Abstract
The skin lesion of early Lyme disease, erythema migrans (EM), is so characteristic that routine practice is to treat all such patients with antibiotics. Because other skin lesions may resemble EM, it is not known whether presumptive treatment of EM is appropriate in regions where Lyme disease is rare. We constructed a decision model to compare the cost and clinical effectiveness of three strategies for the management of EM: Treat All, Observe, and Serology as a function of the probability that an EM-like lesion is Lyme disease. Treat All was found to be the preferred strategy in regions that are endemic for Lyme disease. Where Lyme disease is rare, Observe is the preferred strategy, as presumptive treatment would be expected to produce excessive harm and increased costs. Where Lyme disease is rare, clinicians and public health officials should consider observing patients with EM-like lesions who lack travel to Lyme disease-endemic areas.
Key Words: : Lyme disease, Lyme borreliosis, Erythema migrans, Southern tick-associated rash illness (STARI), Borrelia burgdorferi, Ixodes scapularis, Deer tick, Black-legged tick, Amblyomma americanum, Lone star tick, Antibiotics, Doxycycline, Amoxicillin, Cost-effectiveness, Decision analysis
Introduction
Lyme disease, caused by Borrelia burgdorferi, is the most common vector-borne infection in the United States, affecting tens of thousands of persons annually. Most patients with Lyme disease present with the characteristic erythema migrans (EM) skin lesion. Disseminated sequelae are nearly always averted if the infection is recognized and treated at this stage (Wormser et al. 2003, Kowalski et al. 2010). If EM goes untreated, patients are likely to develop complications such as arthritis, neuroborreliosis, or carditis. Because EM is so specific for early Lyme disease, it is standard practice to empirically treat EM with antibiotics (Wormser et al. 2006).
It has become increasingly recognized that EM-like skin lesions are not pathognomonic for Lyme disease (Tibbles and Edlow 2007, Sharma et al. 2010). Of particular note is the “southern tick-associated rash illness,” or STARI, which occurs primarily in southeastern and southcentral states. STARI is associated with the bite of an Amblyomma americanum tick, which is incapable of transmitting B. burgdorferi (Ryder et al. 1992, Piesman et al. 1997, Ledin et al 2005, Soares et al. 2006, Zeidner et al. 2009). Investigations in southern states have consistently failed to detect B. burgdorferi in EM-like skin lesions, and patients with these skin lesions are seronegative for B. burgdorferi (Kirkland et al. 1997, Piesman and Happ 1997, Felz et al. 1999, Wormser et al. 2005). There are no known sequelae of untreated STARI, and its etiology, natural history, and appropriate treatment remain unknown.
STARI and Lyme disease have geographic distributions that correspond to their respective tick vectors. Thus, the probability that an EM-like skin lesion is Lyme disease, P(Lyme | EM), will vary as a function of geography. If EM is treated with antibiotics to avert disseminated Lyme disease, this strategy would be of low yield in areas where Lyme disease is uncommon and where, consequently, P(Lyme | EM) is low. Here we present a decision analysis study comparing strategies for the management of EM-like lesions across a theoretical range of P(Lyme | EM).
Materials and Methods
Decision model
We compared three strategies for the management of patients presenting with an EM-like skin lesion: (1) Treat All, in which all patients are given a standard course of antibiotics intended to treat EM due to early Lyme disease; (2) Observe, in which patients are observed, and treated only if disseminated Lyme disease develops; (3) Serology, in which patients are tested using standard two-tier serology (enzyme-linked immunosorbent assay [ELISA] followed by western blot [WB] in the case of a positive or equivocal ELISA). Antibiotics are given to patients meeting criteria for seropositivity, whereas patients with negative serologic tests are observed.
The independent variable upon which we based our model was P(Lyme | EM), or the probability that an EM-like lesion is due to Lyme disease. This value was modeled from 0 to 1. Variables incorporated into the model were the probabilities and costs of disseminated Lyme disease; the effectiveness, risks, and costs of antibiotic therapy; and the sensitivity, specificity, and costs of diagnostic tests.
Assumptions
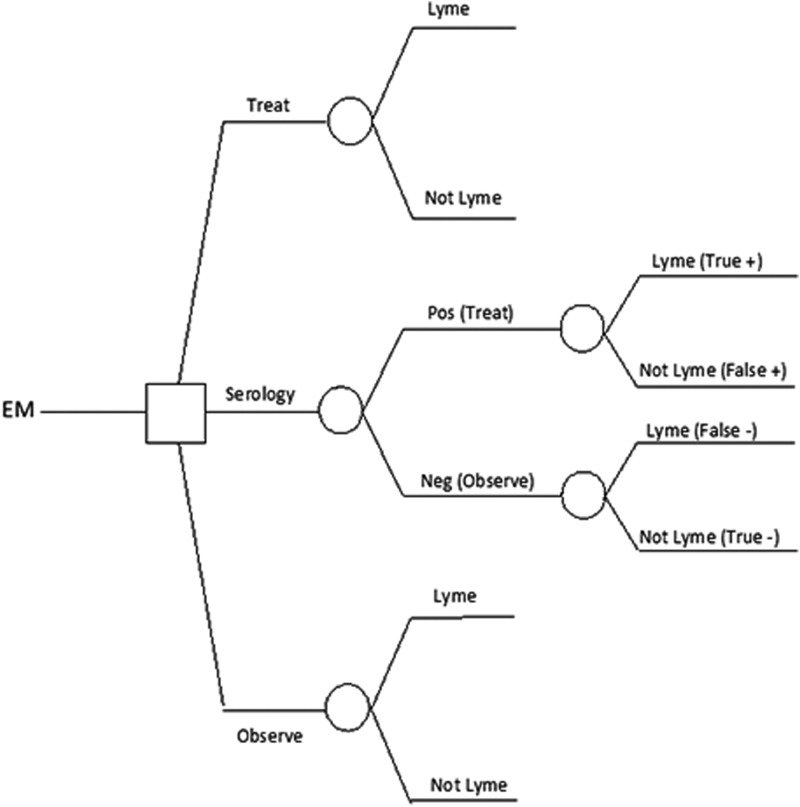
Our model assumes that EM is treated with antibiotics solely to prevent disseminated Lyme disease. This incorporates the subsidiary assumptions that antibiotic treatment directed at B. burgdorferi infection is not justified for the resolution of EM itself or for the treatment of STARI. The decision tree is illustrated in Figure 1.
FIG. 1.
Basic decision tree used in model. The probabilities of disseminated Lyme disease sequelae and of major and minor adverse treatment effects were modeled for each treatment strategy. EM, erythema migrans; Pos, positive; Neg, negative.
Probabilities and costs
The probabilities and costs used in our model are presented in Table 1. The base values represent our best estimates. For sensitivity-analysis purposes, we estimated lower and upper end points of a plausible range for all variables in the model, with the exception of the sensitivity and specificity of serology.
Table 1.
Probabilities and Costs Used in Decision Modela
| Probabilities | Low | Base | High |
|---|---|---|---|
| Probability of sequelae if EM is treated |
0.001 |
0.005 |
0.01 |
| Probability of sequelae if EM is not treated |
0.54 |
0.83 |
0.99 |
| Cardiac |
|
0.05 |
|
| Neurologic |
|
0.15 |
|
| Arthritic |
|
0.6 |
|
| Treatment | |||
| Probability of major adverse reaction |
0.0001 |
0.0005 |
0.001 |
| Probability of minor adverse reaction |
0.03 |
0.05 |
0.1 |
| Serology | |||
| Sensitivity - P(Positive | Lyme) |
|
0.35 |
|
| Specificity - P(Negative | No Lyme) | 0.98 | ||
| Costs (2010 dollars) | Low | Base | High |
|---|---|---|---|
| Cost of amoxicillin or doxycycline for EM |
4 |
4 |
216 |
| Major adverse reaction |
3140 |
5000 |
8258 |
| Minor adverse reaction |
91 |
250 |
429 |
| Cost of Lyme disease sequelae |
2003 |
4000 |
11496 |
| Serology | 37 | 80 | 127 |
Costs are in 2010 US dollars.
EM, erythema migrans.
Several prior cost effectiveness models of Lyme disease have been published (Magid et al. 1992, Nichol et al. 1998, Meltzer et al. 1999, Shadick et al. 2001, Hsia et al. 2002). These studies and their references were reviewed in detail. The range of probabilities modeled in our study was derived from these figures.
The costs of oral doxycycline and oral amoxicillin therapy were based on the average wholesale price. Costs of laboratory testing for Lyme disease were obtained by communication with staff from the Quest, Mayo, and ARUP laboratories, and our cost estimates reflect the range of prices quoted. These particular laboratories were contacted because they are referral laboratories that receive samples from a large market base.
The costs of disseminated Lyme disease and of major adverse medication effects were derived from the studies discussed above (Magid et al. 1992, Nichol et al. 1998, Meltzer et al. 1999, Shadick et al. 2001, Hsia et al. 2002). One exception should be noted: The Meltzer study projected an 11-year duration of costs related to Lyme arthritis and neuroborreliosis (Meltzer et al. 1999). This resulted in an 11-fold multiplier that produced a cost much higher than that used in other models. We felt that this duration of illness was not reflective of the typical patient with Lyme disease. For this reason, we used only the single-year cost estimate from that study. All costs were converted to 2010 dollars using the medical cost inflation component of the consumer price index (Felz et al. 1999). The upper and lower cost estimates for disseminated Lyme disease were determined using an average weighted by the probability of each major disseminated complication. The sensitivity of serology with a solitary EM skin lesion has been demonstrated in the literature to be about 0.35 (Aguero-Rosenfeld et al. 1992, Aguero-Rosenfeld 2005).
Cost-effectiveness analysis
Using the decision tree in Figure 1 as the basis for our computational model, we calculated the expected cost associated with each of the three management strategies. Expected cost is the probability-weighted average cost per patient, considering the possible outcomes for the patient, the cost of each outcome, and their probabilities.
Results
Clinical effectiveness and harm
The expected (i.e., probability-weighted) benefits and harms of each management strategy are presented in Table 2. Treating all patients with EM-like lesions would result in the most averted cases of disseminated Lyme disease. Where EM is synonymous with Lyme disease, i.e., P(Lyme | EM)=1, our model predicts that 78,000 cases of disseminated Lyme disease would be averted for every 100,000 treated patients. This number varied directly with P(Lyme | EM), and in settings where Lyme disease accounts for a progressively smaller proportion of EM-like lesions, presumptive treatment would benefit fewer patients. For example, where P(Lyme | EM)=0.0001, only eight cases of disseminated Lyme disease would be prevented for every 100,000 patients treated.
Table 2.
Clinical Outcomes of Strategies for the Management of EM-Like Lesions As a Function of P(Lyme | EM)
|
Cases averted per 100,000 | |||
|---|---|---|---|
| P(Lyme | EM) | Observe | Treat All | Serology |
| 1 |
0 |
78,000 |
27,300 |
| 0.1 |
0 |
7,800 |
2,730 |
| 0.01 |
0 |
780 |
273 |
| 0.001 |
0 |
78 |
27.3 |
| 0.0001 |
0 |
7.8 |
2.73 |
| 0.00001 | 0 | 0.78 | 0.27 |
|
Disseminated Cases per 100,000 | |||
|---|---|---|---|
| P(Lyme | EM) | Observe | Treat All | Serology |
| 1 |
83,000 |
5,000.00 |
55,700 |
| 0.1 |
8,300 |
500.00 |
5,570 |
| 0.01 |
830 |
50.00 |
557 |
| 0.001 |
83 |
5.00 |
55.7 |
| 0.0001 |
8.3 |
0.05 |
5.57 |
| 0.00001 | 0.83 | 0.01 | 0.56 |
|
Major Adverse Medication Events per 100,000 | |||
|---|---|---|---|
| P(Lyme | EM) | Observe | Treat All | Serology |
| 1 |
0 |
50 |
17.5 |
| 0.1 |
0 |
50 |
2.65 |
| 0.01 |
0 |
50 |
1.17 |
| 0.001 |
0 |
50 |
1.02 |
| 0.0001 |
0 |
50 |
1.0 |
| 0.00001 | 0 | 50 | 1.0 |
|
Major Adverse Medication Events per case averted | |||
|---|---|---|---|
| P(Lyme | EM) | Observe | Treat All | Serology |
| 1 |
n/a |
0.000641 |
0.00064 |
| 0.1 |
n/a |
0.00641 |
0.00097 |
| 0.01 |
n/a |
0.0641 |
0.0043 |
| 0.001 |
n/a |
0.641 |
0.037 |
| 0.0001 |
n/a |
6.41 |
0.37 |
| 0.00001 | n/a | 64.1 | 3.7 |
EM, erythema migrans.
Major adverse medication events were assumed to occur at a constant incidence, regardless of Lyme disease incidence. P(Lyme | EM), however, influenced the number of major adverse medication events per averted case of disseminated Lyme disease. If one were to treat 100,000 patients in a setting where P(Lyme | EM)=1, one would anticipate 0.00064 major adverse medication events per averted case of disseminated Lyme disease. As P(Lyme | EM) decreases, however, a treat-all strategy would result in progressively more major adverse medication events per averted case of disseminated Lyme disease. Ultimately, with P(Lyme | EM) of approximately 0.001, one patient would have a major adverse medication event for every averted case of disseminated Lyme disease. At P(Lyme | EM) of 0.00001, a major adverse medication event would be 64 times more likely than an averted case of disseminated Lyme disease.
The insensitivity of serology in early localized Lyme disease would result in high numbers of false negatives. According to our model, observing 100,000 patients where P(Lyme | EM)=1 would result in 83,000 cases of disseminated Lyme disease, as compared with merely 5000 under a Treat All strategy. In this setting, if serology were used to guide treatment, one would still expect 55,700 disseminated Lyme disease cases. Even in regions with low P(Lyme | EM), one would still anticipate a substantial number of disseminated Lyme disease cases due to false negative testing. With P(Lyme | EM)=0.001, serology-guided treatment would result in 44 cases of disseminated Lyme disease as compared with only 5 with a Treat All strategy. On the other hand, serology would substantially reduce the number of adverse treatment events per averted case of disseminated Lyme disease.
Cost effectiveness
Cost effectiveness was expressed by the following measures: Cost per patient (Fig. 2A), and cost per case averted (Fig. 2B). Both were calculated for P(Lyme | EM) ranging from 0 to 1. Cost per patient was calculated for all three management strategies. Cost per case averted was calculated only for Treat All and Serology (because an Observe strategy would never result in an averted case).
FIG. 2.
Cost effectiveness of management strategies as a function of P(Lyme | EM). (A) At all values of P(Lyme | EM) above 0.0061, Treat All is the least costly strategy per patient. Below 0.0061, Observe becomes the least costly. (B) At all values of P(Lyme | EM), Treat All is associated with the lowest cost per averted case of disseminated Lyme disease. EM, erythema migrans.
Where all EM cases are due to Lyme disease (P(Lyme | EM)=1), the cost for a Treat All strategy would be $219 per patient. Serology-guided care would cost $2315 per patient. The Observe strategy would cost $3320 per patient. Where EM-like lesions are never due to Lyme disease (P(Lyme | EM)=0), Treat All would cost $19 per patient, Serology would cost $80, and Observe would cost $0 per patient. All strategies become more costly as P(Lyme | EM) increases (Fig. 2). Treat All is the least expensive strategy for all values of P(Lyme | EM) greater than 0.0061. Below this value, Observe was the least costly strategy. Serology was never the most cost effective strategy at any value of P(Lyme | EM).
For all P(Lyme | EM)>0.0061, Treat All would result in a net savings per averted case of disseminated Lyme disease. Where P(Lyme | EM)=1, this would save $3976 per case averted (as compared with an Observe strategy). At values of P(Lyme | EM)<0.0061, Treat All would result in a net loss. Treat All resulted in a lower cost per case averted than Serology at all values of P(Lyme | EM) (Fig. 2B). Furthermore, Treat All was cost effective relative to Observe (resulting in net savings) for a wider range of values for P(Lyme | EM) compared to Serology, which was cost effective relative to Observe for values of P(Lyme | EM)>0.074.
Sensitivity analysis
The results described above focus on the impact of P(Lyme | EM) on the preferred strategy. We can, however, consider how varying the other inputs might affect our results. Although we do not present details here, our sensitivity analysis shows the following:
• For values of P(Lyme | EM)>about 0.074, Treat All is the preferred strategy, no matter where the values of the other inputs fall within the ranges shown in Table 1.
• For larger values of P(Lyme | EM), the cost of disseminated Lyme disease has the most impact on cost effectiveness of Treat All; the larger this cost, the more cost effective is Treat All.
• As P(Lyme | EM) decreases below 0.074, the cost of antibiotic treatment used in Treat All becomes more important; the greater the treatment cost, the less cost effective is Treat All.
• The larger the treatment cost, the larger the breakeven value of P(Lyme | EM) where Treat All has no advantage over Observe, and the greater the range of P(Lyme | EM) for which Observe is preferred.
• Despite the ranges in Table 1, none of the other inputs play a major role in determining whether Treat All is cost effective relative to Observe.
Discussion
Despite common teaching that EM is pathognomonic for Lyme disease, STARI has emerged as an alternative diagnosis in regions where Lyme disease is rare. Because the appropriate treatment of STARI is unknown, the primary goal of treating EM remains to prevent disseminated Lyme disease. If so, then achieving this goal will be improbable in settings where STARI is far more likely than Lyme disease, and the expected benefits of therapy may be outweighed by costs and risks. We have constructed a model using clinical and cost variables to determine if there is a threshold of risk below which testing or observation become the preferable to empiric treatment.
Our model affirms that empirically treating patients with EM-like lesions remains the preferred practice not only in highly Lyme-endemic states, but also in transitional states, such as Maryland and Virginia, where both Lyme disease and STARI coexist. On the other hand, where Lyme disease is nonendemic, EM-like lesions are most likely to have an alternative explanation (e.g., STARI) and a Treat All strategy will rarely if ever prevent disseminated Lyme disease. As a consequence, with a declining P(Lyme | EM), Treat All becomes progressively less cost effective and more potentially harmful. Ultimately the risk of adverse events exceeds the likelihood of therapeutic success. Between these two extremes is a transitional zone where both STARI and Lyme disease coexist, and where Treat All generally remains the preferred strategy.
The major question raised by our study is one of geography: Where is the risk of Lyme disease is so low that Observe becomes preferable to Treat All? While P(Lyme | EM) has not been studied systematically over a wide geographic area, several studies have illustrated that Lyme disease is highly improbable among EM patients in southcentral and southeastern states. Of 23 EM patients from Georgia and South Carolina, no patient was seropositive by standardized interpretive criteria; the only patient who was culture positive had Borrelia garinii infection, which was presumably acquired in Europe (Felz et al. 1999). Of 14 patients seen in North Carolina, paired acute and convalescent sera were negative for B. burgdorferi in 13 of 13 patients tested, and five of five patients were negative by biopsy (Kirkland et al. 1997). Only one of 72 patients from Missouri with EM-like lesions developed seroreactivity to the B. burgdorferi C6 peptide, as compared with eight of nine patients from New York (Philipp et al. 2006). B. burgdorferi was not demonstrable from EM-like lesions in Missouri, whereas it is regularly demonstrated in patients from New York (Campbell et al. 1995, Wormser et al. 2005). These findings corroborate Lyme disease surveillance statistics, which consistently demonstrate that Lyme disease is very uncommon in Missouri, and in southeastern states including the Carolinas and Georgia (Centers for Disease Control and Prevention 2010, Centers for Disease Prevention and Control 2005–2010).
The abundance of Ixodes scapularis nymphs, and in particular nymphs infected with B. burgdorferi, correlates strongly with human Lyme disease transmission (Diuk-Wasser et al. 2006, Pepin et al. 2012). I. scapularis nymphs have been very infrequently reported south of the 39th parallel (Goddard and McHugh 1990, Diuk-Wasser et al. 2006), and infected nymphs were virtually absent in this region in recent large-scale sampling studies Diuk-Wasser et al. 2006, Diuk-Wasser et al. 2012). States with high rates of human Lyme disease are characterized by an I. scapularis nymph density that exceeds 5 ticks per 1000 m2 (Table 3) (Diuk-Wasser et al. 2006). By contrast, density is only 0.5 nymphs per 100 m2 in southern and southeastern states. A. americanum, on the other hand, is abundant in these southern states, with densities exceeding seven ticks per 1000 m2 In fact, A. americanum is by far the most abundant human-biting tick in this region of the country (Anigstein and Anigstein 1975, Goddard and McHugh 1990, Campbell and Bowles 1994, Felz et al. 1996, Felz and Durden 1999, Felz et al. 1999, Merten and Durden 2000). This tick is rare, however, in the northeast and upper Midwest. The states where EM-like lesions are unlikely to be Lyme disease are thus characterized by (1) abundance of A. americanum, (2) paucity of I. scapularis nymphs, (3) absence of B. burgdorferi-infected nymphs, and (4) a low surveillance incidence of human Lyme disease. In these states it should be assumed that an P(Lyme | EM) is very low, and an Observe strategy would be most appropriate.
Table 3.
Relative Densities of Amblyomma americanum and Ixodes scapularis in Select States
| State | Number of sampling sites | Average A. americanum density (ticks per 1000 m2) | Average I. scapularis density (ticks per 1000 m2) | Incidence of Lyme disease (cases per 100,000) |
|---|---|---|---|---|
| Massachusetts |
1 |
0 |
6 |
36.3 |
| New York |
1 |
0 |
5.8 |
12.3 |
| Minnesota |
2 |
0 |
8.13 |
24.4 |
| Wisconsin |
3 |
0 |
7.0 |
44 |
| Maryland |
1 |
10.33 |
4.67 |
20.1 |
| Virginia |
1 |
7.6 |
1.40 |
11.4 |
| North Carolina |
2 |
8.92 |
0 |
0.2 |
| South Carolina |
2 |
8.25 |
0.25 |
0.4 |
| Georgia |
2 |
11.67 |
0.8 |
0.1 |
| Kentucky |
2 |
11 |
0 |
0.1 |
| Missouri |
4 |
10.75 |
0.5 |
0.1 |
| Arkansas |
1 |
7 |
0 |
0 |
| Louisiana |
1 |
7.33 |
0 |
0 |
| Oklahoma | 4 | 10.98 | 0.26 | 0 |
Tick data were collected during summer months (May–August) in 2004 and represent all nymphal and adult individuals detected during sampling. Sites were sampled between one and six times per year (mean=3.26, standard deviation [SD]=1.63); densities were first averaged across sampling sessions by site, and then across sites by state Centers for Disease Control and Prevention 2005–2010; Duik-Wasser et al. 2006; Duik-Wasser, et al. unpublished data 2006.)
- • Five independent transects must have been sampled during each 1- to 2-day site visit;
- • Numbers of adult and nymphal ticks were summed across transects during each visit;
- • Numbers of ticks were averaged for each site across the number of site visits;
- • Density estimates for each site were averaged within a state;
- • Data from only 2004 were included.
A. americanum and I. scapularis coexist in mid-Atlantic states, such as Maryland and Virginia (Table 3). Patients in this region are at risk of both Lyme disease and STARI. Despite the abundance of A. americanum and the falling density of I. scapularis as one progresses south, one still finds an appreciable incidence of Lyme disease in these two states. Even if P(Lyme | EM) is only 0.1, the Treat All strategy would prevent 7800 cases of disseminated Lyme disease per 100,000 patients treated, with a cost of only one major adverse medication event per 156 cases averted, and it would be the most cost-effective approach. Thus, it can be concluded that in transitional states where Lyme disease and STARI coexist, the Treat All strategy remains preferred. Statewide recommendations may not be appropriate for states with internally heterogeneous Lyme disease transmission (e.g., Illinois or Virginia).
Because of its poor sensitivity in early Lyme disease, serology-guided treatment proved to be neither cost effective nor clinically effective, regardless of P(Lyme | EM). The likelihood of a false negative serology renders this test unhelpful to rule Lyme disease in when the pretest probability is already high, and uninformative when the pretest probability is low.
Our study makes a critical assumption that merits discussion: That antibiotic treatment of STARI is not useful. In reality this remains unknown. Expert opinions and observations from an uncontrolled cohort study suggest that STARI resolves more quickly once antibiotics are started (Masters et al. 2008, Wormser et al. 2005). In contrast, an individual case report demonstrated lack of response to amoxicillin (Feder et al. 2011). There are no known sequelae of untreated STARI. In the absence of an etiology and without controlled treatment trials, the role of antibiotics in STARI remains undefined.
A second assumption is that EM is not worth treating for its own sake. While this condition is mild in many cases, patients who are ill or febrile can expect quick improvement with effective antibiotics. There also may be some patients who are very anxious about a strategy of observation and follow up, and offering antibiotics may have an additional psychological utility in these cases.
It is worth discussing various factors that we chose not to include in our model. We did not model paired acute and convalescent serology. This strategy would improve the sensitivity of serodiagnosis in early infection, but this could be offset by the potential to develop early disseminated disease before obtaining convalescent sera. We did not model biopsy PCR identification of B. burgdorferi, both because it has performed variably in studies and because it would be cost prohibitive. We did not model the investigational C6 antibody test. We did not model the use of macrolides for the treatment of EM due to the higher risk of treatment failure. We also did not model the potential dental toxicity of doxycycline in young children. Finally, while it is difficult to accurately estimate the cost of a given complication of Lyme disease, our sensitivity analysis attributed less than 10% of the variability in cost effectiveness to these costs.
Conclusions
We have constructed a model of treatment strategies for EM-like lesions. In southern and southeastern states, treatment of EM as if it is Lyme disease may result in an unacceptably high risk of complications with little expected benefit. On the other hand, empiric treatment should remain the standard of care in areas with endemic Lyme disease transmission, including the mid-Atlantic states, where STARI and Lyme disease coexist. In particular, where Lyme disease is not locally transmitted, physicians should obtain a travel history before assuming that an EM-like lesion is synonymous with Lyme disease. Whether STARI requires antibiotics is a question that only research can adjudicate.
Acknowledgment
We thank Maria Diuk-Wasser, Yale University.
Author Disclosure Statement
PML, RJB, RC: No competing financial interests exist. GPW: Research grants from CDC, NIH, Immunetics, Inc., Bio-Rad, DiaSorin, Inc., and BioMerieux; Equity in Abbott; Expert witness in malpractice cases involving Lyme disease; Unpaid board member American Lyme Disease Foundation; Expert witness regarding Lyme disease in a disciplinary action for the Missouri Board of Registration for the Healing Arts. Consultant to Baxter for Lyme vaccine development.
References
- Centers for Disease Control and Prevention: Incidence by State, 2005–2010. Available at www.cdc.gov/lyme/stats/chartstables/incidencebystate.html Last accessed July1, 2012
- Centers for Disease Control and Prevention: Reported Lyme Disease Cases, U.S., 2010. Available at www.cdc.gov/lyme/stats/maps/map2010.html Last accessed July1, 2012
- Aguero-Rosenfeld ME, Nowakowski J, McKenna DF, Carbonaro CA, et al. . Serodiagnosis in early Lyme disease. J Clin Microbiol 1993; 31:3090–3095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguero-Rosenfeld ME, Wang G, Schwartz I, Wormser GP. Diagnosis of Lyme borreliosis. Clin Microbiol Rev 2005; 18:484–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anigstein L, Anigstein D. A review of the evidence in retrospect for a rickettsial etiology in Bullis fever. Tex Rep Biol Med 1975; 33:201–211 [PubMed] [Google Scholar]
- Campbell BS, Bowles DE. Human tick bite records in a United States Air Force population, 1989–1992: Implications for tick-borne disease risk. J Wilderness Med 1994; 5:405–412 [Google Scholar]
- Campbell GL, Paul WS, Schriefer ME, Craven RB, et al. . Epidemiologic and diagnostic studies of patients with suspected early Lyme disease, Missouri, 1990–1993. J Infect Dis 1995; 172:470–480 [DOI] [PubMed] [Google Scholar]
- Diuk-Wasser MA, et al. Unpublished data from Diuk-Wasser, et al. 2006
- Diuk-Wasser MA, Gatewood AG, Cortinas MR, Yaremych-Hamer S, et al. . Spatiotemporal patterns of host-seeking Ixodes scapularis nymphs (Acari: Ixodidae) in the United States. J Med Entomol 2006: 43:166–176 [DOI] [PubMed] [Google Scholar]
- Diuk-Wasser MA, Hoen AG, Cislo P, Brinkerhoff R, et al. . Human risk of infection with Borrelia burgdorferi, the Lyme disease agent, in eastern United States. Am J Trop Med Hyg 2012: 86:320–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feder HM, Jr., Hoss DM, Zemel L, Telford SR 3rd, et al. . Southern tick-associated rash illness (STARI) in the north: STARI following a tick bite in Long Island, New York. Clin Infect Dis 2011; 53:e142–e146 [DOI] [PubMed] [Google Scholar]
- Felz MW, Durden LA, Oliver JH, Jr., Ticks parasitizing humans in Georgia and South Carolina. J Parasitol 1996; 82:505–508 [PubMed] [Google Scholar]
- Felz MW, Chandler FW, Jr., Oliver JH, Jr., Rahn DW, et al. . Solitary erythema migrans in Georgia and South Carolina. Arch Dermatol 1999a; 135:1317–1326 [DOI] [PubMed] [Google Scholar]
- Felz MW, Durden LA. Attachment sites of four tick species (Acari: Ixodidae) parasitizing humans in Georgia and South Carolina. J Med Entomol 1999b; 36:361–364 [DOI] [PubMed] [Google Scholar]
- Goddard J, McHugh CP. Impact of a severe tick infestation at Little Rock AFB, Arkansas on volant scorpion military training. Mil Med 1990; 155:277–280 [PubMed] [Google Scholar]
- Hsia EC, Chung JB, Schwartz JS, Albert DA. Cost-effectiveness analysis of the Lyme disease vaccine. Arthritis Rheum 2002; 46:1651–1660 [DOI] [PubMed] [Google Scholar]
- Kirkland KB, Klimko TB, Meriwether RA, Schriefer M, et al. . Erythema migrans-like rash illness at a camp in North Carolina: A new tick-borne disease? Arch Intern Med 1997; 157:2635–2641 [PubMed] [Google Scholar]
- Kowalski TJ, Tata S, Berth W, Mathiason MA, et al. . Antibiotic treatment duration and long-term outcomes of patients with early Lyme disease from a Lyme disease-hyperendemic area. Clin Infect Dis 2010; 50:512–520 [DOI] [PubMed] [Google Scholar]
- Ledin KE, Zeidner NS, Ribeiro JM, Biggerstaff BJ, et al. . Borreliacidal activity of saliva of the tick Amblyomma americanum. Med Vet Entomol 2005; 19:90–95 [DOI] [PubMed] [Google Scholar]
- Magid D, Schwartz B, Craft J, Schwartz JS. Prevention of Lyme disease after tick bites. A cost-effectiveness analysis. N Engl J Med 1992; 327:534–541 [DOI] [PubMed] [Google Scholar]
- Masters EJ, Grigery CN, Masters RW. STARI, or Masters disease: Lone Star tick-vectored Lyme-like illness. Infect Dis Clin North Am 2008; 22:361–376, viii. [DOI] [PubMed] [Google Scholar]
- Meltzer MI, Dennis DT, Orloski KA. The cost effectiveness of vaccinating against Lyme disease. Emerg Infect Dis 1999; 5:321–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merten HA, Durden LA. A state-by-state survey of ticks recorded from humans in the United States. J Vector Ecol 2000; 25:102–113 [PubMed] [Google Scholar]
- Nichol G, Dennis DT, Steere AC, Lightfoot R, et al. . Test-treatment strategies for patients suspected of having Lyme disease: A cost-effectiveness analysis. Ann Intern Med 1998;128:37–48 [DOI] [PubMed] [Google Scholar]
- Pepin KM, Eisen RJ, Mead PS, Piesman J, et al. . Geographic variation in the relationship between human Lyme disease incidence and density of infected host-seeking Ixodes scapularis nymphs in the eastern United States. Am J Trop Med Hyg 2012; 86:1062–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipp MT, Masters E, Wormser GP, Hogrefe W, et al. . Serologic evaluation of patients from Missouri with erythema migrans-like skin lesions with the C6 Lyme test. Clin Vaccine Immunol 2006; 13:1170–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piesman J, Happ CM. Ability of the Lyme disease spirochete Borrelia burgdorferi to infect rodents and three species of human-biting ticks (blacklegged tick, American dog tick, lone star tick) (Acari:Ixodidae). J Med Entomol 1997; 34:451–456 [DOI] [PubMed] [Google Scholar]
- Ryder JW, Pinger RR, Glancy T. Inability of Ixodes cookei and Amblyomma americanum nymphs (Acari: Ixodidae) to transmit Borrelia burgdorferi. J Med Entomol 1992; 29:525–530 [DOI] [PubMed] [Google Scholar]
- Shadick NA, Liang MH, Phillips CB, Fossel K, et al. . The cost-effectiveness of vaccination against Lyme disease. Arch Intern Med 2001; 161:554–561 [DOI] [PubMed] [Google Scholar]
- Sharma A, Jaimungal S, Basdeo-Maharaj K, Chalapathi Rao AV, et al. . Erythema migrans-like illness among Caribbean islanders. Emerg Infect Dis 2010; 16:1615–1617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares CA, Zeidner NS, Beard CB, Dolan MC, et al. . Kinetics of Borrelia burgdorferi infection in larvae of refractory and competent tick vectors. J Med Entomol 2006; 43:61–67 [DOI] [PubMed] [Google Scholar]
- Tibbles CD, Edlow JA. Does this patient have erythema migrans? JAMA 2007; 297:2617–2627 [DOI] [PubMed] [Google Scholar]
- Wormser GP, Ramanathan R, Nowakowski J, McKenna D, et al. . Duration of antibiotic therapy for early Lyme disease. A randomized, double-blind, placebo-controlled trial. Ann Intern Med 2003; 138:697–704 [DOI] [PubMed] [Google Scholar]
- Wormser GP, Masters E, Liveris D, Nowakowski J, et al. . Microbiologic evaluation of patients from Missouri with erythema migrans. Clin Infect Dis 2005a; 40:423–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wormser GP, Masters E, Nowakowski J, McKenna D, et al. . Prospective clinical evaluation of patients from Missouri and New York with erythema migrans-like skin lesions. Clin Infect Dis 2005b; 41:958–965 [DOI] [PubMed] [Google Scholar]
- Wormser GP, Dattwyler RJ, Shapiro ED, Halperin JJ, et al. . The clinical assessment, treatment, and prevention of lyme disease, human granulocytic anaplasmosis, and babesiosis: Clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2006; 43:1089–1134 [DOI] [PubMed] [Google Scholar]
- Zeidner N, Ullmann A, Sackal C, Dolan M, et al. . A borreliacidal factor in Amblyomma americanum saliva is associated with phospholipase A2 activity. Exp Parasitol 2009; 121:370–375 [DOI] [PubMed] [Google Scholar]