Abstract
Houston, Texas, maintains an environment conducive to dengue virus (DENV) emergence; however, surveillance is passive and diagnostic testing is not readily available. To determine if DENV is present in the area, we tested 3768 clinical specimens (2138 cerebrospinal fluid [CSF] and 1630 serum) collected from patients with suspected mosquito-borne viral disease between 2003 and 2005. We identified 47 immunoglobulin M (IgM)-positive dengue cases, including two cases that were positive for viral RNA in serum for dengue serotype 2. The majority of cases did not report any history of travel outside the Houston area prior to symptom onset. The epidemic curve suggests an outbreak occurred in 2003 with continued low-level transmission in 2004 and 2005. Chart abstractions were completed for 42 of the 47 cases; 57% were diagnosed with meningitis and/or encephalitis, and 43% met the case definition for dengue fever. Two of the 47 cases were fatal, including one with illness compatible with dengue shock syndrome. Our results support local transmission of DENV during the study period. These findings heighten the need for dengue surveillance in the southern United States.
Key Words: : Dengue virus, West Nile virus, Surveillance, Meningitis, IgM enzyme-linked immunosorbent assay, RT-PCR
Introduction
Arthropod-borne viruses can become established as epidemic or endemic agents of disease when imported into a region populated with competent vectors and susceptible amplification hosts. Given the ecological and climatic conditions of Houston, Texas, and the recent emergence of West Nile virus (WNV), there is high potential for the emergence of other arboviruses in this region. Dengue virus (DENV), the causative agent of dengue fever (DF), is of particular concern because it is the most common vector-borne viral infection worldwide, with increasing geographic expansion and an estimated 96 million clinically apparent infections each year (Bhatt et al. 2013). DENV is transmitted primarily by urbanized Aedes aegypti mosquitoes, and DF is due to infection with one of four closely-related serotypes, DENV1–4 (Gubler 1998). The spectrum of illness ranges from a nonspecific acute febrile illness to severe dengue, with symptoms ranging from plasma leakage, hemorrhagic manifestations, hypovolemic shock, and the possibility of death. Illness uncommonly manifests as encephalitis (Lum et al. 1996, Jackson et al. 2008, Wasay et al. 2008, World Health Organization 2009, Soares et al. 2010), with a clinical presentation similar to WNV encephalitis.
Historically, Texas was the site of several dengue epidemics until the early 1900s, and local transmission of DENV has recently occurred in the southern part of the state (Rice 1922, Centers for Disease Control and Prevention 1994, Rawlings et al. 1998, Centers for Disease Control and Prevention 2001, Reiter et al. 2003, Brunkard et al. 2007, Ramos et al. 2008). Houston's proximity to the dengue-endemic areas of southern Texas and northern Mexico puts it at risk for DENV emergence. Other factors also increase the risk for introduction and sustained transmission, including vast shipping, a high volume of both air and ship travel entry, many of its ≈4 million residents who routinely travel to and from dengue-endemic areas, and an abundance of competent vectors, including Ae. aegypti and Ae. albopictus mosquitoes (Sprenger et al. 1986, Brown et al. 2011). On the basis of our hypothesis of risk of DENV emergence, we conducted a retrospective study to determine if any cases of suspected arboviral illness in the Houston area were attributable to DENV infection, and, if so, to determine if there was evidence of locally acquired infection.
Materials and Methods
Ethics statement
All specimens submitted to the City of Houston Department of Health and Human Services (HDHHS) Public Health Laboratory for WNV testing and were found negative were included in this study. This bank of specimens included 1630 serum and 2138 cerebrospinal fluid (CSF) specimens from patients with arboviral-like illness in the Houston area between 2003 and 2005. Specimens were de-identified prior to transfer to the University of Texas Health Science Center (UTHSC) at the Houston School of Public Health. Acquisition of specimens and data for the purpose of this study was reviewed and approved by UTHSC's Committee for the Protection of Human Subjects (HSC-SPH-05-008) and the internal review board of HDHHS. Upon identification of DENV-positive specimens, the HDHHS performed medical chart reviews and conducted interviews as part of their legal mandate to investigate DENV infections, which are reportable in Texas. Because of this legal mandate, informed consent was not obtained. Data from medical records and interviews were de-identified and shared with UTHSC for epidemiological analysis and for inclusion in this manuscript. Acquisition of this additional de-identified data was also reviewed and approved by the UTHSC's Committee for the Protection of Human Subjects (HSC-SPH-09-0183).
Laboratory methods
All 3768 specimens had previously tested negative by enzyme-linked immunosorbent assay (ELISA) for immunoglobulin M (IgM) antibodies to WNV and St. Louis encephalitis virus (SLEV) by the HDHHS. All specimens were tested at the UTHSC–Houston for the presence of anti-DENV IgM antibodies using the Panbio Dengue IgM ELISA Capture Kit (Panbio Inc., Queensland, Australia) according to the manufacturer's instructions, with the exception of an additional positive control per plate. Serum and CSF specimens were tested at a dilution of 1:100 and 1:2, respectively (Prince et al. 2004). Test results with an index value <0.9 were interpreted as negative, equivocal if between 0.9 and 1.1, and positive if >1.1. All positive and equivocal specimens were repeated to verify results. Only those with positive results were included for further study.
Anti-DENV IgM antibody positive specimens were reanalyzed for anti-WNV IgM antibodies according to the Centers for Disease Control and Prevention (CDC) monoclonal antibody capture (MAC) ELISA protocol (Martin et al. 2002b). Briefly, goat anti-human IgM antibody was coated on 96-well plates followed sequentially by patient serum (diluted 1:400) or CSF (no dilution), WNV antigen (Focus Technologies, Cypress, CA), and a horseradish peroxidase–conjugated monoclonal antibody followed by substrate for the conjugate. Samples were run in duplicate with positive and negative controls, and an average absorbance of each was determined at 450 nm. A P/N ratio (positive to negative control or patient sample to negative control) of ≥2.0 was considered positive.
Plaque reduction neutralization titer (PRNT) assays for DENV serotypes 1–4 were performed at the University of Texas Medical Branch on DENV IgM-positive specimens that had sufficient volume in 12-well, Vero microplate cell cultures using a fixed virus inoculum (≈800 focus-forming units [FFU] per well) against varying serum dilutions (1:20–1:1,280) (Vasilakis et al. 2008). Serum specimens were diluted in minimal essential medium (MEM), containing 2% fetal bovine serum (FBS). Virus was mixed with an equal volume of each serum dilution, and the mixture was incubated for 1 h at 37°C. Then, 250 μL of the serum–virus mixture was placed into Vero cell cultures and incubated for 1 h at 37°C. One milliliter of 0.8% methycellulose in Opti-MEM I overlay was placed in each well, and the plates were incubated at 37°C for 4 days. The plates were then fixed with 1:1 methanol:acetone, and foci were stained immunologically and counted to determine the level of virus neutralization, as described previously (Vasilakis 2007). PRNT titers were scored as the reciprocal of the highest dilution of serum that inhibited 60% of foci. Infecting serotype was determined by evidence of two-fold higher titer in one serotype versus others.
Nested reverse transcriptase-polymerase chain reaction (RT-PCR) for DENV serotypes 1–4 was performed at UTHSC–Houston on all sera and CSF in which sufficient volume was available following the method of Lanciotti et al. (1992). Briefly, RNA was extracted from serum using a QIAamp Viral RNA Extraction Kit according to the manufacturer's instructions (Qiagen, Valencia, CA) and amplified using a Qiagen OneStep RT-PCR Kit under the following conditions. The master mix was comprised of 2.0 μL of 10 mM deoxyribonucleotide triphosphates (dNTPs), 10.0 μL of 5× buffer, 10.0 μL of Q solution, 2.0 μL of multi-dengue sense primer, and 2.0 μL of the multi-dengue antisense primer. The final concentration of the primers was 0.6 μM. Finally 2.0 μL of enzyme mix was added as well as 20.0 μL of water for a total volume of 50.0 μL. The volume of template added was 2 μL. The primer sequences and RT-PCR protocol were previously described (Lanciotti et al. 1992). For the nested reaction, 10 μL of a 1:100 dilution of the first-cycle PCR product served as the template. The master mix consisted of 10 μL of 5× buffer, 1 μL of dNTPs, 1.5 μL of primer SD, 1.5 μL of type-specific primer, 1 μL of Taq polymerase, 20.5 μL of water, and 10 μL of template. The reaction was amplified in a thermal cycler, and the product was analyzed by agarose gel electrophoresis for positive bands at the expected gel positions.
Medical record abstraction
Conducted under the mandate given to local health authorities to investigate a notifiable disease, medical records that were available for all DENV IgM-positive cases were obtained by the HDHHS and reviewed retrospectively. A case ascertainment form was used to abstract information pertaining to patient symptoms and clinical findings at the time of their hospital visit or admission. Collected data also included date of symptom onset, laboratory tests performed, diagnoses, treatments, and medical and social history, including travel history. Anticipating that travel history would not be included in many of the medical records, HDHHS also conducted personal telephone interviews with all DENV IgM-positive cases that could be contacted for dates and locations of travel in the 3 weeks prior to illness onset.
Data were entered into Epi-Info (Epi-info 2000, version 3.5, Atlanta, GA). Descriptive statistics were used to describe the case population, and attack rates were calculated on demographic characteristics based on the 2000 Harris County census data (Harris County, Texas 2000).
Clinical findings and case classification
Descriptive statistics on reported symptoms and clinical exam findings including laboratory test results were performed for all cases with positive anti-DENV IgM. Case classification was based on 1997 World Health Organization (WHO) criteria (World Health Organization 1997). DF was defined as a fever and two or more of the following signs/symptoms—retro-orbital or ocular pain, headache, rash, myalgia, arthralgia, leukopenia, or hemorrhagic manifestations. Dengue hemorrhagic fever (DHF) was defined as a case that had fever for 2–7 days, hemorrhagic manifestations, thrombocytopenia, and evidence of plasma leakage. A case of dengue shock syndrome (DSS) was defined as a case presenting with the same clinical characteristics as DHF along with rapid, weak pulse and narrow pulse pressure (<20 mmHg) or hypotension.
Determination of autochthonous transmission versus travel-associated dengue
Travel history was assessed by analyzing data from the medical record abstractions and/or personal interviews. A travel-associated case was defined as one in which an individual traveled to a DENV-endemic area within 3 weeks prior to onset of symptoms. Suspected dengue cases were classified as locally acquired if no history of recent travel was reported or if travel was not to a dengue-endemic area. If travel history was not captured in the medical record abstractions or the interviews, the cases were not considered in the evaluation to determine autochthonous transmission.
Temporal and geospatial trends
Epidemic curves were constructed based on dates of onset of symptoms derived from chart abstractions. We mapped the residence of all suspected cases that had illness onset in 2003 and reported to have no recent travel history to an endemic area using ArcGIS (v. 3.2. Environmental Systems Research Institute, Redlands, CA). Areas were mapped of Ae. aegypti and Ae. albopictus locations as identified through mosquito surveillance by Harris County Public Health and Environmental Services Mosquito Control Division. These maps were created and overlaid in ArcGIS based on historical numbers of Ae. aegypti and Ae. albopictus collected throughout years 2003–2005. Densities were calculated for each operational area by dividing the total number of male and female mosquitos collected by the total number of trap nights and were categorized in graduated levels to depict visually on the map.
Results
Diagnostic findings
We obtained 3768 specimens from patients with suspected arboviral illness; 2138 (56.7%) specimens were CSF and 1630 (43.2%) were serum. We identified 47 (1.2%) with positive detectable anti-DENV IgM antibodies; 38 (81%) specimens were serum and 9 (19%) were CSF. We retested all positive specimens for anti-WNV IgM antibodies by MAC-ELISA to address whether any specimens would cross-react with WNV. Of the 47 positive specimens, 46 (98%) were negative for anti-WNV IgM antibodies, and one (2%) tested equivocal for WNV. Sufficient quantity of sera or CSF was available for 17 IgM-positive specimens to allow for PRNT testing (Table 1). Of those, three were positive, mainly showing neutralization for DENV-1 and DENV-2. One of these patients had a history of travel to San Antonio, Texas, in the 3-week period before onset of symptoms. Because PRNT is primarily positive in convalescing samples (Council of State and Territorial Epidemiologists 2009) and 94% of specimens were collected within a week following onset of symptoms, these results are as expected.
Table 1.
PRNT60 Against All DENV Serotypes of Houston IgM-Positive Specimens with Sufficient Volume for Testing, 2003–2005
| Sample ID | Specimen type | DENV-1 (OBS7690) | DENV-2 (16681) | DENV-3 (JKT85-934) | DENV-4 (H241) | History of recent travel | No. of days sample collected after onset of symptoms |
|---|---|---|---|---|---|---|---|
| CL2072 |
Serum |
<20 |
<20 |
<20 |
NT |
Unknown |
5 |
| CL2841 |
Serum |
>640 |
>640 |
320 |
80 |
San Antonio, TX |
6 |
| CL3261 |
Serum |
<20 |
<20 |
<20 |
<20 |
No |
2 |
| CL2549 |
Serum |
<20 |
<20 |
<20 |
<20 |
Unknown |
2 |
| CL2042 |
Serum |
<20 |
<20 |
<20 |
<20 |
Unknown |
3 |
| CL0039 |
Serum |
40 |
160 |
<20 |
<20 |
No |
2 |
| CL2030a |
Serum |
NT |
20 |
<20 |
NT |
No |
4 |
| CL2832 |
CSF |
<20 |
<20 |
<20 |
<20 |
No |
15 |
| CL1425 |
Serum |
<20 |
<20 |
<20 |
<20 |
Unknown |
1 |
| CL3384 |
Serum |
<20 |
<20 |
<20 |
<20 |
No |
1 |
| CL2022 |
Serum |
<20 |
<20 |
<20 |
<20 |
No |
3 |
| CL3341 |
Serum |
<20 |
<20 |
<20 |
<20 |
Unknown |
1 |
| CL3344 |
Serum |
<20 |
<20 |
<20 |
<20 |
Unknown |
4 |
| CL0861 |
Serum |
<20 |
<20 |
<20 |
<20 |
Unknown |
2 |
| CL3411a |
Serum |
<20 |
<20 |
<20 |
<20 |
Rio Grande Valley, TX |
4 |
| CL1834 |
CSF |
NT |
<20 |
NT |
NT |
Unknown |
Unknown |
| CL0133 | Serum | <20 | <20 | <20 | <20 | Unknown | 7 |
Positive for DENV-2 by PCR.
PRNT60, plaque reduction (60%) neutralization titer; DENV, dengue virus; NT, not tested due to insufficient volume of specimen.
The RT-PCR assay was positive for DENV-2 in the sera from two IgM-positive patients (Table 1). In both patients, specimens were collected 4 days after onset of fever. One patient, a 39-year-old female of undisclosed race/ethnicity, had no history of recent travel and had onset of illness in June 2003. Her illness included fever, headache, photosensitivity, myalgia, nausea, and retro-orbital pain. CSF had both elevated protein and pleocytosis. The second PCR-positive patient was a 25-year-old male of undisclosed race/ethnicity who had reported recent travel to the Rio Grande Valley of Texas, although the exact dates of travel were unknown. His onset of illness was in March, 2004, and consisted of headache, myalgia, nausea, and vomiting. There were no reports of local transmission of DENV in the Rio Grande Valley, Texas, at that time.
Clinical findings
Data from hospital records were available for 42 of the 47 anti-DENV IgM-positive cases. These 42 positive cases presented to 20 different hospitals in the Houston area and were hospitalized for a median of 5 days (range, 1–64 days). Demographic characteristics of the study population shown in Table 2 included equal proportions of males and females. The largest proportion of cases (40%) was among white, non-Hispanic persons. The median age was 29 among all IgM-positive cases and 26 for those who met the WHO case definition for DF. The highest attack rate was in those 20–34 years of age (2.4 per 100,000 population).
Table 2.
Study Population Demographics for 42 IgM-Positive Suspected Acute Dengue Cases
| IgM-positive cases, n=42 (%) | Attack rate per 100,000 populationa | Meet case definition for dengue fever, n=18 (%) | |
|---|---|---|---|
| Gender | |||
| Female |
21 (50) |
1.2 |
9 (50) |
| Male |
21 (50) |
1.2 |
9 (50) |
| Age at admission | |||
| <5 years |
3 (7) |
1.1 |
1 (6) |
| 5–19 years |
5 (12) |
0.6 |
2 (11) |
| 20–34 years |
20 (48) |
2.4 |
9 (50) |
| 35–44 years |
5 (12) |
0.9 |
3 (17) |
| 45–54 years |
2 (5) |
0.5 |
1 (6) |
| 55–64 years |
3 (7) |
1.3 |
1 (6) |
| >65 years |
3 (7) |
1.2 |
1 (6) |
| Unknown |
1 (2) |
— |
0 |
| Race/ethnicity | |||
| African American |
6 (14) |
1.0 |
3 (17) |
| Asian |
1 (2) |
0.6 |
0 |
| Caucasian |
17 (40) |
1.2 |
8 (44) |
| Hispanic |
12 (29) |
1.1 |
2 (11) |
| Other |
1 (2) |
— |
1 (6) |
| Unknown | 5 (12) | — | 4 (22) |
Based on 2000 census population for Harris County (Harris County, Texas 2000).
IgM, immunoglobulin M.
Reported symptoms and clinical findings for the study population are summarized in Tables 3 and 4, respectively. Most of the anti-DENV IgM-positive cases had experienced a suspected viral infection with neurologic and liver involvement. Fever, headache, nausea, and vomiting were the most commonly reported symptoms upon admission. Of the 42 cases, 18 (43%) met the WHO criteria for DF. Most IgM-positive cases (57%) and those who met the WHO case definition for DF (61%) were diagnosed with aseptic or viral meningitis and/or encephalitis. Pleocytosis was found in five of the six cases that had IgM-positive CSF and in all 12 cases that met the case definition of DF and had CSF collected. Other prominent neurological findings included headache, neck and/or back pain, photosensitivity, altered mental status, and nuchal rigidity. Of the 22 cases that had liver enzyme test results recorded, 13 (59%) had elevated alanine transaminase (ALT) and 9 (41%) had elevated aspartate transaminase (AST).
Table 3.
Reported Symptoms As Identified by Medical Chart Review in the Study Population
| Patient-reported symptoms | All DENV IgM+cases, n=42 (%) | Meet case definitionafor dengue fever, n=18 (%) |
|---|---|---|
| Constitutional | ||
| Fever |
35 (83) |
18 (100) |
| Weakness |
5 (12) |
1 (6) |
| Chills/rigors |
5 (12) |
2 (11) |
| Fatigue/lethargy |
4 (10) |
1 (6) |
| Myalgiaa |
6 (14) |
6 (33) |
| Malaise |
3 (7) |
3 (17) |
| Arthralgiaa |
10 (24) |
10 (56) |
| Rash* |
6 (14) |
3 (17) |
| Gastrointestinal | ||
| Nausea |
20 (48) |
8 (44) |
| Vomiting |
21 (50) |
9 (50) |
| Diarrhea |
1 (2) |
0 (0) |
| Abdominal pain |
5 (12) |
2 (11) |
| Neurological | ||
| Headachea |
27 (64) |
13 (72) |
| Neck and/or back pain |
10 (24) |
8 (44) |
| Dizziness/vertigo |
1 (2) |
1 (6) |
| Stiff neck |
3 (7) |
3 (17) |
| Blurred vision |
2 (5) |
1 (6) |
| Photosensitivity/photophobia |
8 (19) |
3 (17) |
| Retro-orbital paina |
3 (7) |
2 (11) |
| Respiratory | ||
| Cough |
1 (2) |
0 (0) |
| Shortness of breath/dyspnea |
1 (2) |
0 (0) |
| Sore throat | 3 (7) | 0 (0) |
Dengue fever was defined as fever and two or more of the following symptoms: retro-orbital or ocular pain, headache, rash, myalgia, arthralgia, leukopenia, or hemorrhagic manifestations.
DENV, dengue virus; IgM, immunoglobulin M.
Table 4.
Clinical Findings Derived from Medical Chart Abstractions among the DENV IgM-Positive Study Population
| Clinical features | All cases, n=no. cases assessed (%) | Meet case definitionafor dengue fever, n=no. cases assessed (%) |
|---|---|---|
| Physical exam findings | ||
| Fever on admission (>100.3°F) |
15/41 (37) |
8/18 (44) |
| Elevated blood pressure on admission (systolic >120 mmHg and/or diastolic >80 mmHg) |
19/35 (54) |
6/14 (43) |
| Lymphadenopathy |
2/42 (5) |
2/18 (11) |
| Neurological exam findings | ||
| Altered mental status |
15/42 (36) |
6/18 (33) |
| Generalized seizures |
2/42 (5) |
1/18 (6) |
| Nuchal rigidity |
10/42 (24) |
4/18 (22) |
| Myoclonus or tremors |
2/42 (5) |
0/18 (0) |
| Laboratory and diagnostic testing | ||
| Abnormal CSF (>5 WBC/mm3 and/or protein >45 mg/dL) |
25/30 (83) |
12/12 (100) |
| Pleocytosis in CSF (>5 WBC/mm3) |
21/30 (70) |
12/12 (100) |
| Elevated protein in CSF (>45 mg/dL) |
19/30 (63) |
9/12 (75) |
| Peripheral leukocytosis (>10,000/mm3) |
19/40 (48) |
8/18 (44) |
| Leukopenia (<5000/mm3)a |
4/40 (10) |
3/18 (17) |
| Anemia (hemoglobin <12 g/dL in females; <14 g/dL in males) |
12/39 (31) |
7/18 (39) |
| Thrombocytopenia (platelets <150×103/μL) |
2/34 (6) |
2/18 (11) |
| Hypoalbuminemia (albumin <3.4 g/dL) |
3/21 (14) |
1/11 (9) |
| Elevated AST/SGOT (>40 U/L) |
9/22 (41) |
4/10 (40) |
| Elevated ALT/SGPT (>40 U/L) |
13/22 (59) |
4/10 (40) |
| Elevated creatinine (>1.4 mg/dL) |
4/37 (11) |
2/17 (12) |
| Abnormal EEG |
6/8 (75) |
3/4 (75) |
| Abnormal EMG |
5/6 (83) |
1/1 (100) |
| Clinical findings | ||
| Loss of consciousness |
1/42 (2) |
0/18 (0) |
| Intubation/ventilatory support |
7/42 (17) |
1/18 (6) |
| Hemorrhagic manifestationsa |
1/42 (2) |
0/18 (0) |
| Diagnosis of viral encephalitis |
5/42 (12) |
2/18 (11) |
| Diagnosis of viral meningitis |
22/42 (52) |
10/18 (56) |
| Death | 2/42 (5) | 0/18 (0) |
Dengue fever was defined as fever and two or more of the following symptoms: retro-orbital or ocular pain, headache, rash, myalgia, arthralgia, leukopenia, or hemorrhagic manifestations.
DENV, dengue virus; IgM, immunoglobulin M; CSF, cerebrospinal fluid; WBC, white blood cells; AST, aspartate transaminase; SGOT, serum glutamic-oxaloacetic transaminase; ALT, alanine transaminase; SGPT, serum glutamic-pyruvic transaminase; EEG, electroencephalogram; EMG, electromyogram.
Two (5%) cases died during hospitalization. One was a 92-year-old African-American woman. The patient was admitted with fever, elevated blood pressure, reported weakness, and unresponsiveness in June of 2003. In the medical record, clinicians noted nuchal rigidity, pleocytosis and elevated protein of the CSF, altered mental status due to unresponsiveness, a downgoing Babinski sign, and seizures. The patient was diagnosed with viral meningitis and encephalitis. Death occurred 10 days after onset of illness. The patient had been bedridden for almost 2 years following a hip replacement surgery; thus, she had no recent travel outside of Houston. CSF that was collected on the first day of hospitalization (5 days post-onset of symptoms) was positive for anti-DENV IgM and negative for WNV IgM antibodies. This case was unique in that a serum sample collected on the first day of hospitalization was also submitted to the public health laboratory. This specimen was found to be positive for anti-DENV IgM and negative for WNV IgM. PRNT assay results on serum were negative for DENV serotypes 1–3 (CL2072, Table 1). There was an insufficient amount of sample remaining for DENV-4 PRNT. According to the medical record, the patient was also negative for California encephalitis complex, eastern equine encephalitis, western equine encephalitis virus, and herpes simplex. This patient's death was likely due to a recent primary DENV infection that manifested as encephalitis.
The second fatal case was a 49-year-old woman who was admitted to the hospital in July of 2004 for septic shock, severe sepsis, hemorrhagic manifestations related to disseminated intravascular coagulopathy, acute renal failure, and acute liver dysfunction. She had complaints of fever, headache, and abdominal pain that began 2 days prior to admission. Prior to loss of consciousness, she had experienced altered mental status, and pleural effusion was noted in the medical record. The final diagnosis was recurrent severe sepsis with anoxic brain injury accompanied by hepatic cirrhosis. She died on the 11th day of hospitalization. The patient had a history of frequent travel to Mexico, including a visit just prior to illness onset. Serum collected on the second day of hospitalization (3 days following onset of symptoms) was positive for anti-DENV IgM and negative for WNV IgM antibodies. Unfortunately, prior exposure to DENV could not be confirmed by PRNT assay because there was insufficient specimen available for testing. Nevertheless, the patient's travel history and clinical characteristics suggest that complications were a result of a severe DENV infection that progressed to DSS.
Aside from the suspected DSS case, none of the remaining members of the study population had clinical characteristics that met the WHO criteria for DHF. Rash was reported in six of the 42 cases (14%), but none were described as petechial; two (6%) patients had thrombocytopenia, both of whom met the case definition for DF. Hemoconcentration was not reported for any of the patients, and no signs or indications of hemorrhagic manifestations or organomegaly were noted in medical records of any patients other than the DSS case.
Epidemiology and evidence of autochthonous transmission of DENV in Houston
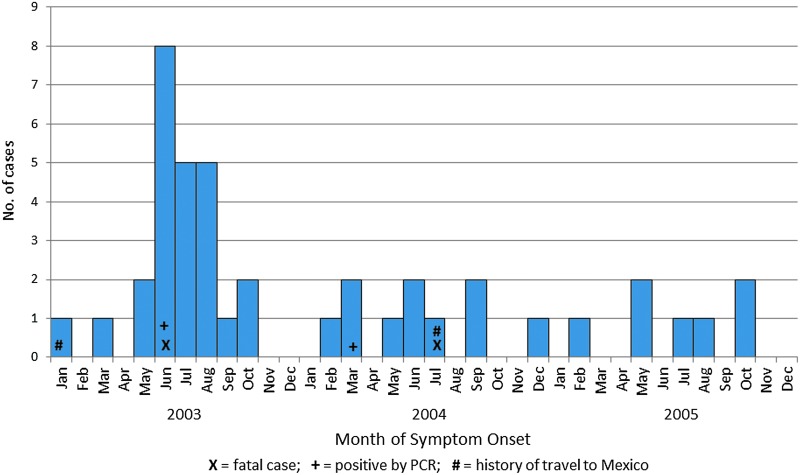
Twenty-five positive cases (60%) had illness onset dates in 2003, 10 (24%) in 2004, and 7 (17%) in 2005. Most of the cases (n=35; 83%) had onset of symptoms between May and November (Fig. 1). The number of cases in Houston in 2003 indicates an increase during the summer months, with the epidemic curve characteristic of an outbreak.
FIG. 1.
Epidemic curve of cases with positive anti-dengue virus (DENV) immunoglobulin M (IgM) antibodies by date of onset of symptoms, Houston, Texas, 2003–2005.
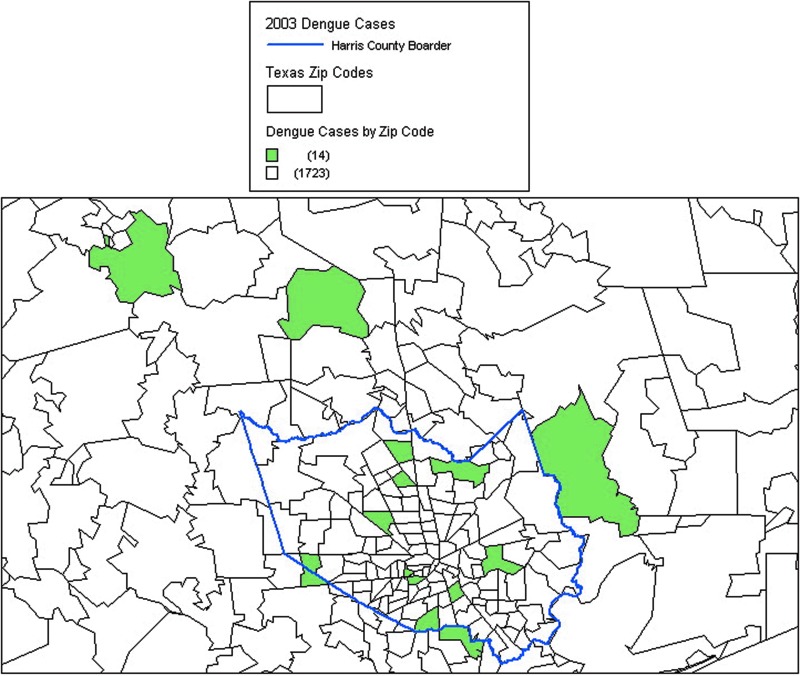
Travel history was obtained on 19 of the 42 (45%) cases via medical records and/or telephone interviews (Table 5). Most of these cases (84%) reported no travel or had not traveled to an area known to be endemic to DENV. Zip codes of the residences of individuals from 2003 who tested positive for anti-DENV IgM antibody who had not traveled to an endemic area (n=14) were mapped (Fig. 2). Each case was in a separate zip code, and there was no evidence of clustering.
Table 5.
Destinations of Recent Travel Anti-DENV IgM-Positive Cases Reporting Travel History
| Destination | Number individuals |
|---|---|
| Mexicoa |
2b |
| Rio Grande Valley, Texasa |
1 |
| San Antonio, Texas |
2 |
| Baton Rouge, Louisiana |
1 |
| Portland, Oregon |
1 |
| College Station, Texas |
2 |
| No travel outside of Houston area | 10 |
Denotes a dengue-endemic area.
Includes possible dengue shock syndrome (DSS) case.
DENV, dengue virus; IgM, immunoglobulin M.
FIG. 2.
Geographic location of immunoglobulin M (IgM)-positive cases of dengue in 2003 with no history of travel (n=14) in the Houston metropolitan area.
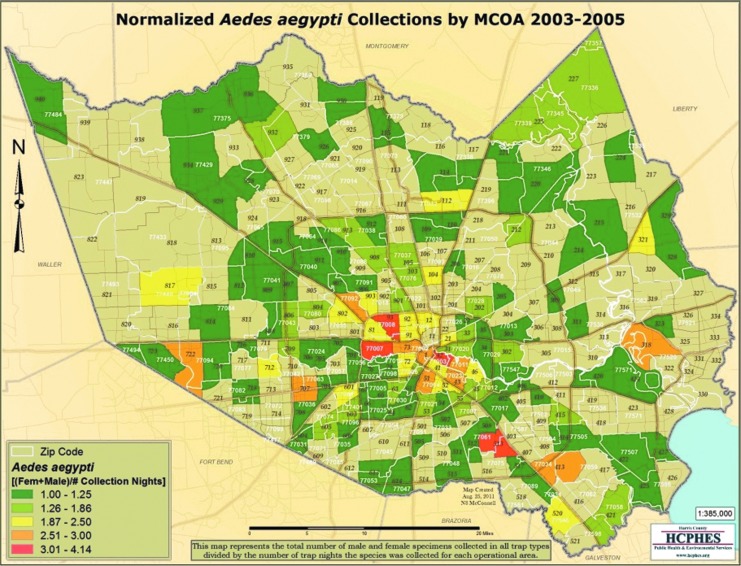
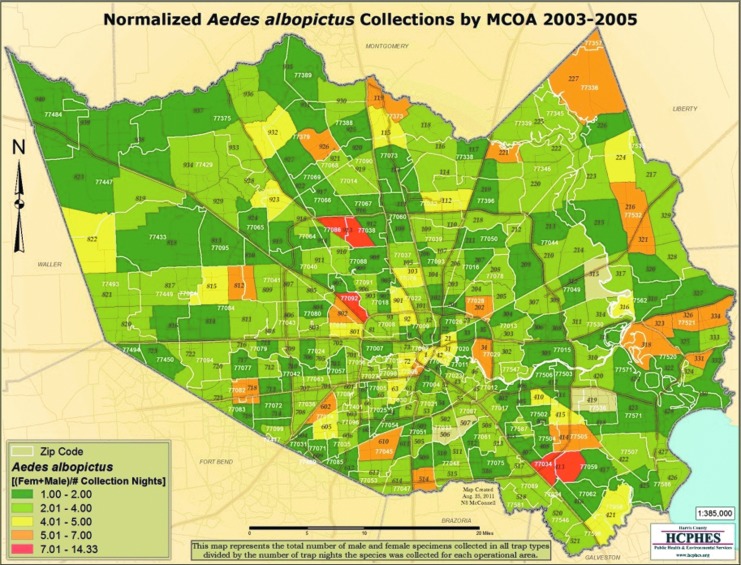
Review of surveillance data for Ae. aegypti, the primary vector for dengue, from 2003 to 2005 showed that the populations were widespread in the Houston area, including in the zip codes where individuals from this study resided. Figures 3 and 4 show the locations of Ae. aegypti and Ae. albopictus collections between 2003 and 2005.
FIG. 3.
Map depicting Ae. aegypti collections by Harris County Mosquito Control Operational Areas (MCOA) from 2003 to 2005.
FIG. 4.
Map depicting Ae. albopictus collections by Harris County Mosquito Control Operational Areas (MCOA) from 2003 to 2005.
Discussion
We report here for the first time data supportive of autochthonous transmission of DENV in Houston, Texas, within the past 10 years. The detection of anti-DENV IgM-positive antibodies in 47 patients with suspected arboviral clinical illness, many with no recent travel outside of the region, indicates that DENV was present and circulating in the Houston area between 2003 and 2005. A high proportion of cases in 2003 occurred between late spring and early fall, when transmission would be expected, and the shape of the epidemic curve was characteristic of an arboviral disease outbreak. Data from medical chart abstractions and travel histories also suggest that an outbreak of dengue occurred in the Houston area in 2003. Because there was no established surveillance for dengue between 2003 and 2005, these cases were not tested for or suspected of DENV infection at the time. Given the lack of active surveillance for dengue, the biasing of specimen submissions toward patients with neurological involvement since physicians suspected WNV infection, and the fact that 50–90% of all DENV infections are asymptomatic (Kyle and Harris 2008), this study most likely underestimates the true number of cases that occurred between 2003 and 2005 in the Houston area.
Because DENV was not known to be circulating in the Houston area, health care providers may not have specifically assessed the signs and symptoms unique to DF, and therefore important indicators of a DENV infection may not have been assessed at the time of patient hospitalization or recorded in the medical records. Interestingly, DF was not included as a differential diagnosis or mentioned at all in any of the abstracted medical record data reviewed here, including the clinically compatible DSS case in a person with a history of recent travel to a dengue endemic area. Because DENV is a reportable disease in the state of Texas, no cases of imported dengue were officially reported to the state from Harris County during this time period, with the exception of one imported case of dengue from Nicaragua in August of 2005 (Jim Schuermann, Texas Department of State Health Services, personal communication). This strongly suggests that recognition and/or reporting of dengue in the Houston area is low, and that efforts should be made to notify and educate Houston-area clinicians of the possibility of locally acquired cases.
The last documented outbreak of dengue fever in the Houston area was the “Galveston outbreak” of 1922 in which over 500,000 cases of DF were found along the Gulf Coast of the United States (Rice 1922). This demonstrates the viability for DENV transmission in this area of Texas; however, we cannot conclude from this study if DENV was reintroduced into Houston at some point in the recent past or if DENV has been endemic at undetectable levels.
Detecting IgM antibodies specific for dengue virus in the CSF of nine patients is highly significant for central nervous system infection because IgM antibodies do not typically cross the blood–brain barrier (Lum et al. 1996, Martin et al. 2002b). Additionally, an IgM-positive CSF specimen is considered confirmatory for diagnosing acute DENV infection (Centers for Disease Control and Prevention 2012). In this study, one CSF sample tested positive for DENV and was equivocal for WNV, which could be attributed to cross-reaction on the WNV ELISA; however, cross-reaction between the DENV serogroup and WNV, along with other viruses in the Japanese encephalitis serogroup, has been found to be low (Martin et al. 2002a).
The IgM MAC-ELISA has been widely used in the Unites States and abroad to diagnose acute cases of arboviral infections using serum and CSF specimens, and it is currently one of the preferred methods for diagnosis by most public health and research laboratories (Centers for Disease Control and Prevention 2003). Anti-DENV IgM typically becomes detectable around 3– 5 days after onset in approximately 50% of patients with acute infections and detectable by day 10 in 99% of patients with acute infections (World Health Organization 2009). The MAC-ELISA has shown to be simple and has a high specificity that reduces the occurrence of cross-reactive antibodies, especially in CSF specimens (Martin et al. 2002a). Disadvantages include the potential for false-negative results when specimens are collected too close to the onset of illness before IgM antibodies are detectable. False-negative IgM results from either serum or CSF specimens could have also occurred if a patient had a secondary DENV infection resulting in a small or undetectable IgM response (World Health Organization 2009). For primary infections, the Panbio Dengue IgM Capture ELISA Kit used in this study has a reported sensitivity and specificity of 96.8% and 99.5%, respectively, and a predictive value positive of 99.3% (Vasquez et al. 2007). This kit has been validated as an excellent test to identify an acute primary DENV infection.
We identified two individuals positive for DENV-2 by RT-PCR. These findings were very helpful in establishing the serotype and evidence of autochthonous transmission in at least one of the cases. There are several possible explanations as to the low number of PCR-positive cases. One is that we mostly focused our PCR testing on IgM-positive cases. Considering the dynamics of the immune response, typically viremia diminishes around the time the patient has detectable IgM antibodies. Additionally, a study in Pakistan found that significant clinical illness was more likely to be associated with being positive for IgM and negative for RNA (Tang et al. 2008). Another possible reason for the low numbers of PCR-positive specimens could be related to specimen storage and the integrity of the samples. The specimens in this study had undergone numerous freeze–thaw cycles (at least five) for ELISA testing before being tested by PCR, and these specimens had spent a considerable amount of time being stored at −20°C. These factors lessened our likelihood of detecting RNA by PCR.
We identified three cases whose sera were positive by PRNT. PRNT can be used to confirm specific flavivirus infections in convalescent samples, particularly when there is concern about cross-reactive antibodies to other flaviviruses (Council of State and Territorial Epidemiologists 2009). In this study, PRNT allowed us to identify the circulating serotype and confirm DENV infection in at least a subset of the study population. Unfortunately, due to the retrospective nature of this study, convalescent specimens were not collected or available for further PRNT testing.
Because the specimens available for this study were from patients suspected of having WNV infection, a high percentage of our study population exhibited clinical signs and had laboratory evidence of central nervous system involvement. Interestingly, over 75% of the individuals who received a nontraumatic lumbar puncture had abnormal CSF characteristic of aseptic viral meningitis/encephalitis. A 2009 case report from Brazil supports the notion that DF can present with a neuroinvasive clinical picture (Soares et al. 2010). The case report describes a 24-year-old woman with a laboratory confirmation of DENV who presented with fever, headache, nuchal rigidity, and pleocytosis. The authors describe the case as one without typical symptoms of DF and suggest that DF can be a cause of meningitis.
Other studies have also observed that neurologic involvement during DENV infection is becoming more recognized with DF (World Health Organization 2009). A study conducted in Jamaica indicated that abnormal neurologic findings in DF cases were common (Jackson et al. 2008). Among 401 patients with an unknown viral infection with central nervous system involvement, 13.5% were identified by confirmatory laboratory tests to be DF. Of the DF cases, 52% and 33% were diagnosed with encephalitis and meningitis, respectively. Neurologic involvement in cases of DF is not unheard of, but nonetheless not the most common manifestation, thus it is possible that there were an even larger number of unrecognized cases of DF in the Houston area between 2003 and 2005.
There are several limitations to this study worth mentioning. Although we have established that the majority of the illnesses in our study population were related to a viral infection, viruses other than DENV could be implicated. It is possible that these cases may not have been DF, but another neuroninvasive febrile illness caused by a flavivirus that induces IgM antibodies that cross-react with DENV. We do, however, have clinical evidence that supports our findings, and laboratory testing confirmed DENV-2 by PCR in two cases. Additionally, we ruled out several arboviruses known to be present in the Houston area, including the nonflavivirus eastern equine encephalitis virus. Interestingly, DENV-2 was also detected along the US/Mexico border in 2005 (Centers for Disease Control and Prevention 2007).
We did not find evidence of geographic clustering among residences of DENV-positive individuals who reported to have not traveled outside of Houston. Additionally it is possible that the residential zip codes used to construct the map were not the zip codes where infection was acquired. Therefore, it is difficult to draw any concrete conclusions about geographic characteristics of DENV transmission in the Houston area. Finally, because we conducted the interviews of all DENV-positive individuals who could be located in 2009, there is a substantial risk of recall bias when asking about their travel histories just prior to their illness onset (up to 6 years later). All histories obtained matched those medical records that did mention travel prior to illness onset, but those records without mention of travel history could not be validated with the interviewee's response.
In this study, we demonstrated evidence of acute DENV infections in at least a subset of cases with clinically compatible DF illness in the Houston area with no history of travel. Dengue is an important emerging disease in Texas that could easily become endemic considering the ecology of the area and abundance of competent vectors. The findings of this study as well as recent findings of DENV transmission in Florida (Centers for Disease Control and Prevention 2010) underscore the need for DENV surveillance in the Houston area and other regions of the southern United States where ecological conditions would allow for emergence of DENV. Additional serosurveys of the Houston population along with prospective active surveillance for human cases and field studies in mosquitoes are needed to determine the existence and location of local viral activity. Furthermore, because no vaccine is currently available against DENV, prevention efforts should focus on educating physicians about the signs and symptoms of DF, ensure availability of diagnostic testing, and educate the public on the importance of avoiding mosquito bites.
Acknowledgments
This paper is dedicated to the memory of Dr. Olushola Adeleye, who provided immense support for this research study. We are grateful to the staff at the City of Houston Department of Health and Human Services public health laboratory, Nathan O'Connell from Harris County Mosquito Control, and Monica Sierra and Jennifer Bigbee from the University of Texas Health Science Center at Houston School of Public Health for their assistance with specimen and data acquisition. We would also like to thank Drs. Tyler Sharp, Jorge Munoz, Kay Tomashek, Harold Margolis from the Dengue Branch at the Centers for Disease Control and Prevention for their review of the data and manuscript. We also want to thank Ms. Amy York and Ms. Debbie Phillips from PanBio Diagnostics for their technical assistance.
Author Disclosure Statement
This study was supported in part by a grant from the United States Department of Defense, Army (grant #W81XWH-04-2-0031) and by National Institutes of Health (NIH) grant AI069145.
References
- Bhatt S, Gething PW, Brady OJ, Messina JP, et al. The global distribution and burden of dengue. Nature 2013; 496:504–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JE, McBride CS, Johnson P, Ritchie S, et al. Worldwide patterns of genetic differentiation imply multiple ‘domestications' of Aedes aegypti, a major vector of human diseases. Proc Biol Sci 2011; 278:2446–2454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunkard JM, Robles López JL, Ramirez J, et al. Dengue fever seoprevalence and risk factors, Texas–Mexico Border, 2004. Emerg Infect Dis 2007; 13:1477–1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Dengue surveillance—United States, 1986–1992. MMWR 1994; 43:7–19 [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Underdiagnosis of dengue—Laredo, Texas, 1999. MMWR 2001; 50:57–59 [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Epidemic/epizootic West Nile virus in the United States: guidelines for surveillance, prevention, and control, 2003. 2003. Available at www.cdc.gov/ncidod/dvbid/westnile/clinicians/surveillance.htm#casedef Last accessed August23, 2013
- Centers for Disease Control and Prevention Dengue hemorrhagic fever—U.S.–Mexico Border, 2005. MMWR 2007; 56:785–789 [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Locally acquired dengue—Key West, Florida, 2009–2010. MMWR 2010; 59:577–581 [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention 2012Nationally Notifiable Diseases and Conditions. Available at www.cdc.gov/nnds/document/2012-case%20defintions.pdf Accessed Oct. 2013
- Gubler DJ. Dengue and dengue hemorrhagic fever. Clin Micro Rev 1998; 11:480–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris County, Texas 2000 Census Data Available at http://factfinder.census.gov/faces/tableservices/jsf/pages/productview.xhtml?src=bkmk Accessed October. 2013
- Jackson ST, Mullings A, Bennett F, Khan C, et al. Dengue infection in patients presenting with neurological manifestations in a dengue endemic population. West Ind Med J 2008; 57:373–376 [PubMed] [Google Scholar]
- Kyle JL, Harris E. Global spread and persistence of dengue. Annu Rev Microbiol 2008; 9:71. [DOI] [PubMed] [Google Scholar]
- Lanciotti RS, Calisher CH, Gubler DJ, Chang GJ, et al. Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase-polymerase chain reaction. J Clin Microbiol 1992; 30:545–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lum LC, Lam SK, Choy YS, George R, et al. Dengue encephalitis: A true entity? Am J Trop Med Hyg 1996; 54:256–259 [DOI] [PubMed] [Google Scholar]
- Martin DA, Biggerstaff BJ, Allen B, Johnson AJ, et al. Use of immunoglobulin M cross-reactions in differential diagnosis of human flaviviral encephalitis infections in the United States. Clin Diagn Lab Immunol 2002a; 9:544–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin DA, Muth DA, Brown T, Johnson AJ, et al. Standardization of immunoglobulin M capture enzyme-linked immunosorbent assays for routine diagnosis of arboviral infections. J Clin Microbiol 2002b; 38:1823–1826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince HE, Lapé-Nixon M, Moore RJ, Hogrefe WR. Utility of the Focus Technologies West Nile virus immunoglobulin M capture enzyme-linked immunosorbent assay for testing cerebrospinal fluid. J Clin Microbiol 2004; 42:12–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos MM, Mohammed H, Zielinski-Gutierrez E, Hayden MH, et al. Epidemic dengue and dengue hemorrhagic fever at the Texas-Mexico border: Results of a household-based seroepidemiologic survey, December 2005. Am J Trop Med Hyg 2008; 78:364–369 [PubMed] [Google Scholar]
- Rawlings JA, Hendricks KA, Burgess CR, Campman RM, et al. Dengue surveillance in Texas, 1995. Am J Trop Med Hyg 1998; 59:95–99 [DOI] [PubMed] [Google Scholar]
- Reiter P, Lathrop S, Bunning M, Biggerstaff B, et al. Texas lifestyle limits transmission of dengue virus. Emerg Infect Dis 2003; 9:86–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice L. Dengue fever, preliminary report of an epidemic at Galveston. Texas State J Med 1922; 189:217–218 [Google Scholar]
- Soares CN, Cabral-Castro MJ, Peralta JM, Freitas MR, et al. Meningitis determined by oligosymptomatic dengue virus type 3 infection: Report of a case. Int J Infect Dis 2010; 14:e150–e152 [DOI] [PubMed] [Google Scholar]
- Sprenger D, Wuithiranyagool T. The discovery and distribution of Aedes albopictus in Harris County, Texas. J Am Mosquito Control Assoc 1986; 2:217–219 [PubMed] [Google Scholar]
- Tang JW, Khanani MR, Zubairi AM, Lam WY, et al. A wide spectrum of dengue IgM and PCR positivity post-onset of illness found in a large dengue 3 outbreak in Pakistan. J Med Virol 2008; 80:2113–2121 [DOI] [PubMed] [Google Scholar]
- Vasilakis N, Shell EJ, Fokam EB, Mason PW, et al. Potential of ancestral sylvatic dengue-2 viruses to re-emerge. Virology 2007; 358:402–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasilakis N, Durbin A, Travassos da Rosa AAP, Munoz-Jordan J, et al. Antigenic Relationships between Sylvatic and Endemic Dengue Viruses. Am J Trop Med Hyg 2008; 79:128–132 [PubMed] [Google Scholar]
- Vazquez S, Hafner G, Ruiz D, Calzada N, et al. Evaluation of immunoglobulin M and G capture enzyme-linked immunosorbent assay Panbio kits for diagnostic dengue infections. J Clin Virol 2007; 39:194–198 [DOI] [PubMed] [Google Scholar]
- Wasay M, Channa R, Jumani M, Shabbir G, et al. Encephalitis and myelitis associated with dengue viral infection: Clinical and neuroimaging features. Clin Neurol Neurosurg 2008; 110:635–640 [DOI] [PubMed] [Google Scholar]
- World Health Organization Clinical diagnosis. Dengue hemorrhagic fever: Diagnosis, Treatment, Prevention and Control, 2nd ed. Geneva, Switzerland: World Health Organizatio, 1997a [Google Scholar]
- World Health Organization Dengue: Guidelines for Diagnosis, Treatment, Prevention, and Ccontrol. 2009. Available at http://whqlibdoc.who.int/publications/2009/9789241547871_eng.pdf Last accessed August23, 2013 [PubMed]