 was similar. At Day 7, Mmp28−/− mice had slightly fewer neutrophils and more macrophages in the alveolar compartment, and at Day 14, Mmp28−/− mice continued to have greater macrophage influx compared with WT mice (Fig. 4G–I), suggesting that MMP28 regulates the recruitment of neutrophils and macrophages.
In isolated cells from the alveolar compartment, we found significantly less Arg1 and Fizz1 expression and greater Il6 expression in adhesion-isolated Mmp28−/− macrophages (Fig. 5A–C), indicating that Mmp28−/− pulmonary macrophages had impaired M2 polarization. These differences in Arg1 expression were also seen in BAL cells at Day 14, along with decreased expression of Tgfb1 in this cellular compartment (Fig. 5D and E), indicating that MMP28-dependent M2 polarization contributes to the profibrotic responses in the lung.
Figure 5. Analysis of gene expression of isolated cells or whole lung from bleomycin-treated WT and Mmp28−/− mice.
(A–C) At Day 7, adhesion-purified Mmp28−/− pulmonary macrophages (Mac) had reduced M2 gene expression (Arg1, Fizz1) and greater expression of the M1 marker, Il6, as assessed by qRT-PCR when compared with WT macrophages. (D and E) At Day 14, the alveolar cells had reduced expression of the M2 marker, Arg1, and the profibrotic gene, Tgfb1, in Mmp28−/− compared with WT cells. (F) At Day 14, there was also a significant reduction in Col1a1 gene expression in the Mmp28−/− lung. *P < 0.05.
To address whether collagen synthesis is impaired, we assessed the gene expression of Col1a1 in the whole lung of bleomycin-treated Mmp28−/− and WT mice. Compared with WT, we found a small reduction (23.4%) in Col1a1 transcription in Mmp28−/− lungs at Day 14 (Fig. 5F). We also saw a modest (25.9%) reduction in fibronectin-1 gene expression in Mmp28−/− lungs (data not shown). Although the difference in collagen transcripts was small, they would predict a reduced accumulation of collagen proteins with time. Indeed, whereas total collagen deposition rose and then resolved in WT mice, we saw an overall modest fibrotic response in Mmp28−/− mice (Fig. 4E and F).
DISCUSSION
We have uncovered a novel regulatory role for Mmp28 in shaping macrophage polarization in vivo, and these changes are associated with alterations in fibrotic responses in the lung. We reported previously altered chemotaxis and accelerated macrophage influx in macrophages lacking Mmp28. As monocytes are the recruited precursors to these differentiated cells, we sought to determine whether monocytes could be a significant source of Mmp28. We report that specific subsets of circulating monocytes express Mmp28, specifically the Ly6chigh population of inflammatory cells that are the predominant recruited monocytes during tissue injury and inflammation. In the lung, we show that Mmp28 is expressed by recruited macrophages and that absence of Mmp28 inhibits M2 polarization, characterized by reduced Arg1 and Ym1 expression and with greater Il6 expression. Our previous studies indicate that resident and recruited macrophages can polarize toward M2 cells during ALI resolution but that the majority of M2 cells in the lung is derived from recruited cells [17]. Our findings highlight that Mmp28 may be involved not only in recruitment of these cells but also in coordinating their function within the tissue.
We found that TLR agonists that induce M1 polarization as well as cytokines that induce M2 polarization up-regulate Mmp28 gene expression. With the use of transcriptional profiling and qRT-PCR, we found a novel, regulatory role for Mmp28 in dampening proinflammatory responses and promoting M2 gene expression—an observation corroborated by our analysis of macrophages isolated during lung injury. In contrast, Mmp28−/− M0 cells did not share these changes in inflammatory gene up-regulation.
In addition to augmented changes in M1 polarization seen in Mmp28−/− cells, we found that Mmp28−/− cells failed to fully polarize toward M2 in vivo. Although we observed decreased M2 polarization of recruited Mmp28−/− pulmonary macrophages in P. aeruginosa pneumonia, there was no defect in lung-injury resolution at later time-points (not shown), and greater M1 polarization appeared to favor the infected host with faster bacterial clearance, as reported previously [12]. We hypothesized that sterile injury models would be more revealing of the role of Mmp28 in modulating macrophage function and lung-repair mechanisms. As such, we examined the role of Mmp28 in bleomycin-induced pulmonary fibrosis, as the M2 phenotype is associated with pulmonary fibrosis [23]. Furthermore, macrophages, as well as Ly6chigh monocytes, have been associated with the pathogenesis of pulmonary fibrosis [21].
In our model, Mmp28−/− mice were modestly protected from bleomycin-induced fibrosis, and weight loss after Day 7—a surrogate marker for lung injury—was more pronounced in WT mice. At Days 7 and 14, we observed impaired M2 polarization, and at Day 14, we observed reduced, profibrotic gene expression in Mmp28−/− mice, including reduced Tgfb1 expression in the alveolar compartment. Importantly, these changes were associated with decreased collagen synthesis at Days 14 and 21. These results suggest that Mmp28 regulation of macrophage function has important implications in lung biology and wound-repair mechanisms. These findings can be extended to injury models outside of the lung, as we have found impaired M2 polarization and fibrotic responses in the heart postinfarction [31].
A limitation of our studies is that we have not yet confirmed a causal role of impaired M2 polarization to the reduced fibrogenic response. However, there is growing evidence of an association of M2 cells and fibrosis, both within the lung and other tissues [20–23]. Furthermore, macrophages with greater M2 phenotypes, including Arg-1 expression, are associated with enhanced fibrotic responses, and one mechanism of action is via a secreted factor(s) that promotes fibroblast expression of extracellular matrix (ECM) proteins [32]. Others have shown increased Arg1 expression in bleomycin-induced pulmonary fibrosis in mice [28] and in macrophages from patients with idiopathic pulmonary fibrosis [29]. However, in bleomycin-induced fibrosis, the direct role of Arg1 in promoting fibrosis is unknown, and in our studies, it may be a marker of other M2-associated gene-expression changes that regulate the fibrotic milieu in the lung, including Tgfb1. Hence, Mmp28-dependent regulation of macrophage polarization represents a novel and unexpected mechanism for a MMP to regulate fibrogenic responses.
We reported previously that Mmp28 expression by epithelial cells protects against apoptosis [30], and epithelial cell apoptosis is know to be an important driver of pulmonary fibrosis [33, 34]. Based on these observations, one would predict that Mmp28−/− mice would be more susceptible to fibrosis. However, the net effect of Mmp28 knockdown is reduced fibrotic responses, suggesting that the macrophage contribution of Mmp28 may be more significant. In other models of lung injury, we observed down-regulation of epithelial-derived Mmp28 and up-regulation of macrophage-derived Mmp28 [12]. Hence, its antiapoptotic effects on the epithelium may be limited in this chronic model.
One strength of our findings is that we validate observed, in vitro macrophage phenotypes in vivo, using two different models of lung injury. In P. aeruginosa-infected Mmp28−/− mice, we find that enhanced M1 polarization may be of benefit to the host during infection to allow for more rapid host immune response [12]. In contrast, in bleomycin-induced lung fibrosis, we find that Mmp28-dependent regulation of M2 polarization may regulate wound-repair responses. Tight regulation of these functions is critical for balanced responses to acute inflammation and tissue repair, and Mmp28 may serve critical roles in regulating these processes. These findings are consistent with reports published previously of the disparate functions of macrophage populations in injury and repair mechanisms [18] and suggest broad relevance of Mmp28.
Our previous results indicate that Mmp28 restrains macrophage recruitment, and coupled with these new findings, Mmp28 may have evolved to alter recruitment of cells skewed toward a reparative phenotype. Consistent with what we observed in P. aeruginosa, there was greater macrophage influx into the lung in Mmp28−/− mice. We have not yet uncovered how Mmp28 alters macrophage recruitment or polarization, but our studies indicate that Mmp28 dampens proinflammatory responses to several stimuli beyond LPS (not shown), suggesting that these effects may share a common inhibitory pathway that is activated by Mmp28. As these findings are observed in macrophage cultures, and MMP28 is a secreted proteinase, they suggest an intrinsic mechanism occurring via a change in a secreted or membrane protein.
Supplementary Material
ACKNOWLEDGMENTS
Research was supported by the following: University of Washington Research Royalty Fund (to A.M.M.), Howard Hughes Medical Institute Early Physician Scientist Award (to A.M.M.), and U.S. National Institutes of Health HL-084385 and HL-098067.
SEE CORRESPONDING EDITORIAL ON PAGE 1
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
- ALI
- acute lung injury
- Arg-1
- arginase-1
- BAL
- bronchoalveolar lavage
- BM
- bone marrow
- BMDM
- bone marrow-derived macrophage
- Col1a1
- collagen type 1, α 1
- Ct
- threshold cycle
- EGR1
- early growth response 1
- FDR
- false discovery rate
- Fizz-1
- found in inflammatory zone protein
- GADD45A/B
- growth arrest and DNA damage-inducible, α/β
- GO
- Gene Ontology
- M0
- unstimulated macrophages
- M1
- classically activated macrophage
- M2
- alternatively activated macrophage
- MMP
- matrix metalloproteinase
- Mmp28−/−
- matrix metalloproteinase 28-deficient
- NFKBIA
- NF of κ light polypeptide gene enhancer in B cells inhibitor, α
- Poly(I:C)
- polyinosinic:polycytidylic acid
- qRT-PCR
- quantitative RT-PCR
- Ym-1
- T-lymphocyte-derived eosinophil chemotactic factor
AUTHORSHIP
S.A.G. performed gene array analysis and contributed to the manuscript writing. L.K.J. and T.P.B. performed in vitro macrophage studies. I.H. performed bleomycin studies and collagen assays. J.H. performed bleomycin studies. Y.W. maintained mice and collected BM cells. W.C.P. contributed to the manuscript review and Mmp28−/− generation. A.M.M. conceived of experiments; performed macrophage and in vivo studies, and prepared the manuscript.
REFERENCES
- 1. Manicone A. M., McGuire J. K. (2008) Matrix metalloproteinases as modulators of inflammation. Semin. Cell. Dev. Biol. 19, 34–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Li Q., Park P. W., Wilson C. L., Parks W. C. (2002) Matrilysin shedding of syndecan-1 regulates chemokine mobilization and transepithelial efflux of neutrophils in acute lung injury. Cell 111, 635–646 [DOI] [PubMed] [Google Scholar]
- 3. McQuibban G. A., Butler G. S., Gong J. H., Bendall L., Power C., Clark-Lewis I., Overall C. M. (2001) Matrix metalloproteinase activity inactivates the CXC chemokine stromal cell-derived factor-1. J. Biol. Chem. 276, 43503–43508 [DOI] [PubMed] [Google Scholar]
- 4. McQuibban G. A., Gong J. H., Tam E. M., McCulloch C. A., Clark-Lewis I., Overall C. M. (2000) Inflammation dampened by gelatinase a cleavage of monocyte chemoattractant protein-3. Science 289, 1202–1206 [DOI] [PubMed] [Google Scholar]
- 5. McQuibban G. A., Gong J. H., Wong J. P., Wallace J. L., Clark-Lewis I., Overall C. M. (2002) Matrix metalloproteinase processing of monocyte chemoattractant proteins generates CC chemokine receptor antagonists with anti-inflammatory properties in vivo. Blood 100, 1160–1167 [PubMed] [Google Scholar]
- 6. Illman S. A., Keski-Oja J., Parks W. C., Lohi J. (2003) The mouse matrix metalloproteinase, epilysin (MMP-28), is alternatively spliced and processed by a furin-like proprotein convertase. Biochem. J. 375, 191–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Illman S. A., Keski-Oja J., Lohi J. (2001) Promoter characterization of the human and mouse epilysin (MMP-28) genes. Gene 275, 185–194 [DOI] [PubMed] [Google Scholar]
- 8. Lohi J., Wilson C. L., Roby J. D., Parks W. C. (2001) Epilysin, a novel human matrix metalloproteinase (MMP-28) expressed in testis and keratinocytes and in response to injury. J. Biol. Chem. 276, 10134–10144 [DOI] [PubMed] [Google Scholar]
- 9. Rodgers U. R., Kevorkian L., Surridge A. K., Waters J. G., Swingler T. E., Culley K., Illman S., Lohi J., Parker A. E., Clark I. M. (2009) Expression and function of matrix metalloproteinase (MMP)-28. Matrix Biol. 28, 263–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Illman S. A., Lohi J., Keski-Oja J. (2008) Epilysin (MMP-28)—structure, expression and potential functions. Exp. Dermatol. 17, 897–907 [DOI] [PubMed] [Google Scholar]
- 11. Ma Y., Chiao Y. A., Zhang J., Manicone A. M., Jin Y. F., Lindsey M. L. (2012) Matrix metalloproteinase-28 deletion amplifies inflammatory and extracellular matrix responses to cardiac aging. Microsc. Microanal. 18, 81–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Manicone A. M., Birkland T. P., Lin M., Betsuyaku T., van Rooijen N., Lohi J., Keski-Oja J., Wang Y., Skerrett S. J., Parks W. C. (2009) Epilysin (MMP-28) restrains early macrophage recruitment in pseudomonas aeruginosa pneumonia. J. Immunol. 182, 3866–3876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gordon S., Taylor P. R. (2005) Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 5, 953–964 [DOI] [PubMed] [Google Scholar]
- 14. Mantovani A., Sica A., Locati M. (2005) Macrophage polarization comes of age. Immunity 23, 344–346 [DOI] [PubMed] [Google Scholar]
- 15. Gordon S., Martinez F. O. (2010) Alternative activation of macrophages: mechanism and functions. Immunity 32, 593–604 [DOI] [PubMed] [Google Scholar]
- 16. Varin A., Gordon S. (2009) Alternative activation of macrophages: immune function and cellular biology. Immunobiology 214, 630–641 [DOI] [PubMed] [Google Scholar]
- 17. Johnston L. K., Rims C. R., Gill S. E., McGuire J., Manicone A. M. (2012) Pulmonary macrophage subpopulations in induction and resolution of acute lung injury. Am. J. Respir. Cell Mol. Biol. 47, 417–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Duffield J. S., Forbes S. J., Constandinou C. M., Clay S., Partolina M., Vuthoori S., Wu S., Lang R., Iredale J. P. (2005) Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J. Clin. Invest. 115, 56–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Arnold L., Henry A., Poron F., Baba-Amer Y., van Rooijen N., Plonquet A., Gherardi R. K., Chazaud B. (2007) Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J. Exp. Med. 204, 1057–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Braga T. T., Correa-Costa M., Guise Y. F., Castoldi A., De Oliveira C. D., Hyane M. I., Cenedeze M. A., Teixeira S. A., Muscara M. N., Perez K. R., Cuccovia I. M., Pacheco-Silva A., Gonçalves G. M., Camara N. O. (2012) MyD88 signaling pathway is involved in renal fibrosis by favoring a Th2 immune response and activating alternative M2 macrophages. Mol. Med. 18, 1231–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gibbons M. A., MacKinnon A. C., Ramachandran P., Dhaliwal K., Duffin R., Phythian-Adams A. T., van Rooijen N., Haslett C., Howie S. E., Simpson A. J., Hirani N., Gauldie J., Iredale J. P., Sethi T., Forbes S. J. (2011) Ly6Chi monocytes direct alternatively activated profibrotic macrophage regulation of lung fibrosis. Am. J. Respir. Crit. Care Med. 184, 569–581 [DOI] [PubMed] [Google Scholar]
- 22. López-Navarrete G., Ramos-Martínez E., Suárez-Álvarez K., Aguirre-García J., Ledezma-Soto Y., León-Cabrera S., Gudiño-Zayas M., Guzmán C., Gutiérrez-Reyes G., Hernández-Ruíz J., Camacho-Arroyo I., Robles-Díaz G., Kershenobich D., Terrazas L. I., Escobedo G. (2011) Th2-associated alternative Kupffer cell activation promotes liver fibrosis without inducing local inflammation. Int. J. Biol. Sci. 7, 1273–1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pechkovsky D. V., Prasse A., Kollert F., Engel K. M., Dentler J., Luttmann W., Friedrich K., Muller-Quernheim J., Zissel G. (2010) Alternatively activated alveolar macrophages in pulmonary fibrosis-mediator production and intracellular signal transduction. Clin. Immunol. 137, 89–101 [DOI] [PubMed] [Google Scholar]
- 24. Johnston L. K., Rims C. R., Gill S. E., McGuire J. K., Manicone A. M. (2012) Pulmonary macrophage subpopulations in the induction and resolution of acute lung injury. Am. J. Respir. Cell Mol. Biol. 47, 417–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Geissmann F., Jung S., Littman D. R. (2003) Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 19, 71–82 [DOI] [PubMed] [Google Scholar]
- 26. Zhang B., Kirov S., Snoddy J. (2005) WebGestalt: an integrated system for exploring gene sets in various biological contexts. Nucleic Acids Res. 33 (Web server issue), W741–W748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ashburner M., Ball C. A., Blake J. A., Botstein D., Butler H., Cherry J. M., Davis A. P., Dolinski K., Dwight S. S., Eppig J. T., Harris M. A., Hill D. P., Issel-Tarver L., Kasarskis A., Lewis S., Matese J. C., Richardson J. E., Ringwald M., Rubin G. M., Sherlock G. (2000) Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 25, 25–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Endo M., Oyadomari S., Terasaki Y., Takeya M., Suga M., Mori M., Gotoh T. (2003) Induction of arginase I and II in bleomycin-induced fibrosis of mouse lung. Am. J. Physiol. Lung Cell. Mol. Physiol. 285, L313–L321 [DOI] [PubMed] [Google Scholar]
- 29. Mora A. L., Torres-Gonzalez E., Rojas M., Corredor C., Ritzenthaler J., Xu J., Roman J., Brigham K., Stecenko A. (2006) Activation of alveolar macrophages via the alternative pathway in herpesvirus-induced lung fibrosis. Am. J. Respir. Cell Mol. Biol. 35, 466–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Manicone A. M., Harju-Baker S., Johnston L. K., Chen A. J., Parks W. C. (2011) Epilysin (matrix metalloproteinase-28) contributes to airway epithelial cell survival. Respir. Res. 12, 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ma Y., Halade G. V., Zhang J., Ramirez T. A., Levin D., Voorhees A., Jin Y. F., Han H. C., Manicone A. M., Lindsey M. L. (2013) Matrix metalloproteinase-28 deletion exacerbates cardiac dysfunction and rupture after myocardial infarction in mice by inhibiting M2 macrophage activation. Circ. Res. 112, 675–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Minami E., Castellani C., Malchodi L., Deem J., Bertko K., Meznarich J., Dishmon M., Murry C. E., Stempien-Otero A. (2010) The role of macrophage-derived urokinase plasminogen activator in myocardial infarct repair: urokinase attenuates ventricular remodeling. J. Mol. Cell. Cardiol. 49, 516–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gunther A., Korfei M., Mahavadi P., von der Beck D., Ruppert C., Markart P. (2012) Unravelling the progressive pathophysiology of idiopathic pulmonary fibrosis. Eur. Respir. Rev. 21, 152–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bhandary Y. P., Shetty S. K., Marudamuthu A. S., Gyetko M. R., Idell S., Gharaee-Kermani M., Shetty R. S., Starcher B. C., Shetty S. (2012) Regulation of alveolar epithelial cell apoptosis and pulmonary fibrosis by coordinate expression of components of the fibrinolytic system. Am. J. Physiol. Lung Cell. Mol. Physiol. 302, L463–L473 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.