This article reviews the clinical evidence supporting the benefits of targeted agents in the treatment of metastatic renal cell carcinoma, discusses survival endpoints used in their pivotal clinical trials, and outlines future research directions.
Keywords: Angiogenesis inhibitor, Combination drug therapy, Mammalian target of rapamycin, Renal cell carcinoma, Vascular endothelial growth factor
Abstract
Although systemic therapy for patients with metastatic renal cell carcinoma (mRCC) was once limited to the cytokines interleukin-2 and interferon (IFN)-α, in recent years several targeted therapies have become available for first- and second-line use. These include sorafenib, sunitinib, bevacizumab (plus IFN-α), temsirolimus, everolimus, and, most recently, pazopanib. This expanded list of treatment options arose from molecular biological research that revealed aberrant signal transduction activities in RCC, enabling the identification of specific molecular targets for therapy. Molecular-targeted therapies have better efficacy and tolerability than cytokine therapy, and many are administered orally. The superior outcomes achieved with molecular-targeted agents are prompting investigators to reconsider overall survival as a primary endpoint in clinical trials, given the inherent complications of a required long duration of follow-up, a required large population, and confounding caused by crossover trial designs or effects of subsequent therapy after progression on the agent of interest. In mRCC trials, progression-free survival has become a popular primary endpoint and has served as the basis of approval for several targeted therapies. In addition to the identification of new agents, current research is focused on the evaluation of combination therapy with targeted agents. As more information regarding mechanisms of disease and drug resistance becomes available, new targets, new targeted agents, and new combinations will be studied with the goal of providing maximal efficacy with minimal toxicity. This article reviews the clinical evidence supporting the benefits of targeted agents in mRCC treatment, discusses survival endpoints used in their pivotal clinical trials, and outlines future research directions.
Introduction
In the past 5 years, treatment options have expanded considerably for patients with metastatic renal cell carcinoma (mRCC) [1]. Previously, systemic treatment was limited to cytokine therapy with interleukin (IL)-2 or interferon (IFN)-α, because mRCC is largely resistant to chemotherapy [2]. Cytokine use is based on the rationale that immune system stimulation kills cancer cells. However, in patients with mRCC, cytokine therapy is associated with low rates of response and high rates of toxicity in the first-line setting [2]. In the second-line setting (in patients who have progressed on one cytokine), even fewer responses are observed while toxicity remains similar to that of first-line use [3]. Consequently, new therapies were needed to improve outcomes in patients with mRCC.
As information regarding aberrant activities of signal transduction pathways in RCC became available, specific molecular targets for potential therapies were identified and analyzed pharmacologically in a variety of in vitro and preclinical studies. As detailed in this supplement by Finley et al. [4], molecular-targeted therapies directed at the vascular endothelial growth factor (VEGF) and the mammalian target of rapamycin (mTOR) signaling pathways evolved from such research, because both VEGF and mTOR activities were shown to be involved in the pathogenesis of mRCC [5].
Today, six targeted therapies have been evaluated in randomized, controlled phase III clinical trials of patients with mRCC and approved by regulatory authorities. The objectives of this article are to review the clinical evidence supporting the benefits of these agents, discuss the survival endpoints used in their pivotal clinical trials, and identify future research directions with these targeted therapies.
Efficacy of Currently Approved Molecular-Targeted Agents for mRCC
The six molecular-targeted agents approved in the U.S. for the treatment of mRCC are sorafenib, sunitinib, bevacizumab (in combination with IFN-α), temsirolimus, everolimus, and pazopanib. With the exception of pazopanib, which is currently under regulatory agency review in Europe with conditional approval pending mature data, all these agents are approved in Europe as well. Figure 1 summarizes their chronology and use in the U.S. and Europe, and Table 1 summarizes key efficacy data from their pivotal trials, which are reviewed in detail below [6–16].
Figure 1.
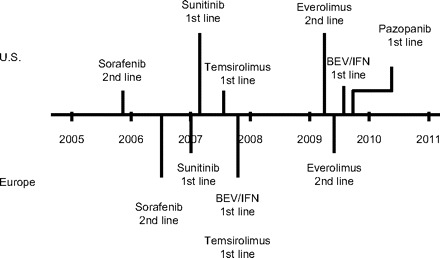
Chronology and uses of available molecular-targeted agents in metastatic renal cell carcinoma in the U.S. and Europe.
Abbreviations: BEV, bevacizumab; IFN, interferon-α.
Table 1.
Efficacy of approved targeted therapies for mRCC in pivotal phase III clinical trials
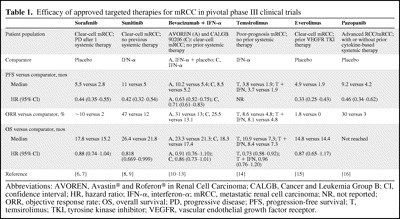
Abbreviations: AVOREN, Avastin® and Roferon® in Renal Cell Carcinoma; CALGB, Cancer and Leukemia Group B; CI, confidence interval; HR, hazard ratio; IFN-α, interferon-α; mRCC, metastatic renal cell carcinoma; NR, not reported; ORR, objective response rate; OS, overall survival; PD, progressive disease; PFS, progression-free survival; T, temsirolimus; TKI, tyrosine kinase inhibitor; VEGFR, vascular endothelial growth factor receptor.
Stage migration did not significantly affect enrollment in the pivotal clinical trials that included patients with mRCC. A phenomenon of survival migration (patients living longer as a result of more effective front-line therapy) was observed and may influence outcomes in second-line treatment and beyond (i.e., one sequence of agents may be superior to another sequence with regard to progression-free survival [PFS] and overall survival [OS]). Ongoing clinical trials in the refractory setting may shed more light on whether this ultimately will affect patient management or the ability to interpret ongoing and recently completed phase III trials; however, it may not, because randomization may negate this effect.
Sorafenib
Sorafenib is an oral multikinase inhibitor that inhibits signaling by Ras, VEGF receptors (VEGFRs), and platelet-derived growth factor receptors (PDGFRs). It was approved by the U.S. Food and Drug Administration (FDA) in December 2005 and by the European Medicines Agency (EMA) in July 2006 for the treatment of patients with cytokine-refractory, advanced RCC or mRCC.
The pivotal trial that led to the approval of sorafenib, the Treatment Approaches in Renal Cancer Global Evaluation Trial (TARGET), was a randomized, double-blind, placebo-controlled, international phase III study of single-agent sorafenib in 903 cytokine-refractory patients [6]. Patients enrolled in TARGET were of favorable or intermediate risk for survival as determined by the Memorial Sloan-Kettering Cancer Center (MSKCC) risk score [6, 17]. They received continuous oral sorafenib, 400 mg twice daily (n = 451), or placebo (n = 452) in 6-week cycles for the first 24 weeks and in 8-week cycles thereafter; treatment was continued until disease progression or patient withdrawal from the study. The primary endpoint was OS, and the secondary endpoint was PFS [6]. The study design included assessments of OS at two planned interim analyses and one final analysis and an assessment of PFS at a planned interim analysis [7].
The interim analysis of PFS, carried out in January 2005, demonstrated a significantly longer median PFS interval with sorafenib than with placebo, 5.5 months versus 2.8 months, respectively (p < .001) [6]. The PFS time was longer with sorafenib regardless of age, MSKCC risk score, prior cytokine therapy, the presence of lung or liver metastases at baseline, and time since diagnosis [6]. Based on these promising results, patients assigned to receive placebo were allowed to cross over to the other study arm to receive sorafenib in May 2005. In the first interim analysis of OS, carried out just before crossover, the median OS time had not been reached in the sorafenib group and was 14.7 months in the placebo group. Also at that time, assessment of the objective response rate (ORR) found rates of 10% with sorafenib and 2% with placebo [6]. The second interim analysis of OS was carried out in November 2005. Although the median OS times were 19.3 months with sorafenib and 15.9 months with placebo, the difference did not achieve statistical significance [6]. The final analysis of OS was carried out in September 2006; the median OS times were 17.8 months with sorafenib and 15.2 months with placebo, but the difference was not statistically significant [7]. However, a preplanned secondary analysis that accounted for the confounding effects of crossover showed that the OS time was significantly longer with sorafenib than with placebo (17.8 months versus 14.3 months, respectively; p = .0287) [7].
Sunitinib
Sunitinib is an oral multitargeted receptor tyrosine kinase inhibitor (RTKI) that inhibits signaling by VEGFRs, PDGFRs, and c-Kit. The FDA granted sunitinib accelerated approval in January 2006 based on responses in patients with mRCC who had failed cytokine therapy and regular approval in February 2007 based on results obtained in the first-line treatment of patients with advanced RCC or mRCC. Sunitinib received conditional approval in July 2006 and full approval in January 2007 from the EMA.
The trial that led to the full approval of sunitinib was a randomized phase III study that compared single-agent sunitinib with IFN-α in 750 previously untreated patients with mRCC [9]. Patients received oral sunitinib, 50 mg, once daily in 6-week cycles (4 weeks of treatment and 2 weeks of no treatment; n = 350) or s.c. IFN-α three times weekly, escalated in weekly increments from 3 MU to 6 MU to 9 MU per dose (n = 350). Treatment was continued until disease progression, unacceptable toxicity, or consent withdrawal. PFS was the primary endpoint. OS was a secondary endpoint, along with the ORR and health-related quality of life (QOL), which was assessed with the Functional Assessment of Cancer Therapy–General (FACT-G) and the FACT–Kidney Symptom Index (FKSI) [9]. Three analyses were scheduled, and the results of the second and third were completed and reported [8, 9].
At the second analysis, the median PFS interval was significantly longer with sunitinib than with IFN-α (11 months versus 5 months; p < .001), and this result was unaffected by patient age, sex, or MSKCC risk score [9]. The median OS time had not been reached in either group [9]. The ORRs at the second analysis were 31% with sunitinib and 6% with IFN-α (p < .001); at the final analysis, the ORRs were 47% and 12%, respectively (p < .001) [8, 9]. In addition, QOL was superior with sunitinib than with IFN-α; scores indicated clinically meaningful differences in kidney cancer–related symptoms and overall QOL (p < .001) [9].
The PFS benefit demonstrated at the second analysis enabled patients who had progressed on IFN-α to cross over to receive sunitinib. Results of the final survival analysis showed a marginally greater median OS time with sunitinib than with IFN-α (26.4 months versus 21.8 months, respectively; p = .051) [8]. An exploratory analysis that accounted for the confounding effects of crossover showed that the OS time was significantly longer with sunitinib than with IFN-α (26.4 months versus 20.0 months, respectively; p = .036). In addition, a separate exploratory analysis of patients who did not receive poststudy cancer treatment showed that the median OS time with sunitinib was double that with IFN-α (28.1 months versus 14.1 months, respectively; p = .003) [8].
Bevacizumab plus IFN-α
Bevacizumab is an i.v. administered anti-VEGF monoclonal antibody given in combination with s.c. injected IFN-α. This combination was approved by the EMA in November 2007 and by the FDA in August 2009 for the first-line treatment of patients with advanced RCC or mRCC.
The pivotal trials, carried out in previously untreated patients with mRCC, were a randomized, double-blind study of bevacizumab plus IFN-α versus placebo plus IFN-α (Avastin® and Roferon® in Renal Cell Carcinoma [AVOREN]; n = 649) [10, 11] and a randomized, open-label study of bevacizumab plus IFN-α versus IFN-α (Cancer and Leukemia Group B [CALGB] 90206; n = 732) [12, 13]. Both evaluated the same doses of bevacizumab (10 mg/kg every 2 weeks) and IFN-α (9 MU three times weekly) and had OS as the primary endpoint [10, 13]. In both, bevacizumab treatment was continued until disease progression, unacceptable toxicity, or consent withdrawal; in AVOREN, IFN-α was administered for a maximum of 52 weeks. Each has reported results of an interim analysis of PFS and a final analysis of OS.
In both the AVOREN and CALGB 90206 trials, interim analyses showed that median the PFS interval was significantly longer with bevacizumab plus IFN-α than with the comparator (AVOREN: 10.2 months versus 5.4 months, respectively; p = .0001; CALGB 90206: 8.5 months versus 5.2 months, respectively; p < .0001) [10, 13]. In the AVOREN trial, the ORRs were 31% with bevacizumab plus IFN-α and 13% with placebo plus IFN-α (p = .0001) [10]; in the CALGB 90206 trial, the ORRs were 25.5% with bevacizumab plus IFN-α and 13.1% with IFN-α (p < .0001) [13].
In both trials, bevacizumab plus IFN-α showed trends toward a longer OS time than with the comparator, but the differences were not statistically significant [11, 12]. However, the OS results of both trials were likely confounded. In the AVOREN trial, after favorable PFS results were obtained for bevacizumab plus IFN-α, compared with placebo plus IFN-α, patients in the placebo arm were allowed to cross over to the bevacizumab arm [10]. In addition, more than half the patients in each arm who discontinued because of disease progression or other reasons received subsequent therapy, which included sorafenib and sunitinib [11]. In the CALGB 90206 trial, crossover was not permitted for patients who received IFN-α monotherapy, but poststudy data showed that most of these patients received subsequent therapy, and almost half received sorafenib or sunitinib [13].
Temsirolimus
Temsirolimus is an mTOR inhibitor that was approved by the FDA in May 2007 and by the EMA in November 2007 for the first-line treatment of patients with poor-prognosis mRCC.
The pivotal trial of temsirolimus was a randomized phase III study that compared temsirolimus or temsirolimus plus IFN-α with IFN-α alone in 626 patients newly diagnosed with mRCC who had at least three of six predictors of short survival. These predictors included serum lactate dehydrogenase >1.5× the upper limit of normal, hemoglobin level below the lower limit of normal, corrected serum calcium level >10 mg/dl, <1 year from initial diagnosis to randomization, Karnofsky performance status score of 60 or 70, and multiple organ metastases [14]. Patients received a 30-minute i.v. infusion of 25 mg temsirolimus (temsirolimus-only group) or 15 mg temsirolimus plus s.c. IFN-α three times weekly (3 MU for the first week, 6 MU thereafter) (combination therapy group). Patients in the IFN-α monotherapy group received IFN-α three times weekly, escalated in weekly increments from 3 MU to 9 MU to 18 MU. Treatment was continued until disease progression, symptomatic deterioration, or unacceptable toxicity. The primary endpoint was OS [14].
Per investigator assessments, the median PFS intervals were 3.8 months with temsirolimus alone, 1.9 months with IFN-α alone, and 3.7 months with the combination [14]. The ORR was 4.8% with IFN-α alone, 8.6% with temsirolimus alone, and 8.1% with the combination. The median OS time was significantly longer with temsirolimus alone than with IFN-α alone (10.9 months versus 7.3 months, respectively; p = .008), and combination therapy with temsirolimus and IFN-α did not lead to a significantly longer median OS time than with IFN-α alone (8.4 months versus 7.3 months, respectively) [14].
Everolimus
Everolimus is an oral mTOR inhibitor that was approved by the FDA in March 2009 for the treatment of patients with advanced RCC after failure of sorafenib or sunitinib, and by the EMA in May 2009 for the treatment of patients with advanced RCC that has progressed on or after treatment with VEGF-targeted therapy.
The pivotal trial, Renal Cell cancer treatment with Oral RAD001 given Daily (RECORD)-1, was a randomized, double-blind, placebo-controlled phase III study of single-agent everolimus versus placebo in 416 patients with mRCC who had progressed on VEGFR TKI therapy [15, 18]. Prior bevacizumab and cytokine therapy also were allowed. Patients received continuous oral everolimus, 10 mg (n = 277), or placebo (n = 139) once daily [18]. Treatment was continued until disease progression, unacceptable toxicity, death, or discontinuation. Patients who progressed on placebo were allowed to cross over to receive everolimus. The primary endpoint was PFS. Secondary endpoints included OS and the ORR, as well as disease-related symptoms and health-related QOL, which were assessed with the FKSI–Disease-Related Symptoms and the European Organization for the Research and Treatment of Cancer QLQ-30 questionnaires [18]. The study design included two planned interim analyses and a final analysis [18].
The double-blind phase of the study was terminated early (February 2008) after results of the second interim analysis of data collected through mid-October 2007 showed that the median PFS interval was significantly longer with everolimus (4.0 months) than with placebo (1.9 months; p < .0001) [18]. The PFS time was significantly longer with everolimus regardless of previous treatment, MSKCC risk score, age, or sex [18]. The study was then unblinded, and all patients who were receiving placebo were offered open-label everolimus [15]. The ORRs at the second interim analysis were 1% with everolimus and 0% with placebo; disease stabilization was observed in 63% of patients treated with everolimus and 32% of patients receiving placebo. No differences between groups were noted in time to definitive deterioration of symptoms or in QOL [18].
The final analysis was carried out with data collected through February 2008, when the double-blind phase was terminated; OS data were collected through November 2008. In the final analysis, the PFS benefit with everolimus remained, with a median PFS time of 4.9 months, versus 1.9 months with placebo [15]. The rates of disease stabilization with everolimus and placebo were 66.8% and 32.4%, respectively [15, 18]. Everolimus led to a significantly longer time to definitive deterioration in Karnofsky performance status and time to definitive deterioration of symptoms than with placebo [15]. Although results of the final survival analysis showed no significant difference in the median OS time between everolimus and placebo, a post hoc exploratory analysis that accounted for the confounding effects of crossover (the rank-preserving structural failure time model) showed that everolimus provided a 1.9-fold longer survival time than placebo [15].
Pazopanib
Pazopanib is an oral multikinase angiogenesis inhibitor that inhibits signaling by VEGFRs, PDGFRs, and c-Kit. It was approved by the FDA in October 2009 for the first-line treatment of patients with advanced RCC.
The pivotal trial was a randomized, double-blind, placebo-controlled phase III study of single-agent pazopanib in 435 cytokine-pretreated or treatment-naïve patients with locally advanced RCC and/or mRCC [16]. Patients received continuous oral pazopanib, 800 mg (n = 290), or placebo (n = 145) once daily. Treatment continued until disease progression, death, unacceptable toxicity, or consent withdrawal. The primary endpoint was PFS; other endpoints included OS, ORR, duration of response, and health-related QOL. Patients who progressed on placebo were eligible to receive open-label pazopanib [16].
The median PFS duration was significantly longer with pazopanib than with placebo in the entire population (9.2 months versus 4.2 months, respectively; p < .0001) and in the cytokine-pretreated (7.4 months versus 4.2 months, respectively; p < .001) and treatment-naïve (11.1 months versus 2.8 months, respectively; p < .0001) subpopulations [16]. Pazopanib led to a significantly longer PFS time regardless of MSKCC risk score, age, sex, or performance status. The ORR was 30% with pazopanib, versus 3% with placebo (p < .001), and QOL with pazopanib did not differ significantly from that with placebo. Final OS results are pending maturity of the data [16].
Other Agents
Axitinib (AG-013736)
Axitinib is an orally bioavailable VEGFR TKI. In a phase II open-label, single-arm study, axitinib monotherapy was evaluated in 52 patients with cytokine-refractory mRCC, 30 of whom had at least one poor prognostic factor per MSKCC risk criteria [19]. The ORR was 44.2%. The median time to progression was 15.7 months and the median OS time was 29.9 months [19]. A subsequent phase II, open-label, single-arm trial assessed the activity of axitinib in 62 patients with sorafenib-refractory disease [20]. MSKCC risk status was not determined in this population. The ORR was 22.6%, median PFS time was 7.4 months, and median OS time was 13.6 months [20]. When data from patients refractory to sorafenib alone (n = 15), sunitinib and sorafenib (n = 14), and cytokines and sorafenib (n = 29) were analyzed post hoc, the ORRs were 27%, 7%, and 28%, respectively, and the median PFS times were 7.7 months, 7.1 months, and 9 months, respectively, suggesting that total crossresistance does not exist between axitinib and these agents [21].
Tivozanib
Tivozanib, also an orally bioavailable VEGFR TKI, was investigated in a phase II randomized, placebo-controlled, discontinuation trial of 272 patients naïve to VEGF-targeted therapy. Interim results indicated a 28% ORR [22]. Trial data collected at a later time point showed a 25.4% ORR and a median PFS interval of 11.8 months; a subgroup analysis of these data suggested that patients with clear cell RCC and prior nephrectomy appear to respond best to tivozanib, with an ORR of 29.6% and a median PFS time that was not reached [23].
Dovitinib (TKI258)
Dovitinib is a selective oral inhibitor of fibroblast growth factor receptors and VEGFRs. Published phase I data from the phase I/II trial of dovitinib in 20 heavily pretreated patients indicated that the ORR was 10% and the preliminary median PFS duration was 5.5 months. The maximum-tolerated dose for further study in the phase II portion of the trial was 500 mg daily [24].
Survival Endpoints Used in Pivotal Clinical Trials of Approved Molecular-Targeted Agents
Optimal endpoints for clinical trials of oncology agents have been the subject of much discussion in recent literature. OS is considered the gold standard for efficacy evaluation; however, inherent difficulties are present with the use of OS. These difficulties include the length of time required for its measurement, the requirement for a large sample size, the confounding effects of subsequent therapies after discontinuation of study therapy, and the confounding effects of crossover trial designs [25]. In contrast, PFS requires a shorter length of time and a smaller population for measurement, and it is not influenced by the effects of subsequent therapy [26].
OS was the primary endpoint for the pivotal trials of sorafenib, bevacizumab plus IFN-α, and temsirolimus. As described above, in the TARGET trial, the primary intent-to-treat analysis of OS did not meet the prespecified statistical threshold for significance, suggesting no survival benefit with sorafenib versus placebo. However, a crossover effect was demonstrated when the results of the preplanned secondary analysis that censored patients receiving placebo revealed a survival benefit with sorafenib [7]. This result was obtained with data from September 2006; in contrast, the longer PFS interval with sorafenib was evident at the January 2005 interim analysis. The longer median PFS time seen in the TARGET trial was the basis for the regulatory approval of sorafenib.
In the AVOREN trial, concern for the poststudy effects of other new targeted therapies on OS led to the use of PFS as the basis for regulatory approval of bevacizumab plus IFN-α. The trial was conducted during the time period that sorafenib and sunitinib first became available, creating the potential for patients with disease progression to receive these new agents as second-line therapy, which would confound OS data from the AVOREN trial. The investigators made an agreement with regulatory authorities to use the PFS data obtained prior to the final OS data as the basis for approval [10].
The temsirolimus pivotal trial did not include a crossover design; in addition, this poor-prognosis population required a shorter length of time for measurement of OS. In such a situation, OS is an appropriate endpoint because it is achievable [26]. However, as reviewed above, most pivotal trials in mRCC patients include a crossover design, and most patient populations are more heterogeneous with respect to the MSKCC risk score, making OS a more difficult endpoint to use.
PFS was the primary endpoint for the pivotal trials of sunitinib, everolimus, and pazopanib, and the basis for their regulatory approval. In mRCC patients, treatment effects on PFS are associated with treatment effects on OS; therefore, PFS has been proposed as an alternative endpoint to OS [26]. The validity of PFS as a surrogate for OS was supported by recent retrospective studies of patients with RCC treated with VEGFR-targeted therapies, which demonstrated a positive relationship between OS and PFS at 3 and 6 months [27, 28].
Future Directions: Combination Therapy with Targeted Agents
Sequential therapy with targeted agents is the current standard of care [1]; however, the strategy of combination therapy with targeted agents is under active investigation. Combination therapy provides the potential for additive or synergistic effects as a result of a more complete blockade of aberrant signaling. In addition, it has the potential to delay or circumvent the development of resistance that would eventually arise with single-agent therapy from the redundancies in signaling pathways. The goal is to use each agent at its full dose, but efficacy gains must be balanced with the potential for greater toxicity.
Sorafenib Combinations
Sorafenib was investigated in combination with cytokine therapy in several phase II trials. Sorafenib (400 mg twice daily) plus IL-2 (4.5 MU five times weekly) for six consecutive weeks was safe, feasible, and more active than monotherapy with sorafenib (400 mg twice daily) in a phase II trial of 128 previously untreated patients with mRCC. The median PFS duration, the primary endpoint, was 38 weeks with the combination and 30 weeks with sorafenib monotherapy [29]. In two phase II trials of sorafenib plus IFN-α, response rates were higher than expected for either drug alone, but high rates of toxicity and required dose reduction limited therapy [30, 31]. In contrast, sorafenib (400 mg twice daily) plus low-dose IFN-α (3 MU five times weekly) was active and well tolerated in a phase II trial of previously untreated patients with mRCC, prompting a recommendation for further investigation [32].
The combination of two anti-VEGF therapies, sorafenib and bevacizumab, was evaluated in a phase I trial of patients with a variety of advanced solid tumors but resulted in an unexpected level of toxicity at lower doses. Neither agent could be escalated to its full dose [33]. A combination with reduced doses (sorafenib, 200 mg; bevacizumab, 5 mg/kg) is being evaluated in the phase II Bevacizumab, Sorafenib and Temsirolimus in advanced renal cell carcinoma (BeST) trial (Table 2) [34–37].
Table 2.
Ongoing trials of combination therapy with targeted agents in advanced RCC or mRCC

Abbreviations: BeST, Bevacizumab, Sorafenib and Temsirolimus in advanced renal cell carcinoma; IFN-α, interferon-α; INTORACT, INvestigation of TORisel and Avastin Combination Therapy; mRCC, metastatic RCC; PFS, progression-free survival; RCC, renal cell carcinoma; RECORD, Renal Cell cancer treatment with Oral RAD001 given Daily; TORAVA, TORisel and AVAstin.
Sunitinib Combinations
In a phase I trial in patients with previously treated advanced RCC, VEGFR tyrosine kinase inhibition with sunitinib plus downstream mTOR inhibition with temsirolimus resulted in substantial toxicity at low starting doses of both agents (sunitinib, 25 mg daily; temsirolimus, 15 mg once weekly), causing trial termination [38].
The combination of sunitinib and bevacizumab was studied in a phase I trial of patients with advanced solid tumors [39], a phase I trial of patients with mRCC [40], and a case series of patients who had failed prior sunitinib [41]. It was feasible and active in RCC, although toxicities at higher doses and with longer durations of therapy require additional investigation [39–41].
Temsirolimus Combinations
Combination therapy with full doses of i.v. temsirolimus (25 mg weekly) and bevacizumab (10 mg/kg every 2 weeks in 4-week cycles) was active and well tolerated in a phase II study of patients with RTKI-refractory RCC. Interim results showed an ORR of 16% and a disease stabilization rate of 72% [42], and prompted comparison of this regimen with bevacizumab plus IFN-α in a phase III study [INvestigation of TORisel and Avastin Combination Therapy (INTORACT)] (Table 2) [34–37]. This combination also is being evaluated in the BeST and TORisel and AVAstin (TORAVA) trials (Table 2) [34–37].
Everolimus Combinations
Combination therapy with everolimus and the VEGFR TKIs is showing promise in initial studies. Everolimus (5 mg daily) plus sorafenib (400 mg daily) was safe and feasible in a phase I trial of patients with advanced RCC, prompting phase II study [43]. In a phase I trial of patients with mRCC, a maximum-tolerated dose of everolimus of 20 mg weekly plus sunitinib at a dose of 37.5 mg daily was identified for phase II study [44]. In contrast, everolimus (2.5 mg daily) plus imatinib (600 mg daily) was not recommended for further development after results of a phase II study in previously treated patients with mRCC demonstrated a 3-month PFS rate of 49%, which did not meet the prespecified criteria for continuation [45].
In a phase II study of two groups of patients with mRCC, one with and one without prior TKI treatment, everolimus (10 mg daily) plus bevacizumab (10 mg/kg every 2 weeks) was active and well tolerated. The median PFS times were 9.1 months in previously untreated patients and 7.1 months in previously treated patients; the ORRs were 30% and 23%, respectively [46]. This regimen, which uses full doses of each agent, is being evaluated as first-line therapy in a phase II study, RECORD-2 (Table 2) [34–37]. Ongoing clinical trials of combination therapy with targeted agents in patients with mRCC are summarized in Table 2 [34–37].
Conclusions
The development of molecular-targeted therapies has greatly increased the treatment options available for and outcomes achieved by patients with mRCC. Since 2005, six targeted agents have been approved by regulatory authorities for various uses in advanced RCC or mRCC patients. Clinical trial data demonstrate better prognoses with these agents, because the PFS and OS times achieved with targeted agents have been shown to be superior to those seen with IFN-α in head-to-head comparisons, and second-line therapies have achieved PFS and OS times superior to those with placebo. The greater availability of more effective agents has complicated the use of OS as a primary efficacy endpoint in mRCC clinical trials, leading to proposals for alternative endpoints. Efforts to enhance efficacy and combat resistance with targeted agents include investigations of combination therapy. At present, combination therapy strategies have not been proven to be beneficial, with many combinations showing excessive toxicity with marginal or inferior efficacy to that seen with the sequential use of agents. Combination therapy strategies that incorporate new agents that target pathways important in drug resistance are undergoing early evaluation in clinical trials. As more information regarding mechanisms of disease and drug resistance becomes available, new targets, new targeted agents, and new combinations will be studied with the goal of providing maximal efficacy with manageable toxicity.
Acknowledgments
The author takes full responsibility for the content of the paper but thanks Stephanie Leinbach, Ph.D., and Amy Zannikos, Pharm.D., supported by Novartis Pharmaceuticals Corporation, for their assistance in manuscript writing and editing.
References
- 1.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology™. Kidney Cancer. [accessed March 26, 2010]. V. 2.2010. Available at http://www.nccn.org/professionals/physician_gls/PDF/kidney.pdf.
- 2.Oudard S, George D, Medioni J, et al. Treatment options in renal cell carcinoma: Past, present and future. Ann Oncol. 2007;18(suppl 10):x25–x31. doi: 10.1093/annonc/mdm411. [DOI] [PubMed] [Google Scholar]
- 3.Escudier B, Chevreau C, Lasset C, et al. Cytokines in metastatic renal cell carcinoma: Is it useful to switch to interleukin-2 or interferon after failure of a first treatment? J Clin Oncol. 1999;17:2039–2043. doi: 10.1200/JCO.1999.17.7.2039. [DOI] [PubMed] [Google Scholar]
- 4.Finley DS, Pantuck AJ, Belldegrun AS. Tumor biology and prognostic factors in renal cell carcinoma. The Oncologist. 2011;16(suppl 2):4–13. doi: 10.1634/theoncologist.2011-S2-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mulders P. Vascular endothelial growth factor and mTOR pathways in renal cell carcinoma: Differences and synergies of two targeted mechanisms. BJU Int. 2009;104:1585–1589. doi: 10.1111/j.1464-410X.2009.08987.x. [DOI] [PubMed] [Google Scholar]
- 6.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 7.Escudier B, Eisen T, Stadler WM, et al. Sorafenib for treatment of renal cell carcinoma: Final efficacy and safety results of the phase III treatment approaches in renal cancer global evaluation trial. J Clin Oncol. 2009;27:3312–3318. doi: 10.1200/JCO.2008.19.5511. [DOI] [PubMed] [Google Scholar]
- 8.Motzer RJ, Hutson TE, Tomczak P, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:3584–3590. doi: 10.1200/JCO.2008.20.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 10.Escudier B, Pluzanska A, Koralewski P, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: A randomised, double-blind phase III trial. Lancet. 2007;370:2103–2111. doi: 10.1016/S0140-6736(07)61904-7. [DOI] [PubMed] [Google Scholar]
- 11.Escudier B, Bellmunt J, Négrier S, et al. Phase III trial of bevacizumab plus interferon alfa-2a in patients with metastatic renal cell carcinoma (AVOREN): Final analysis of overall survival. J Clin Oncol. 2010;28:2144–2150. doi: 10.1200/JCO.2009.26.7849. [DOI] [PubMed] [Google Scholar]
- 12.Rini BI, Halabi S, Rosenberg JE, et al. Phase III trial of bevacizumab plus interferon alfa versus interferon alfa monotherapy in patients with metastatic renal cell carcinoma: Final results of CALGB 90206. J Clin Oncol. 2010;28:2137–2143. doi: 10.1200/JCO.2009.26.5561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rini BI, Halabi S, Rosenberg JE, et al. Bevacizumab plus interferon alfa compared with interferon alfa monotherapy in patients with metastatic renal cell carcinoma: CALGB 90206. J Clin Oncol. 2008;26:5422–5428. doi: 10.1200/JCO.2008.16.9847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 15.Motzer RJ, Escudier B, Oudard S, et al. Phase 3 trial of everolimus for metastatic renal cell carcinoma: Final results and analysis of prognostic factors. Cancer. 2010;116:4256–4265. doi: 10.1002/cncr.25219. [DOI] [PubMed] [Google Scholar]
- 16.Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: Results of a randomized phase III trial. J Clin Oncol. 2010;28:1061–1068. doi: 10.1200/JCO.2009.23.9764. [DOI] [PubMed] [Google Scholar]
- 17.Motzer RJ, Mazumdar M, Bacik J, et al. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol. 1999;17:2530–2540. doi: 10.1200/JCO.1999.17.8.2530. [DOI] [PubMed] [Google Scholar]
- 18.Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma: A double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372:449–456. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 19.Rixe O, Bukowski RM, Michaelson MD, et al. Axitinib treatment in patients with cytokine-refractory metastatic renal-cell cancer: A phase II study. Lancet Oncol. 2007;8:975–984. doi: 10.1016/S1470-2045(07)70285-1. [DOI] [PubMed] [Google Scholar]
- 20.Rini BI, Wilding G, Hudes G, et al. Phase II study of axitinib in sorafenib-refractory metastatic renal cell carcinoma. J Clin Oncol. 2009;27:4462–4468. doi: 10.1200/JCO.2008.21.7034. [DOI] [PubMed] [Google Scholar]
- 21.Dutcher JP, Wilding G, Hudes GR, et al. Sequential axitinib (AG-013736) therapy of patients (pts) with metastatic clear cell renal cell cancer (RCC) refractory to sunitinib and sorafenib, cytokines and sorafenib, or sorafenib alone [abstract 5127] J Clin Oncol. 2008;26(15 suppl):281s. [Google Scholar]
- 22.Bhargava P, Esteves B, Lipatov ON, et al. Activity and safety of AV-951, a potent and selective VEGFR1, 2 and 3 kinase inhibitor, in patients with renal cell carcinoma (RCC): Interim results of a phase II randomized discontinuation trial [abstract 283]. Presented at the 2009 Annual Genitourinary Cancers Symposium; February 26–28, 2009; Orlando, FL. [Google Scholar]
- 23.Bhargava P, Esteves B, Al-Adhami M, et al. Activity of tivozanib (AV-951) in patients with renal cell carcinoma (RCC): Subgroup analysis from a phase II randomized discontinuation trial (RDT) [abstract 4599] J Clin Oncol. 2010;28(15 suppl):366s. [Google Scholar]
- 24.Angevin E, Lin C, Pande AU, et al. A phase I/II study of dovitinib (TKI258), a FGFR and VEGFR inhibitor, in patients (pts) with advanced or metastatic renal cell cancer: Phase I results [abstract 3057] J Clin Oncol. 2010;28(15 suppl):247s. [Google Scholar]
- 25.Pazdur R. Endpoints for assessing drug activity in clinical trials. The Oncologist. 2008;13(suppl 2):19–21. doi: 10.1634/theoncologist.13-S2-19. [DOI] [PubMed] [Google Scholar]
- 26.Lebwohl D, Kay A, Berg W, et al. Progression-free survival: Gaining on overall survival as a gold standard and accelerating drug development. Cancer J. 2009;15:386–394. doi: 10.1097/PPO.0b013e3181b9c5ec. [DOI] [PubMed] [Google Scholar]
- 27.Heng DY, Park T, Bjarnason GA, et al. Progression-free survival (PFS) as a predictor of overall survival (OS) in metastatic renal cell carcinoma (mRCC) treated with vascular endothelial growth factor (VEGF)-targeted therapy [abstract 329]. Presented at the 2009 Annual Genitourinary Cancers Symposium; March 5–7, 2010; San Francisco, CA. [Google Scholar]
- 28.Halabi S, Rini BI, Stadler WM, et al. Use of progression-free survival (PFS) to predict overall survival (OS) in patients with metastatic renal cell carcinoma (mRCC) [abstract 385]. Presented at the 2009 Annual Genitourinary Cancers Symposium; March 5–7, 2010; San Francisco, CA. [Google Scholar]
- 29.Procopio G, Verzoni E, Bracarda S, et al. A randomized, open label, prospective study comparing the association between sorafenib (So) and interleukin-2 (IL-2) versus So alone in advanced untreated renal cell cancer (RCC): Rosorc Trial [abstract 5099] J Clin Oncol. 2009;27(15 suppl):258s. [Google Scholar]
- 30.Ryan CW, Goldman BH, Lara PN, Jr, et al. Sorafenib with interferon alfa-2b as first-line treatment of advanced renal cell carcinoma: A phase II study of the Southwest Oncology Group. J Clin Oncol. 2007;25:3296–3301. doi: 10.1200/JCO.2007.11.1047. [DOI] [PubMed] [Google Scholar]
- 31.Gollob JA, Rathmell WK, Richmond TM, et al. Phase II trial of sorafenib plus interferon alfa-2b as first- or second-line therapy in patients with metastatic renal cell cancer. J Clin Oncol. 2007;25:3288–3295. doi: 10.1200/JCO.2007.10.8613. [DOI] [PubMed] [Google Scholar]
- 32.Bracarda S, Porta C, Boni C, et al. Randomized prospective phase II trial of two schedules of sorafenib daily and interferon-α2a (IFN) in metastatic renal cell carcinoma (RAPSODY): GOIRC Study 0681 [abstract 5100] J Clin Oncol. 2007;25(18 suppl):259s. [Google Scholar]
- 33.Azad NS, Posadas EM, Kwitkowski VE, et al. Combination targeted therapy with sorafenib and bevacizumab results in enhanced toxicity and antitumor activity. J Clin Oncol. 2008;26:3709–3714. doi: 10.1200/JCO.2007.10.8332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.ClinicalTrials.gov. NCT00631371. Study Comparing Bevacizumab + Temsirolimus vs Bevacizumab + Interferon-Alfa in Advanced Renal Cell Carcinoma Subjects (INTORACT) [accessed April 16, 2010]. Available at http://www.clinicaltrials.gov/ct2/show/NCT00631371?term=NCT00631371&rank=1.
- 35.ClinicalTrials.gov. NCT00378703. Bevacizumab, Sorafenib, and Temsirolimus in Treating Patients With Metastatic Kidney Cancer. [accessed April 16, 2010]. Available at http://www.clinicaltrials.gov/ct2/show/NCT00378703?term=NCT00378703&rank=1.
- 36.ClinicalTrials.gov. NCT00619268. Combination of Temsirolimus and Bevacizumab in Patients with Metastatic Renal Cell Carcinoma (TORAVA) [accessed April 16, 2010]. Available at http://www.clinicaltrials.gov/ct2/show/NCT00619268?term=NCT00619268&rank=1.
- 37.ClinicalTrials.gov. NCT00719264. Safety and Efficacy of Bevacizumab Plus RAD001 Versus Interferon Alfa-2a and Bevacizumab in Adult Patients With Kidney Cancer. [accessed April 18, 2010]. Available at http://www.clinicaltrials.gov/ct2/show/NCT00719264?term=NCT00719264&rank=1.
- 38.Fischer P, Patel P, Carducci MA, et al. Phase I study combining treatment with temsirolimus and sunitinib malate in patients with advanced renal cell carcinoma [abstract 16020] J Clin Oncol. 2008;26(15 suppl):672s. doi: 10.3816/CGC.2009.n.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rini BI, Garcia JA, Cooney MM, et al. A phase I study of sunitinib plus bevacizumab in advanced solid tumors. Clin Cancer Res. 2009;15:6277–6283. doi: 10.1158/1078-0432.CCR-09-0717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feldman DR, Kondagunta GV, Ronnen EA, et al. Phase I trial of bevacizumab plus sunitinib in patients (pts) with metastatic renal cell carcinoma (mRCC) [abstract 5099] J Clin Oncol. 2007;25(18 suppl):259s. [Google Scholar]
- 41.Medioni J, Banu E, Helley D, et al. Salvage therapy with bevacizumab-sunitinib combination after failure of sunitinib alone for metastatic renal cell carcinoma: A case series. Eur Urol. 2009;56:207–211. doi: 10.1016/j.eururo.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 42.Merchan JR, Pitot HC, Qin R, et al. Phase I/II trial of CCI 779 and bevacizumab in advanced renal cell carcinoma (RCC): Safety and activity in RTKI refractory RCC patients [abstract 5039] J Clin Oncol. 2009;27(15 suppl):244s. [Google Scholar]
- 43.Harzstark AL, Rosenberg JE, Weinberg VK, et al. A phase I study of sorafenib and RAD001 for metastatic clear cell renal cell carcinoma [abstract 5104] J Clin Oncol. 2009;27(15 suppl):259s. [Google Scholar]
- 44.Kroog GJ, Feldman DR, Kondagunta GV, et al. Phase I trial of RAD001 (everolimus) plus sunitinib in patients with metastatic renal cell carcinoma [abstract 5037] J Clin Oncol. 2009;27(15 suppl):243s. [Google Scholar]
- 45.Ryan CW, Vuky J, Chan JS, et al. Phase II study of everolimus (E) with imatinib (IM) in patients with previously-treated renal carcinoma (RCC) [abstract e16075] J Clin Oncol. 2009;27(15 suppl):714s. [Google Scholar]
- 46.Hainsworth JD, Spigel DR, Burris HA, 3rd, et al. Phase II trial of bevacizumab and everolimus in patients with advanced renal cell carcinoma. J Clin Oncol. 2010;28:2131–2136. doi: 10.1200/JCO.2009.26.3152. [DOI] [PubMed] [Google Scholar]


