Abstract
Background: Injectable antispasticity agents have been utilized for the reduction of pain. However, there are no reports of its use for end-of-life pain.
Patient Case: A 62-year-old female with a history of progressive left frontotemporal glioblastoma status post gross total resection, radiation, and chemotherapy presented to the physical medicine and rehabilitation (PM&R) clinic for management of spastic quadriplegia and pain. At the time of presentation to the PM&R clinic she was no longer eligible for further cancer treatment. The patient had been declining neurologically with cognitive changes, weakness, and increasing spasticity. The patient had an Edmonton Symptom Assessment Scale (ESAS) pain score of 8/10 at her visit, as reported by her husband. She exhibited mild to moderate spasticity during the exam. Cognitively, she was unable to follow commands and would fluctuate between being awake for a few minutes and sleeping during the exam. She was not on any oral muscle relaxants and none were started due to her state of hypoarousal. Nine days after the initial consultation she received 700 units of onabotulinum toxin into her bilateral upper limbs and left thigh and a phenol nerve block to her left tibial nerve. At a follow-up visit 28 days later in the palliative care clinic, the ESAS pain score was 0. The patient died 51 days post-injection.
Conclusion: The case report demonstrates the use of injectable antispasticity agents in the reduction of end-of-life pain in a glioblastoma patient.
Introduction
Botulinum toxin and phenol have been effective in the treatment of many musculoskeletal and neuromuscular conditions. Botulinum toxin (BT) works at the presynaptic region of the neuromuscular junction by blocking the release of acetylcholine from the motor nerve terminals.1,2 Via this chemical denervation, BT causes muscle weakness in overactive, hypertonic muscles. Additionally, research suggests that it may cause analgesia by affecting other neurotransmitters. The effects of BT last three to four months.1 In the literature there are numerous reports on the effectiveness of BT in treating painful conditions of varying etiologies, including neuropathic pain, myofascial pain, and other soft-tissue pain syndromes, such as fibromyalgia.3 Phenol nerve blocks have been used long before BT. Phenol denatures protein and thus causes generalized neurolysis that affects both motor and sensory nerve fibers.4 Thus it reduces muscle tone by reducing abnormal neural signals. Phenol is effective in spasticity of large proximal leg muscles or as a nerve block in spastic foot drop.5 In this case we present a patient who had severe pain from her central nervous system (CNS) mediated spasticity and the efficacy of a combination of onabotulinum toxin and phenol in providing pain relief.
Case Presentation
This was a 62-year-old right-handed female with a history of Meige's Syndrome and tetraparesis due to glioblastoma who was referred to the PM&R clinic at a tertiary care cancer center for the management of spasticity. Of note, she had a long history of treatment of the Meige's Syndrome associated facial dystonia and blepharospasm with BT but had not had an injection in years. The patient underwent a left frontotemporal craniotomy for resection of this mass, and pathology confirmed glioblastoma multiforme 18 months prior to physiatry presentation. Two months later she completed a total radiation dose of 60 Gy in 30 fractions with concurrent temazolamide chemotherapy. After failure of temozolamide, thalidomide and an experimental agent, ANG1005, were used. Chemotherapy was discontinued six months before PM&R clinic presentation due to myelosuppression, disease progression, and poor functional status. No further chemotherapy or radiation treatment would be offered; however, the patient was provided with ongoing symptom management. The patient was on modafinil to treat her hypoarousal. She relied on tube feeds for nutrition.
She presented to the PM&R clinic for spastic quadriplegia management (left greater than right). Her spasticity was constantly present but was accompanied by “spasm attacks,” which were very painful and could last for several minutes at a time. Although the patient was unable to verbalize her pain, the husband would notice signs of discomfort including moaning and cringing. The Edmonton Symptom Assessment Scale (ESAS) pain score was 8 at the initial visit, as reported by her husband.
On physical examination she exhibited fluctuating levels of arousal and responsiveness. For brief moments she was awake and would visually scan the room without maintaining persistent eye contact. This alternated with periods of drowsiness. She was unable to follow commands or respond verbally to any questions. She had increased muscle tone in all four extremities, left greater than right. Modified Ashworth scores (out of 4) were: right elbow flexors, 1; right ankle plantar flexors, 2; left elbow flexors, 2; left third, fourth, fifth finger flexors, 2; left knee extensors, 2; left ankle plantar flexors, 3.
She was diagnosed with spasticity due to her progressive glioblastoma. She was not on any oral muscle relaxants or opioid analgesics and none were started due to her state of hypoarousal. She had a mixed delirium with hypoactive features with fluctuating consciousness. She was already on dexamethasone to manage the presumptive diagnosis of cerebral edema and had electroencephalography with no evidence of epileptiform discharges. It was determined that the best option to reduce her painful muscle spasms given her decreased alertness would be injection with BT. It was also quite evident that her prognosis was poor. The patient returned to the clinic nine days after the initial consultation and received 700 units of onabotulinum toxin into her bilateral upper limbs and left lower limb and a phenol nerve block of the right lower limb tibial nerve. The phenol nerve block consisting of 3 ml of 6% phenol at 0.3 mA of electrical stimulation was used to avoid overdosing with the botulinum toxin. Home health occupational therapy was also requested.
The following muscles were injected with onabotulinum toxin:
• Right flexor carpi ulnaris: 50 units
• Right pronator teres: 50 units
• Left flexor digitorum superficialis: 50 units
• Left flexor digitorum profundus: 50 units
• Right bicep brachii: 50 units
• Left bicep brachii: 150 units
• Left brachialis: 50 units
• Left latissimus dorsi: 100 units
• Left quadriceps: 150 units
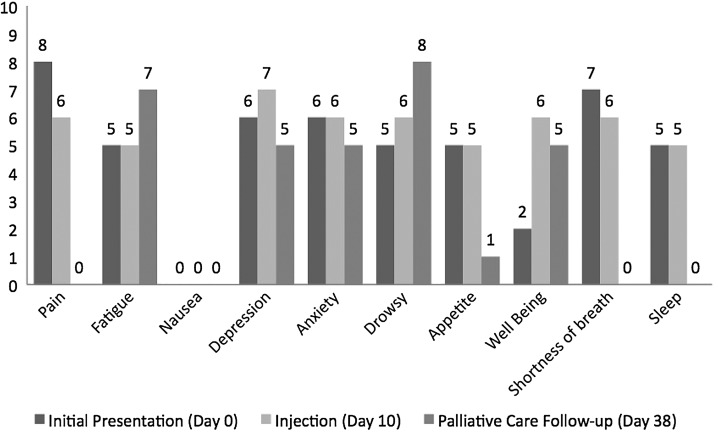
An ESAS pain score of 6 was reported on the day of the procedure prior to the injections. At a follow-up visit 28 days later in the palliative care clinic, the ESAS pain score was 0, with notable improvement in her spastic muscle pain reported by both the palliative care specialist and her husband. Furthermore, her ESAS scores were improved at this time (28 days post-injection) in depression, anxiety, appetite, shortness of breath, and sleep (see Figure 1). ESAS totals were 49 at initial physiatry clinic presentation, 52 on the day of injection (10 days after clinic presentation), and 31 on the day of palliative care follow-up (38 days after clinic presentation). The patient died 61 days after her initial physiatry clinic presentation.
FIG. 1.
Edmonton Symptom Assessment Scale (ESAS) scores from initial PM&R clinic visit (Day 0), day of botulinum toxin injection (Day 10), and during a follow up clinic visit (Day 38).
Discussion
We report the successful use of injectable spasticity agents for treatment of end-of-life pain related to poorly controlled muscle spasms. The patient's ESAS pain scores were high and her husband reported a high level of distress due to her pain. After receiving her injections there was tremendous improvement in her pain (per her husband's report), and this alleviated her symptom burden as a whole as demonstrated by the decrease in her total ESAS score.
Traditionally, it has been understood that BT delivers pain relief by reducing hypertonic muscle activity.2 Interruption of neuromuscular transmission leads to a decrease in the excessive muscle contraction that causes pain. However, the pain relief is not attributed solely to the muscle relaxant effects of BT, as BT may have direct and indirect analgesic properties. Pain relief from BT injections for spastic conditions outweighs the degree of muscle relaxation or improvement in function.7,8 Studies on the antinociceptive effects of BT in animal models have shown that onabotulinumtoxinA inhibits the release of glutamate and excitatory neurotransmitters such as substance P from nociceptive neurons, which reduces peripheral pain nerve sensitization.6 There is also a concomitant indirect reduction in central sensitization, which is associated with a reduction in chronic pain.5,8 In this patient's case the pain relief was likely due to a combination of the analgesic effects of the medication and the relaxation of the uncomfortable spastic muscles.
The first application of BT for providing pain relief was demonstrated in the treatment of cervical dystonia.1 BT is clearly effective and safe for cervical dystonia, as injections into cervical muscles at the appropriate doses are well tolerated.10 However optimal treatment intervals, long term efficacy, and quality of life issues need to be explored further.10 There have also been numerous studies on the use of BT for hemiplegic stroke patients with shoulder pain. Botulinum injections into the pectoralis major muscle yielded substantial decreases in pain in hemiplegic patients with spasticity and shoulder pain.11 Another randomized, double-blind study comparing intramuscular onabotulinum toxin injections versus intraarticular triamcinolone acetonide injections showed significant improvement in pain and improved shoulder range-of-motion in the group treated with onabotulinum toxin.12 A Cochrane Review on BT injections for shoulder pain highlighted the pain reduction, improved shoulder function, and shoulder range of motion associated with BT injections versus placebo.13 However the results of these studies should be interpreted with caution as there are only a few studies and they have small sample sizes, which results in a high risk of bias.13 An evidence-based review of BT for spasticity showed Level A evidence for use of BT for spasticity treatment in adults and children, especially in the injection of calf muscles for cerebral palsy equinus varus deformities and to improve muscle tone and passive function in adults with focal spasticity.1 Pilot studies on the use of BT in patients with neuropathic pain due to complex regional pain syndrome Type 1, spinal cord injury, and brachial plexopathies have demonstrated effective pain relief; however, the sample sizes in these studies were small.14,15
BT has also been reported to be effective in the treatment of myofascial pain. A Cochrane Review on the effectiveness of treating myofascial pain syndrome (excluding head and neck muscles) found four randomized, controlled studies comparing BT injections versus placebo.7 Only one of the four studies reported significant improvement in pain intensity scores. Thus, there is inconclusive evidence to support the use of BT for myofascial pain syndrome. Treatment of multiple neuromuscular and musculoskeletal conditions with BT has been examined in recent years with mixed evidence in the literature.
BT management of sialorrhea can be utilized in the supportive care setting. Much of the BT data on sialorrhea have been focused on cerebral palsy, Parkinson's, and amyotrophic lateral sclerosis patient populations.16,17 The direct anticholinergic effects of the toxin on the salivary glands decrease the secretion of water and electrolytes.16 Studies on the use of BT in head and neck cancer patients include reports of BT injections pre- and postoperatively to limit salivation and to maintain oral hygiene to limit the risk of infection, including the risk of aspiration pneumonia.18–20 The xerostomia-induced effects of BT in salivary gland injections potentially can be utilized in end-of-life care to provide oral hygiene for patients who are no longer able to control their oral secretions and who are not candidates for systemic pharmacological therapies.
The use of neurolytic agents such as phenol and ethanol for pain has also been well studied. Improved pain in the right lower limb below the knee could be due to reduced efferent and afferent nerve signals from the nerve block resulting in reduced tone and sensation respectively. The addition of a nerve block may be indicated when there are multiple regions of spasticity and there is not enough BT to adequately treat all of the areas, as there is a maximal dose per session.
A limitation in this case is that the patient's pain was assessed with the ESAS reported by her husband. Patients' ratings of their symptoms should be the “gold standard;”21,22 however, our patient was too sedated to provide an assessment of her symptoms. In the palliative care unit or other settings where patients may be unresponsive or delirious, it is common practice for the nursing staff or family members to perform the ESAS. Research shows that family-reported ESAS scores are accurate in the assessment of patient distress.22–24 Moreover, agreement in ratings of symptoms from proxies were more often accurate with physical symptoms, such as with pain.24 Thus, family-reported assessments should be obtained only if the patient is clearly unable to report his or her own symptom assessments because of a cognitive impairment.
Delirium and cognitive impairment are common symptoms among patients at the end of life. The symptoms can be exacerbated by systemic muscle relaxants and analgesic agents. Use of injectable spasticity reducing agents in the treatment of end-of-life pain can reduce painful symptoms with minimal systemic side effects. This is the first case report of the use of such agents for end-of-life pain.
Conclusions
This is the first case report demonstrating the successful use of injectable antispasticity agents used in the relief of end-of-life pain in a patient with progressive glioblastoma. Because of the minimal systemic side effects of these agents, they may be a useful tool reducing pain in terminally ill patients. More studies, specifically high-quality randomized, controlled studies, need to be done in order to provide conclusions on the effectiveness of the medication and to further explore the study safety profile of BT.
Acknowledgments
This study was supported in part by M.D. Anderson Cancer Center support grant CA 016672. Eduardo Bruera is supported in part by NIH grants RO1NRO10162-01A1, RO1CA122292-01, and RO1CA124481-01.
Author Disclosure Statement
No conflicting financial interests exist.
References
- 1.Simpson DM, Gracies JM, Graham HK, et al.: Assessment: Botulinum neurotoxin for the treatment of spasticity (an evidence-based review): Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurol 2008;70:1691–1698 [DOI] [PubMed] [Google Scholar]
- 2.Aoki KR: Evidence for antinociceptive activity of botulinum toxin type A in pain management. Headache 2003;43Suppl 1:S9–S15 [DOI] [PubMed] [Google Scholar]
- 3.Smith HS, Audette J, Royal MA: Botulinum toxin in pain management of soft tissue syndromes. Clin J Pain 2002;18:S147–S154 [DOI] [PubMed] [Google Scholar]
- 4.Horn LJ, Singh G, Drabowski ER. Chemoneurolysis with phenol and alcohol: A “dying art” that merits revival. In: Brashear A, Elovic E. (eds): Spasticity Diagnosis and Management. New York: Demos Medical, 2011, pp. 102–103 [Google Scholar]
- 5.Kheder A, Nair KP: Spasticity: Pathophysiology, evaluation and management. Pract Neurol 2012;12:289–298 [DOI] [PubMed] [Google Scholar]
- 6.Aoki KR, Francis J: Updates on the antinociceptive mechanism hypothesis of botulinum toxin A. Parkinsonism Relat Disord 2011;17Suppl 1:S28–S33 [DOI] [PubMed] [Google Scholar]
- 7.Soares A, Andriolo RB, Atallah AN, da Silva EM: Botulinum toxin for myofascial pain syndromes in adults. Cochrane Database Syst Rev 2012;4:CD007533. [DOI] [PubMed] [Google Scholar]
- 8.Wissel J, Muller J, Dressnandt J, et al.: Management of spasticity associated pain with botulinum toxin A. J Pain Symptom Manage 2000;20:44–49 [DOI] [PubMed] [Google Scholar]
- 9.Smith HS, Audette J, Royal MA: Botulinum toxin in pain management of soft tissue syndromes. Clin J Pain 2002;18:S147–S154 [DOI] [PubMed] [Google Scholar]
- 10.Costa J, Espirito-Santo C, Borges A, et al.: Botulinum toxin type B for cervical dystonia. Cochrane Database Syst Rev 2005:CD004315. [DOI] [PubMed] [Google Scholar]
- 11.Marco E, Duarte E, Vila J, et al.: Is botulinum toxin type A effective in the treatment of spastic shoulder pain in patients after stroke? A double-blind randomized clinical trial. J Rehab Med 2007;39:440–447 [DOI] [PubMed] [Google Scholar]
- 12.Lim JY, Koh JH, Paik NJ: Intramuscular botulinum toxin-A reduces hemiplegic shoulder pain: A randomized, double-blind, comparative study versus intraarticular triamcinolone acetonide. Stroke 2008;39:126–131 [DOI] [PubMed] [Google Scholar]
- 13.Singh JA, Fitzgerald PM: Botulinum toxin for shoulder pain. Cochrane Database Syst Rev 2010:CD008271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Argoff CE: A focused review on the use of botulinum toxins for neuropathic pain. Clin J Pain 2002;18:S177–S181 [DOI] [PubMed] [Google Scholar]
- 15.Ranoux D, Attal N, Morain F, Bouhassira D: Botulinum toxin type A induces direct analgesic effects in chronic neuropathic pain. Ann Neurol 2008;64:274–283 [DOI] [PubMed] [Google Scholar]
- 16.Ellies M, Laskawi R, Tormahlen G, Gotz W: The effect of local injection of botulinum toxin A on the parotid gland of the rat: An immunohistochemical and morphometric study. J Oral Maxillofac Surg 2000;58:1251–1256 [DOI] [PubMed] [Google Scholar]
- 17.Ellies M, Laskawi R, Rohrbach-Volland S, Arglebe C: Up-to-date report of botulinum toxin therapy in patients with drooling caused by different etiologies. J Oral Maxillofac Surg 2003;61:454–457 [DOI] [PubMed] [Google Scholar]
- 18.Bomeli SR, Desai SC, Johnson JT, Walvekar RR: Management of salivary flow in head and neck cancer patients: A systematic review. Oral Oncol 2008;44:1000–1008 [DOI] [PubMed] [Google Scholar]
- 19.Corradino B, Di Lorenzo S, Moschella F: Botulinum toxin A for oral cavity cancer patients: In microsurgical patients BTX injections in major salivary glands temporarily reduce salivary production and the risk of local complications related to saliva stagnation. Toxins 2012;4:956–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laskawi R, Ellies M: The role of botulinum toxin in the management of head and neck cancer patients. Curr Opin Otolaryngol Head Neck Surg 2007;15:112–116 [DOI] [PubMed] [Google Scholar]
- 21.Bruera E, Watanabe S: New developments in the assessment of pain in cancer patients. Support Care Cancer 1994;2:312–318 [DOI] [PubMed] [Google Scholar]
- 22.Nekolaichuk CL, Bruera E, Spachynski K, MacEachern T, Hanson J, Maguire TO: A comparison of patient and proxy symptom assessments in advanced cancer patients. Palliat Med 1999;13:311–323 [DOI] [PubMed] [Google Scholar]
- 23.Akin S, Durna Z: A comparative descriptive study examining the perceptions of cancer patients, family caregivers, and nurses on patient symptom severity in Turkey. Eur J Oncol Nurs 2013;17:30–37 [DOI] [PubMed] [Google Scholar]
- 24.Wennman-Larsen A, Tishelman C, Wengstrom Y, Gustavsson P: Factors influencing agreement in symptom ratings by lung cancer patients and their significant others. J Pain Symptom Manage 2007;33:146–155 [DOI] [PubMed] [Google Scholar]