PAF binds to its receptor on mast cells inducing them to migrate from the skin to the lymph nodes, where they mediate immune suppression.
Keywords: immune suppression, inflammatory mediators, cell trafficking, UV radiation
Abstract
The UVB (290–320 nm) radiation in sunlight is responsible for inducing skin cancer. Exposure to UV radiation is also immunosuppressive, and the systemic immune suppression induced by UV is a well-recognized risk factor for cancer induction. As UVB radiation is absorbed within the upper layers of the skin, indirect mechanisms must play a role in activating systemic immune suppression. One prominent example is mast cell migration, which from the skin to the draining LN is an essential step in the cascade of events leading to immune suppression. What triggers mast cell migration is not entirely clear. Here, we tested the hypothesis that PAF, a lipid mediator of inflammation produced by the skin in response to UV exposure, is involved. Mast cell-deficient mice (KitW-sh/W-sh) are resistant to the suppressive effect of UV radiation, and reconstituting mast cell-deficient mice with normal bone marrow-derived mast cells restores susceptibility to immunosuppression. However, when mast cells from PAFR−/− mice were used, the reconstituted mice were not susceptible to the suppressive effects of UV. Furthermore, PAFR−/− mice showed impaired UV-induced mast cell migration when compared with WT mice. Finally, injecting PAF into WT mice mimicked the effect of UV irradiation and induced mast cell migration but not in PAFR−/− mice. Our findings indicate that PAFR binding induces mast cells to migrate from the skin to the LNs, where they mediate immune suppression.
Introduction
The UV radiation found in sunlight, particularly UVB (290–320 nm), is a common environmental factor that has adverse effects on human health. UV radiation is the primary cause of skin cancer. Both nonmelanoma skin cancer, the most common type of human cancer, and melanoma, the most deadly form of skin cancer are induced by UV radiation [1–3]. UV radiation is also immunosuppressive, and the systemic immune suppression caused by UV exposure is a major risk factor for skin cancer induction [4–6]. In addition to suppressing the immune response to skin cancers [7], UV exposure suppresses classic DTH reactions [8] and suppresses antibody production to T-dependent antigens [9]. In view of the fact that most of the UVB energy present in sunlight is absorbed in the upper layers of the skin, it is not entirely clear how dermal exposure to UV radiation activates systemic immune suppression. One potential mechanism involves the migration of immune regulatory cells from the skin to the draining LNs. Two cells that have been shown to transmit the suppressive signal from the skin to the immune system are epidermal Langerhans cells [10–12] and dermal mast cells [13, 14].
Mast cells are considered to be fundamental regulators of type I hypersensitivity; however, it is increasingly evident that mast cells participate in other immune phenomena [15, 16]. One example is the role of mast cells in UV-induced suppression of cellular and humoral immune reactions [9, 13, 17]. Mast cells are essential for the development of UV suppression, as evidenced by the fact that mast cell-deficient mice are resistant to the immunosuppressive effects of UV radiation [13]. More recent findings indicate that unconventional mast cell migration from UV-irradiated skin to the draining LNs represents a crucial step in the induction of suppression. Blocking mast cell migration, by using a CXCR4 antagonist, prevented the induction of immune suppression in UV-irradiated mice [14].
What remains unclear, however, is the identity of the molecular signal(s) that trigger mast cell migration. A variety of chemical mediators produced in the skin in response to UV exposure, including cis-urocanic acid [18, 19], vitamin D3 [20], and PAF [21, 22], has been implicated in the induction of immune suppression by UV radiation. PAF is a lipid mediator of inflammation that is produced by keratinocytes in response to UV exposure [23–25]. PAF exerts its effects through a G-coupled receptor that is widely expressed in different cell populations [26]. PAFR antagonists block UV-induced immune suppression [21], and no immune suppression is found in UV-irradiated PAFR−/− mice [22]. Although keratinocytes have been implicated as a main target for PAF produced after UV irradiation [27], it has been shown that the PAFR expression by bone marrow-derived cells is critical for UV-induced suppression of CHS [28]. Moreover, recent evidence indicates that mast cells express this receptor and are able to respond to PAF [29].
From the literature, it is readily apparent that mast cells and PAF are critical players in the induction of immune suppression following UV exposure. In view of the fact that PAF can affect the migration of a variety of cell types [30–32], we decided to test the hypothesis that the activation of the PAFR on mast cells triggers their migration from the skin to the LN, where they contribute to immune suppression. Our findings indicate that activating the PAFR on mast cells is critical to induce mast cell migration and represent an essential step in the cascade of events that lead to UV-induced systemic immune suppression.
MATERIALS AND METHODS
Mice
Eight- to 10-week-old female, mast cell-deficient (KitW-sh/W-sh) and C57BL/6 mice were obtained from The Jackson Laboratory (Bar Harbor, ME, USA). PAFR−/− mice, backcrossed onto a C57BL/6 background, were developed by Ishii and Shimizu [33] and provided to us, with Dr. Shimizu's permission, by Dr. Jeffrey B. Travers (Indiana University School of Medicine, Indianapolis, IN, USA). The mice were maintained in facilities approved by the Association for Assessment and Accreditation of Laboratory Animal Care International, in accordance with current regulations and standards of the U.S. Department of Agriculture, Department of Health and Human Services, and National Institutes of Health. All animal procedures were reviewed and approved by the MD Anderson Cancer Center Animal Care and Use Committee.
Mast cell-deficient mice were reconstituted with BMMCs, as described previously [9, 14, 34]. Briefly, bone marrow cells were obtained from the femurs and tibias of 6- to 8-week-old C57BL/6 or PAFR−/− mice and then cultured at a concentration of 106 cells/ml in complete RPMI 1640, supplemented with murine rIL-3 (10 ng/mL; PeproTech, Rocky Hill, NJ, USA) and SCF (10 ng/mL; PeproTech). Nonadherent cells were transferred to fresh culture medium twice a week for 4–6 weeks, at which point, >95% of the viable cells were mast cells, as verified by flow cytometry (CD117+ FcϵRIα+). A total of 1 × 106 BMMCs was injected into eight sites underlying the dorsal skin of mast cell-deficient mice. Six weeks later, the mice were exposed to UV radiation.
UV exposure
The shaved backs of mice were exposed to an immunosuppressive dose of UV radiation (15 kJ/m2 UVB; 290–320 nm), supplied by a 1000-W xenon arc solar simulator (Oriel, Stratford, CT, USA), as described previously [35]. During the irradiation, the ears were covered with light opaque black cloth to block UV exposure. The intensity and spectral output of the solar simulator were measured with an Optronic Model OL-754 scanning spectrophotometer (Optronic Laboratories, Orlando, FL, USA). Control mice were shaved but not irradiated.
Induction of CHS in vivo
Mast cell-deficient mice were reconstituted with BMMC isolated from PAFR−/− or C57BL/6 mice. Six weeks after reconstitution, the mice were UV-irradiated and 4 days later, were sensitized by applying 50 μl 0.5% DNFB solution in acetone/olive oil (4:1, v/v) to their shaved abdomens. Six days later, ear thickness was measured with an engineer's micrometer (Mitutoyo, Tokyo, Japan), and 10 μl 0.2% DNFB solution was applied to both ear surfaces. On Day 7, the mice were euthanized, and the thickness of each ear was measured again. The data are expressed as the mean change in ear swelling [(left ear+right ear)/2] for each mouse. Generally, there were 10 mice/group.
Antibodies and reagents
c-PAF, a biologically potent, nonmetabolizable analog of PAF, the PAFR antagonist, PCA-4248, and PGE2were obtained from Enzo Life Sciences (Plymouth Meeting, PA, USA). Antibodies specific for CXCR4 were purchased from BD Biosciences (San Jose, CA, USA). Antibodies specific for CD45, FcϵRIα, CD117, CD4, and CD44 were purchased from eBioscience (San Diego, CA, USA). Goat and rabbit anti-PAFR was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-rabbit Alexa 488 and anti-goat Alexa 488 were acquired from Invitrogen (Carlsbad, CA, USA). Cell-surface staining was measured with a FACSCalibur flow cytometer (BD Biosciences), and results were analyzed with FlowJo (Tree Star, Ashland, CA, USA). Mast cell numbers in the LNs were calculated using the percentage of CD45+CD117+FcϵRI+ positive cells detected by flow cytometry and the total cell counts for the inguinal LNs of individual mice.
Quantitative real-time PCR
Total RNA was extracted with Trizol (Invitrogen) and purified further by using the RNeasy RNA cleanup protocol (Qiagen, Germantown, MD, USA). cDNA was reverse-transcribed from total RNA using a high-capacity cDNA RT kit (Applied Biosystems, Carlsbad, CA, USA). cDNA (25 ng) was subjected to real-time RT-PCR using a sequence detector (Model ABI Prism 7500) and target mixes for PAFR, CXCR4, CXCL12, and β-actin (Taqman Gene Expression Assay, Applied Biosystems). Ct values for target genes were normalized to β-actin using the following equation: 1.8(actin−target gene) × 1000, where actin is the Ct of the β-actin control, target gene is the Ct of the gene being evaluated, and 1000 is an arbitrary factor to bring all values above 1 [14].
Chemotaxis assay
Chemotaxis assays were performed as described previously [36]. Briefly, BMMCs from C57BL/6 or PAFR−/− mice were left unstimulated or stimulated with 1000 nM c-PAF. After washing, cells were resuspended to a cell density of 3 × 106/ml in HEPES buffer containing 0.5% BSA; 0.1 ml was seeded in the Transwell-permeable support with 5.0 μM pore polycarbonate membranes on 6.5 mm inserts (Costar, Corning, NY, USA) and preincubated with the lower chamber containing 600 μl HEPES buffer-BSA 0.5% for 30 min. Upper chambers were then placed in lower chambers that contained 100 ng/ml SCF (PeproTech) or CXCL12 (PeproTech) and incubated for 5 h at 37°C. Cells that migrated into the lower chamber were counted by microscopy.
Histological analysis of mast cells
Skin samples from mice were embedded in paraffin and 7 μm sections cut, fixed, and then stained for mast cells using toluidine blue. Dermis area was calculated with NIH ImageJ software (http://rsb.info.nih.gov/nih-image/). Results are presented as mast cells/mm2 [34, 37].
Draining LNs from control or UV-irradiated mice were placed in the same orientation for different groups in Tissue-Tek optimal cutting temperature solution (Sakura Finetek, Torrance, CA, USA) and snap-frozen in liquid nitrogen. Frozen sections (4 μm) were cut, fixed, and then stained for mast cells using toluidine blue [14, 37]. Three to five different sections covering each LN were used for analysis. The sections started at the proximal area in relation to the efferent lymph vessels, and care was taken to ensure that sectioning occurred in the same area of each LN and that the different groups of mice had a similar LN area for analysis. Mast cell density in the LN was determined by counting the total number of toluidine blue-positive cells/LN section and dividing it by the area of the LN calculated with NIH ImageJ software.
Data analysis and statistics
Statistical difference between the control group and experimental groups was determined using a one-way ANOVA, followed by Bonferroni's multiple comparison test (GraphPad Prism software V4; GraphPad Software, San Diego, CA, USA). Representative experiments are shown; each experiment was repeated at least three times.
RESULTS
Mast cells express the PAFR
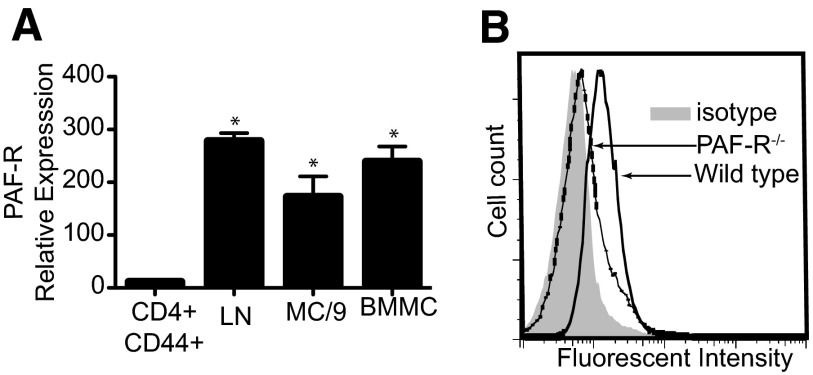
The initial experiments were designed to confirm PAFR expression on mast cells. Two different populations of murine mast cells—the mouse mast cell line MC/9 and B6 BMMC mice—were used. As others have shown that resting and activated T cells do not express the PAFR [38], we used sorted CD4+ CD44+ as the negative control. Total LN cells were used as the positive control [33]. MC/9 and BMMCs expressed mRNA for the PAFR (Fig. 1A). We confirmed this observation by performing flow cytometry to measure cell-surface expression of the PAFR on BMMCs from WT and PAFR−/− mice, as described previously [39]. We observed marked staining of the WT cells, which was not evident when BMMCs, derived from PAFR−/− mice were used (Fig. 1B). These results confirm that murine mast cells express the PAFR.
Figure 1. Mast cells express the PAFR.
(A) PAFR mRNA expression was analyzed by quantitative real-time PCR. Whole LN cells were used as a positive control. Sorted activated T cells (CD4+ CD44+) were used as a negative control. Results are expressed as means ± sem. *P = 0.001 versus negative control. (B) PAFR protein on cell surface was analyzed by flow cytometry. BMMCs derived from PAFR−/− mice were used as a negative control.
PAFR expression on mast cells is essential for UV-induced immune suppression
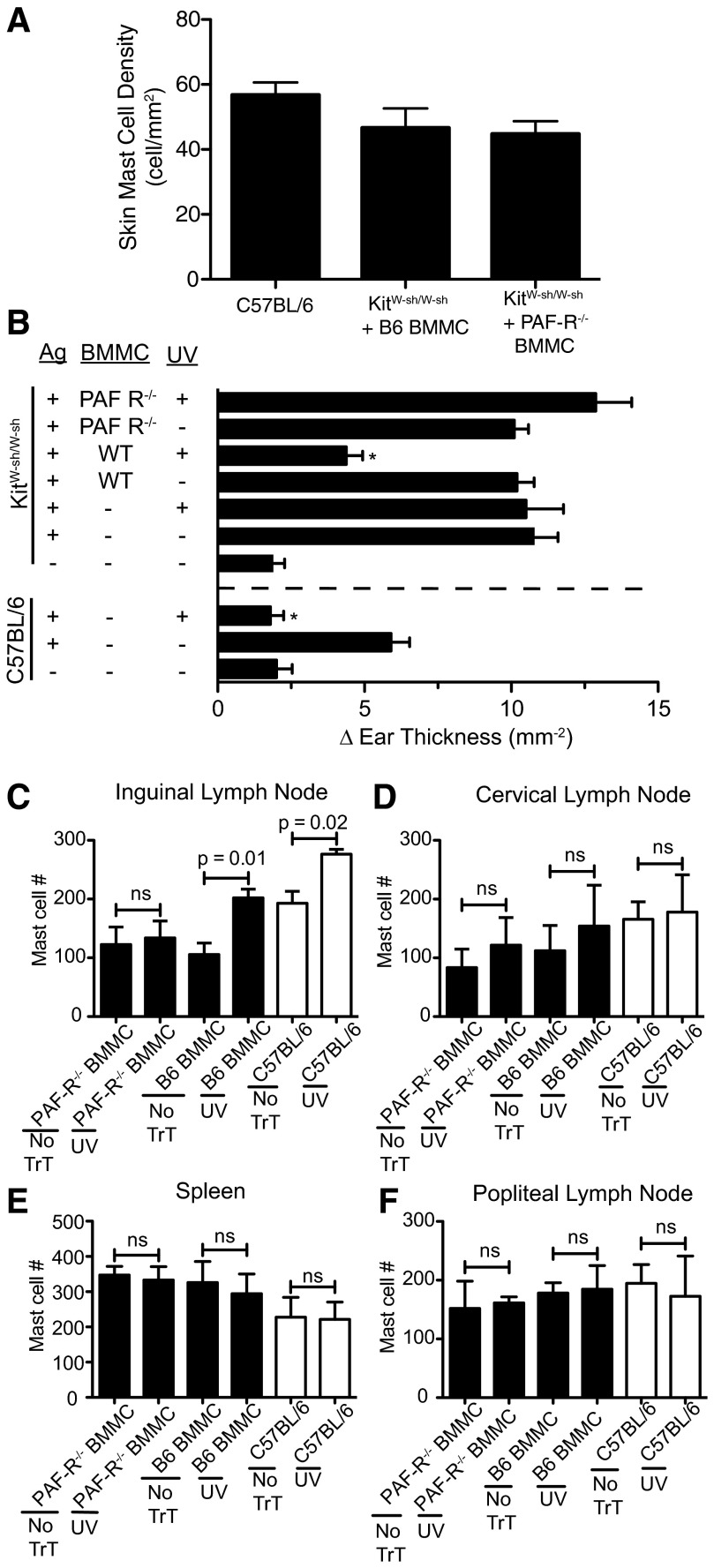
Mast cells and mast cell migration from the skin to the draining LN are essential for UV-induced immune suppression [13, 14]. To test the hypothesis that PAFR expression on mast cells is essential for immune suppression, we reconstituted mast cell-deficient mice with BMMCs derived from C57BL/6 or PAFR−/− mice. As the reconstitution technique involved injecting BMMCs into the skin of mast cell-deficient mice, 6 weeks prior to UV exposure, we first determined if there was any difference in the ability of WT or PAFR−/− BMMCs to reconstitute the skin. The data are found in Fig. 2A. We saw no difference in skin mast cell density after KitW-sh/W-sh mice were reconstituted with B6 BMMC (KitW-sh/W-sh+B6 BMMC) or PAFR−/− BMMC (KitW-sh/W-sh+PAFR−/− BMMC). For the sake of comparison, mast cell density in unmanipulated, normal WT mice (C57BL/6) is also shown. We also measured the expression of CD117 and FcϵRI on BMMCs derived from C57BL/6 and PAFR−/− mice to ascertain if a difference existed between these two cell populations. As measured by CD117 and FcϵRI surface expression, mast cells derived from WT and PAFR−/− mice were identical (Supplemental Fig. 1).
Figure 2. PAFR on mast cells is essential for UV-induced immune suppression.
(A) Mast cell-deficient mice were injected intradermally with B6 BMMC (KitW-sh/W-sh+B6 BMMC) or PAFR−/− BMMC (KitW-sh/W-sh+PAFR−/− BMMC). Six weeks after reconstitution, mast cell density was determined by toluidine blue staining. Mast cell density in normal mice (C57BL/6) is also shown. (B) Mast cell-deficient mice were reconstituted with B6 BMMC or PAFR−/− BMMC. Six weeks later, the mice were exposed to 15 kJ/m2 UVB radiation. The effect of UV on CHS in C57BL/6 mice is shown for comparison. Positive controls are mice that were sensitized and challenged but were not exposed to UV. Negative control refers to mice that were not sensitized but were challenged. Results are expressed as mean change (δ) in ear swelling ± sem; there were 10 mice/group. *P < 0.05 compared with relevant positive control. (C–F) Mast cell-deficient mice (black bars) were reconstituted with WT (B6 BMMC) or PAFR−/− BMMC. Six weeks later, the mice were exposed to UV (15 kJ/m2 UVB). Twenty-four hours later, the inguinal LNs (C), cervical LNs (D), spleen (E), and popliteal LNs (F) of the irradiated mice or nonirradiated controls (no TrT) were isolated and mast cell numbers determined by flow cytometry. The positive control consisted of exposing WT C57BL/6 mice (open bars) to UV.
We then tested the hypothesis that PAFR expression on mast cells is required for the induction of immune suppression. To address this issue, we reconstituted mast cell-deficient mice with BMMCs isolated from PAFR−/− or C57BL/6 mice. The mast cell-reconstituted mice were then exposed to an immunosuppressive dose (15 kJ/m2) of UVB radiation. The effect that UV exposure had on CHS is shown in Fig. 2B. As reported previously, mast cell-deficient mice are capable of generating a vigorous CHS reaction when a contact allergen is applied to their skin [13, 14]. Similarly, exposing mast cell-deficient KitW-sh/W-sh mice to UV radiation did not suppress CHS, whereas the same dose of UV radiation significantly suppressed CHS when applied to C57BL/6 mice. When the mast cell-deficient mice were reconstituted with B6 BMMCs, they became susceptible to the immunosuppressive effect of UV radiation (KitW-sh/W-sh mice; *P=0.05, antigen+WT BMMC+UV vs. antigen+UV). Mice that were reconstituted with BMMCs from PAFR−/− mice and then exposed to UV radiation were not immunosuppressed.
Previously, we reported that UV irradiation of the skin promotes mast cell migration to the draining inguinal LN but not to the distant popliteal node [14]. In the next series of experiments, we determined if the presence or absence of the PAFR on mast cells would affect migration. Bone marrow-derived mast cells isolated from WT or PAFR−/− mice were injected in the back skin of mast cell-deficient mice, as described previously [13]. Six weeks later, the mice were exposed to UV radiation, and 24 h after UV exposure, their spleens, cervical, inguinal, and popliteal LNs were removed and mast cell density determined. As a positive control C57BL/6 mice were also irradiated with 15 kJ/m2 UVB radiation (Fig. 2C). In agreement with previous findings, we saw that UV exposure of the WT C57BL/6 mice induced mast cell migration to the skin draining inguinal LNs but not to the spleen cervical or popliteal LN. Similarly, when mast cell-deficient mice were reconstituted with B6 BMMCs and then UV-irradiated, we noted increased mast cell migration to the inguinal LN but not to the spleen or other LNs. On the other hand, when the mast cell-deficient mice were reconstituted with BMMCs from PAFR−/− mice and then exposed to UV, no mast cell migration to the inguinal LN was noted. These data indicate that PAFR expression on mast cells is required for UV-induced mast cell migration and UV-induced immune suppression.
c-PAF up-regulates CXCR4 expression on mast cells
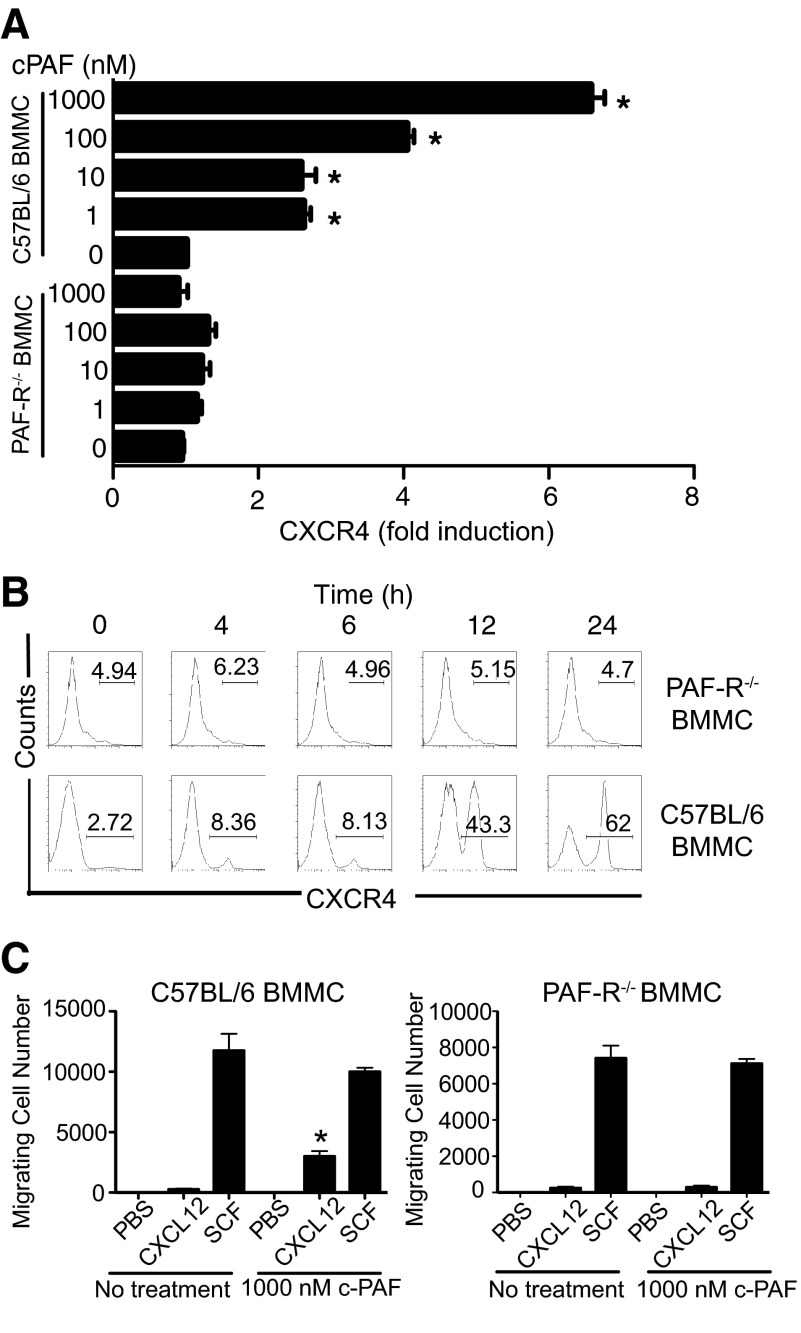
Previously, we demonstrated that UV-induced mast cell migration was CXCR4-dependent [14]. We next evaluated whether treating mast cells with PAF up-regulated CXCR4 expression. B6 BMMC mice were cultured with c-PAF. After 24 h in culture, the cells were isolated, and CXCR4 expression was measured by quantitative real-time PCR. The treatment of B6 BMMC with c-PAF induced a significant transcription of CXCR4, whereas little expression of CXCR4 above baseline was found when BMMCs, derived from PAFR−/− mice, were treated with c-PAF (Fig. 3A). To confirm this observation, we evaluated CXCR4 surface expression on B6 BMMC and PAFR−/− BMMC mice. When WT BMMCs were treated with c-PAF, we saw up-regulation of surface expression of CXCR4 as early as 4 h of poststimulation, and at 12 h after stimulation, almost 50% of BMMCs were positive for CXCR4. Peak expression was noted, 24-h post-treatment (Fig. 3B; C57BL/6). Contrasting with this, BMMCs from PAFR−/− mice did not show any increase in CXCR4 expression after culture with c-PAF (Fig. 3B; PAFR−/−). Furthermore, PAF-treated B6 BMMC, migrated in response to CXCL12, versus the minimal migration observed with PAF-treated BMMCs from PAFR−/− mice, were used (Fig. 3C).
Figure 3. PAF induces mast cell CXCR4 expression.
(A) B6 BMMC and PAFR−/− BMMC were cultured for 24 h with different concentrations of c-PAF, and CXCR4 mRNA was measured by quantitative real-time PCR. Results are expressed as means ± sem. *P = 0.001 versus baseline level. (B) BMMCs were cultured with 1000 nM c-PAF, cells were collected at different times poststimulation, and the CXCR4 surface expression was detected by flow cytometry. (C) BMMCs were cultured overnight with medium (No treatment) or 1000 nM c-PAF and washed, and 3 × 105 cells were added to upper chambers. Migrating cells were counted 5 h later. Results are expressed as means ± sem. *P < 0.05; Migration of cells with no treatment versus migration of cells pretreated with c-PAF. SCF was used as a positive control.
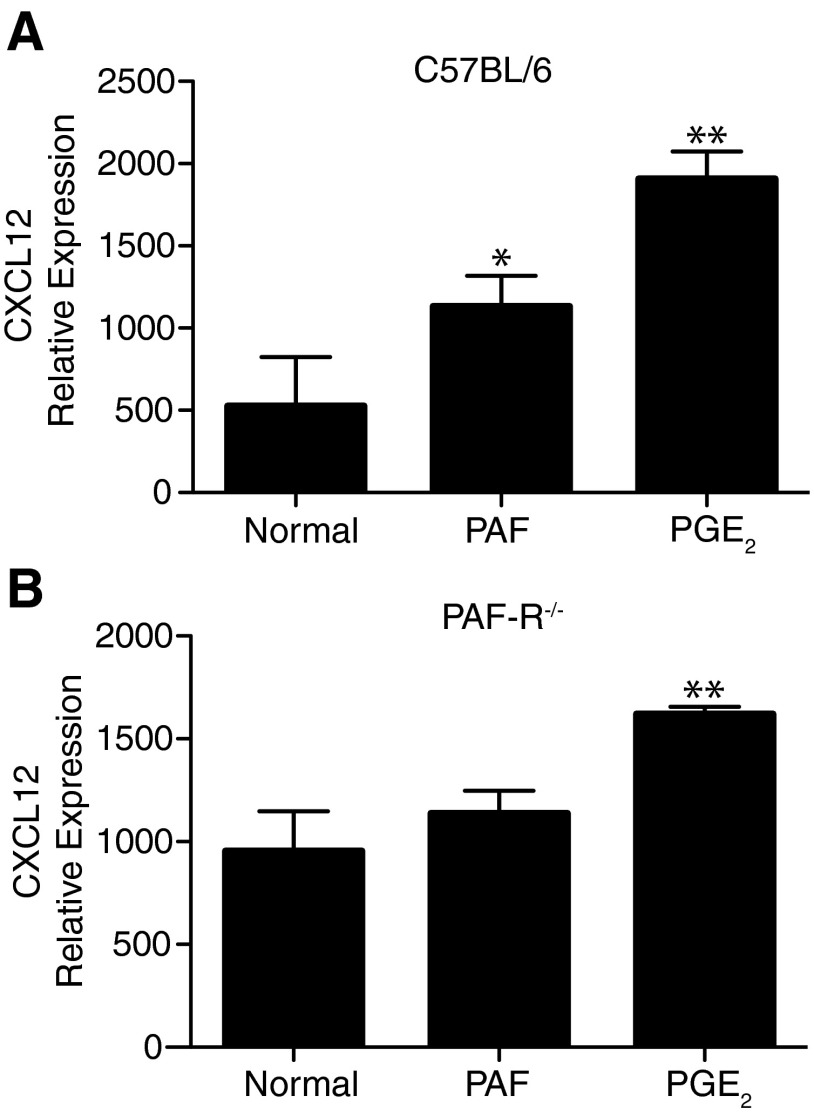
Next, we determined if PAF affects the expression of CXCL12, the ligand for CXCR4, by LN cells. Injecting an immunosuppressive dose of PAF (1000 picomoles) [21] into C57BL/6 mice resulted in a significant (P<0.05 vs. normal) increase in CXCL12 expression by LN cells. As others have shown that PGE2 up-regulates CXCL12 expression [40], PGE2 was used as a positive control in this experiment (Fig. 4A). No increase in CXCL12 expression was observed when PAF was injected in PAFR−/− mice (Fig. 4B). These findings indicate that PAF up-regulates the expression of CXCR4 on mast cells and the expression of its ligand, CXCL12, on draining LN cells.
Figure 4. PAF induces LN CXCL12 expression.
Mice were injected i.p. with 1000 picomoles c-PAF or 1 μg PGE2. Twenty-four hours after treatment, the inguinal LNs were collected. CXCL12 expression was measured by quantitative real-time PCR. Results are expressed as means ± sem. *P < 0.05; **P = 0.01 versus normal.
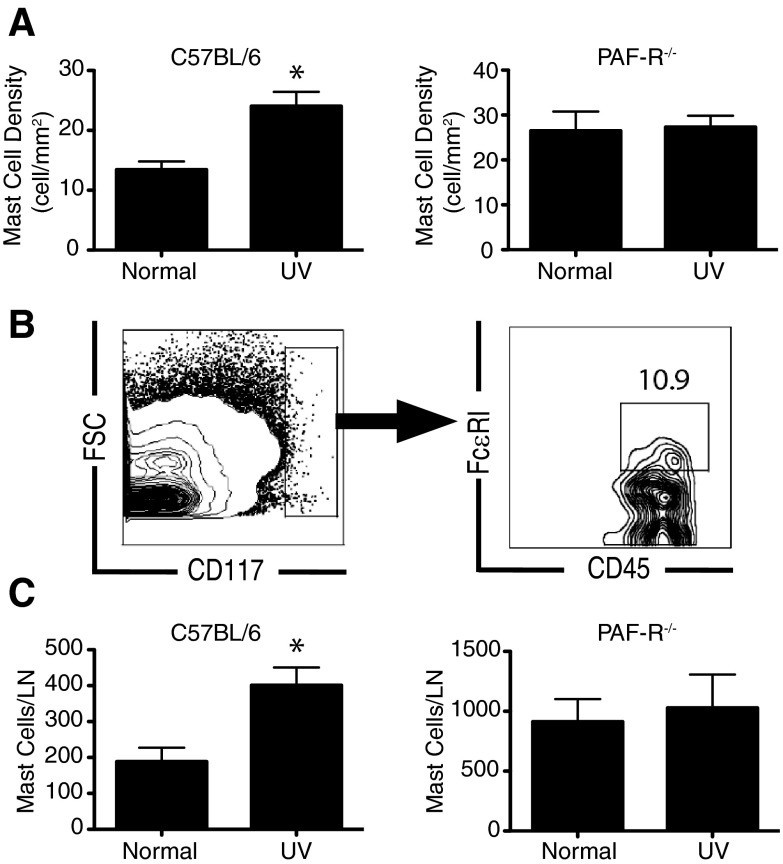
Fewer mast cells accumulate in the draining LNs of PAFR−/− mice in response to UV irradiation
We exposed C57BL/6 and PAFR−/− mice to an immunosuppressive dose of UV radiation (15 kJ/m2), and 24 h later, we measured mast cell numbers in the draining LN (Fig. 5A, toluidine blue staining; Fig. 5B and C, flow cytometry). As expected [14], we observed a significant accumulation of mast cells in the LNs of UV-irradiated C57BL/6 mice but not in UV-irradiated PAFR−/− mice. These results suggest that PAFR expression on mast cells is essential to trigger their migration to the LNs following UV exposure.
Figure 5. UV-induced mast cell migration is suppressed in PAFR−/−mice.
C57BL/6 or PAFR−/− mice were exposed to UV radiation, and their draining LNs were removed 24 h later. (A) Mast cell density was evaluated by toluidine blue staining. (B) Gating strategy for detecting mast cells by flow cytometry (double-stained FcϵRIα+, CD117+ cells in the CD45 gate). FSC, Forward-scatter. (C) Mast cell density was evaluated by flow cytometry. Results are expressed as means ± sem. *P = 0.01 vs. normal.
To confirm this observation, we treated C57BL/6 mice with an immunosuppressive dose of c-PAF [21]. We noted an accumulation of mast cells in the LNs of WT mice, 24 h post-i.p. administration of c-PAF (Fig. 6A), which was not observed in PAFR−/− mice (Fig. 6B). In addition, WT mice that were pretreated with 500 nmoles PCA-4248, a PAFR antagonist [21], and then exposed to UV radiation did not show an increase in mast cell density in the draining LNs (Fig. 6A). To rule out the possibility that this defect in mast cell migration is a result of decreased mast cell numbers in the skin of PAFR−/−, we measured dermal mast cell density in the WT and PAFR−/− mice. The mast cell density in C57BL/6 and PAFR−/− mice was identical, and as expected, no mast cells were found in the skin of KitW-sh/W-sh mice (Supplemental Fig. 2). We also measured skin mast cell density, 24 h after i.p. injection of c-PAF. The numbers of mast cells in the skin of PAF-injected mice decreased, supporting the hypothesis that that PAF is an important mediator in driving mast cell migration (Fig. 6C), which was also measured following intradermal injection of c-PAF. Unlike the data reported above, we never observed significant mast cell migration to any LN or to the spleen after intradermal PAF administration (Fig. 6D–G).
Figure 6. Administration (i.p.) of c-PAF induces mast cell migration.
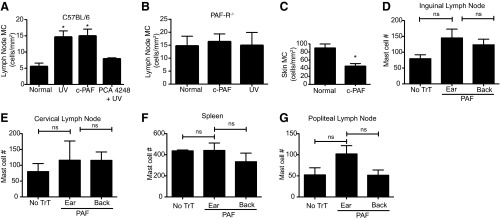
(A) C57BL/6 or (B) PAFR−/− mice were exposed to UV radiation or injected with 1000 picomoles c-PAF (i.p.). One group of mice was injected with 500 nmoles of the PAFR antagonist PCA-4248, 1 h before UV exposure. Twenty-four hours after treatment, the inguinal LNs were collected, and mast cells (MC) were detected by toluidine blue staining. (C) WT mice were injected with 1000 picomoles c-PAF (i.p.), and then skin mast cell density was measured 24 h later. Results are expressed as means ± sem. *P = 0.05 versus normal. (D–G) Intradermal injection of c-PAF does not activate mast cell migration. WT mice were injected with 1000 picomoles c-PAF intradermally into the pinna of the ear or multiple sites on the back. Twenty-four hours later, the LNs and spleen of the injected mice were isolated, and mast cell numbers were determined by flow cytometry (mean±sem); (D) inguinal LNs; (E) cervical LNs; (F) spleen; (G) popliteal LNs.
DISCUSSION
As UV radiation is the carcinogen primarily responsible for inducing skin cancer, and in view of the fact that UV-induced immune suppression is an important risk factor for skin cancer induction, a major focus of our research is to understand the mechanisms involved. A role for mast cells in UV-induced immune suppression was first implicated by Streilein and coworkers [41] and then formally proven by Hart and colleagues [13], who demonstrated no immune suppression in UV-irradiated mast cell-deficient mice and restoration of immune suppression when mast cell-deficient mice were reconstituted with BMMCs. Since these initial reports, evidence from a variety of laboratories has confirmed the essential role of mast cells in UV-induced immune suppression [9, 14, 17, 42]. It has become apparent recently that mast cells are important regulatory cells that suppress a variety of adaptive immune reactions [15, 16].
Although almost all of the energy contained with UVB radiation is absorbed in the upper layers of the skin, UVB exposure induces system-wide immune suppression. How the immunosuppressive signal is transmitted from the skin to the immune system is not entirely clear, but previous findings from this lab indicated that mast cell migration from the UV-irradiated skin to the draining LNs was a critical step for the induction of immune suppression [14]. Here, we tested the hypothesis that PAF mediates the migration of mast cells from the skin to the draining LNs. We focused our attention on PAF for a number of reasons. PAF is produced by keratinocytes following acute UV irradiation [23–25], and over the years, a variety of studies has demonstrated that it plays a critical role in UV-induced immune suppression and skin cancer induction [21]. Injecting c-PAF into mice activates systemic immune suppression [21, 22, 28, 43]. PAF also regulates the migration of many cell types, including tumors cells [31, 44], neutrophils [32], and PMN leukocytes [45]. In vitro studies have also indicated that PAF mediates the migration of mast cells [46, 47]. Our findings confirm these observations and support the hypothesis that UV-induced PAF activates mast cell migration in vivo.
The findings presented here indicate that PAF treatment up-regulates CXCR4 expression on mast cells. Similarly, we find a significant increase in CXCL12 expression by draining LN cells following PAF injection. It is interesting that we noticed that only a proportion of B6 BMMC overexpressed CXCR4 after c-PAF stimulation (Fig. 3). The most reasonable explanation for this finding is the heterogeneity of BMMCs, as reflected by the differential expression of integrins during cytokine induced growth and differentiation of BMMCs [48]. Although the increase in CXCL12 expression was only twofold, it mimics the up-regulation of CXCL12 found in the draining LNs of UV-irradiated mice [14]. From these findings, we conclude that UV-induced PAF contributes to mast cell migration by up-regulating the expression of CXCR4 on mast cells and its ligand, CXCL12, on LN cells.
We also find increased accumulation of mast cells in the draining LNs of UV-irradiated WT mice but not in the draining LNs of UV-irradiated PAFR−/− mice. When mast cell-deficient mice were reconstituted with PAFR−/− mast cells, UV exposure did not activate mast cell migration to the skin draining LNs nor were we able to reconstitute UV-induced immune suppression (Fig. 2). Similarly, treating WT mice with c-PAF activated mast cell migration, and injecting PAFR antagonists into UV-irradiated WT mice interfered with the migration of mast cells into the draining LN. The dose of PAF that was used here is based on previous work, where we showed that injecting 1000 picomoles induced significant in vivo immune suppression [21]. Others have shown that UV irradiation of mouse and/or human skin generates PAF and PAFR agonists [24, 25]. Moreover, depending on the dose of UV applied, Marathe and colleagues [24] report that UV-irradiated keratinocytes release ∼4000 picomoles PAF/106 cells, suggesting that the dose used here to induce immune suppression is physiological. It should be noted, however, that following intradermal/s.c. injection of PAF (either into the back skin or the ears), we did not find any statistical difference in the numbers of mast cells in the PBS-treated controls versus the spleen, inguinal, cervical, or popliteal LNs of PAF-treated mice. Moreover, in our hands, intradermal/s.c. injections of PAF have been uniformly negative in inducing immune suppression (data not shown) or mast cell migration (Fig. 6). This is probably related to the production of PAF-acetylhydrolase by normal keratinocytes [49] and/or by bacteria that reside on the skin [50].
Although initial mast cell density and mast cell numbers were higher in LNs isolated from PAFR−/− mice compared with C57BL/6 mice, the PAFR−/− mice are resistant to UV-induced immune suppression, and we were unable to observe any further increase in LN mast cell density following UV exposure. The reason for the increased basal level of mast cells in the LNs of PAFR−/− mice is not entirely clear but could be related to an altered recruitment of mast cell progenitors to the node [47].
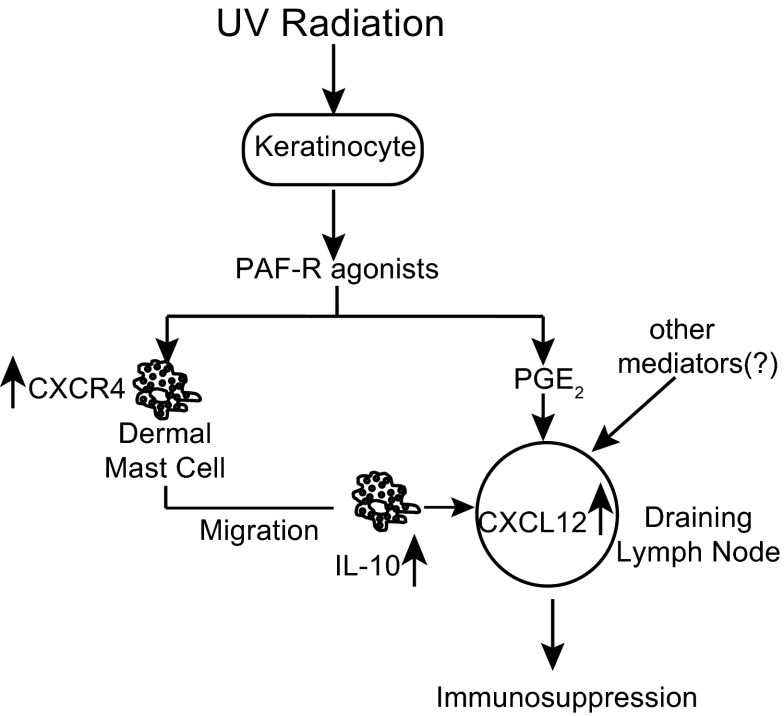
Based on the findings reported here and those presented previously in the literature, we propose the following scenario (Fig. 7). UV irradiation of the skin induces epidermal keratinocytes to release PAF. UV-induced PAF then binds to the PAFR on dermal mast cells and triggers the up-regulation of CXCR4. PAF also up-regulates CXCL12 expression on LN cells. Based on the short half-life of PAF, its rapid clearance from the circulation [51], and its degradation by PAF-acetylhydrolase [52], it is doubtful that keratinocyte-derived PAF gets to the LN. However, as PAF promotes PGE2 production [53], we suggest that PAF-induced PGE2 up-regulates CXCL12 expression in the node, thus setting up the gradient needed for mast cell migration [14]. This is supported, in part, by our observation that we can over-ride the inability of PAF to up-regulate CXCL12 expression in PAFR−/− mice by injecting PGE2 (Fig. 4B), although other UV-induced chemical mediators could also be involved in this phenomenon. The mast cells then migrate from the skin to the draining LNs. We propose that mast cells that migrate to the draining LNs suppress the immune response by secreting IL-10. A number of older reports in the literature indicate that UV-induced IL-10 is essential for the induction of immune suppression [54, 55]. Recently, we reported that exposing mice to UV radiation suppressed germinal-center formation, antibody secretion, and follicular Th cell function. No immune suppression was noted in mast cell-deficient mice, and the immune suppression could be restored when mast cell-deficient mice were reconstituted with normal BMMCs. However, when mast cell-deficient mice were reconstituted with BMMCs derived from IL-10−/− mice and then exposed to UV radiation, no suppression of antibody formation, germinal-center formation, or follicular Th cell function was observed [9]. This suggests that UV-activated mast cells are an important source of the IL-10 that mediates UV-induced immune suppression.
Figure 7. Model for the role of PAFR agonists during immune suppression induced by UV radiation.
Skin damage induced by UV irradiation leads to secretion of PAF by keratinocytes and oxidation of phopsholipids that bind to the PAFR on mast cells. This induces the surface expression of CXCR4. UV irradiation of the skin also leads to the up-regulation of CXCL12 by LN cells [14]. This facilitates the migration of CXCR4-positive mast cells from the dermis to the draining LNs. We propose that IL-10-secreting mast cells [9] migrate to the skin draining LNs and mediate immune suppression.
These findings may have implications beyond UV-induced immune regulation. Applying the aromatic hydrocarbons found in jet fuel to the skin also induces immune suppression [56, 57]. Injecting jet fuel-treated mice with PAFR antagonists overcomes the induction of immune suppression [58]. No immune suppression is observed in mast cell-deficient mice, and reconstituting mast cell-deficient mice with normal BMMCs restores immune suppression. Moreover, jet fuel treatment activates mast cell migration from the skin to the draining LNs, and blocking this migration with a CXCR4 antagonist prevents immune suppression [37]. We suggest that a PAF-induced mast cell migration may be a common reaction to dermal trauma and is not unique to UV-induced skin damage. Whether other agents that cause immune suppression by damaging the skin, such as the thermal trauma found in burn patients [59], use a similar mechanism remains to be seen, but we suggest that PAF-triggered mast cell migration may be a common feature.
In addition to mast cells, migrating Langerhans cells play an essential role in transmitting the immunosuppressive signal from the skin to the immune system following in vivo UV exposure [11]. Loser and colleagues [10] report that in vivo, UV exposure up-regulates the expression of RANKL on irradiated keratinocytes and suggest that the up-regulation of RANKL in the inflamed skin “rewires” local Langerhans cells to activate regulatory T cells. On the other hand, we reported previously that the UV irradiation of the skin promotes the migration of Langerhans cells to the LNs, where they activate NK T cells to secrete IL-4 and become immune-suppressive [12]. It is interesting to note that applying a contact allergen to WT mice activates Langerhans cell migration. However, no Langerhans cell migration was observed when the same contact allergen was applied to the skin of PAFR−/− mice [30]. This suggests that UV-induced PAF, upon binding to its receptor on Langerhans cells, may play a role in the migration of Langerhans cells to the LN and the subsequent activation of immune suppression. Experiments are in progress to test this possibility.
Some immune reactions, including CHS, are generally enhanced in mast cell-deficient mice compared with the response found in WT mice (see Fig. 2B for an example), implying an immunosuppressive role for mast cells [15, 16]. A recent paper presented by Dudeck and colleagues [60], using a new method of depleting mast cells (injecting diphtheria toxin into mice that only express the diphtheria toxin receptor on mast cells), challenges the paradigm that mast cells are immunosuppressive. In their study, depleting mast cells did not result in enhanced CHS, but rather, contact allergy was suppressed ∼50% in these mice, indicating that mast cells promote CHS. How these findings affect the conclusions arrived at in this manuscript, in which mast cell-deficient mice were used, is not clear. However, it should be noted that in the experiments presented by Dudeck and colleagues [60], the inherent immunoregulatory potential of mast cells was examined. This is considerably different from the situation described here, in which a potent immunosuppressive insult, UV radiation, was used to activate the mast cells. The effect of mast cells on antibody formation may be illustrative here. McLachlan and colleagues [61] report that mast cells serve as adjuvants for antibody formation in vivo. In their studies, compound 48/80-activated mast cells secreted TNF and enhanced antibody production. On the other hand, a previous study from this laboratory, in which UV radiation was used to activate mast cells, resulted in the up-regulation of mast cell IL-10 secretion, which suppressed follicular Th cell function, depressed germinal center formation, and suppressed antibody secretion in vivo [9]. We suggest that the agent used to activate the mast cell may result in different biological effects.
In summary, our data support a role for PAF in triggering mast cell migration following UV irradiation in vivo. We suggest that PAF produced in the skin in response to UV radiation activates mast cells to up-regulate CXCR4 expression, favoring their migration to the draining LNs. Once there, we suggest that mast cells secrete immunoregulatory products, such as IL-10, thus promoting immune suppression.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by a grant from the National Cancer Institute, U.S. National Institutes of Health, Bethesda, MD, USA, (CA131207) and the Cancer Prevention and Research Institute of Texas (RP120777). The animal, histology, and flow cytometry facilities at the MD Anderson Cancer Center are supported, in part, by a core grant from the National Cancer Institute (CA16672). Funding from Secretaría de Investigación y Posgrado, Instituto Politécnico Nacional, and Consejo Nacional de Ciencia y Tecnología, “Red Desarrollo de Fármacos y Métodos Diagnósticos”, and 157100 C.B. supported R.C-S.
We thank Nasser Kazimi for technical support.
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
- B6-BMMC
- C57BL/6 BMMC
- c-PAF
- carbamyl-modified platelet-activating factor
- CHS
- contact hypersensitivity
- Ct
- threshold cycle
- DNFB
- 2,4-dinitro-1-flurobenzene
- PAF
- platelet-activating factor
- PAFR−/−
- platelet-activating factor receptor-deficient
- SCF
- stem cell factor
AUTHORSHIP
R.C-S. and S.E.U. designed the study. R.C-S., L.C., A.D.C-B., A.Y.L-F., and Y.M. performed the experiments. R.C-S. and S.E.U. wrote the paper. All of the authors approved the final version of the manuscript.
REFERENCES
- 1. Boscoe F. P., Schymura M. J. (2006) Solar ultraviolet-B exposure and cancer incidence and mortality in the United States, 1993–2002. BMC Cancer 6, 264–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Landi M. T., Baccarelli A., Tarone R. E., Pesatori A., Tucker M. A., Hedayati M., Grossman L. (2002) DNA repair, dysplastic nevi, and sunlight sensitivity in the development of cutaneous malignant melanoma. J. Natl. Cancer Inst. 94, 94–101 [DOI] [PubMed] [Google Scholar]
- 3. Wang Y., Digiovanna J. J., Stern J. B., Hornyak T. J., Raffeld M., Khan S. G., Oh K. S., Hollander M. C., Dennis P. A., Kraemer K. H. (2009) Evidence of ultraviolet type mutations in xeroderma pigmentosum melanomas. Proc. Natl. Acad. Sci. USA 106, 6279–6284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fisher M. S., Kripke M. L. (1982) Suppressor T lymphocytes control the development of primary skin cancers in ultraviolet-irradiated mice. Science 216, 1133–1134 [DOI] [PubMed] [Google Scholar]
- 5. Yoshikawa T., Rae V., Bruins-Slot W., Van den Berg J. W., Taylor J. R., Streilein J. W. (1990) Susceptibility to effects of UVB radiation on induction of contact hypersensitivity as a risk factor for skin cancer in humans. J. Invest. Dermatol. 95, 530–536 [DOI] [PubMed] [Google Scholar]
- 6. Vajdic C. M., van Leeuwen M. T., Webster A. C., McCredie M. R., Stewart J. H., Chapman J. R., Amin J., McDonald S. P., Grulich A. E. (2009) Cutaneous melanoma is related to immune suppression in kidney transplant recipients. Cancer Epidemiol. Biomarkers Prev. 18, 2297–2303 [DOI] [PubMed] [Google Scholar]
- 7. Kripke M. L. (1974) Antigenicity of murine skin tumors induced by ultraviolet light. J. Natl. Cancer Inst. 53, 1333–1336 [DOI] [PubMed] [Google Scholar]
- 8. Ullrich S. E. (2005) Mechanisms underlying UV-induced immune suppression. Mutat. Res. 571, 185–205 [DOI] [PubMed] [Google Scholar]
- 9. Chacon-Salinas R., Limon-Flores A. Y., Chavez-Blanco A. D., Gonzalez-Estrada A., Ullrich S. E. (2011) Mast cell-derived IL-10 suppresses germinal center formation by affecting T follicular helper cell function. J. Immunol. 186, 25–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Loser K., Mehling A., Loeser S., Apelt J., Kuhn A., Grabbe S., Schwarz T., Penninger J. M., Beissert S. (2007) Epidermal RANKL controls regulatory T-cell numbers via activation of dendritic cells. Nat. Med. 12, 1372–1379 [DOI] [PubMed] [Google Scholar]
- 11. Schwarz A., Noordegraaf M., Maeda A., Torii K., Clausen B. E., Schwarz T. (2010) Langerhans cells are required for UVR-induced immunosuppression. J. Invest. Dermatol. 130, 1419–1427 [DOI] [PubMed] [Google Scholar]
- 12. Fukunaga A., Khaskhely N. M., Ma Y., Sreevidya C. S., Taguchi K., Nishigori C., Ullrich S. E. (2010) Langerhans cells serve as immunoregulatory cells by activating NKT cells. J. Immunol. 185, 4633–4640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hart P. H., Grimbaldeston M. A., Swift G. J., Jaksic A., Noonan F. P., Finlay-Jones J. J. (1998) Dermal mast cells determine susceptibility to ultraviolet B-induced systemic suppression of contact hypersensitivity responses in mice. J. Exp. Med. 187, 2045–2053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Byrne S. N., Limon-Flores A. Y., Ullrich S. E. (2008) Mast cell migration from the skin to the draining lymph nodes upon ultraviolet irradiation represents a key step in the induction of immune suppression. J. Immunol. 180, 4648–4655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Galli S. J., Kalesnikoff J., Grimbaldeston M. A., Piliponsky A. M., Williams C. M., Tsai M. (2005) Mast cells as “tunable” effector and immunoregulatory cells: recent advances. Annu. Rev. Immunol. 23, 749–786 [DOI] [PubMed] [Google Scholar]
- 16. Kalesnikoff J., Galli S. J. (2008) New developments in mast cell biology. Nat. Immunol. 9, 1215–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Alard P., Kurimoto I., Niizeki H., Doherty J. M., Streilein J. W. (2001) Hapten-specific tolerance induced by acute, low-dose ultraviolet B radiation of skin requires mast cell degranulation. Eur. J. Immunol. 31, 1736–1746 [DOI] [PubMed] [Google Scholar]
- 18. De Fabo E. C., Noonan F. P. (1983) Mechanism of immune suppression by ultraviolet irradiation in vivo. I. Evidence for the existence of a unique photoreceptor in skin and its role in photoimmunology. J. Exp. Med. 157, 84–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Walterscheid J. P., Nghiem D. X., Kazimi N., Nutt L. K., McConkey D. J., Norval M., Ullrich S. E. (2006) Cis-urocanic acid, a sunlight-induced immunosuppressive factor, activates immune suppression via the 5-HT2A receptor. Proc. Natl. Acad. Sci. USA 103, 17420–17425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gorman S., Kuritzky L. A., Judge M. A., Dixon K. M., McGlade J. P., Mason R. S., Finlay-Jones J. J., Hart P. H. (2007) Topically applied 1,25-dihydroxyvitamin D3 enhances the suppressive activity of CD4+CD25+ cells in the draining lymph nodes. J. Immunol. 179, 6273–6283 [DOI] [PubMed] [Google Scholar]
- 21. Walterscheid J. P., Ullrich S. E., Nghiem D. X. (2002) Platelet-activating factor, a molecular sensor for cellular damage, activates systemic immune suppression. J. Exp. Med. 195, 171–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wolf P., Nghiem D. X., Walterscheid J. P., Byrne S., Matsumura Y., Matsumura Y., Bucana C., Ananthaswamy H. N., Ullrich S. E. (2006) Platelet-activating factor is crucial in psoralen and ultraviolet A-induced immune suppression, inflammation, and apoptosis. Am. J. Pathol. 169, 795–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Alappatt C., Johnson C. A., Clay K. L., Travers J. B. (2000) Acute keratinocyte damage stimulates platelet-activating factor production. Arch. Dermatol. Res. 292, 256–259 [DOI] [PubMed] [Google Scholar]
- 24. Marathe G. K., Johnson C., Billings S. D., Southall M. D., Pei Y., Spandau D., Murphy R. C., Zimmerman G. A., McIntyre T. M., Travers J. B. (2005) Ultraviolet B radiation generates platelet-activating factor-like phospholipids underlying cutaneous damage. J. Biol. Chem. 280, 35448–35457 [DOI] [PubMed] [Google Scholar]
- 25. Travers J. B., Berry D., Yao Y., Yi Q., Konger R. L. (2010) Ultraviolet B radiation of human skin generates platelet-activating factor receptor agonists. Photochem. Photobiol. 86, 949–954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Izumi T., Shimizu T. (1995) Platelet-activating factor receptor: gene expression and signal transduction. Biochim. Biophys. Acta 1259, 317–333 [DOI] [PubMed] [Google Scholar]
- 27. Travers J. B., Huff J. C., Rola-Pleszczynski M., Gelfand E. W., Morelli J. G., Murphy R. C. (1995) Identification of functional platelet-activating factor receptors on human keratinocytes. J. Invest. Dermatol. 105, 816–823 [DOI] [PubMed] [Google Scholar]
- 28. Zhang Q., Yao Y., Konger R. L., Sinn A. L., Cai S., Pollok K. E., Travers J. B. (2008) UVB radiation-mediated inhibition of contact hypersensitivity reactions is dependent on the platelet-activating factor system. J. Invest. Dermatol. 128, 1780–1787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kajiwara N., Sasaki T., Bradding P., Cruse G., Sagara H., Ohmori K., Saito H., Ra C., Okayama Y. (2010) Activation of human mast cells through the platelet-activating factor receptor. J. Allergy Clin. Immunol. 125, 1137.e6–1145.e6 [DOI] [PubMed] [Google Scholar]
- 30. Fukunaga A., Khaskhely N. M., Sreevidya C. S., Byrne S. N., Ullrich S. E. (2008) Dermal dendritic cells, and not Langerhans cells, play an essential role in inducing an immune response. J. Immunol. 180, 3057–3064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bussolati B., Biancone L., Cassoni P., Russo S., Rola-Pleszczynski M., Montrucchio G., Camussi G. (2000) PAF produced by human breast cancer cells promotes migration and proliferation of tumor cells and neo-angiogenesis. Am. J. Pathol. 157, 1713–1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Moreno S. E., Alves-Filho J. C., Rios-Santos F., Silva J. S., Ferreira S. H., Cunha F. Q., Teixeira M. M. (2006) Signaling via platelet-activating factor receptors accounts for the impairment of neutrophil migration in polymicrobial sepsis. J. Immunol. 177, 1264–1271 [DOI] [PubMed] [Google Scholar]
- 33. Ishii S., Shimizu T. (2000) Platelet-activating factor (PAF) receptor and genetically engineered PAF receptor mutant mice. Prog. Lipid Res. 39, 41–82 [DOI] [PubMed] [Google Scholar]
- 34. Grimbaldeston M. A., Chen C. C., Piliponsky A. M., Tsai M., Tam S. Y., Galli S. J. (2005) Mast cell-deficient W-sash c-kit mutant kit W-sh/W-sh mice as a model for investigating mast cell biology in vivo. Am. J. Pathol. 167, 835–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nghiem D. X., Walterscheid J. P., Kazimi N., Ullrich S. E. (2002) Ultraviolet radiation-induced immunosuppression of delayed-type hypersensitivity in mice. Methods 28, 25–33 [DOI] [PubMed] [Google Scholar]
- 36. Kuehn H. S., Jung M. Y., Beaven M. A., Metcalfe D. D., Gilfillan A. M. (2011) Prostaglandin E2 activates and utilizes mTORC2 as a central signaling locus for the regulation of mast cell chemotaxis and mediator release. J. Biol. Chem. 286, 391–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Limon-Flores A. Y., Chacon-Salinas R., Ramos G., Ullrich S. E. (2009) Mast cells mediate the immune suppression induced by dermal exposure to JP-8 jet fuel. Toxicol. Sci. 112, 144–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Simon H. U., Tsao P. W., Siminovitch K. A., Mills G. B., Blaser K. (1994) Functional platelet-activating factor receptors are expressed by monocytes and granulocytes but not by resting or activated T and B lymphocytes from normal individuals or patients with asthma. J. Immunol. 153, 364–377 [PubMed] [Google Scholar]
- 39. Marrache A. M., Gobeil F., Jr., Bernier S. G., Stankova J., Rola-Pleszczynski M., Choufani S., Bkaily G., Bourdeau A., Sirois M. G., Vazquez-Tello A., Fan L., Joyal J. S., Filep J. G., Varma D. R., Ribeiro-Da-Silva A., Chemtob S. (2002) Proinflammatory gene induction by platelet-activating factor mediated via its cognate nuclear receptor. J. Immunol. 169, 6474–6481 [DOI] [PubMed] [Google Scholar]
- 40. Katoh H., Hosono K., Ito Y., Suzuki T., Ogawa Y., Kubo H., Kamata H., Mishima T., Tamaki H., Sakagami H., Sugimoto Y., Narumiya S., Watanabe M., Majima M. (2010) COX-2 and prostaglandin EP3/EP4 signaling regulate the tumor stromal proangiogenic microenvironment via CXCL12-CXCR4 chemokine systems. Am. J. Pathol. 176, 1469–1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Niizeki H., Alard P., Streilein J. W. (1997) Cutting edge: calcitonin gene-related peptide is necessary for ultraviolet B-impaired induction of contact hypersensitivity. J. Immunol. 159, 5183–5186 [PubMed] [Google Scholar]
- 42. Ullrich S. E., Nghiem D. X., Khaskina P. (2007) Suppression of an established immune response by UVA—a critical role for mast cells. Photochem. Photobiol. 83, 1095–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sreevidya C. S., Khaskhely N. M., Fukunaga A., Khaskina P., Ullrich S. E. (2008) Inhibition of photocarcinogenesis by platelet-activating factor or serotonin receptor antagonists. Cancer Res. 68, 3978–3984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Melnikova V. O., Mourad-Zeidan A. A., Lev D. C., Bar-Eli M. (2006) Platelet-activating factor mediates MMP-2 expression and activation via phosphorylation of cAMP-response element-binding protein and contributes to melanoma metastasis. J. Biol. Chem. 281, 2911–2922 [DOI] [PubMed] [Google Scholar]
- 45. Lefebvre J. S., Marleau S., Milot V., Levesque T., Picard S., Flamand N., Borgeat P. (2010) Toll-like receptor ligands induce polymorphonuclear leukocyte migration: key roles for leukotriene B4 and platelet-activating factor. FASEB J. 24, 637–647 [DOI] [PubMed] [Google Scholar]
- 46. Nilsson G., Metcalfe D. D., Taub D. D. (2000) Demonstration that platelet-activating factor is capable of activating mast cells and inducing a chemotactic response. Immunology 99, 314–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rastogi P., White M. C., Rickard A., McHowat J. (2008) Potential mechanism for recruitment and migration of CD133 positive cells to areas of vascular inflammation. Thromb. Res. 123, 258–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fehlner-Gardiner C. C., Uniyal S., von Ballestrem C. G., Chan B. M. (1996) Differential utilization of VLA-4 (alpha 4 beta 1) and -5 (alpha 5 beta 1) integrins during the development of mouse bone marrow-derived mast cells. Differentiation 60, 317–325 [DOI] [PubMed] [Google Scholar]
- 49. Marques M., Pei Y., Southall M. D., Johnston J. M., Arai H., Aoki J., Inoue T., Seltmann H., Zouboulis C. C., Travers J. B. (2002) Identification of platelet-activating factor acetylhydrolase II in human skin. J. Invest. Dermatol. 119, 913–919 [DOI] [PubMed] [Google Scholar]
- 50. Liu M., Zhu H., Li J., Garcia C. C., Feng W., Kirpotina L. N., Hilmer J., Tavares L. P., Layton A. W., Quinn M. T., Bothner B., Teixeira M. M., Lei B. (2012) Group A Streptococcus secreted esterase hydrolyzes platelet-activating factor to impede neutrophil recruitment and facilitate innate immune evasion. PLoS Pathog. 8, e1002624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Liu J., Chen R., Marathe G. K., Febbraio M., Zou W., McIntyre T. M. (2011) Circulating platelet-activating factor is primarily cleared by transport, not intravascular hydrolysis, by lipoprotein-associated phospholipase A2/PAF acetylhydrolase. Circ. Res. 108, 469–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Prescott S. M., Zimmerman G. A., Stafforini D. M., McIntyre T. M. (2000) Platelet-activating factor and related lipid mediators. Annu. Rev. Biochem. 69, 419–445 [DOI] [PubMed] [Google Scholar]
- 53. Pei Y., Barber L. A., Murphy R. C., Johnson C. A., Kelley S. W., Dy L. C., Fertel R. H., Nguyen T. M., Williams D. A., Travers J. B. (1998) Activation of the epidermal platelet-activating factor receptor results in cytokine and cyclooxygenase-2 biosynthesis. J. Immunol. 161, 1954–1961 [PubMed] [Google Scholar]
- 54. Rivas J. M., Ullrich S. E. (1992) Systemic suppression of delayed-type hypersensitivity by supernatants from UV-irradiated keratinocytes. An essential role for keratinocyte-derived IL-10. J. Immunol. 149, 3865–3871 [PubMed] [Google Scholar]
- 55. Beissert S., Hosoi J., Kühn R., Rajewsky K., Müller W., Granstein R. D. (1996) Impaired immunosuppressive response to ultraviolet radiation in interleukin-10-deficient mice. J. Invest. Dermatol. 107, 553–557 [DOI] [PubMed] [Google Scholar]
- 56. Ramos G., Limon-Flores A. Y., Ullrich S. E. (2007) Dermal exposure to jet fuel suppresses delayed-type hypersensitivity: a critical role for aromatic hydrocarbons. Toxicol. Sci. 100, 415–422 [DOI] [PubMed] [Google Scholar]
- 57. Ullrich S. E., Lyons H. J. (2000) Mechanisms involved in the immunotoxicity induced by dermal application of JP-8 jet fuel. Toxicol. Sci. 58, 290–298 [DOI] [PubMed] [Google Scholar]
- 58. Ramos G., Kazimi N., Nghiem D. X., Walterscheid J. P., Ullrich S. E. (2004) Platelet activating factor receptor binding plays a critical role in jet fuel-induced immune suppression. Toxicol. Appl. Pharmacol. 195, 331–338 [DOI] [PubMed] [Google Scholar]
- 59. Miller A. C., Rashid R. M., Elamin E. M. (2007) The “T” in trauma: the helper T-cell response and the role of immunomodulation in trauma and burn patients. J. Trauma 63, 1407–1417 [DOI] [PubMed] [Google Scholar]
- 60. Dudeck A., Dudeck J., Scholten J., Petzold A., Surianarayanan S., Kohler A., Peschke K., Vohringer D., Waskow C., Krieg T., Muller W., Waisman A., Hartmann K., Gunzer M., Roers A. (2011) Mast cells are key promoters of contact allergy that mediate the adjuvant effects of haptens. Immunity 34, 973–984 [DOI] [PubMed] [Google Scholar]
- 61. McLachlan J. B., Shelburne C. P., Hart J. P., Pizzo S. V., Goyal R., Brooking-Dixon R., Staats H. F., Abraham S. N. (2008) Mast cell activators: a new class of highly effective vaccine adjuvants. Nat. Med. 14, 536–541 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.