Abstract
The NKG2D activating receptor has been implicated in numerous autoimmune diseases. We tested the role of NKG2D in models of autoimmunity and inflammation using NKG2D knockout mice and antibody blockade experiments. The severity of experimental autoimmune encephalitis (EAE) was decreased in NKG2D-deficient mice when the disease was induced with a limiting antigen dose, but unchanged with an optimal antigen dose. Surprisingly, however, NKG2D deficiency had no detectable effect in several other models, including two models of type 1 diabetes, and a model of intestinal inflammation induced by poly(I:C). NKG2D antibody blockade in normal mice also failed to inhibit disease in the NOD diabetes model or the intestinal inflammation model. Published evidence using NKG2D knockout mice demonstrated a role for NKG2D in mouse models of atherosclerosis and liver inflammation, as well as in chronic obstructive pulmonary disease. Therefore, our results suggest that NKG2D plays selective roles in inflammatory diseases.
Keywords: NKG2D, NK cells, EAE, NOD, type 1 diabetes, intestinal inflammation
1. Introduction
The NKG2D activating receptor is employed by NK cells for recognition of tumor cells and virus-infected cells. NKG2D is expressed by all NK cells and various T cell subsets, including CD8+ T cells, and fractions of NK1.1+ T cells and γδ T cells. On NK cells, NKG2D can provide a sufficient signal for target recognition. On T cells, the receptor provides coactivating signals in some cases but may also suffice for triggering in the case of T cells exposed to cytokines associated with inflammatory stimuli such as IL-15 [1-4].
NKG2D binds to each of several cell surface protein ligands, which are self-proteins related to MHC class I molecules. The ligands include MICA, MICB and ULBP1-6 in humans, and RAE-1α-ε, MULT1 and H60a, b and c in mice [1, 2, 5]. Notably, several of the NKG2D ligands can be cleaved from the cell surface by proteases including matrix metalloproteinases (MMPs), which results in the accumulation of soluble ligands in sera from some cancer patients [6, 7].
Inappropriate expression of NKG2D ligands, if it were to occur in uninfected and untransformed tissue, could cause inflammation and promote autoimmunity. Various lines of evidence suggest roles of NKG2D or its ligands in inflammatory and autoimmune diseases, including rheumatoid arthritis [8, 9], colitis, celiac disease and other forms of intestinal inflammation [4, 10-14], multiple sclerosis [15], alopecia areata [16], type 1 diabetes [17-19], chronic obstructive pulmonary disease [20] and atherosclerosis and metabolic syndrome associated with type 2 diabetes [21]. In many of these studies, the evidence for NKG2D’s role was based on genetic associations or other correlative criteria including the expression of NKG2D ligands and NKG2D+ cells in the relevant lesions. In a few cases using mouse models, however, more direct evidence was obtained for a causal role of NKG2D in inflammatory diseases. For example, it was reported that type 1 diabetes was prevented when mice were exposed to antibodies that block NKG2D [17]. Similarly, collagen-induced arthritis was prevented in mice treated with NKG2D antibodies [9]. Disease was also inhibited by NKG2D antibody in two models of inflammatory bowel diseases, including a model of inflammation of the small intestine induced by injection of high doses of poly(I:C) [22] and a model of colitis induced by adoptive transfer of naïve CD4+ T cells devoid of regulatory T cells [12, 13]. Two studies have used knockout mice to investigate the role of NKG2D in inflammatory diseases. In one, atherosclerosis and liver inflammation associated with a high fat diet or ablation of pancreatic islet cells in apoE-deficient mice was prevented in knockout mice lacking NKG2D as well as by treating the mice with NKG2D antibodies [21]. In another, severe inflammation accompanying influenza virus infections of mice with cigarette smoke-induced chronic obstructive pulmonary disease was significantly ameliorated in knockout mice, and evidence implicated NKG2D expressed by NK cells in the inflammatory process [20].
Recently, we generated a mutant mouse in which the NKG2D gene was disrupted by gene targeting [23]. The knockout strain was employed to demonstrate that NKG2D-deficiency results in an increased incidence or acceleration of malignancy in two models of spontaneous cancer, but not in all cancer models studied. As already mentioned, the gene-targeted mice were also employed to demonstrate a major requirement for NKG2D in the development of atherosclerosis and metabolic syndrome, including liver inflammation, in a mouse model of the disease [21]. In the present study, we have employed the gene-targeted mice, and in some cases antibody blockade, to examine the role of NKG2D in several mouse models of inflammatory diseases. A modest role for NKG2D was detected in experimental autoimmune encephalitis (EAE), a mouse model sharing some features with multiple sclerosis. Surprisingly, using knockout mice and in some cases antibody blockade, no role for NKG2D was detected in two models of type 1 diabetes or a model of inflammatory bowel disease, contradicting previous reports.
2. Methods
2.1. Mice
C57BL6/J (B6) mice were purchased from Jackson Laboratories and in some cases bred within the laboratory. NKG2D-deficient mice on the B6 genetic background (B6-Klrk1−/−) were generated in our laboratory [23], NKD mice (C57BL/6 mice with an NK deficiency due to the integration of a Ly49A transgene) were previously described [24] and kindly provided by Dr. Wayne Yokoyama (Washington University, St. Louis, MO). C57BL/6 mice harboring the BDC2.5 Transgene were previously described [25] and kindly provided by Drs. Diane Mathis and Christophe Benoist (Harvard Medical School, Boston, MA). Seven week old female NOD/ShiLt mice were purchased from Jackson Laboratories (Bar Harbor, Maine). B6-Klrk1−/− mice were backcrossed to the NOD.NK1.1 strain (NOD.B6-(D6Mit254-D6Mit339)/CarJ stock number 004482, Jackson Laboratories, Bar Harbor, ME) as described in the text. Mice were screened for the presence and/or the absence of the targeted Klrk1 gene by PCR as previously described [23]. The presence of the BDC transgene was detected using the primers: BDC 2.5a for: CATGTTTCCCTGCACATCAG, BDC 2.5a rev: CCAGATCCAAAGATGAGTTGC. The presence of the H2d allele was determined using the following primers: LP40: TCTAGAATTCACAGCGACATGGGCGAGC; LP41: TCTAGAATTCCGTAGTTGTGTCTGCACA.
All mice were bred and maintained under pathogen-specific free conditions at the University of California, Berkeley in compliance with institutional guidelines. Mice were euthanized by CO2 inhalation in accord with the policies of the office of Laboratory Animal and Care (OLAC) at UC Berkeley.
2.2. Antibodies
MI-6 antibody was prepared in the laboratory. The MI-6 hybridoma was grown in a CELLine CL1000 bioreactor (Argos Technologies, Elgin, IL) per manufacturer’s instructions and culture supernatants were harvested. After two rounds of ammonium sulfate precipitation and dialysis with a 10K MWCO Slide-A-Lyzer (Thermo Scientific, Rockford, IL), antibody was purified with Melon Gel per the manufacturer’s instructions (Thermo Scientific, Rockford, IL). CX5 antibody was a gift from Novo Nordisk (Copenhagen, Denmark). Control rat IgG was purchased from Jackson ImmunoResearch. The endotoxin content of all antibody preps was <0.01 ng/mg as tested with the QCL-1000 assay kit from Lonza Inc (Allendale, NJ). For in vivo blocking, mice were injected i.p. with 200 μg of mAb (CX5 or MI-6) per dose. Treatment regimens are described separately below.
2.3. Experimental autoimmune encephalomyelitis
Mice were immunized subcutaneously in two locations on the back with 20 or 100 μg of MOG35-55 peptide in incomplete Freund’s Adjuvant (Sigma-Aldrich, St. Louis, MO) complemented with0.5mg/ml of Mycobacterium tuberculosis H37RA (Difco Laboratories, Franklin Lakes, NJ). MOG:35-55 peptide (MEVGWYRSPFSRVVHLYRNGK) was kindly provided by the Howard Hughes Medical Institute Mass Spectrometry Facility (UC Berkeley, Berkeley, CA). In addition, 200 ng of pertussis toxin (List Biological Laboratories, Hornby, ONT, Canada) was administered i.p. immediately following immunization and again 48 hours later. Clinical assessment of EAE was performed daily according to the following scoring system: 0 = no disease, 1 = limp tail, 2 = hind limb weakness, 3 = partial or complete hind limb paralysis, 4 = hind limb paralysis plus forelimb weakness, and 5 = moribund or dead. Mice that were in between gradations were scored in increments of 0.5. p<0.05 denotes significance. The two-tailed Wilcoxon signed rank test was used to compare the average clinical scores observed in groups of experimental and control mice.
2.4. Poly(I:C) Treatment
Male mice between 6 and 8 weeks of age were weighed and then injected i.p. with 30 μg/g body weight of HMW (high molecular weight) poly(I:C) (Invivogen, San Diego, CA) in sterile PBS. Mice were monitored and weighed every six hours for 36-100 hours. Mice were euthanized if weight loss exceeded 15%.
2.5. Type 1 diabetes models
In all of the mouse models studied, blood glucose levels were monitored weekly with a BD Logic blood glucose monitor (Walgreens, Deerfield, IL). Mice were considered diabetic if blood glucose measurements were greater than or equal to 300 mg/dL on two consecutive weekly readings. NOD/ShiLtJ, NOD.NK1.1 and BDC2.5Tg mice were followed until 40, 50 and 30 weeks of age respectively. For NKG2D antibody treatments, NOD/ShiLtJ female mice were injected i.p. twice weekly with 200 μg of antibody or isotype control rat IgG starting at eight weeks of age until the mice were 32 (Fig. 2) or 25 (data not shown) weeks of age .
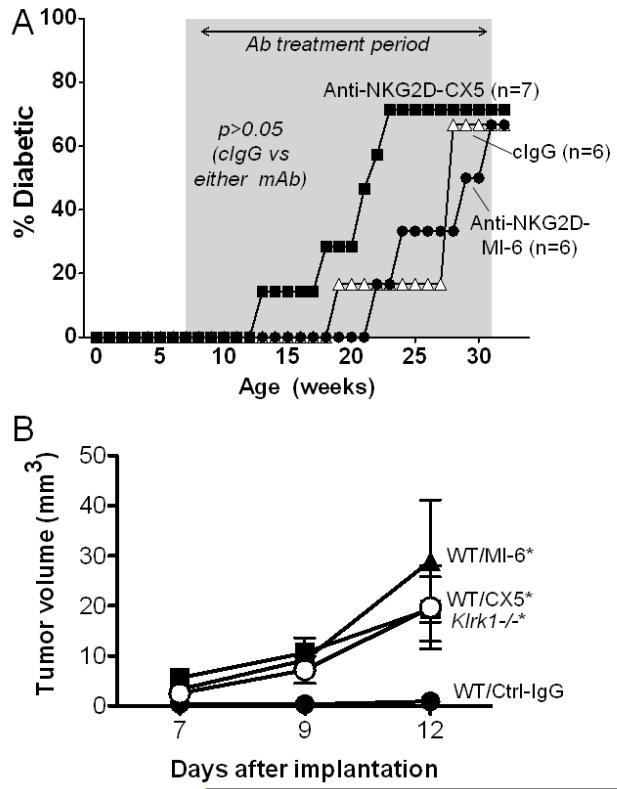
Figure 2.
Treatment of NOD mice with NKG2D antibody does not depress the incidence of T1D. (A) Female NOD/ShiLtJ mice were treated twice weekly between the ages of 7 and 32 weeks of age (indicated by the shaded area) with 200 μg control IgG (open triangles, n=6), MI-6 NKG2D antibody (closed circles, n=6) or CX5 NKG2D antibody (closed squares, n=7). Mice received 200 μg of antibody twice weekly, i.p. Diabetes was diagnosed when the blood glucose levels reached 300 mg/dl on two consecutive readings. As assessed with the Log-Rank test, the treated groups did not differ significantly from the control IgG group. (B) Control experiment demonstrating that NKG2D antibodies MI-6 (closed triangles) and CX5 (closed squares) block rejection of tumor cells. Control IgG treated mice (closed circles) and untreated Klrk1−/− mice (closed inverted triangles) are shown for comparison. Mice were injected subcutaneously with 1 × 105 RMA-RAE-1β tumor cells on day 0. Antibodies or control IgG (200 μg/injection) were injected on days -1, 5, 8 and 12, and tumor sizes were determined on the days shown. p=0.0038, p=0.0047, and 0.0001 comparing control IgG to MI-6, CX5 and Klrk1−/− groups by two-way analysis of variance (ANOVA).
3. Results
3.1. Role of NKG2D in Type 1 diabetes models
To determine whether the absence of NKG2D affects the course of type 1 diabetes development in the NOD genetic background, we generated NKG2D knockout mice on the congenic NOD.NK1.1 background. NOD.NK1.1 mice [26] have NOD genes at nearly all the loci, except for the genes in or near the NK gene complex (NKC), which are derived from B6 mice. The NKC contains the gene for NKG2D (called Klrk1) as well as many polymorphic genes that influence NK cell functions. We used NOD.NK1.1 for these crosses because the mutant Klrk1 allele in NKG2D knockout mice is from the B6 strain, and we wished to compare Klrk1+/+ and Klrk1−/− mice with the same (B6) NKC genes. It was important to do so, because previous studies showed a slightly lower (but still high) incidence of type 1 diabetes in NOD.NK1.1 mice in comparison to conventional NOD mice [26]. Thus, we crossed B6-Klrk1−/− mice to NOD.NK1.1 congenic mice and backcrossed an additional eight generations. NOD.NK1.1-Klrk1+/− offspring were then selected and intercrossed. Klrk1−/− and Klrk1+/+ NOD.NK1.1 littermates, all of which have B6 type NKC genes, were grouped from numerous matings and used for the subsequent experiments.
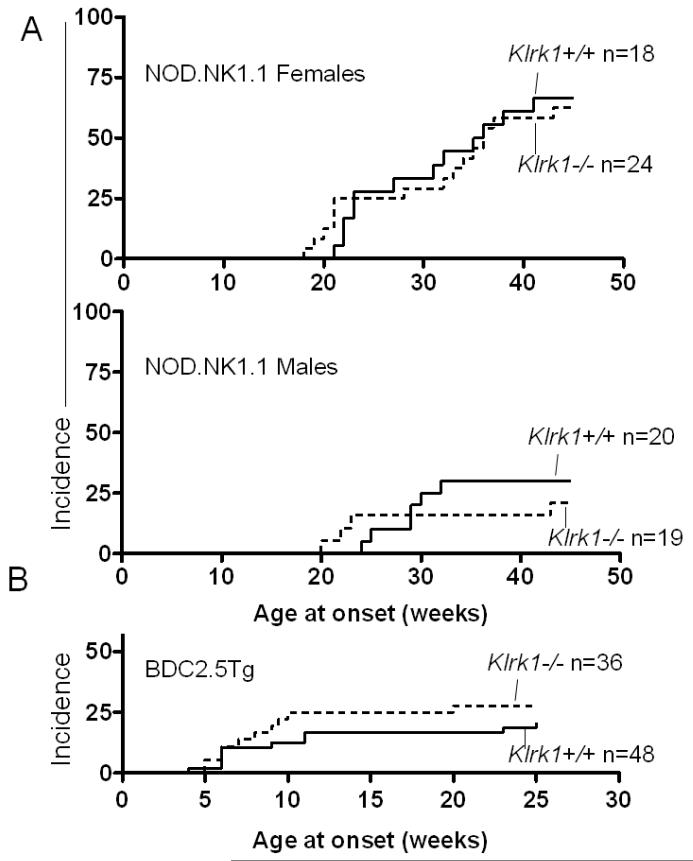
T1 diabetes was assessed in each cohort by measuring blood glucose level starting at the age of 12 weeks. As shown in Figure 1A, neither female nor male NOD.NK1.1 Klrk1−/− mice exhibited a significantly elevated incidence of disease compared to NOD.NK1.1 Klrk1+/+ littermates of the same sex (p=0.86 and p=0.6 respectively, by logrank test). Blood glucose levels were similar in the two groups of mice, as well (Supplementary Fig. 1). The age at onset for both genders was also comparable in the presence or absence of NKG2D. As previously reported, NOD.NK1.1 females exhibited a generally higher incidence of disease compared to males [26], and this was true regardless of the status of the Klrk1 gene (Fig. 1A). Thus, the absence of Klrk1 did not appreciably alter diabetes development in the NOD.NK1.1 background.
Figure 1. NKG2D-deficiency is not associated with lower rates of type 1 diabetes.
(A) Incidence of T1D in NOD.NK1.1 Klrk1−/− and NOD.NK1.1-Klrk1+/+ littermates was assessed over time by weekly measurement of blood glucose levels. Females are shown in the upper panel (p=0.86, using the Log-Rank test) and males in the lower panel (p=0.60). The numbers of mice in each group are indicated (n). (B) Incidence of T1D in BDC2.5Tg-Klrk1−/− and BDC2.5Tg-Klrk1+/+ mice (both sexes) was assessed over time (p=0.31).
To extend the analysis of type 1 Neither antibody delayed the onset of T1 diabetesdiabetes, we examined whether NKG2D-deficiency impacts disease development in the BDC2.5 transgenic model [27, 28]. These mice express a diabetogenic T cell receptor transgene, as well as the corresponding H-2g7 MHC complex, on the B6 genetic background. BDC2.5 transgenic mice carrying one copy of the transgene and one copy of the H-2g7 allele (BDC2.5 H-2g7/b) were crossed to Klrk1−/− mice to generate BDC2.5 H-2g7/b Klrk1+/− mice. These mice were intercrossed to generate a first generation of BDC2.5 H-2g7/b Klrk1+/+ and Klrk1−/− mice that were included in the study. Some of these mice were also used for further crosses to generate more BDC2.5 H-2g7/b Klrk1+/+ and Klrk1−/− mice, also included in the study. As previously reported for H-2g7/b heterozygous BDC2.5 mice [27], the incidence of diabetes in the Klrk1+/+ mice was relatively low, approximately 21% (Fig. 1B). In Klrk1−/− mice, disease incidence was not significantly different (approximately 28%, p=0.31) (Fig. 1B), nor was the pattern of blood glucose levels (Supplementary Fig. 2). Hence, we observed no indication that NKG2D deficiency alters the course of diabetes development in either the BDC2.5 or NOD.NK1.1 models.
Given the discrepancy between our results on a NOD.NK1.1 background with those obtained in a model of NOD mice using NKG2D antibody blockade [29], we compared the incidence of diabetes in conventional NOD mice treated with two different clones of NKG2D monoclonal antibodies, MI-6 [30] and CX5 [29], the latter corresponding to the same antibody used in the earlier study [29]. NOD/ShiLtJ female mice were treated between 7 and 32 weeks of age with NKG2D antibody or control rat IgG, using high doses of antibody and the same treatment regimen to that used previously [17]. Neither antibody delayed the onset of T1 diabetes, caused a decrease in the overall incidence of type 1 diabetes (Fig. 2A), or reduced blood glucose levels (Supplementary Fig. 3) compared to mice treated with control IgG. A repeat experiment also failed to detect reduced disease with NKG2D antibody blockade (data not shown). A control experiment using B6 mice demonstrated that treatments with either the MI-6 or CX5 antibodies against NKG2D blocked rejection of a subcutaneously transferred tumor cell line transductant expressing RAE-1β (Fig. 2B). The tumor cells were rejected in mice treated with control IgG, but grew in antibody-treated mice with the delayed kinetics similar to tumor growth in Klrk1−/− mice. Therefore, the antibodies were effective in blocking NKG2D under similar in vivo conditions. In conclusion, these analyses failed to detect a role for NKG2D in promoting type 1 diabetes and were therefore in agreement with the results from analysis of Klrk1−/− mice.
3.2. Role of NKG2D in Poly(I:C)-induced injury of the small intestine
Intraperitoneal injection of mice with high doses of poly(I:C) induces acute mucosal injury of the small intestine, manifested by rapid weight loss and villus atrophy [22, 31]. The process is partially dependent on IL-15 and was associated with increased expression of the NKG2D ligand RAE-1 by intestinal epithelial cells. Reportedly, intestinal injury was inhibited by treating mice with NKG2D antibody, suggesting a role for NKG2D in the process [22].
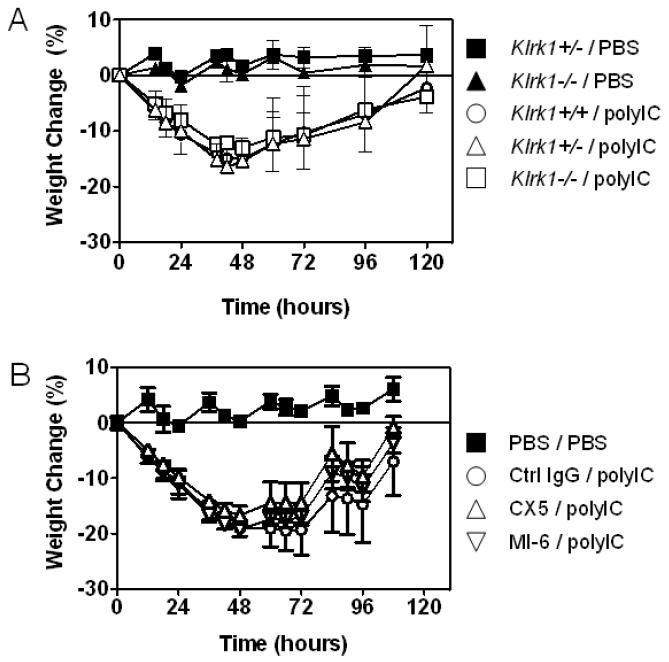
To address whether poly(I:C)-induced disease is altered in the absence of NKG2D, B6-Klrk1−/− and B6-Klrk1+/+ littermates were treated with poly(I:C) and assessed for weight loss. Poly(I:C) treatment induced 10-20% weight loss in the Klrk1−/− mice, which was not significantly different than the weight loss observed in Klrk1+/+ control mice (Fig. 3A). Occasionally, mice in one or another group in this and other experiments had to be euthanized because of severe weight loss, but this occurred equally in both the Klrk1+/+ and Klrk1−/− groups and did not bias the data. The remaining mice recovered by 96 hours after treatment. In conclusion, poly(I:C)-induced injury did not require the intact NKG2D gene.
Figure 3.
NKG2D is unnecessary for weight loss associated with inflammation of the bowel induced by poly(I:C). (A) 6-8 week old male Klrk1−/− or Klrk1+/+ mice were injected intraperitoneally with either 30 μg/g poly(I:C) or a similar volume of PBS, and body weight was monitored 3 times a day for 4-5 days. n=3 for all groups except PBS-treated Klrk1−/− mice, n=2. Data represent means ± SDs. (B) 6 week old male C57BL/6 mice (n=5/group) were injected i.p. with poly(I:C) or PBS on day 0, and with 200 μg control IgG or NKG2D antibody (MI-6 or CX5) on days -1 and 3. Data represent means ± SD.
To address whether NKG2D antibody blockade prevents poly(I:C)-induced intestinal injury, groups of B6 mice were treated with high doses of either MI-6 or CX5 NKG2D antibodies, or control rat IgG, during and after treatment with poly(I:C). Considerable weight loss occurred in response to poly(I:C) treatment, whether or not the mice were treated with either of the NKG2D antibodies (Fig. 3B). Similar to the analysis of NKG2D knockout mice, the antibody blockade experiments failed to detect a role for NKG2D in promoting poly(I:C) induced disease.
3.3. NKG2D contributes to the severity of experimental autoimmune encephalomyelitis (EAE) under limiting conditions
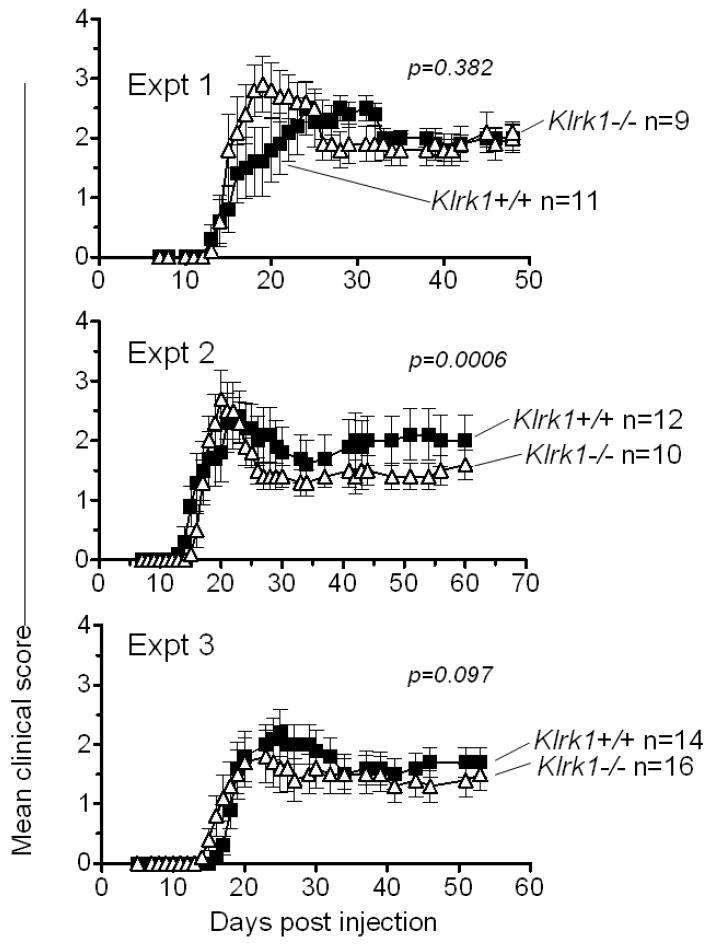
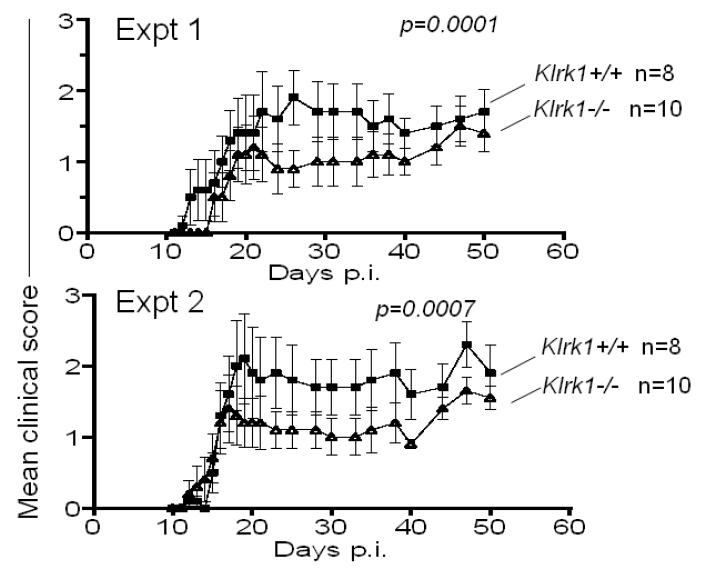
To assess whether NKG2D deficiency influences the development of EAE, groups of Klrk1−/− and Klrk1+/+ females, both from intercrosses of Klrk1+/− parents, were subjected to a standard EAE induction protocol, in which they were immunized with 100 ug MOG peptide in CFA and pertussis toxin. In three independent experiments, we obverved little or no difference in clinical score between the experimental groups (Fig. 4), although there was a small but statistically significant decrease in clinical score in the Klrk1−/− mice (Fig. 4, Expt 2, p=0.0006). The possibility that NKG2D plays a minor role was suggested by the results of a pilot experiment using a suboptimal preparation of pertussis toxin, where we observed substantially lower overall clinical scores and significantly less disease in the Klrk1−/− mice (data not shown). To determine whether NKG2D plays a role in disease under limiting conditions, we repeated the induction protocol with the optimal pertussis toxin preparation, but with a limiting dose of MOG peptide (20 ug/injection) (Fig. 5). Under these conditions we observed lower overall disease scores over the course of the analyses in wildtype mice, as expected. Importantly, in both experiments performed, we observed significantly lower disease scores in the Klrk1−/− mice compared to Klrk1+/+ littermates, showing that NKG2D plays a role in EAE under limiting conditions (Fig. 5, Expt 1 and 2, p<0.0001 and p=0.0007, respectively).
Figure 4.
Experimental Autoimmune Encephalitis (EAE) severity is similar in Klrk1+/+ and Klrk1−/− mice. Clinical scores of Klrk1−/− (triangles) and Klrk1+/+ mice (squares) immunized with 100 μg of MOG peptide. Numbers of mice/group are indicated. Each panel represents an independent experiment performed with the indicated number of mice for each genotype. Statistical significance (Wilcoxon signed rank test comparing each data set) is indicated in each panel. Data are represented as means ± SD.
Figure 5.
Klrk1−/− mice exhibit lower EAE severity when limiting antigen doses are used to induce the disease. Clinical scores of Klrk1−/− (triangles) and Klrk1+/+ (squares) mice when immunized with 20 μg of MOG peptide, in two independent experiments. Statistical significance (Wilcoxon signed rank test comparing each data set) is indicated in each panel. Data are represented as means ± SD.
In an attempt to corroborate the role of NKG2D in EAE under limiting conditions, we attempted to block NKG2D in vivo with antibodies during the disease induction protocol. When we injected antibodies intraperitoneally during the EAE induction protocol we observed substantial mortality, even in groups treated with control IgG. Independent batches of both control rat IgG (from different companies) or NKG2D antibodies had the same effects (30%-70% mortality), arguing against contamination of the antibodies with toxins (data not shown). A published report suggested that the toxicity may result from anaphylaxis when mice are exposed to foreign proteins in the presence of pertussis toxin and adjuvant [32]. In any case, these toxic effects precluded an unbiased analysis of the effects of NKG2D antibodies on the development of EAE.
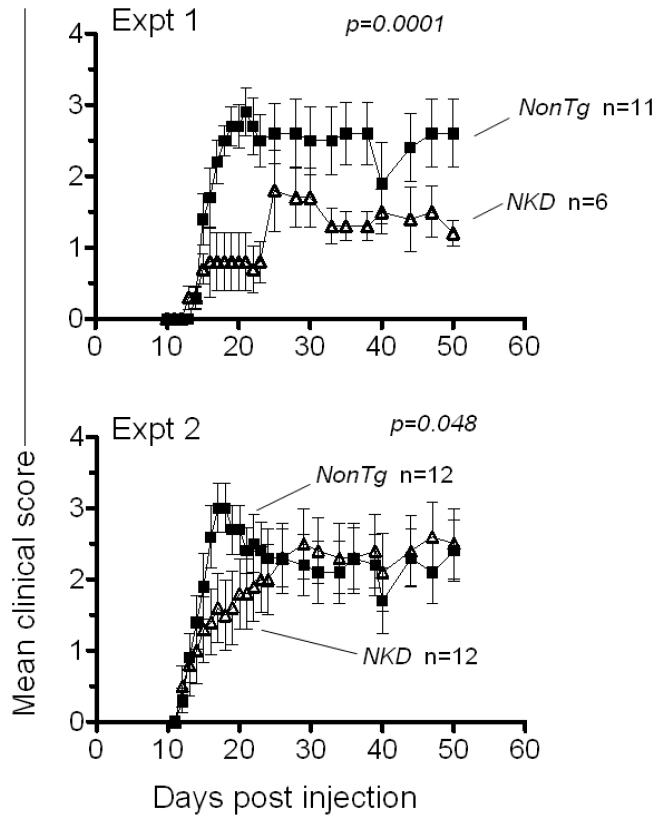
Toxicity accompanying injection of antibodies suggest that antibody-mediated depletions may result in unreliable findings in the context of the EAE protocol, which may account for contradictory conclusions of different studies that NK cells either promote or inhibit EAE [33-35]. As an alternative approach to test the role of NK cells in EAE, we compared WT mice with the NKD transgenic mouse strain generated in the Yokoyama lab, which is nearly devoid of mature NK cells [24]. Using the optimal EAE induction protocol (100 μg MOG peptide) in two replicate experiments, EAE developed with significantly slower kinetics in NKD mice compared to nonTg littermates (Fig. 6). These data support the conclusion that NK cells contribute to the severity of EAE, rather than inhibiting it.
Figure 6.
Lower EAE severity in mice lacking NK cells. Clinical scores of NKD mice and control littermates when immunized with 100 μg of MOG peptide, in two independent experiments. Statistical significance (Wilcoxon signed rank test comparing each data set) is indicated in each panel. Data are represented as means ± SD.
4. Discussion
The studies herein have investigated the role of NKG2D in several mouse models of inflammatory and autoimmune disease. The results, when taken together with previously published results, suggest that NKG2D plays a selective role in inflammatory diseases. Specifically, we failed to detect a role for NKG2D in two models of type 1 diabetes, or in a model of inflammatory intestinal injury induced by poly(I:C). In contrast, a significant role for NKG2D was detected in the EAE model, but only under limiting conditions. With our collaborators, we previously employed NKG2D knockout mice and antibody blockade studies to demonstrate a substantial role of NKG2D in atherosclerosis and metabolic syndrome [21], as well as in chronic obstructive pulmonary disease [20]. Other groups, using antibody blockade, reported a role for NKG2D in a transfer model of colitis [12, 13] and in the collagen-induced arthritis model [9].
Why NKG2D interactions are necessary to promote some inflammatory diseases but not others remains unclear. One possibility is that it reflects redundancy in activating signals that induce disease, such that other activating signals suffice to promote disease even in the absence of NKG2D interactions. Such redundancy may also apply or not depending on the induction protocol, which could explain why NKG2D has little influence on EAE incidence under optimal induction conditions (100 μg MOG) but a greater role under limiting conditions (20 μg MOG peptide). Another possibility is that although ligand expression occurs in certain inflammatory conditions, immune cells are not activated via NKG2D because they are not recruited efficiently or are exposed to suppressive conditions in the local environment. Notably, however, results in a recent paper using transgenic mice expressing NKG2D ligands ectopically in the pancreas suggested that NKG2D ligands themselves have the potential to recruit NKG2D+ CD8 T cells into the pancreatic islets [36]. A third possibility is that ligand induction occurs in some diseases or conditions, and not others. In the atherosclerosis/metabolic syndrome model, for example, it was proposed that specific metabolites that accumulate in these conditions, such as oxidized low density lipoproteins and advanced glycation endproducts, are responsible for inducing ligand expression. Such inducers may be absent in the context of certain other inflammatory diseases. It was reported, however, that ligand expression can be detected in both the NOD type 1 diabetes model [17] and in the poly(I:C) intestinal injury model [22], arguing against this explanation. A fourth possibility is that the release of soluble NKG2D ligands from inflamed tissues differs in these different syndromes, resulting in the differential modulation of NKG2D-dependent responses [8, 15, 37]. Finally, in the presence of NKG2D ligands, dysfunctions in the signaling pathways of NKG2D-expressing cells could impair the functionality of the cells as has been proposed in other studies [18, 29].
It remains unclear why our results differed from published results in the mouse NOD diabetes model and the poly(I:C)-induced intestinal injury model. In those models we observed no inhibition of disease in NKG2D knockout mice or in mice treated with NKG2D antibodies, whereas the previously published results depended on antibody blockade only [17, 22]. It is possible that differences in the microbiota or the housing of the mice can account for the discrepancies [38]; if so it would suggest that NKG2D’s role is incidental to the main processes leading to autoimmunity. Undetected infections could also play a role, given recent evidence that viral infections may interact with other diabetes-inducing conditions to promote diabetes[19]. Finally, it is conceivable that the antibody preparations led to misleading results because they contained trace amounts of microbial or other products, or antibody aggregates that crosslink NKG2D and therefore cause activation of the receptor and unanticipated biological effects. Our antibody preparations failed to induce detectable activation of NK cells in vivo, as determined by assessing NK cell activation ex vivo (data not shown), but other preparations may behave differently.
The finding that EAE is diminished in NKG2D-deficient mice under limiting conditions suggests that NKG2D may play a role in that disease. This conclusion fits with genetic and in vitro studies that have provided suggestive evidence for a role of NKG2D in multiple sclerosis in humans [15, 39, 40]. NKG2D-deficient mice showed reduced disease only under limiting conditions, suggesting the possibility that other activating immune receptors or stimuli can substitute for NKG2D in initiating disease in this model, or that NKG2D plays a secondary role that can amplify inflammation.
We were unable to test the role of NKG2D in EAE using the alternative method of antibody blockade, due to toxicity that may be related to the necessity of exposing mice to pertussis toxin in that disease model. Therefore, it remains possible that the effect of NKG2D-deficiency is indirect. A previous study demonstrated that reduced EAE also occurred in mice lacking the DAP12 signaling adapter molecule [41], which, along with DAP10, can mediate NKG2D signaling in mice [42]. However, DAP12 signaling also mediates signaling by numerous other NK receptor and myeloid cell receptors [43], so the relevance of this finding to the role of NKG2D in the disease is uncertain.
Toxicity accompanying injection of antibodies suggests that antibody blockade is an unreliable method in the context of the EAE protocol, and may therefore account for contradictory results that have come from such studies in attempts to determine the consequence of NK cell depletions in the context of EAE [33-35]. Our new data with the NKD mice suggest that NK cells do in fact exacerbate EAE, as suggested by results in one of the previous publications, and to a similar extent as was observed in that study [35]. In light of the fact that virtually all NK cells express NKG2D, and that NKG2D is a potent activating receptor for NK cells, it is plausible that NKG2D participates in the exacerbation of the disease by directly activating NK cells. However, NKG2D may also act in part in other NKG2D+ cell types, including CD8 T cells and NK1.1+ T cells. The relatively minor role of NKG2D in this EAE model makes it difficult to identify the relevant cell types experimentally. Even if NKG2D acts exclusively in NK cells to promote EAE, the data as a whole suggest that other modes of NK cell activation may also play a role, since the effects of NKG2D-deficiency were seen primarily with limiting MOG doses, whereas the effects of NK depletion were seen with optimal MOG doses.
In conclusion, the results argue that NKG2D does not play a substantial role in several models of inflammation, in contradiction to some previous reports, but does play a modest role in EAE under limiting conditions. Previous publications demonstrate an important role for NKG2D in at least two other inflammatory disease models in mice. Further studies comparing these inflammatory conditions should help elucidate specific conditions that determine when NKG2D plays an inductive role in autoimmunity. The results will aid in evaluating which human conditions are good candidates for therapeutic interventions to block NKG2D or its ligands.
Supplementary Material
Highlights.
Experimental autoimmune encephalitis was less severe in NKG2D knockout mice.
Type 1 diabetes is unchanged in NKG2D-deficient NOD mice and BDC2.5 transgenic mice.
NKG2D antibody treatment of NOD mice did not prevent type I diabetes.
Poly(I:C)-induced intestinal inflammation was unchanged in NKG2D knockout mice.
NKG2D antibody treatment did not prevent poly(I:C)-induced intestinal inflammation.
Acknowledgements
The authors thank Birgitte Urso, Clause Haase and Nicolai Wagtmann of Novo Nordisk for providing CX5 antibody and fruitful discussions, and we thank Novo Nordisk for research support and the NIH for research grant support. We thank Lily Zhang for managing the laboratory and our colleages for helpful critiques of the results.
Funding
The work was supported by a research grant from Novo Nordisk, SA and grants from the National Institutes of Health.
Abbreviations
- RAE-1
Retinoic Acid Early Inducible Gene-1
- NK cells
Natural Killer cells
- NKG2D
Natural Killer Group 2, member D
- poly(I:C)
polyinosinic:polycytidylic acid.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
D.R. received a research grant from Novo Nordisk, SA.
References
- [1].Raulet DH. Roles of the NKG2D immunoreceptor and its ligands. Nat Rev Immunol. 2003;3:781–90. doi: 10.1038/nri1199. [DOI] [PubMed] [Google Scholar]
- [2].Champsaur M, Lanier LL. Effect of NKG2D ligand expression on host immune responses. Immunol Rev. 2010;235:267–85. doi: 10.1111/j.0105-2896.2010.00893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Groh V, Rhinehart R, Randolph-Habecker J, Topp MS, Riddell SR, Spies T. Costimulation of CD8 αβ T cells by NKG2D via engagement by MIC induced on virus-infected cells. Nat Immunol. 2001;2:255–60. doi: 10.1038/85321. [DOI] [PubMed] [Google Scholar]
- [4].Meresse B, Chen Z, Ciszewski C, Tretiakova M, Bhagat G, Krausz TN, et al. Coordinated induction by IL15 of a TCR-independent NKG2D signaling pathway converts CTL into lymphokine-activated killer cells in celiac disease. Immunity. 2004;21:357–66. doi: 10.1016/j.immuni.2004.06.020. [DOI] [PubMed] [Google Scholar]
- [5].Eagle RA, Trowsdale J. Promiscuity and the single receptor: NKG2D. Nat Rev Immunol. 2007;7:737–44. doi: 10.1038/nri2144. [DOI] [PubMed] [Google Scholar]
- [6].Salih HR, Rammensee HG, Steinle A. Cutting edge: down-regulation of MICA on human tumors by proteolytic shedding. Journal of immunology. 2002;169:4098–102. doi: 10.4049/jimmunol.169.8.4098. [DOI] [PubMed] [Google Scholar]
- [7].Groh V, Wu J, Yee C, Spies T. Tumour-derived soluble MIC ligands impair expression of NKG2D and T- cell activation. Nature. 2002;419:734–8. doi: 10.1038/nature01112. [DOI] [PubMed] [Google Scholar]
- [8].Groh V, Bruhl A, El-Gabalawy H, Nelson JL, Spies T. Stimulation of T cell autoreactivity by anomalous expression of NKG2D and its MIC ligands in rheumatoid arthritis. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:9452–7. doi: 10.1073/pnas.1632807100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Andersson AK, Sumariwalla PF, McCann FE, Amjadi P, Chang C, McNamee K, et al. Blockade of NKG2D ameliorates disease in mice with collagen-induced arthritis: A potential pathogenic role in chronic inflammatory arthritis. Arthritis Rheum. 2011;63:2617–29. doi: 10.1002/art.30460. [DOI] [PubMed] [Google Scholar]
- [10].Hue S, Mention JJ, Monteiro RC, Zhang S, Cellier C, Schmitz J, et al. A direct role for NKG2D/MICA interaction in villous atrophy during celiac disease. Immunity. 2004;21:367–77. doi: 10.1016/j.immuni.2004.06.018. [DOI] [PubMed] [Google Scholar]
- [11].Allez M, Tieng V, Nakazawa A, Treton X, Pacault V, Dulphy N, et al. CD4+NKG2D+ T cells in Crohn’s disease mediate inflammatory and cytotoxic responses through MICA interactions. Gastroenterology. 2007;132:2346–58. doi: 10.1053/j.gastro.2007.03.025. [DOI] [PubMed] [Google Scholar]
- [12].Kjellev S, Haase C, Lundsgaard D, Urso B, Tornehave D, Markholst H. Inhibition of NKG2D receptor function by antibody therapy attenuates transfer-induced colitis in SCID mice. Eνr J Immunol. 2007;37:1397–406. doi: 10.1002/eji.200636473. [DOI] [PubMed] [Google Scholar]
- [13].Ito Y, Kanai T, Totsuka T, Okamoto R, Tsuchiya K, Nemoto Y, et al. Blockade of NKG2D signaling prevents the development of murine CD4+ T cell-mediated colitis. Am J Physiol Gastrointest Liver Physiol. 2008;294:G199–207. doi: 10.1152/ajpgi.00286.2007. [DOI] [PubMed] [Google Scholar]
- [14].Lu M, Xia B, Ge L, Li Y, Zhao J, Chen F, et al. Role of major histocompatibility complex class I-related molecules A*A5.1 allele in ulcerative colitis in Chinese patients. Immunology. 2008 doi: 10.1111/j.1365-2567.2008.02953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Fernandez-Morera JL, Rodriguez-Rodero S, Lahoz C, Tunon A, Astudillo A, Garcia-Suarez O, et al. Soluble MHC class I chain-related protein B serum levels correlate with disease activity in relapsing-remitting multiple sclerosis. Hum Immunol. 2008;69:235–40. doi: 10.1016/j.humimm.2008.01.021. [DOI] [PubMed] [Google Scholar]
- [16].Petukhova L, Duvic M, Hordinsky M, Norris D, Price V, Shimomura Y, et al. Genome-wide association study in alopecia areata implicates both innate and adaptive immunity. Nature. 2010;466:113–7. doi: 10.1038/nature09114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ogasawara K, Hamerman JA, Ehrlich LR, Bour-Jordan H, Santamaria P, Bluestone JA, et al. NKG2D Blockade Prevents Autoimmune Diabetes in NOD Mice. Immunity. 2004;20:757–67. doi: 10.1016/j.immuni.2004.05.008. [DOI] [PubMed] [Google Scholar]
- [18].Qin H, Lee IF, Panagiotopoulos C, Wang X, Chu AD, Utz PJ, et al. Natural killer cells from children with type 1 diabetes have defects in NKG2D-dependent function and signaling. Diabetes. 2011;60:857–66. doi: 10.2337/db09-1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Van Belle TL, Ling E, Haase C, Bresson D, Urso B, von Herrath MG. NKG2D blockade facilitates diabetes prevention by antigen-specific Tregs in a virus-induced model of diabetes. J Autoimmun. 2013;40:66–73. doi: 10.1016/j.jaut.2012.08.001. [DOI] [PubMed] [Google Scholar]
- [20].Wortham BW, Eppert BL, Motz GT, Flury JL, Orozco-Levi M, Hoebe K, et al. NKG2D mediates NK cell hyperresponsiveness and influenza-induced pathologies in a mouse model of chronic obstructive pulmonary disease. Journal of immunology. 2012;188:4468–75. doi: 10.4049/jimmunol.1102643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Xia M, Guerra N, Sukhova GK, Yang K, Miller CK, Shi GP, et al. Immune activation resulting from NKG2D/ligand interaction promotes atherosclerosis. Circulation. 2011;124:2933–43. doi: 10.1161/CIRCULATIONAHA.111.034850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Zhou R, Wei H, Sun R, Zhang J, Tian Z. NKG2D recognition mediates Toll-like receptor 3 signaling-induced breakdown of epithelial homeostasis in the small intestines of mice. Proc Natl Acad Sci U S A. 2007;104:7512–5. doi: 10.1073/pnas.0700822104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Guerra N, Tan YX, Joncker NT, Choy A, Gallardo F, Xiong N, et al. NKG2D-deficient mice are defective in tumor surveillance in models of spontaneous malignancy. Immunity. 2008;28:571–80. doi: 10.1016/j.immuni.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kim S, Iizuka K, Aguila HL, Weissman IL, Yokoyama WM. In vivo natural killer cell activities revealed by natural killer cell-deficient mice. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:2731–6. doi: 10.1073/pnas.050588297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Katz JD, Wang B, Haskins K, Benoist C, Mathis D. Following a diabetogenic T cell from genesis through pathogenesis. Cell. 1993;74:1089–100. doi: 10.1016/0092-8674(93)90730-e. [DOI] [PubMed] [Google Scholar]
- [26].Carnaud C, Gombert J, Donnars O, Garchon H, Herbelin A. Protection against diabetes and improved NK/NKT cell performance in NOD.NK1.1 mice congenic at the NK complex. J Immunol. 2001;166:2404–11. doi: 10.4049/jimmunol.166.4.2404. [DOI] [PubMed] [Google Scholar]
- [27].Luhder F, Katz J, Benoist C, Mathis D. Major histocompatibility complex class II molecules can protect from diabetes by positively selecting T cells with additional specificities. J Exp Med. 1998;187:379–87. doi: 10.1084/jem.187.3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Poirot L, Benoist C, Mathis D. Natural killer cells distinguish innocuous and destructive forms of pancreatic islet autoimmunity. Proc Natl Acad Sci U S A. 2004;101:8102–7. doi: 10.1073/pnas.0402065101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ogasawara K, Hamerman JA, Hsin H, Chikuma S, Bour-Jordan H, Chen T, et al. Impairment of NK Cell Function by NKG2D Modulation in NOD Mice. Immunity. 2003;18:41–51. doi: 10.1016/s1074-7613(02)00505-8. [DOI] [PubMed] [Google Scholar]
- [30].Jamieson AM, Diefenbach A, McMahon CW, Xiong N, Carlyle JR, Raulet DH. The role of the NKG2D immunoreceptor in immune cell activation and natural killing. Immunity. 2002;17:19–29. doi: 10.1016/s1074-7613(02)00333-3. [DOI] [PubMed] [Google Scholar]
- [31].Zhou R, Wei H, Sun R, Tian Z. Recognition of double-stranded RNA by TLR3 induces severe small intestinal injury in mice. J Immunol. 2007;178:4548–56. doi: 10.4049/jimmunol.178.7.4548. [DOI] [PubMed] [Google Scholar]
- [32].Pedotti R, Mitchell D, Wedemeyer J, Karpuj M, Chabas D, Hattab EM, et al. An unexpected version of horror autotoxicus: anaphylactic shock to a self-peptide. Nat Immunol. 2001;2:216–22. doi: 10.1038/85266. [DOI] [PubMed] [Google Scholar]
- [33].Matsumoto Y, Kohyama K, Aikawa Y, Shin T, Kawazoe Y, Suzuki Y, et al. Role of natural killer cells and TCR gamma delta T cells in acute autoimmune encephalomyelitis. European journal of immunology. 1998;28:1681–8. doi: 10.1002/(SICI)1521-4141(199805)28:05<1681::AID-IMMU1681>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- [34].Zhang B, Yamamura T, Kondo T, Fujiwara M, Tabira T. Regulation of experimental autoimmune encephalomyelitis by natural killer (NK) cells. J Exp Med. 1997;186:1677–87. doi: 10.1084/jem.186.10.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Winkler-Pickett R, Young HA, Cherry JM, Diehl J, Wine J, Back T, et al. In vivo regulation of experimental autoimmune encephalomyelitis by NK cells: alteration of primary adaptive responses. J Immunol. 2008;180:4495–506. doi: 10.4049/jimmunol.180.7.4495. [DOI] [PubMed] [Google Scholar]
- [36].Kupz A, Scott TA, Belz GT, Andrews DM, Greyer M, Lew AM, et al. Contribution of Thy1+ NK cells to protective IFN-gamma production during Salmonella Typhimurium infections. Proceedings of the National Academy of Sciences of the United States of America. 2013 doi: 10.1073/pnas.1222047110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Holdenrieder S, Eichhorn P, Beuers U, Samtleben W, Stieber P, Nagel D, et al. Soluble NKG2D ligands in hepatic autoimmune diseases and in benign diseases involved in marker metabolism. Anticancer Res. 2007;27:2041–5. [PubMed] [Google Scholar]
- [38].Markle JG, Frank DN, Mortin-Toth S, Robertson CE, Feazel LM, Rolle-Kampczyk U, et al. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science. 2013;339:1084–8. doi: 10.1126/science.1233521. [DOI] [PubMed] [Google Scholar]
- [39].Darlington PJ, Podjaski C, Horn KE, Costantino S, Blain M, Saikali P, et al. Innate immune-mediated neuronal injury consequent to loss of astrocytes. J Neuropathol Exp Neurol. 2008;67:590–9. doi: 10.1097/NEN.0b013e3181772cf6. [DOI] [PubMed] [Google Scholar]
- [40].Saikali P, Antel JP, Newcombe J, Chen Z, Freedman M, Blain M, et al. NKG2D-mediated cytotoxicity toward oligodendrocytes suggests a mechanism for tissue injury in multiple sclerosis. J Neurosci. 2007;27:1220–8. doi: 10.1523/JNEUROSCI.4402-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Bakker AB, Hoek RM, Cerwenka A, Blom B, Lucian L, McNeil T, et al. DAP12-deficient mice fail to develop autoimmunity due to impaired antigen priming. Immunity. 2000;13:345–53. doi: 10.1016/s1074-7613(00)00034-0. [DOI] [PubMed] [Google Scholar]
- [42].Diefenbach A, Tomasello E, Lucas M, Jamieson AM, Hsia JK, Vivier E, et al. Selective associations with signaling proteins determine stimulatory versus costimulatory activity of NKG2D. Nat Immunol. 2002;3:1142–9. doi: 10.1038/ni858. [DOI] [PubMed] [Google Scholar]
- [43].Lanier LL. DAP10- and DAP12-associated receptors in innate immunity. Immunological reviews. 2009;227:150–60. doi: 10.1111/j.1600-065X.2008.00720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.