Abstract
Objective: The aim of this study was to investigate the effect of laser irradiation on root surface demineralization caused by local drug delivery systems (DDS), and to evaluate the effect of sealing on drug retention. Background data: The duration of supportive periodontal treatment (SPT) has increased with increasing life expectancy. Repeated root planing and DDS application during SPT should be reconsidered with regard to their effects on the root surface. Methods: Extracted human teeth were collected, cut into 3×3×2 mm root dentin specimens, and divided randomly into eight groups with various combinations of Nd:YAG laser power (0, 0.5, 1, and 2 W), with and without DDS (minocycline HCl). Specimen microhardness and calcium (Ca) solubility were measured after treatment. The specimens (control and laser and DDS groups) were examined by scanning electron microscopy. Forty SPT patients were recruited, to assess the effect of periodontal pocket sealing on drug retention. Results: Laser irradiation increased the microhardness of root specimens in an energy-dependent manner. Calcium solubilities decreased from the 0 W+DDS group to the 2.0 W+DDS group. The mean Ca solubilities in the 1.0 W+DDS and 2.0 W+DDS groups were significantly lower than in the 0 W+DDS group (p<0.01, p<0.001, respectively). Laser irradiation counteracted the softening effect of DDS. Morphologic change was observed in the 2W+DDS group; however, no morphologic changes were observed in the control and the 1W+DDS groups. The mean concentration of minocycline in the periodontal pocket 24 h after application was 252.79±67.50 μg/mL.Conclusions: Laser irradiation of the root surface inhibited the softening and decalcification caused by minocycline HCl. Sealing the periodontal pockets effectively improved drug retention. These results suggest that the combination of laser irradiation and DDS could benefit patients receiving repeated SPT.
Introduction
The goals of periodontal treatment are reduction of probing pocket depth, and establishment of good plaque control and stable occlusion. However, residual pockets often remain in patients even after active periodontal treatment. In these cases, repeated supportive periodontal treatment (SPT) is needed to prevent recurrence of periodontitis and further tooth loss.1 Currently, SPT programs consist primarily of scaling and root planing (SRP), or a combination of SRP with adjunctive antibacterial ointment application to the periodontal pockets to achieve a synergistic debridement effect. Supportive periodontal treatment should be scheduled repeatedly every 3–4 months, often long-term,2 because life expectancy in many countries is increasing.
SRP has some disadvantages. Scalers cannot easily reach the bottom of pockets;3–5 therefore, residual plaque and calculus can remain in the deep pockets after treatment. Furthermore, a single light pass with a scaler can shave 4.8–20.6 μm from the root surface.6 Repeated use of mechanical instruments may lead to cumulative scuffing of the root surface.6,7 The use of SRP in SPT should be reconsidered with regard to changes in the root surface, including surface morphology. To avoid these changes, an alternative method with less effect on the root surface is required.
Local drug delivery systems (DDS) offer advantages over systemic antibiotic administration: (1) a relatively small amount of drug can produce a high concentration in the periodontal pocket; and (2) complications associated with systemic administration are generally reduced. However, DDS also has some disadvantages. Many DDS agents have been used, including tetracycline fiber,8 minocycline ointment, doxycycline polymer, metronidazole gel,9 and chlorhexidine chip.10 Of these, the tetracycline series in particular is used in hydrochloride form, which has a low pH. Short-term application of a low pH tetracycline HCl solution can demineralize the root surface,11–13 softening the root. Another disadvantage of DDS is that most of the injected drug disappears within 6–7 h.14 To overcome this disadvantage, Polson et al.15 modified the copolymer base material (polylactic acid and D-L-lactide dissolved in a biocompatible carrier) to slow the release rate. Although achieving high local drug concentrations for 2–5 days helps eradicate periodontal pathogens in the pocket,16 this duration is considered too long with regard to the demineralizing effect on the root surface, especially when using the tetracycline HCl series.
Laser irradiation is known to inhibit the effect of demineralization by acid solution. In particular, Nd:YAG laser alone and/or in combination with acidulated phosphate fluoride (APF) or fluoride has been shown to enhance the acid-resistance of enamel.17–19 Therefore, we proposed a new SPT program using a combination of laser irradiation of the root surface instead of SRP, with effective DDS concentration achieved against biofilm by the application of a sealer.
The purpose of this preliminary study was to investigate the effect of irradiation with a Nd:YAG laser on root surface demineralization, and to establish a high drug concentration in the periodontal pocket by applying a sealer to the pocket opening.
Materials and Methods
Sample collection and disc specimen preparation
Thirty-four non-carious, extracted human premolar and molar teeth were used. After extraction, teeth were immediately rinsed with distilled water and brushed gently with a soft toothbrush. They were stored in distilled water at −4°C until the next step.
The root surfaces of the sample teeth were scaled with Gracey curets #1-2 (Hu-Friedy, Chicago, IL) to remove remaining soft tissues and calculus, providing a clean surface. The 34 prepared tooth roots were used and cut into 100 pieces measuring 3×3×2 mm with a water-cooled, low-speed diamond cutter (Fig. 1B), and embedded in acrylic resin (thickness 4 mm) setting the root surface upper (Fig. 1C). One hundred root disk specimens with a diameter of 15 mm and a thickness of 3 mm cutting upper surface were prepared using a Zegen microtome SP1600 (Leica, Tokyo, Japan) to expose the root dentin (Fig. 1D). Root dentin surfaces were polished and finished with waterproof abrasive 800-grit sandpaper (Sanko Co. Japan). Specimen discs were placed into the ultrasonic bath sonicator for 30 min to remove extraneous matter left over from the procedure. All prepared disk specimens were gathered and shuffled.
FIG. 1.
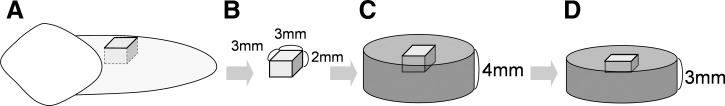
Preparation procedure of sample species. (A) Extracted sample teeth (B) cut into cubic piece (3×3×2 mm), (C) embedded in acrylic resin 4 mm thick. (D) To make a flattened root dentin surface, 1 mm embedded acrylic resin specimen from upper surface (cementum) was cut and polished.
Laser application
An Nd:YAG laser system (Neocure 7200®, SOKKIA, Kyoto, Japan) was used in this study. This system emits photons at a wavelength of 1064 nm with pulse duration of 90 μsec and pulse rate of 10–100 pulse/sec. The power output settings of the Nd:YAG laser used in this study were 0.5 W (50 mJ/pulse), 1.0 W (100 mJ/pulse), and 2.0 W (200 mJ/pulse).
Microhardness assessment and calcium solubility
The disc specimens were divided randomly into eight groups. The control group received no laser irradiation or drug application (n=10). The laser alone group (n=30) received laser irradiation treatment only, and was subdivided randomly into three subgroups based on laser power outputs (0.5 W, n=10; 1.0 W, n=10; 2.0 W, n=10). The DDS group (n=60) received DDS with or without laser treatment and was subdivided randomly into four subgroups (0 W+DDS, n=15; 0.5 W+DDS, n=15; 1.0 W+DDS, n=15; 2.0 W+DDS, n=15).
The laser fiber tip was placed in contact with the exposed root surface and moved slowly at an inclination of 45 degrees relative to the tooth surface, with saline on the root surface to simulate a periodontal pocket. A single operator irradiated the exposed root surface of all experimental disk specimens for 30 sec at approximately the same speed. After laser irradiation, 20 mg of minocycline HCl ointment (Periocline®; Sunstar, Osaka, Japan) was placed on the specimens in the four DDS subgroups, and the specimens were stored at 37°C for 24 h.
The microhardness of each specimen was measured using microhardness testing equipment (MVK-C6; AKASHI, Japan). The Knoop hardness (Hk) of each segment was measured five times with a 100 g load for 30 sec and the value was determined as follows:
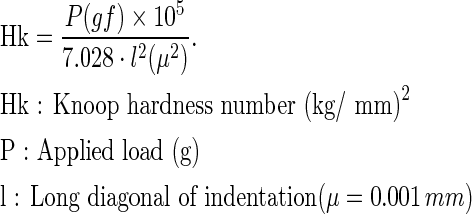 |
To analyze calcium solubility, minocycline HCl ointment was dissolved with 2 mL of N,N-dimethylformamide (Kanto Chemical, Tokyo, Japan) on the laser and DDS disk specimens. Ca solubilities in the solutions were determined by a polarized Zeeman atomic absorption spectrophotometer (Z-8200; Hitachi, Japan). The area of the root surface was photographed with a scale and measured using imaging software (ImageJ, National Institutes of Health, Bethesda, MD). Acid resistance was expressed as dissolved Ca per area (μg/mm2).
Morphologic studies (scanning electron microscopic [SEM] examination)
After DDS application followed by laser irradiation, specimens were fixed with a 2% glutaraldehyde solution in phosphate buffered saline for 3–4 h at room temperature. Fixed specimens were treated with 1% osmium tetroxide (buffered at pH 6.5 with 0.1 M sodium cacodylate) for 2 h. After being rinsed several times in a phosphate buffer, the specimens were dehydrated with a graded series of aqueous ethanol (50%, 70%, 80%, 90%, 95%, and 100%) for 30 min at each concentration and dried in hexamethyldisilazane at room temperature. All specimens were sputter-coated with gold to a thickness of 10 nm using an ion coater (JFC-1500, JEOL, Tokyo, Japan) and observed under an SEM (EVO 40, ZEISS, Tokyo, Japan) at 15 kV to examine themorphologic features and microstructural alterations on the treated root surfaces.
Assessment of the effect of periodontal pocket sealing
Forty patients (mean age 60.9±11.7 years) seeking periodontal care at Aichi-Gakuin University Dental Hospital were recruited for assessment of drug retention 24 h after DDS application. The purpose and design of the study were explained and patients signed an informed consent form. Samples were taken from the subjects' periodontal pockets 24 h after DDS injection, following the guidelines of the Aichi-Gakuin University Code of Ethics (section 39). All patients had been receiving SPT for>1 year, had inactive periodontal pocket depth≥4 mm, had≥15 teeth, and had not used antibiotics during the 6 months prior to the SPT phase. Among these 40 patients, 7 were excluded from the study because their temporary seal agents had detached before the sampling visit. An additional eight patients were excluded because sampling was inappropriate (contamination with saliva during sample collection). The clinical characteristics of the patients are shown in Table 1.
Table 1.
Clinical Characteristics of Patients (40 Patients, 40 Sites)
| Sex |
16 males and 24 females |
| Age (years, mean±SD) |
60.9±11.7 |
| Probing depth (mm, mean±SD) |
6.28±1.49 |
| Clinical attachment level (mm, mean±SD) |
8.12±2.13 |
| Bleeding on probing (%) |
44 |
| Suppuration (%) |
8 |
| Incisors (n) |
8 |
| Canines (n) |
1 |
| Premolars (n) |
9 |
| Molars (n) | 22 |
The root surface in the patients' periodontal pockets was irradiated with a laser at 2 W (200 mJ, 10 pps) for an appropriate time (60 sec/site). The optical fiber was inserted into the pocket along the root surface to a depth 1 mm less than the probing depth, without local anesthesia. After laser treatment, 1 mg of minocycline HCl was applied into the pocket, and the orifice of pocket was sealed with a temporary seal agent (DuraSeal®, Reliance, IL). Twenty-four hours later, the seal was removed, and paper strips (Periopaper®, Pro Flow, Inc., Amityville, NY) were inserted into the pocket for 30 sec at each site to collect a gingival crevicular fluid (GCF) sample. The concentration of minocycline in the GCF sample was determined using high performance liquid chromatography (JASCO, Japan).
Statistical Analysis
The microhardness data and the Ca solubility data were examined with the Shapiro–Wilk test on a normal distribution. One-way ANOVA was used to evaluate the effect of irradiation on the microhardness of the root and on Ca solubility. Post-hoc comparisons were made among laser power and control groups using Dunnett's test.
Statistical analysis was performed using SPSS 15.0 (SPSS Inc., Chicago, IL).
Results
Microhardness and Ca solubility
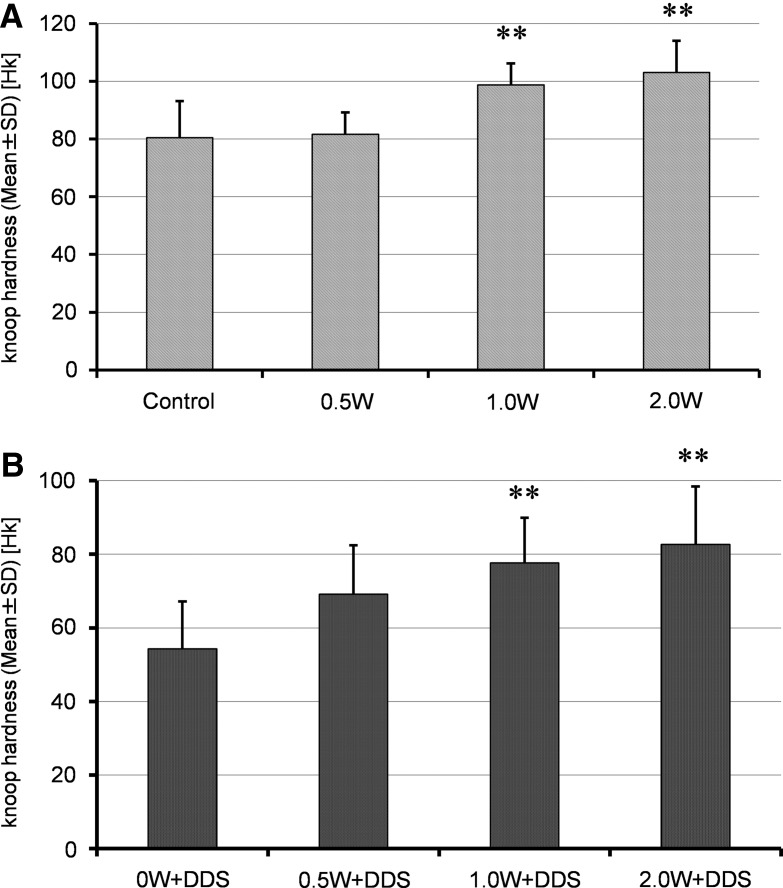
The Knoop hardness (mean±SD) of the laser group was 80.60±6.62 Hk in the control group, 81.73±4.39 Hk at 0.5 W, 98.90±4.23 Hk at 1.0 W, and 103.11±7.38 Hk at 2.0 (Fig. 2A). The mean Knoop hardness values in the 1.0 and 2.0 W groups were significantly higher than in the control group (p<0.001). The Knoop hardness (mean±SD) of the DDS groups was 54.33±9.00 Hk in the 0 W+DDS group, 69.17±10.15 Hk in the 0.5 W+DDS group, 77.69±6.66 Hk in the 1.0 W+DDS group, and 82.73±10.31 Hk in the 2.0 W+DDS group (Fig. 2B). The mean Knoop hardness values in the 0.5 W+DDS group, the 1.0 W+DDS group, and the 2.0 W+DDS group were significantly higher than in the 0 W+DDS group (p<0.001).
FIG. 2.
Knoop hardness. The effect of laser irradiation on the Knoop hardness of root specimens was investigated. Knoop hardness in the laser group increased with increasing laser power from 0 W to 2.0 W. Knoop hardness in 1.0 W and 2.0 W groups was significantly higher than in the control group (p<0.001; A). The Knoop hardness in the groups with laser irradiation followed by minocycline application increased significantly with increasing laser power (1.0 W and 2.0 W; p<0.001; B). The softening effect of minocycline was counteracted by laser irradiation.
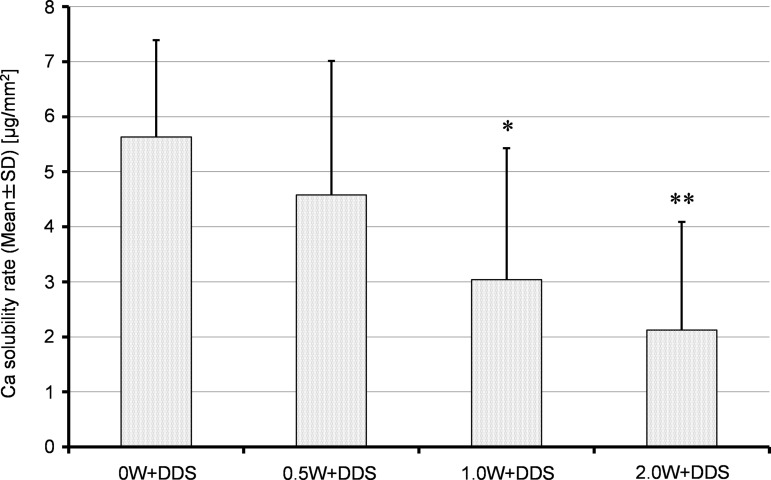
The dissolved Ca per area (μg/mm2) was 5.632±1.762 μg/mm2 in the 0 W+DDS group, 4.578±2.435 μg/mm2 in the 0.5 W+DDS group, 3.041±2.390 μg/mm2 in the 1.0 W+DDS group, and 2.122±1.965 μg/mm2 in the 2.0 W+DDS group (Fig. 3). The mean Ca solubilities in the 1.0 W+DDS and 2.0 W+DDS groups were significantly lower than in the 0 W+DDS group (p<0.01, p<0.001, respectively) whereas no significant difference was found at 0.5 W.
FIG. 3.
Calcium concentration in the minocycline HCl ointment placed on the root specimen for 24 h. In both the 2.0 W+DDS and 1.0 W+DDS groups, Ca solubility was significantly less than in the 0 W+DDS group. 0.5 W+DDS: minocycline was applied to a 0.5 W laser-irradiated root specimen. 1.0 W+DDS: minocycline was applied to a 1.5 W laser-irradiated root specimen. 2.0 W+DDS: minocycline was applied to a 2.0 W laser-irradiated root specimen. (*p<0.01, **p<0.001).
Morphologic studies (SEM examination)
Morphologic alterations of control and treated with laser+DDS specimens are shown in Fig. 4.
FIG. 4.

Scanning electron microscopic (SEM) image of the dentin specimen (×500). Most of dentinal tubules were thought to be occluded by shavings in the process of polishing (A). In the laser+DDS (B,C) group, the orifice of dentinal tubules appeared. The morphologic alterations caused by laser were not observed in the 1W+DDS group. The straight-line, laser-treated site showed a groove-like appearance in the 2W+DDS group, and dentinal tubules occlusion were observed at the lased area (arrow) (C).
The most of dentinal tubules (small and shallow niche) were thought to be occluded by shavings in the process of polishing in the control (0W+without DDS) group (Fig. 4A). Dentinal tubules were clearly observed overall in the 1W+DDS group; however, the morphologic alterations by laser irradiation were not observed clearly (Fig. 4B). The straight-line, laser-treated site showed a groove-like appearance in the 2W+DDS group, and dentinal tubule occlusion was observed at the lased area (Fig. 4C). Between lased lines (unlased area) there was an orifice of dentinal tubules with shiny appearance.
Minocycline concentrations in periodontal pockets
Table 1 shows the clinical characteristics of the subjects who participated in this study. The concentration of minocycline in GCF samples obtained from 25 subjects 24 h after application was 252.79±67.50 μg/mL (mean±SE).
Discussion
In this study, we investigated changes in the root surface (demineralization and decreased microhardness) caused by the application of minocycline HCl ointment for 24 h and the protective effect of laser irradiation before DDS application. The extent of root surface alteration was measured in terms of the microhardness of the root specimen and the Ca dissolved in the ointment.
The smoothness of the specimen was important factor of this kind of study. Therefore, we used waterproof abrasive 800 grit sandpaper to obtain the flattened smooth surface as previously described,20 and that could create the surface roughness (Ra) with 0.56 μm equivalent to that of manual scaling as other investigator described.21,22
The hardening effect of laser irradiation on the root specimens was seen in the 1.0 and 2.0 W groups. A significant protective effect of laser irradiation followed by minocycline application was observed in the 0.5 W+DDS, 1.0 W+DDS, and 2.0 W+DDS groups. White et al.23 obtained similar results, reporting that laser irradiation of dentin before or after acid treatment increased dentin microhardness.
We also analyzed calcium solubility from the disk specimens of the DDS groups. The mean Ca solubility in the 1.0 W+DDS and 2.0 W+DDS groups was significantly lower than in the 0 W+DDS group (p<0.01, p<0.001, respectively). These results agreed with previous studies.24,25
Considering both the microhardening effect and improved acid resistance after laser irradiation, irradiation>2.0 W might be recommended for clinical use.
Although the mechanism of increased microhardness and suppressed decalcification with laser treatment is not completely clear, one possible explanation is that irradiation alters the composition of the hard tissues. A previous study showed that Nd:YAG laser irradiation of a root specimen reduced the intensity of amide peaks III and IV compared with the peaks of an unirradiated control.26 Similar alterations of hard tissue composition have been observed for enamel and dentin. The levels of water, carbonate, and organic substances were reduced in irradiated versus unirradiated enamel. SEM observation showed the melting and resolidification of lased enamel and the dentin surface.27–30 In this study, the dentinal tubule occlusion was observed along with the straight-line, groove-like appearance area, and this phenomenon was thought to be caused by micromelting and recrystallization by laser irradiation. Similar morphologic changes that reduced the diameter of dentinal tubules have been reported previously.31–33 This micromelting and recrystallization within the hard tissues were associated with relatively high mineral contents for the root surface, which may cause increased microhardness and acid resistance.34
Oosterwaal et al. demonstrated that the half-life of fluorescein gel applied to periodontal pockets was 12.5 min.14 To prevent the early loss of the applied drug, we used a sealer at the periodontal pocket opening after DDS application. As a result, 252 μg/mL of minocycline was observed in the GCF 24 h after application. With this long retention time, we assume that the drug spread throughout the entire pocket by passive diffusion, even where a scaler could not reach. Eick and colleagues reported that different drug levels had different effects on biofilm.35,36 They compared the minimum inhibitory concentration (MIC), 5-fold MIC, 10-fold MIC, 50-fold MIC, and 100-fold MIC of a drug and found that concentrations greater than 50-fold MIC at 24 h were effective against biofilm. In another study, Abu-Fanas et al.37 investigated the susceptibility to minocycline of the most commonly isolated gram-negative bacteria taken from periodontal pockets. They showed that MICs of minocycline for these bacteria were<2 μg/mL. Moubareck et al.38 reported that MICs of minocycline against Bacteroides fragilis and Prevotella spp. were 0.06–8 and 0.6–16 μg/mL, respectively. Based on these studies, we believe that the concentration of minocycline we obtained at 24 h (252 μg/mL) is sufficient for clinical treatment, though further studies are needed to test other types of biofilms, similar to the study of Noiri et al.39
For repeated SPT procedures, the combination of laser irradiation with antibiotic therapy appears to be superior to SRP using a scaler with antibiotic therapy, in minimizing changes to the root surface.
Conclusions
Within the limitations of this study, we propose a new approach to SPT using laser irradiation and local antibiotic therapy. To overcome the disadvantages of conventional SPT that includes SRP (in which the root surface is shaved off with each stroke) and the application of adjunctive minocycline HCl (which decalcifies and softens the root surface), laser irradiation of the root surface may be useful. The lased root surface shows greater microhardness than unlased roots, and exhibits acid resistance against the 24 h application of minocycline HCl. Sealing the periodontal pocket opening was shown to be useful for improving local retention of the applied drug. In summary, combining laser irradiation with local antibiotic treatment in supportive periodontal therapy is recommended to preserve the condition of the root surface.
Acknowledgments
This work was supported in part by grants-in-aid from Strategic Research Aichi Gakuin University (AGU)-platform Formation (2008–2012).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Eickholz P., Kim T.-S., Schacher B., Reitmeir P., Bürklin T., and Ratka-Kru″ger P. (2005). Subgingival topical doxycycline versus mechanical debridement for supportive periodontal therapy: a single blind randomized controlled two-center study. Am. J. Dent. 18, 341–346 [PubMed] [Google Scholar]
- 2.Lang N.P., Bragger U., Salvi G.E., Tonetti M.S. (2008). Supportive periodontal therapy (SPT), in: Clinical Periodontology and Implant Dentistry, 5th ed. Lang N.P. and Lindhe J. (eds.). Oxford, UK: Blackwell Munksguard, pp. 1313–1314 [Google Scholar]
- 3.Rabbani G.M., Ash M.M., and Caffesse R.G. (1981). The effectiveness of subgingival scaling and root planing in calculus removal. J. Periodontol. 52, 119–123 [DOI] [PubMed] [Google Scholar]
- 4.Stambaugh R.V., Dragoo M., Smith D.M., and Carasali L. (1981). The limits of subgingival scaling. Int. J. Periodontics Restorative Dent. 1, 30–41 [PubMed] [Google Scholar]
- 5.Waerhaug J. (1978). Healing of the dento-epithelial junction following subgingival plaque control. II. As observed on extracted teeth. J. Periodontol. 49, 119–134 [DOI] [PubMed] [Google Scholar]
- 6.Zappa U., Smith B., Simona C., Graf H., Case D., and Kim W. (1991). Root substance removal by scaling and root planning. J. Periodontol. 62, 750–754 [DOI] [PubMed] [Google Scholar]
- 7.Schmidlin P.R., Beuchat M., Busslinger A., Lehmann B., and Lutz F. (2001). Tooth substance loss resulting from mechanical, sonic and ultrasonic root instrumentation assessed by liquid scintillation. J. Clin. Periodontol. 28, 1058–1066 [DOI] [PubMed] [Google Scholar]
- 8.Bonito A.J., Lux L., and Lohr K.N. (2005). Impact of local adjuncts to scaling and root planing in periodontal disease therapy: a systematic review. J. Periodontol. 76, 1227–1236 [DOI] [PubMed] [Google Scholar]
- 9.Jansson H., Bratthall G., and Soderholm G. (2003). Clinical outcome observed in subjects with recurrent periodontal disease following local treatment with 25% metronidazole gel. J. Periodontol. 74, 372–377 [DOI] [PubMed] [Google Scholar]
- 10.Soskolne W.A., Heasman P.A., Stabholz A., Smart G.J., Palmer M., Flashner M., et al. (1997). Sustained local delivery of chlorhexidine in the treatment of periodontitis: a multi-center study. J. Periodontol. 68, 32–38 [DOI] [PubMed] [Google Scholar]
- 11.Işik G., Ince S., Sağlam F., Onan U. (1997). Comparative SEM study on the effect of different demineralization methods with tetracycline HCl on healthy root surfaces. J. Clin. Periodontol. 24, 589–594 [DOI] [PubMed] [Google Scholar]
- 12.Isik A.G., Tarim B., Hafez A.A., Yalçin F.S., Onan U., and Cox C.F. (2000). A comparative scanning electron microscopic study on the characteristics of demineralized dentin root surface using different tetracycline HCl concentrations and application times. J. Periodontol. 71, 219–225 [DOI] [PubMed] [Google Scholar]
- 13.Sterrett J.D., Simmons J., Whitford G., and Russell C.M. (1997). Tetracycline demineralization of dentin: the effects of concentration and application time. J Clin. Periodontol. 24, 457–463 [DOI] [PubMed] [Google Scholar]
- 14.Oosterwaal P.J., Mikx F.H., and Renggli H.H. (1990). Clearance of a topically applied fluorescein gel from periodontal pockets. J. Clin. Periodontol. 17, 613–615 [PubMed] [Google Scholar]
- 15.Polson A.M., Garrett S., Stoller N.H., Bandt C.L., Hanes P.J., Killoy , et al. (1997). Multi-center comparative evaluation of subgingivally delivered sanguinarine and doxycycline in the treatment of periodontitis. I. Study design, procedures, and management. J. Periodontol. 68, 110–118 [DOI] [PubMed] [Google Scholar]
- 16.Stoller N.H., Johnson L.R., Trapnell S., Harrold C.Q., and Garrett S. (1998). The pharmacokinetic profile of a biodegradable controlled-release delivery system containing doxycycline compared to systemically delivered doxycycline in gingival crevicular fluid, saliva, and serum. J. Periodontol. 69, 1085–1091 [DOI] [PubMed] [Google Scholar]
- 17.Oho T., and Morioka T. (1990). A possible mechanism of acquired acid resistance of human dental enamel by laser irradiation. Caries Res. 24, 86–92 [DOI] [PubMed] [Google Scholar]
- 18.Banda N.R., Vanaja Reddy G., and Shashikiran N.D. (2011). Evaluation of primary tooth enamel surface morphology and microhardness after Nd:YAG laser irradiation and APF gel treatment–an in vitro study. J. Clin. Pediatr. Dent. 35, 377–382 [DOI] [PubMed] [Google Scholar]
- 19.Rios D., Magalhães A.C., Machado M.A., da Silva S.M., Lizarelli Rde, F., Bagnato V.S., and Buzalaf M.A. (2009). In vitro evaluation of enamel erosion after Nd:YAG laser irradiation and fluoride application. Photomed Laser Surg. 27, 743–747 [DOI] [PubMed] [Google Scholar]
- 20.Ting C.-C., Fukuda M., Watanabe T., Aoki T., Sanaoka A., and Noguchi T. (2007). Effects of Er,Cr:YSGG laser irradiation on the root surface: morphologic analysis and efficiency of calculus removal. J. Periodontol. 78, 2156–2164 [DOI] [PubMed] [Google Scholar]
- 21.Ota-Tsuzuki C., Martins F.L., Giorgetti A.P., de Freitas P.M., and Duarte P.M. (2009). In vitro adhesion of Streptococcus sanguinis to dentine root surface after treatment with Er:YAG laser, ultrasonic system, or manual curette. Photomed. Laser Surg. 27, 735–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Folwaczny M., George G., Thiele L., Mehl A., and Hickel R. (2002). Root surface roughness following Er:YAG laser irradiation at different radiation energies and working tip angulations. J. Clin. Periodontol. 29, 598–603 [DOI] [PubMed] [Google Scholar]
- 23.White J.M., and Adams G.L. (1996). Microhardness and scanning electron microscopy analysis of Nd:YAG laser and acid treatment effects in dentin. Scanning Microsc. 10, 329–336 [PubMed] [Google Scholar]
- 24.Tagomori S., and Morioka T. (1989). Combined effects of laser and fluoride on acid resistance of human dental enamel. Caries Res. 23, 225–231 [DOI] [PubMed] [Google Scholar]
- 25.Hossain M., Nakamura Y., Kimura Y., Yamada Y., Kawanaka T., and Matsumoto K. (2001). Effect of pulsed Nd:YAG laser irradiation on acid demineralization of enamel and dentin. J. Clin. Laser Med. Surg. 19, 105–108 [DOI] [PubMed] [Google Scholar]
- 26.Gaspirc B., and Skaleric U. (2001). Morphology, chemical structure and diffusion processes of root surface after Er:YAG and Nd:YAG laser irradiation. J. Clin. Periodontol. 28, 508–516 [DOI] [PubMed] [Google Scholar]
- 27.Konishi N., Fried D., Staninec M., and Featherstone J.D. (1999). Artificial caries removal and inhibition of artificial secondary caries by pulsed CO2 laser irradiation. Am. J. Dent. 12, 213–216 [PubMed] [Google Scholar]
- 28.Marquez F., Quintana E., Roca I., and Salgado J. (1993). Physical–mechanical effects of Nd:YAG laser of sound dentine and enamel. Biomaterials 14, 313–316 [DOI] [PubMed] [Google Scholar]
- 29.Jeng J.H., Chen K.W., Lin C.P., Chou H.G., and Lan W.H. (1999). Ultrastructural changes of the tooth root surface by Nd:YAG laser irradiation followed by citric acid and tetracycline. J. Formos. Med. Assoc. 98, 242–247 [PubMed] [Google Scholar]
- 30.Gómez C., Bisheimer M., Costela A., García–Moreno I., García A., and García J.A. (2009). Evaluation of the effects of Er:YAG and Nd:YAG lasers and ultrasonic instrumentation on root surfaces. Photomed Laser Surg. 27, 43–48 [DOI] [PubMed] [Google Scholar]
- 31.Kumar N.G., and Mehta D.S. (2005). Short-term assessmentof the Nd:YAG laser with and without sodium fluoride varnish in the treatment of dentine hypersensitivity: a clinical and scanning electron microscopy study. J. Periodontol. 76, 1140–1147 [DOI] [PubMed] [Google Scholar]
- 32.Gholami G.A., Fekrazad R., Esmaiel-Nejad A., Katayoun A.M., and Kalhori K.A. (2011). An evaluation of the occluding effects of Er;Cr:YSGG, Nd:YAG, CO2 and diode lasers on dentinal tubules: a scanning electron microscope in vitro study. Photomed. Laser Surg. 29115–121 [DOI] [PubMed] [Google Scholar]
- 33.Palazon M.T., Scaramucci T., Aranha A.C., Prates R.A., Lachowski K.M., Hanashiro F.S., and Youssef M.N. (2013). Immediate and short-term effects of in-office desensitizing treatments for dentinal tubule occlusion. Photomed. Laser Surg. 31, 274–282 [DOI] [PubMed] [Google Scholar]
- 34.Spencer P., Trylovich D.J., and Cobb C.M. (1992). Chemical characterization of lased root surfaces using Fourier transform infrared photoacoustic spectroscopy. J. Periodontol. 63, 633–636 [DOI] [PubMed] [Google Scholar]
- 35.Eick S.T., Seltmann T., and Pfister W. (2004). Efficacy of antibiotics to strains of periodontopathogenic bacteria within a single species biofilm – an in vitro study. J. Clin. Periodontol. 31, 376–383 [DOI] [PubMed] [Google Scholar]
- 36.Wright T.L., Ellen R.P., Lacroix J.M., Sinnadurai S., and Mittelman M.W. (1997). Effects of metronidazole on Porphyromonas gingivalis biofilms. J. Periodontal. Res. 32, 473–477 [DOI] [PubMed] [Google Scholar]
- 37.Abu–Fanas S.H., Drucker D.B., Hull P.S., Reeder J.C., and Ganguli L.A. (1991). Identification, and susceptibility to seven antimicrobial agents, of 61 gram-negative anaerobic rods from periodontal pockets. J. Dent. 19, 46–50 [DOI] [PubMed] [Google Scholar]
- 38.Moubareck C., Gavini F., Vaugien L., Butel M.J., and Doucet–Populaire F. (2005). Antimicrobial susceptibility of bifidobacteria. J. Antimicrob. Chemother. 55, 38–44 [DOI] [PubMed] [Google Scholar]
- 39.Noiri Y., Okami Y., Narimatsu M., Takahashi Y., Kawahara T., and Ebisu S. (2003). Effects of chlorhexidine, minocycline, and metronidazole on Porphyromonas gingivalis strain 381 in biofilms. J. Periodontol. 74, 1647–1651 [DOI] [PubMed] [Google Scholar]