Abstract
Background: Health-related quality of life (HRQoL) has been recognized as an important target and health outcome in obesity research. The current study aimed to examine HRQoL in overweight or obese children after a 10-week primary-care–based weight management program, Parent-Led Activity and Nutrition for Healthy Living, in southern Appalachia.
Methods: Sixty-seven children (ages 5–12 years) and their caregivers were recruited from four primary care clinics, two of which were randomized to receive the intervention. Caregivers in the intervention groups received two brief motivational interviewing visits and four group sessions led by providers as well as four phone follow-ups with research staff. Caregivers completed the PedsQL and demographic questionnaires at baseline and at 3, 6, and 12 months postintervention. Child height and weight were collected to determine standardized BMI.
Results: Caregivers of children receiving the weight control intervention reported no statistically significant improvements in child total HRQoL, as compared to the control group, across the course of treatment (β=0.178; 95% confidence interval, −0.681, 1.037; p=0.687). Additionally, no statistically significant improvements were found across other HRQoL domains.
Conclusions: Future studies examining HRQoL outcomes in primary care may consider treatment dose as well as methodological factors, such as utilization of multiple informants and different measures, when designing studies and interpreting outcomes.
Introduction
Pediatric obesity rates remain high among children, with approximately 33% of the nation's 6- to 11-year-old children classified as overweight or obese.1 Prevalence is higher in specific geographical areas2 and ethnic groups.1 These rates are of importance not only because of physical consequences, but also psychosocial consequences, including lower health-related quality of life (HRQoL).3,4 In fact, HRQoL is currently considered an important outcome and public health goal in obesity research.5,6 A focus on HRQoL allows researchers to extend beyond a narrow view of health as only physical (e.g., BMI) to a more holistic, multi-dimensional view of health that includes mental and social domains and a method for assessing improving the quality of lives.5
Whereas the majority of weight intervention studies have had, as a primary focus, weight loss, there has been increasingly more recognition of the importance of improvements in daily functioning and well-being. To date, several studies have begun to examine the impact of weight management programs on HRQoL. Overall, these studies show positive findings across many HRQoL domains, particularly physical/general and emotional/psychological health.3,4 For instance, Yackobovitch-Gavan and colleagues7 implemented a diet, exercise, or diet plus exercise intervention with 162 obese Israeli 6- to 11-year-olds. Regardless of treatment condition, children significantly improved their total HRQoL, as reported by parents. A more recent study8 examined the impact of a 10-week family-based nutrition, physical activity, and behavioral modification intervention (Positively Fit; PF) versus a brief family-based intervention on self- and parent-reported HRQoL in a sample of overweight and obese children and adolescents seeking treatment. Children in the PF intervention demonstrated a significant and minimal clinically important difference (MCID; defined as the smallest difference that patients perceive as beneficial and would thus mandate change in management9 in parent-reported total HRQoL at postintervention and self-reported HRQoL at 12-month follow-up, as compared to those in the brief intervention).
Although interventions for addressing HRQoL in overweight and obese children are demonstrating some positive outcomes, overweight and obesity rates themselves remain high. Primary care settings are well positioned to address child overweight and obesity, although providers report numerous barriers to treatment implementation,10 and studies examining programs in the primary care setting are limited.11 Parent-Led Activity and Nutrition for Healthy Living was a cluster-randomized pilot trial to evaluate a parent-mediated approach utilizing providers to implement brief motivational interviewing (MI) and parent group sessions to treat child overweight and obesity in primary care in southern Appalachia, a potentially higher risk group based on geographical location and lower socioeconomic status (SES) evidenced in the current sample. The intervention was developed based on the scientific literature outlined in the Expert Committee Recommendations for Treatment of Child and Adolescent Overweight and Obesity.12,13 Specifically, this primary-care–based study included brief MI and behavioral modification, targeted parents, and incorporated provider-led group visits as a treatment delivery design consistent with the Chronic Care Model.12 Individual visits utilizing brief MI incorporated the latest evidence-based tools recommended by the American Academy of Pediatrics and other organizations that have been listed and described in detail previously.14 Group sessions utilized the National Institutes of Health (NIH) Ways to Enhance Children's Activity and Nutrition (We Can!) curricula15 that has been encouraged for use by the public. The current study builds on the limited pediatric obesity treatment literature in primary care settings by assessing HRQoL as an outcome. Similar to previous studies that have documented significant improvements in weight, it was hypothesized that parent perceptions of child HRQoL would be significantly higher in the treatment, as compared to control, group postintervention.
Methods
Procedures
Caregivers of children ages 5–11 years and ≥85th percentile (overweight or obese) were recruited from four primary care clinics (two family medicine and two pediatric). Because of a time lag between initial screening by clinic staff, some (n=25) participants had reached 12 years of age at the official enrollment date. The project coordinator or research staff obtained written caregiver consent during face-to-face visits at primary care clinics, and child assent was obtained on the same or at a later date, as described previously.14 After recruitment, family medicine and pediatric clinics were separately randomized by a coin toss to either treatment or control sites. Providers, specifically, physicians at the family medicine and pediatric treatment clinics, received approximately 8 hours of online and face-to-face training in brief MI and utilization of the NIH We Can! Curricula, which is a family-based approach designed to help children ages 8–13 years maintain a healthy weight.
The intervention was delivered to caregivers only, without children, over approximately 9 weeks. Caregivers in the intervention group participated in two brief (approximately 15 minutes) individual visits, one during the first few weeks of the intervention and one toward the end of the intervention. Additionally, caregivers participated in four group sessions every other week with providers and four phone follow-ups conducted by the project coordinator or project registered dietitian, assessing progress as well as providing more individualized treatment on alternating weeks. The four group sessions (We Can! Energize our Families: Getting Started; Maintain a Healthy Weight: The Energy Balance Equation; What to Feed My Family: Manage ENERGY IN; and Less Sit, More Fit: ENERGY OUT) were based on the NIH We Can! four-lesson curriculum,15 which includes a 1.5-hour session consisting of a warm-up, didactics, group activities, stretch and snack breaks, and goal setting. At least one member of the research team attended each group session, and project staff were available to providers, as needed, for further consultation. The control group received a copy of the NIH We Can! “Families Finding the Balance: A Parent Handbook”15 and routine care. The study was approved by a university institutional review board, and the design and methods of the larger study have been described in detail previously.14
Measures
Health-related quality of life (PedsQL)
Parent-reported HRQoL was assessed by the PedsQL 4.0 Generic Core Scales16 at baseline and at 3, 6, and 12 months. The Parent Report for Young Children (ages 5–7 years)17 was chosen to ensure standardization across measurement procedures and capture parents' perception of the youngest children in the study. This survey includes 23 items assessing parents' perception of his or her child's HRQoL among three summary (Total, Physical, and Psychosocial) and three subscale (Emotional, Social, and School) domains. Previous studies have demonstrated feasibility, reliability, and validity for this measure.17 Cronbach's alphas for the domains at baseline were 0.85 (Total), 0.80 (Physical), 0.81 (Psychosocial), 0.67 (Emotional), 0.74 (Social), and 0.73 (School). Higher scores indicate greater HRQoL.
Demographics and anthropometrics
Demographic variables included child age and gender, parent/caregiver age, gender, relationship to child, race, and education as well as number living in household and enrollment in public and private insurance. Self-reported height and weight were obtained for caregivers and used to calculate BMI. Child height and weight were obtained using a standardized procedure described previously,14 and sex-specific BMI-for-age standardized scores (zBMI) and percentiles were calculated based on the 2000 CDC growth charts.18
Statistical Analysis
A multi-level growth model19 was selected to model the change in HRQoL over time. The multi-level growth model was selected because it provides correct estimates and standard errors when data are nonindependent, such as in repeated-measures designs. Further, the multi-level growth model yields unbiased estimates under longitudinal missingness (i.e., loss to follow-up) as long as the missingness mechanism is at least missing at random. Although treatment was assigned at the site level, it was not possible to model clustering of individuals in sites because of the small number of sites. As an alternative, sites were entered as fixed effects in the model. They were coded such that the model intercept represented the weighted mean HRQoL at baseline for the two control sites, weighted by the number of subjects recruited from each site, whereas the main effect of treatment represented the difference between the weighted mean of the treated sites and the weighted mean of the control sites. Therefore, clustering of subjects into sites was handled by fixed, rather than random, effects.20 Because of collinearity with the treatment indicator variable, indicator variables for only two sites needed to be included in the model.
We conceptualized the treatment effect as a difference in rate of change in HRQoL between treatment and control groups. This treatment effect is consistent with intent-to-treat, because the treatment indicator variable was based on the group to which the participant was assigned, not the compliance of the participant. This approach precluded the possibility of nonequivalence in groups at baseline resulting from incomplete randomization affecting the estimate of the treatment effect. Data were structured in the repeated-measures format with up to four time points per subject. Time was centered at zero and was coded 0, 3, 6, and 12, reflecting the number of months from the beginning of the study. The model was specified as follows:
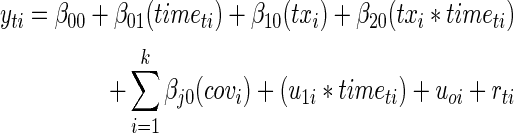 |
where t indexes time points, i indexes subjects, j indexes covariates, and k is the total number of covariates. Additionally, u1i is the random slope, u0i is the random intercept, and rti is the within-subject residual. The interpretation of the fixed-effects (β) parameters is as follows: β00 is the initial HRQoL for the control group and β01 is the average monthly rate of change in HRQoL for the control group. The parameter β10 is the difference between treatment and control HRQoL at baseline, and β20 is the difference in rate of change of HRQoL for treatment versus control. The model included a set of covariates guided by previous literature. These included dummy-coded and within-treatment-group–centered center variables, as well as child age, child zBMI measured at baseline, number in household, and caregiver BMI (all grand-mean centered), dummy-coded indicators of child gender, whether the primary caregiver is the child's grandparent rather than parent, caregiver race, caregiver educational attainment, participation in public health insurance (i.e., TennCare), and participation in private health insurance. The covariates were included in the model to increase statistical power and provide additional protection from confounding resulting from incomplete randomization, but they were not of substantive interest. The parameter β20 represents the effect size of treatment; its corresponding statistical test represents the test of the treatment effect.
Results
Sample Characteristics
The sample consisted of 67 children (58% male) ranging in age from 5 to 12 [mean (M), 8.96; standard deviation (SD), 1.98], with 76% above the 97th percentile for weight (M, 97.80; SD, 2.51). Caregivers ranged in age from 24 to 64 (M, 35.89; SD, 8.53) and were primarily Caucasian mothers (89%). Sixty-four percent of caregivers had a high school education or less, and 71% were enrolled in public health insurance, whereas 38% were enrolled in private insurance when assessed separately. Caregivers had a mean BMI of 34.07 (SD, 7.10), and a mean of 3.91 (1.55) individuals resided in the household. At baseline, caregivers across both the intervention (n=28) and control groups (n=39) reported significantly lower total child HRQoL (M, 67.15; SD, 13.61), as compared to parent reports in a nationally healthy sample (M, 82.70; SD, 15.40; t(66), −9.35; p<0.001) and an obese sample (M, 75; SD, 14.50; t(66), −4.72; p<0.001), both (samples) of which have been described previously.21 Among the intervention group (n=28), approximately 75% of caregivers completed 7 of 10 treatment encounters by individual visits, group sessions, or phone follow-up contacts, including one opportunity for a make-up group session and phone follow-up.
Health-Related Quality-of-Life Outcomes
HRQoL domain scores are shown in Table 1. Examination of data preceding analysis revealed the presence of ceiling effects. Ceiling effects occur when an instrument has insufficient range to measure the full variability of the construct being measured at the upper end of the measurement range22 and are problematic because they can induce bias in the results of statistical procedures. Ceiling effects can be evident even when means are not excessively high, so long as a substantial fraction of the sample has the maximum possible value for the outcome, which is the case for our sample. The multi-level growth model analysis that we planned is not robust to ceiling effects. Therefore, as a sensitivity check, we analyzed our data using a multi-level censored normal model, which is robust to ceiling effects, if certain assumptions are met, using the software package, MPlus version 6.23 The results of the censored normal model were not substantively different from the results obtained from the multi-level model, the results of which are presented in Table 2, indicating that our results are not unduly influenced by censoring. Exposure to treatment was not associated with statistically significant change in HRQoL for any of the domains across the course of treatment.
Table 1.
HRQoL Domain Range, Means, and Standard Deviations
| |
Randomization |
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Control |
Treatment |
Overall |
|||||||||
| N | Range | Mean | SD | N | Range | Mean | SD | N | Range | Mean | SD | |
| Total | ||||||||||||
| Baseline |
39 |
35.87–92.39 |
65.02 |
14.85 |
28 |
53.26–91.30 |
70.11 |
11.26 |
67 |
35.87–92.39 |
67.15 |
13.61 |
| 3 months |
31 |
33.70–92.39 |
68.27 |
15.88 |
22 |
46.74–97.83 |
74.28 |
13.71 |
53 |
33.70–97.83 |
70.76 |
15.17 |
| 6 months |
25 |
33.70–97.83 |
68.89 |
18.00 |
20 |
46.74–97.83 |
78.59 |
15.24 |
45 |
33.70–97.83 |
73.20 |
17.34 |
| 12 months |
18 |
23.91–98.91 |
72.80 |
20.80 |
18 |
59.78–94.57 |
79.66 |
12.24 |
36 |
23.91–98.91 |
76.23 |
17.17 |
| Physical | ||||||||||||
| Baseline |
39 |
18.75–96.88 |
64.89 |
21.54 |
28 |
31.25–100.00 |
69.05 |
16.55 |
67 |
18.75–100.00 |
66.63 |
19.58 |
| 3 months |
31 |
21.88–100.00 |
69.24 |
23.66 |
22 |
28.13–100.00 |
75.00 |
20.25 |
53 |
21.88–100.00 |
71.63 |
22.29 |
| 6 months |
25 |
18.75–96.88 |
68.00 |
23.04 |
20 |
31.25–100.00 |
74.22 |
21.14 |
45 |
18.75–100.00 |
70.76 |
22.19 |
| 12 months |
18 |
15.63–96.88 |
72.40 |
21.87 |
18 |
40.63–100.00 |
77.88 |
17.54 |
36 |
15.63–100.00 |
75.14 |
19.73 |
| Psychosocial | ||||||||||||
| Baseline |
39 |
36.67–91.67 |
65.09 |
15.23 |
28 |
53.33–95.00 |
70.55 |
13.16 |
67 |
36.67–95.00 |
67.37 |
14.55 |
| 3 months |
31 |
38.33–91.67 |
67.80 |
15.99 |
22 |
48.33–98.33 |
73.79 |
14.67 |
53 |
38.33–98.33 |
70.28 |
15.60 |
| 6 months |
25 |
41.67–100.00 |
69.37 |
17.90 |
20 |
50.00–96.67 |
80.92 |
13.74 |
45 |
41.67–100.00 |
74.50 |
17.03 |
| 12 months |
18 |
21.67–100.00 |
73.15 |
21.86 |
18 |
61.67–96.67 |
80.65 |
11.80 |
36 |
21.67–100.00 |
76.90 |
17.73 |
| Emotional | ||||||||||||
| Baseline |
39 |
25.00–100.00 |
62.56 |
18.31 |
28 |
35.00–100.00 |
70.36 |
14.01 |
67 |
25.00–100.00 |
65.82 |
16.98 |
| 3 months |
31 |
35.00–100.00 |
67.10 |
18.11 |
22 |
45.00–95.00 |
74.55 |
13.27 |
53 |
35.00–100.00 |
70.19 |
16.55 |
| 6 months |
25 |
30.00–100.00 |
68.20 |
21.35 |
20 |
20.00–100.00 |
79.75 |
17.28 |
45 |
20.00–100.00 |
73.33 |
20.28 |
| 12 months |
18 |
5.00–100.00 |
71.11 |
26.49 |
18 |
50.00–100.00 |
76.11 |
13.56 |
36 |
5.00–100.00 |
73.61 |
20.89 |
| Social | ||||||||||||
| Baseline |
39 |
15.00–100.00 |
66.28 |
21.85 |
28 |
45.00–100.00 |
72.32 |
18.28 |
67 |
15.00–100.00 |
68.81 |
20.51 |
| 3 months |
31 |
0.00–100.00 |
67.26 |
23.97 |
22 |
45.00–100.00 |
76.82 |
19.97 |
53 |
0.00–100.00 |
71.23 |
22.70 |
| 6 months |
25 |
0.00–100.00 |
68.00 |
28.10 |
20 |
35.00–100.00 |
84.00 |
18.25 |
45 |
0.00–100.00 |
75.11 |
25.28 |
| 12 months |
18 |
20.00–100.00 |
78.06 |
20.37 |
18 |
45.00–100.00 |
83.61 |
15.13 |
36 |
20.00–100.00 |
80.83 |
17.91 |
| School | ||||||||||||
| Baseline |
39 |
0.00–100.00 |
66.41 |
21.09 |
28 |
30.00–100.00 |
68.93 |
18.78 |
67 |
0.00–100.00 |
67.46 |
20.04 |
| 3 months |
31 |
30.00–100.00 |
69.03 |
21.19 |
21 |
30.00–100.00 |
69.29 |
20.08 |
52 |
30.00–100.00 |
69.13 |
20.55 |
| 6 months |
25 |
30.00–100.00 |
72.20 |
20.72 |
20 |
45.00–100.00 |
79.00 |
17.06 |
45 |
30.00–100.00 |
75.22 |
19.28 |
| 12 months | 18 | 0.00–100.00 | 70.28 | 26.65 | 18 | 45.00–100.00 | 82.22 | 16.38 | 36 | 0.00–100.00 | 76.25 | 22.63 |
SD, standard deviation; HRQoL, health-related quality of life.
Table 2.
Multi-Level Growth Model Results
| |
HRQoL domains |
|||||
|---|---|---|---|---|---|---|
| Parameter | Total | Physical | Psychosocial | Emotional | Social | School |
| Intercept |
76.003 (9.912)*** |
86.789 (13.120)*** |
67.034 (10.275)*** |
50.064 (11.891)*** |
88.327 (14.758)*** |
56.360 (11.940)*** |
| Family medicine site 1 |
−7.247 (4.810) |
−5.052 (6.308) |
−9.273 (4.989) |
0.745 (5.760) |
−13.09 (7.132) |
−13.48 (5.759)* |
| Family medicine site 2 |
−2.285 (6.187) |
−6.317 (8.092) |
−2.360 (6.416) |
−6.046 (7.400) |
−6.355 (9.151) |
−0.194 (7.406) |
| Caregiver grandparent |
1.063 (8.916) |
9.574 (11.620) |
−4.496 (9.256) |
−5.033 (10.659) |
3.538 (13.206) |
−12.70 (10.616) |
| Child age |
−0.024 (0.286) |
0.017 (0.376) |
0.010 (0.297) |
−0.050 (0.343) |
−0.003 (0.425) |
0.115 (0.342) |
| No high school |
−5.129 (5.322) |
−6.136 (6.947) |
−5.054 (5.520) |
−7.630 (6.367) |
−8.024 (7.866) |
−2.912 (6.359) |
| Associate degree |
3.187 (4.376) |
4.628 (5.805) |
1.413 (4.536) |
1.145 (5.251) |
2.970 (6.533) |
−1.153 (5.273) |
| Bachelor degree or more |
3.143 (5.643) |
13.771 (7.319) |
−2.918 (5.851) |
−3.645 (6.733) |
−1.664 (8.280) |
−4.164 (6.812) |
| Caregiver BMI |
0.145 (0.238) |
0.417 (0.317) |
−0.016 (0.247) |
−0.184 (0.286) |
0.236 (0.356) |
−0.140 (0.288) |
| Caregiver black |
−12.48 (7.349) |
−19.62 (9.347)* |
−7.790 (7.636) |
13.202 (8.739) |
−17.60 (10.698) |
−18.27 (8.640)* |
| Caregiver Hispanic |
10.759 (9.003) |
3.336 (11.758) |
18.087 (9.351) |
14.171 (10.775) |
21.424 (13.384) |
16.377 (10.687) |
| Number in household |
0.320 (1.059) |
−0.306 (1.375) |
0.673 (1.099) |
2.982 (1.266)* |
−1.740 (1.562) |
1.876 (1.259) |
| TennCare |
−6.589 (5.375) |
−12.18 (7.079) |
−2.693 (5.573) |
−6.674 (6.439) |
−2.593 (7.990) |
1.589 (6.455) |
| Private insurance |
−2.429 (4.626) |
−8.227 (6.104) |
1.029 (4.799) |
−8.991 (5.545) |
−0.571 (6.900) |
8.383 (5.540) |
| Child baseline zBMI |
−2.706 (3.170) |
−3.600 (4.168) |
−1.046 (3.287) |
4.540 (3.798) |
−6.654 (4.707) |
0.739 (3.803) |
| Child male |
4.874 (8.650) |
−0.016 (10.987) |
8.368 (8.995) |
9.509 (10.295) |
13.388 (12.629) |
2.015 (10.125) |
| Time |
0.664 (0.308)* |
0.554 (0.451) |
0.731 (0.311)* |
0.326 (0.370) |
1.185 (0.382)** |
0.664 (0.461) |
| Treatment |
4.284 (3.851) |
0.917 (5.523) |
5.965 (3.970) |
7.428 (4.700) |
8.982 (5.903) |
2.101 (4.931) |
| Time*treatment | 0.178 (0.439) | 0.280 (0.647) | 0.099 (0.442) | 0.020 (0.527) | –0.138 (0.544) | 0.394 (0.660) |
Time refers to monthly change in health-related quality of life (HRQoL) for control group. Treatment refers to difference in HRQoL for treatment versus control group at baseline. Time*treatment refers to difference in rate of change for treatment group versus control group. The time*treatment parameter is the estimated treatment effect.
p<0.001; **p<0.01; *p<0.05.
zBMI, standardized BMI.
Discussion
The current study examined HRQoL outcomes as part of a weight management program targeting overweight and obese children in the primary care setting. In contrast to our hypothesis, parent-reported HRQoL was not significantly higher in the intervention, compared to the control, group postintervention. Whereas only the intervention group demonstrated an increase in mean total HRQoL (+8.48) greater than the MCID of 4.509 from baseline to 6 months, both the intervention and control groups exceeded this cutoff from baseline to 12 months (+9.55 and +7.78, respectively). These latter comparisons must be interpreted with consideration of a decreasing n over time points. Several explanations and directions for future research are outlined below.
There are two potential methodological causes for our finding of no treatment-associated changes in HRQoL. Either the population effect size is zero, implying that there is no treatment effect to find, or our analysis has resulted in a type II error, in which our study was not sufficiently powered to detect the treatment effect as a result of its small magnitude. To produce a lower-bound estimate of the effect size, which may inform future research efforts, we performed a post-hoc power analysis. Rather than using the observed effect size to compute the achieved power, a less-attractive approach resulting from the imprecision of the estimated effect size, we used only our sample size, missing data pattern, and information regarding the variances at the within- and between-subject levels to compute the minimum detectable effect size at 80% power. Using a custom R script, we determined that the minimum detectable effect size for this study was approximately 1.5 units per month. In other words, for us to have had a reasonable chance at rejecting the null hypothesis, exposure to treatment would have needed to cause at least a 1.5-point increase in HRQoL scores per month relative to change observed in the control group. Our failure to reject the null hypothesis suggests that the true effect size is likely smaller than this. In fact, the largest estimated effect size (for School HRQoL) from our analysis was approximately 0.4 points per month, less than one third as large as it would have needed to be to have been reliably detectable. Clearly, future studies of this type will require larger sample sizes or better covariance selection to increase statistical power. In addition to methodological causes, other explanations may be used to explain our findings.
Reviews of the literature identified several pediatric obesity intervention studies assessing changes in HRQoL and demonstrating significant change.3,4 The treatment dose in these interventions ranged from a single surgical procedure to 10–12 behavioral treatment sessions. Based on a recent review of weight management interventions in children,11 larger treatment doses have been found to be associated with greater reductions in weight, although this is unclear as related to change in HRQoL. The current study provided a relatively low treatment dose (approximately 7.5 hours of face-to-face treatment) of intervention potentially contributing to nonsignificant changes in HRQoL; however, our study did find statistically significant reductions in zBMI (β=−0.018; 95% confidence interval, −0.032, −0.003; p=0.019) across the course of treatment by multi-level growth modeling. Another low-dose study conducted in the primary care setting in Australia24 found no significant changes in sustained improvement in BMI or child HRQoL at 9 or 15 months following a secondary prevention approach to child overweight consisting of four brief individual visits with a provider over the course of 12 weeks. Whereas our study builds on the small literature documenting the effectiveness of brief interventions (<10 hours) for significant weight reduction11 as well as those conducted in primary care, demonstrating significant change in HRQoL should remain an important priority and larger treatment doses may be necessary. Further, the magnitude of weight loss and treatment factors (e.g., intervention design, treatment components, and targeting parents vs. parents and children) may be important considerations in HRQoL outcomes research. It may be that a certain amount of weight change is needed to observe an effect on HRQoL. However, review articles to date have failed to document this amount more globally.3,4 Additionally, the NIH We Can! program was initially developed for families of children ages 8–13 years, whereas our sample included families with younger children. Though the group format offered opportunities for problem solving among parents, it is unclear whether additional modifications would contribute to a greater impact among families with younger children. Future intervention development may also benefit from identifying treatment components that more specifically target HRQoL, as opposed to weight, with the assumption that weight loss should contribute to improved HRQoL.
The current study utilized a parent-proxy measure as a result of the research design and aim of the larger intervention study. Other studies have also utilized this measure as part of evaluating child weight interventions.8,25 However, previous literature examining the agreement between child self- and parent-proxy report in nonclinical populations has documented a tendency for parents to report higher child HRQoL than their children.26 On the other hand, parents of children with health conditions, including obesity, have been found to report lower child HRQoL than their children.27 Though cross-informant variance is out of the scope of this article, it is important to note that there are potential factors influencing parental estimation of child HRQoL, such as parental distress and whether the parent is estimating a more- versus less-observable domain (e.g., physical and emotional, respectively).28 Additionally, parental concern about a child's weight may be another factor that could influence parental perception of HRQoL. Based on concerns about ceiling effects and high scores in our current sample, it may be that parents overestimated HRQoL at each time point, although this remains unknown. Future studies may benefit from inclusion of both parent and child measures. Additionally, future research may consider use of other measures assessing related constructs (e.g., daily functioning, mood, weight teasing, and so on).
In an effort to decrease ceiling effects and yield a more-demonstrative change in HRQoL, Varni and colleagues16 have suggested utilization of a more-sensitive, condition-specific instrument. A recent meta-analysis of 34 randomized, controlled trials of weight-loss programs in adults found that HRQoL did not consistently improve in one or more domains.29 However, this review noted that more-positive treatment effects were found in those studies utilizing a condition-specific versus generic measure. It was suggested that condition-specific measures may be more responsive to change in HRQoL as well as less prone to ceiling and floor effects, but that generic measures may have more utility, such as comparisons across interventions and populations. Use of both generic and condition-specific measures may be important for future research in this area.
Finally, research suggests that degree of overweight is inversely associated with HRQoL, such that more-severely overweight children experience poorer HRQoL than less-overweight children.21,28 The majority of children in the current study were severely obese (≥97th percentile). The amount of change in HRQoL that could realistically be achieved in children who are severely obese is unclear. A recent intervention study with 8- to 12-year-old children ≥97th percentile demonstrated significant improvements in HRQoL at 6, but not 12, months postintervention.30 However, this study included 20 group meetings and six booster sessions as well as having utilized a different measure of HRQoL (i.e., the general health perceptions and global health scores of the Child Health Questionnaire, Parent Version), making comparisons to the current study difficult.
The primary strengths of the study included an evaluation of a brief intervention delivered by providers in the primary care setting. Additionally, the inclusion of a lower SES sample (i.e., majority enrolled in public health insurance) and utilization of a nationally recommended program (i.e., NIH We Can!) may also be considered strengths. Some limitations included a smaller sample, missing data across time points, and lack of variation in race and ethnicity, which may limit the generalizability of the findings.
Conclusions
The lack of significant change in HRQoL in the current study highlights the need for additional research in this area, especially in the primary care setting. In addition to larger samples and increased covariant selection, researchers should carefully consider the treatment dose of the intervention when examining HRQoL outcomes. Further, researchers should weigh the pros and cons of utilizing child- versus parent-reported HRQoL as well as generic versus condition-specific measures and consider degree of overweight when interpreting findings. Changes in HRQoL should remain a top priority in pediatric obesity intervention, but researchers should be thoughtful and careful when designing future studies with the aforementioned issues in mind.
Acknowledgment
This project was funded by the NIH (1R15HD054950-01A2).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Ogden CL, Carroll MD, Kit BK, et al. . Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. JAMA 2012;13:1337–1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lutfiyya MN, Lipsky MS, Wisdom-Behounek J, et al. . Is rural residency a risk factor for overweight and obesity for US children? Obesity 2007;15:2348–2356 [DOI] [PubMed] [Google Scholar]
- 3.Griffiths LJ, Parsons TJ, Hill AJ.Self-esteem and quality of life in obese children and adolescents: A systematic review. Int J Pediatr Obes 2010;5:282–304 [DOI] [PubMed] [Google Scholar]
- 4.Tsiros MD, Olds T, Buckley JD, et al. . Health-related quality of life in obese children and adolescents. Int J Obes (Lond) 2009;33:387–400 [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention Health-Related Quality of Life (HRQoL). 2013. Available at www.cdc.gov/hrqol/concept.htm Last accessed August7, 2013
- 6.US Department of Health and Human Services National Institutes of Health. Strategic plan for NIH obesity research. A report of the NIH Obesity Research Task Force (NIH publication no. 11-5493). 2011. Available at http://obesityresearch.nih.gov/about/StrategicPlanforNIH_Obesity_Research_Full-Report_2011.pdf Last accessed August7, 2013
- 7.Yackobovitch-Gavan M, Naglberg N, Phillip M, et al. . The influence of diet and/or exercise and parental compliance on health-related quality of life in obese children. Nutr Res 2009;29:397–404 [DOI] [PubMed] [Google Scholar]
- 8.Steele RG, Aylward BS, Jensen CD, et al. . Comparison of a family-based group intervention for youths with obesity to a brief individual family intervention: A practical clinical trial of Positively Fit. J Pediatr Psychol 2012;37:53–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Varni JW, Burwinkle TM, Seid M, et al. . The PedsQL 4.0 as a pediatric population health measure: Feasibility, reliability, and validity. Ambul Pediatr 2003;3:329–341 [DOI] [PubMed] [Google Scholar]
- 10.Holt N, Schetzina KE, Dalton WT, et al. . Primary care practice addressing child overweight and obesity: A survey of primary care physicians at four clinics in southern Appalachia. South Med J 2011;104:14–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whitlock EP, O'Connor EA, Williams SB, et al. . Effectiveness of weight management interventions in children: A targeted systematic review for the USPSTF. Pediatrics 2010;12:e396–e418 [DOI] [PubMed] [Google Scholar]
- 12.Barlow SE; Expert Committee Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: Summary report. Pediatrics 2007;120: S164–S192 [DOI] [PubMed] [Google Scholar]
- 13.Spear BA, Barlow SE, Ervin C, et al. . Recommendations for treatment of child and adolescent overweight and obesity. Pediatrics 2007;120:S254–S288 [DOI] [PubMed] [Google Scholar]
- 14.Dalton WT, III, Schetzina KE, Holt N, et al. . Parent-Led Activity and Nutrition (PLAN) for healthy living: Design and methods. Contemp Clin Trials 2007;32:882–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.US Department of Health and Human Services National Institutes of Health. National Heart, Lung, and Blood Institute. We Can! Ways to Enhance Children's Activity & Nutrition: Tools and resources: Curricula and toolkits. 2013. Available at www.nhlbi.nih.gov/health/public/heart/obesity/wecan/Last accessed August7, 2013
- 16.Varni JW, Seid M, Kurtin PS, et al. . PedsQL 4.0: Reliability and validity of the Pediatric Quality of Life Inventory Version 4.0 Generic Core Scales in healthy and patient populations. Med Care 2001;39:800–812 [DOI] [PubMed] [Google Scholar]
- 17.Varni JW, Limbers CA, Burwinkle TM, et al. . Parent proxy-report of their children's health-related quality of life: An analysis of 13, 878 parents' reliability and validity across age subgroups using the PedsQL 4.0 Generic Core Scales. Health Qual Life Outcomes 2007;5:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuczmarski RJ, Ogden CL, Guo SS, et al. . 2000 CDC growth charts for the United States: Methods and development. Vital Health Stat 2002;11:1–203 [PubMed] [Google Scholar]
- 19.Raudenbush SW, Bryk AS. Hierarchical Linear Models: Applications and Data Analysis Methods (2nd ed.). Sage: Newbury Park, CA, 2002 [Google Scholar]
- 20.Allison PD. Fixed Effects Regression Models. Sage: Thousand Oaks, CA, 2009 [Google Scholar]
- 21.Varni JW, Limbers CA, Burwinkle TM. Impaired health-related quality of life in children and adolescents with chronic conditions: A comparative analysis of 10 disease clusters and 33 disease categories/severities utilizing the PedsQL 4.0 Generic Core Scales. Health Qual Life Outcomes 2007;5:1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Long S. Regression Models for Categorical and Limited Dependent Variables. Sage: Thousand Oaks, CA, 2007 [Google Scholar]
- 23.MPlus (Version 6) [computer software]. Muthén & Muthén: Los Angeles, CA [Google Scholar]
- 24.McCallum Z, Wake M, Gerner B, et al. . Outcome data from the LEAP (Live, Eat and Play) trial: A randomized controlled trial of a primary care intervention for childhood overweight/mild obesity. Int J Obes (Lond) 2007;31:630–636 [DOI] [PubMed] [Google Scholar]
- 25.Hughes AR, Stewart L, Chapple J, et al. . Randomized, controlled trial of a best-practice individualized behavioral program for treatment of childhood overweight: Scottish Childhood Overweight Treatment Trial (SCOTT). Pediatrics 2008;121:e539–e546 [DOI] [PubMed] [Google Scholar]
- 26.Upton P, Lawford J, Eiser C. Parent-child agreement across child health-related quality of life instruments: A review of the literature. Qual Life Res 2008;17:895–913 [DOI] [PubMed] [Google Scholar]
- 27.Hughes AR, Farewell K, Harris D, et al. . Quality of life in a clinical sample of obese children. Int J Obes (Lond) 2007;31:39–44 [DOI] [PubMed] [Google Scholar]
- 28.Zeller MH, Modi AC. Predictors of health-related quality of life in obese youth. Obesity 2006;14:122–130 [DOI] [PubMed] [Google Scholar]
- 29.Maciejewski ML, Patrick ML, Patrick DL. A structured review of randomized controlled trials of weight loss showed little improvement in health-related quality of life. J Clin Epidemiol 2005;58:568–578 [DOI] [PubMed] [Google Scholar]
- 30.Kalarchian MA, Levine MD, Arslanian SA, et al. . Family-based treatment of severe pediatric obesity: Randomized, controlled trial. Pediatrics 2009;24:1060–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]


