Abstract
Hypothermia is neuroprotective against many acute neurological insults, including ischemic stroke. We and others have previously shown that protection by hypothermia is partially associated with an anti-inflammatory effect. Phagocytes are thought to play an important role in the clearance of necrotic debris, paving the way for endogenous repair mechanisms to commence, but the effect of cooling and phagocytosis has not been extensively studied. Triggering receptor expressed on myeloid cells-2 (TREM2) is a newly identified surface receptor shown to be involved in phagocytosis. In this study, we examined the effect of therapeutic hypothermia on TREM2 expression. Mice underwent permanent middle cerebral artery occlusion (MCAO) and were treated with one of the two cooling paradigms: one where cooling (30°C) began at the onset of MCAO (early hypothermia [eHT]) and another where cooling began 1 hour later (delayed hypothermia [dHT]). In both groups, cooling was maintained for 2 hours. A third group was maintained at normothermia (NT) as a control (37°C). Mice from the NT and dHT groups had similar ischemic lesion sizes and neurological performance, but the eHT group showed marked protection as evidenced by a smaller lesion size and less neurological deficits up to 30 days after the insult. Microglia and macrophages increased after MCAO as early as 3 days, peaked at 7 days, and decreased by 14 days. Both hypothermia paradigms were associated with decreased numbers of microglia and macrophages at 3 and 7 days, with greater decreases in the early paradigm. However, the proportion of the TREM2-positive microglia/macrophages was actually increased among the eHT group at day 7. eHT showed a long-term neurological benefit, but neuroprotection did not correlate to immune suppression. However, hypothermic neuroprotection was associated with a relative increase in TREM2 expression, and suggests that TREM2 may serve a beneficial role in brain ischemia.
Introduction
Hypothermia is recognized as one of the most potent neuroprotectants studied in the laboratory and clinical settings (Busto et al., 1989; Feuerstein et al., 1997). Recent clinical studies have established a role for therapeutic cooling in neuroprotection in some clinical conditions, including anoxic brain injury due to cardiac arrest and hypoxic ischemic neonatal encephalopathy (Yenari and Han, 2012). In contrast to many other neuroprotective treatment strategies, hypothermia influences multiple aspects of brain pathophysiology in the acute, subacute, and even chronic stages of ischemia. It affects pathways leading to excitotoxicity, apoptosis, inflammation, and free radical production, as well as blood flow, metabolism, and blood–brain barrier integrity (Yenari and Han, 2012). Thus, hypothermia could be viewed as a model of neuroprotection, whereby potential therapeutic targets may be identified. Postischemic phagocytosis is thought to be involved in the clearance of necrotic debris, paving the way for endogenous repair mechanisms to commence. The triggering receptor expressed by myeloid cells-2 (TREM2) is a newly indentified molecule described on activated microglia and macrophage (Daws et al., 2001; Sessa et al., 2004; Takahashi et al., 2005), involved in innate immunity and is considered to play an important role in the phagocytosis of microbes (Daws et al., 2001). Its ligand has also been detected in the brain (Daws et al., 2003), and has been postulated to mediate phagocytosis of injured or apoptotic neurons (Daws et al., 2003; Takahashi et al., 2005; Hsieh et al., 2009; Stefano et al., 2009). In this study, we compared paradigms where hypothermia was protective and where hypothermia was not protected in a mouse stroke model to characterize the inflammatory response and TREM2 expression.
Materials and Methods
All animal experiments were approved by the San Francisco VA Medical Center's Institutional Animal Care and Use Committee (IACUC), and were in accordance with NIH guidelines.
Stroke model
A focal cerebral infarct was induced by permanent occlusion of the left middle cerebral artery (MCA) as described previously with slight modifications (Kawabori et al., 2012a, 2012b). All surgical techniques were performed under aseptic conditions. Male C57BL/6 mice (Simonson Labs), weighing 20–25 g, and 8–12 weeks old were used. Mice were allowed free access to food and water and housed in a climate-controlled environment (25°C). Anesthesia was induced by inhalation of 4.0% isoflurane in N2/O2 (80:20%); a surgical procedure was performed under spontaneous ventilation in 1.5–2.0% isoflurane in N2/O2 (80:20%).
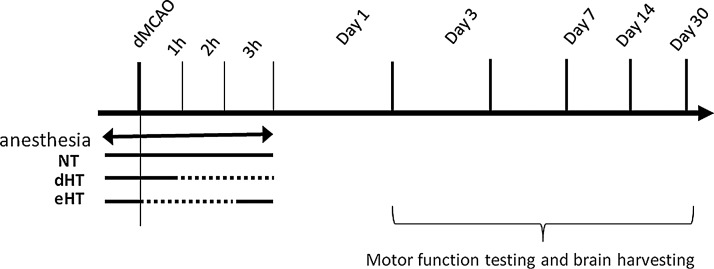
The animals were placed on the heating pad and a 1-cm skin incision was created between the left margin of the orbit and the tragus, and the temporalis muscle was incised. A small craniotomy was made above the proximal segment of MCA, and the MCA was exposed after the dura was opened and retracted. The MCA was occluded by short coagulation with a bipolar at the MCA segment just proximal to the olfactory branch, which was consistently present. The rectal temperature was maintained between 36.5°C and 37.5°C during the procedures, by using a thermometer connected to a heating pad (Harvard Apparatus). A total of 75 mice were subjected to distal MCA occlusion (dMCAO). Mice were randomized into three groups: early hypothermia (eHT), delayed hypothermia (dHT), and normothermia (NT). In the eHT group, cooling began at the time of dMCAO and maintained for 2 hours, with a rectal temperature of 29.5–30.5°C, followed by rewarming. In the dHT group, cooling began 1 hour after dMCAO and continued for 2 hours, with a rectal temperature of 29.5–30.5°C. In the NT group, the rectal temperature was maintained in the normal range (36.5–37.5°C) throughout the experiment. We previously showed that rectal temperatures of 37°C and 30°C correspond to brain temperatures of 38°C and 33°C, respectively (Yenari et al., 2000). The duration of anesthesia was maintained the same for all three groups (Fig. 1). Animals with no observable deficits at the time of recovery from anesthesia were removed from the experiment. The animals were sacrificed at 1, 3, 7, 14, and 30 days after ischemia for the subsequent studies (n=5/group).
FIG. 1.
Schematic of the experimental paradigms. Mice were randomly allocated into three groups. In the normothermia (NT) group, body temperature was maintained between 36.5°C and 37.5°C during the course of the experiment. In the delayed hypothermia (dHT) group, hypothermia (29.5–30.5°C) was induced 1 hour after the ischemic insult (distal middle cerebral artery occlusion [dMCAO]) and maintained for 2 hours. In the early hypothermia (eHT) group, cooling began at the time of dMCAO and continued for 2 hours followed by rewarming to the normal temperature. The duration of the anesthesia was maintained the same for all three groups. Dotted lines show the period of cooling.
Behavior studies
Focal neurological deficits were evaluated at the above-mentioned time points; 1, 3, 7, 14, and 30 days after dMCAO using a neurological score based on that developed by Bederson et al., and modified for use in mice as previously described (Tang et al., 2008; Tang et al., 2011; Zheng et al., 2008): grade 0=no observable neurological deficits, grade 1=fails to extend right forepaw, grade 2=circles to the right, grade 3=falls to the right, and grade 4=cannot walk spontaneously.
In addition, the elevated body swing test was conducted to evaluate asymmetrical motor behavior at each time point as previously described (Borlongan et al., 1995). Briefly, animals were held by the tail and elevated ∼10 cm above the bench top. The direction of the body swing, defined as an upper body turn of >10° to either side, was recorded for each 30 trials. The numbers of left and right turns were counted, and the percentage of turns made to the side contralateral to the ischemic hemisphere (right side bias) was determined.
Histological examination
After animals were perfused with ice-cold phosphate-buffered saline (PBS), the brain was harvested. The brains were then sunk in 20% sucrose for overnight, frozen at −80°C, and stored until use. Fresh frozen sections were cryosectioned in the coronal plane (25 μm thick).
Infarct size
A series of 25-μm-thick coronal sections from six brain regions (between 1.5 mm anterior and 1.5 mm posterior to the bregma) were collected at 500 μm intervals throughout the ischemic lesion and stained with cresyl violet staining. The infarct areas in each section were measured using an image analysis system (ImageJ), and calculated by subtracting the normal ipsilateral area from that of the contralateral hemisphere to reduce errors due to cerebral swelling as described previously (Kawabori et al., 2012a, 2012b).
Histochemistry and fluorescent microscopy
Microglia were identified using lectin histochemistry. Brain sections were treated for endogenous peroxidases with 1.0% hydrogen peroxidase. Sections were then incubated with peroxidase-labeled Griffonia simplicifolia isolectin-B4 (IB4) (10 μg/mL, catalogue no. L5391; Sigma-Aldrich) overnight at 4°C, followed by diaminobenzidine (Vector Laboratories), and then counterstained with hematoxylin. Positive cells were countered in five random nonoverlapping×400 high-power fields within the cortex adjacent to the outer boundary of the infarct as delineated by hematoxylin as previously described (Tang et al., 2007, 2008, 2011). The section 1-mm anterior to the bregma was used to quantify cell counts. Counting was carried out by an investigator blinded to the experimental conditions. Immunopositive cells were counted from five random nonoverlapping ×400 high-power fields within the cortex adjacent to the outer boundary of the infarct as delineated by hematoxylin and eosin staining in four mice for each group.
Adjacent sections were stained for CD68 to identify phagocytes. The CD68 rat anti-mouse monoclonal antibody (1:200, catalogue no. ab53444; Abcam) was used as a primary antibody, followed by biotin-conjugated goat anti-rat (1:1000, catalogue no. sc-2041; Santa Cruz). Elite Vectastain ABC kit (Vector Laboratories) and diaminobenzidine was done to visualize the proteins and hematoxylin staining was undergone as a counterstain. To visualize TREM2, sections were incubated with biotinylated rat monoclonal anti-mouse TREM2 (20 μg/mL, catalogue no. BAF1729; R&D Systems) and the antigen detected using an Elite Vecstain ABC kit, followed by diaminobenzidine, and then counterstained with hematoxylin. To elucidate the proportion of TREM2-positive phagocytic cells, tissues were double labeled for CD68 and TREM2. Brain sections were blocked with normal goat serum and a Streptavidin blocking solution (Vector Laboratories). The section was incubated in antibodies against TREM2 (20 μg/mL, catalogue no. BAF1729; R&D Systems) overnight, followed by Streptavidin Alexa 594 (1:1000, catalogue no. S-11227; Molecular Probe). After the sections were rinsed twice for 5 minutes with PBS, the sections were then incubated with rat monoclonal anti-mouse CD68 (1:1000, catalogue no. ab53444; Abcam) at room temperature for 1 hour, followed by donkey anti-rat Alexa Fluor 488 at room temperature for 1 hour. The sections were mounted on glass slides using the Vector-Shield mounting medium. Slides analysis was conducted by microscopy (Zeiss Aviovert 40 CFL) and the numbers of immunopositive cells were counted as described above.
Since the 60 kD heat shock protein (HSP60) has been implicated as potential ligand of TREM2 (Stefano et al., 2009), brains were stained for this protein. HSP60 was previously described on neurons and astrocytes; thus, brains were double labeled for HSP60, the proposed ligand for TREM2, plus MAP-2 to identify neurons and GFAP to identify astrocytes. Brain sections were reacted with mouse antibodies against HSP60 (1:50, catalogue no. SPA-807; Stressgen), and rabbit polyclonal anti MAP2 (1:100, catalogue no. NG1723932; Millipore) or rabbit polyclonal anti GFAP (1:1000, catalogue no. ab63366; Abcam) at room temperature for 1 hour. After the sections were rinsed twice for 5 minutes with PBS, Alexa 594-conjugated goat anti-mouse IgG (H+L) (1:200, catalogue no. 835723; Invitrogen) and Alexa 488-conjugated goat anti-rabbit IgG (H+L) (1:200, catalogue no. 792513; Invitrogen) were added and incubated at room temperature for 1 hour, and then mounted on glass slides using the Vector-Shield mounting medium. Slides analysis was conducted by microscopy (Zeiss Aviovert 40 CFL). Immunopositive cells were counted as described above.
Statistical analysis
All experiments were carried out in a random fashion, and ratings were carried out by investigators blinded to the experimental manipulations. All statistical analyses were performed using Sigma Stat 3.1 (Systat Software). Quantitative data were presented as mean±S.E. Multiple comparisons were performed using one-way ANOVA followed by the Bonferroni post hoc test. p<0.05 was considered statistically significant.
Results
eHT but not dHT ameliorated neurological deficits
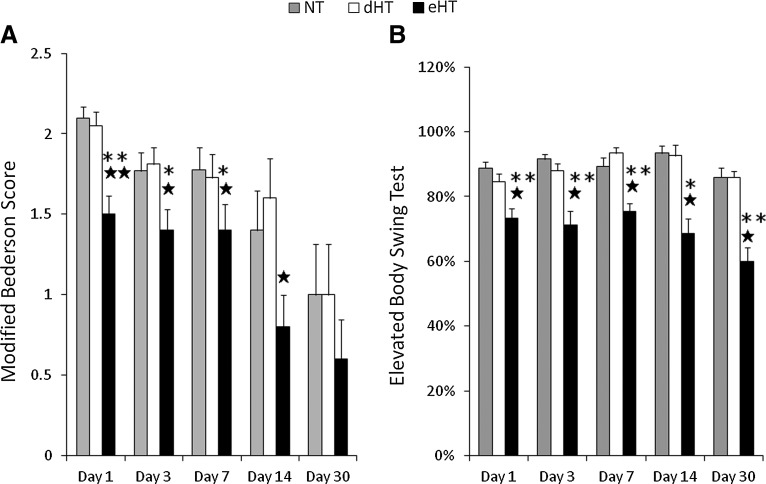
Neurological deficits were significantly reduced only in the eHT group in both the modified Bederson Score (Fig. 2A) and elevated body swing test (Fig. 2B) compared with the NT group and dHT hypothermia group. The eHT group showed better neurological recovery compared with the NT group between day 1 and 14 as estimated by the modified Bederson Score, and between day 1 and 30 using the elevated body swing test. eHT also showed better neurological recovery compare with dHT groups between day 1 and 7 using the modified Bederson Score and between day 1 and 30 on the elevated body swing test. However, the dHT group did not show any significant neurological recovery compared with other groups.
FIG. 2.
eHT showed significantly less neurological deficit compared with NT and dHT. (A) Animals exposed to early cooling (eHT) had improved, and thus, lower modified Bederson scores compared with animals exposed to delayed cooling (dHT) or maintained normothermia (NT). A lower score indicates less deficit. Scores range from 0 to 4 (see text). (B) Animals exposed to eHT showed less swing bias than dHT or NT groups on the elevated body swing test. No deficit would be expected to result in ∼50% swing bias. Thus, a higher % indicates more swing bias, and thus, more neurological deficits (★p<0.05 vs. NT, ★★p<0.01 vs. NT, *p<0.05 vs. dHT, **p<0.01 vs. dHT).
eHT but not dHT decreased infarct size
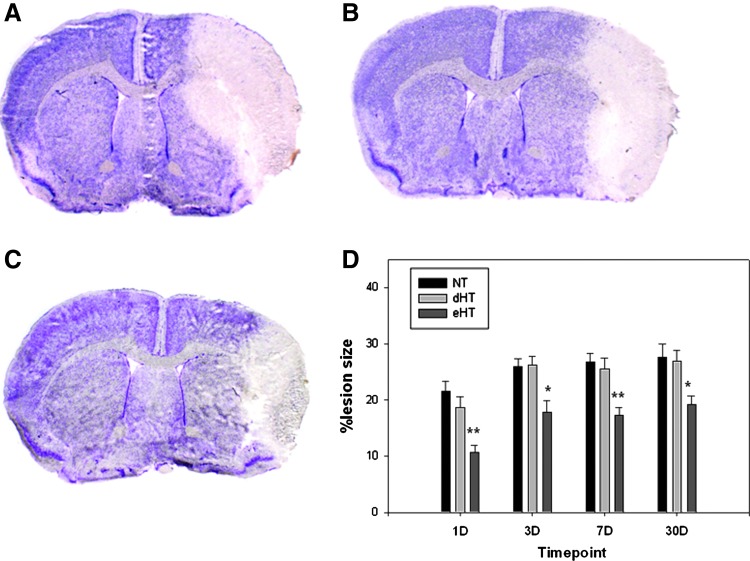
The eHT group showed reduced infarct size by about 30% (Fig. 3) compared with the NT and dHT groups. The infarct appears to expand in size between 1 and 30 days; however, the eHT group showed consistent reduction of the infarct compared with the other groups. Representative photos of cresyl violet-stained brain sections at day 7 are shown in Figure 3A–C. Notably, eHT protected peri-infarct regions.
FIG. 3.
Early, but not dHT is protective. Images of representative cresyl violet-stained brain sections from the (A) NT, (B) dHT, and (C) eHT groups are shown at 7 days post-MCAO. Only the eHT group showed significant reduction of the infarction by about 30% compared with NT and dHT (D) (★p<0.05 vs. NT and dHT; ★★p<0.01 vs. NT and dHT). Color images available online at www.liebertpub.com/ther
Hypothermia decreased microglial activation
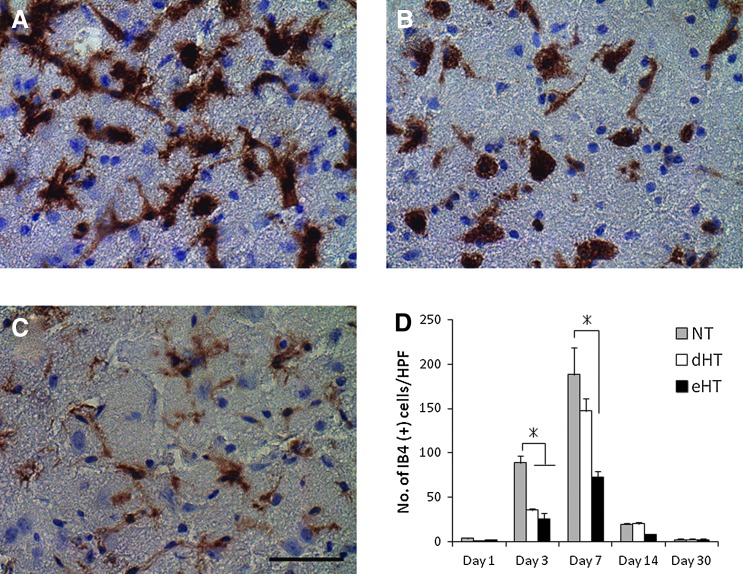
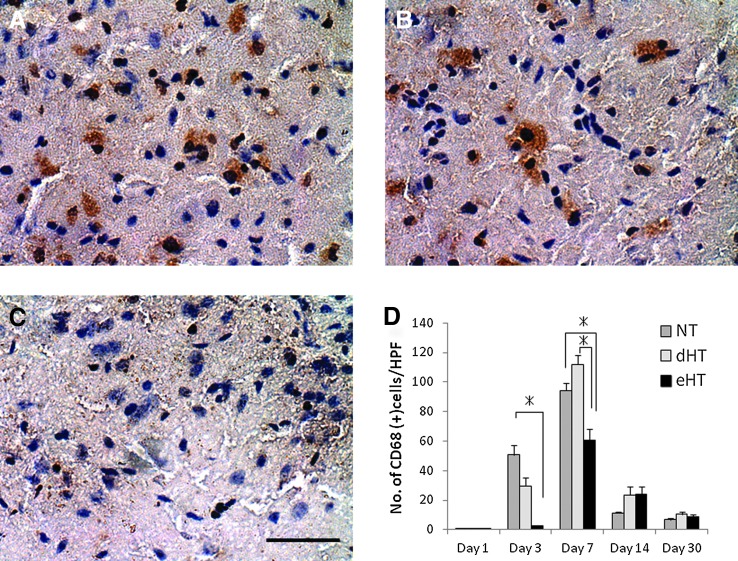
Hypothermia significantly decreased the numbers of activated microglia at day 3 and 7 (Fig. 4). Both the eHT and dHT groups showed significant reduction of the activated microglia at day 3 by about 30–40% (NT; 88.8±22.8 cells/hpf, dHT; 35.5±4.2 cells/hpf, eHT; 25.3±14.4 cells/hpf, p<0.01), while only the eHT group showed significant reduction of activated microglia at day 7 by 40% (NT; 188.3±91.5 cells/hpf, dHT; 147.3±33.6 cells/hpf, eHT; 72.9±13.3 cells/hpf, p<0.01) compared with the NT group. There was no difference of IB4-positive cell counts after day 14. Representative images of NT, dHT, and eHT brains and IB4 staining at day 7 can be seen in Figure 4A–C, respectively.
FIG. 4.
Hypothermia decreased numbers of microglia and macrophages post-MCAO. Representative micrographs of isolectin B4 (IB4) microglia/macrophages 7 days post-MCAO: (A) NT, (B) delayed normothermia, (C) eHT. Both the (B) dHT and (C) eHT showed significant reduction of the activated microglia at day 3 (D), while only the eHT group showed marked reduction of them at day 7 compared with the other groups (*p<0.01). Scale bar=40 μm. Color images available online at www.liebertpub.com/ther
eHT decreased phagocytic activation of myeloid cells
Similar to that observed in IB4-positive microglia, eHT significantly decreased the expression of CD68-positive phagocytes at day 3 and at day 7 by 4% (NT; 50.6±6.5 cells/hpf, dHT; 29.6±5.9 cells/hpf, eHT; 2.4±0.1 cells/hpf, p<0.01) and 64% (NT; 94.4±4.9 cells/hpf, dHT; 112.0±6.0 cells/hpf, eHT; 60.4±7.6 cells/hpf, p<0.01) compared with the NT group, respectively (Fig. 5). Representative images of CD68-stained brain sections from NT, dHT, and eHT at day 3 are shown in Figure 5A–C.
FIG. 5.
Early, but not dHT decreased the number of macrophages. Representative photographs of CD68-positive phagocytes at day 7 (A) NT, (B) delayed normothermia, (C) eHT. The eHT group showed marked reduction at day 3 and at day 7 compared with the other two groups (*p<0.01) (D). Scale bar=40 μm. Color images available online at www.liebertpub.com/ther
Proportionately higher TREM2 expression when hypothermia is protective
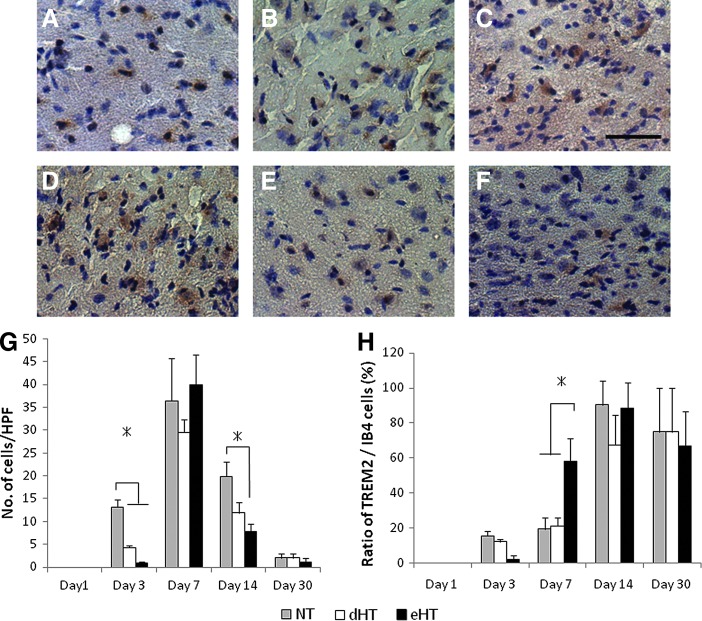
TREM2 is located on the surface of the microglia and macrophages and its activation has been shown to lead to phagocytosis. Recent work indicates that TREM2 may be involved in the phagocytosis of injured or apoptotic brain cells (Takahashi et al., 2005; Hsieh et al., 2009). We first characterized the temporal expression of TREM2 after MCAO, and the effect of cooling. TREM2 expression among the normothermic controls showed little to no staining at 1 day, but markedly increased by day 3, peaking at 7 days, with a few positive cells remaining by day 30 (Fig. 6A–D). Hypothermia, whether early or delayed decreased the overall numbers of TREM2-positive cells at 3 and 14 days, but little difference was noticed at 7 and 30 days. Since TREM2 is only seen on a fraction of myeloid cells, and the overall microglial/macrophage numbers were decreased by cooling, we then normalized TREM2 counts to those of total IB4 counts and found that the proportion of myeloid cells that expressed TREM2 were actually increased in the eHT group compared with the other groups at day 7, a time when IB4-positive cells were at a peak (Fig. 6E). However, this pattern did not persist at days 14 and 30, but interestingly, the proportion of TREM2-positive microglia reached over 80%.
FIG. 6.
Hypothermia decreased the overall triggering receptor expressed on myeloid cells-2 (TREM2) expression, but increased the proportion of TREM2-positive macrophages. Representative photographs of TREM2-positive cells at days 7 (A) NT, (B) delayed normothermia, (C) eHT, 14 (D) NT, (E) delayed normothermia, and (F) eHT. (G) TREM2 expression among the NT group showed little to no staining at day 1, but markedly increased by day 3, peaking at day 7, and with a few positive cells remaining by 30 days. Hypothermia, whether early or delayed, decreased the numbers of TREM2-positive cells at day 3 and 14, but little difference was noticed at day 7 and 30. (H) TREM2 counts normalized to total IB4 counts suggest that the proportion of TREM2 microglia/macrophages is higher in the eHT group compared with the other two groups at day 7 (*p<0.01). Scale bar=40 μm. Color images available online at www.liebertpub.com/ther
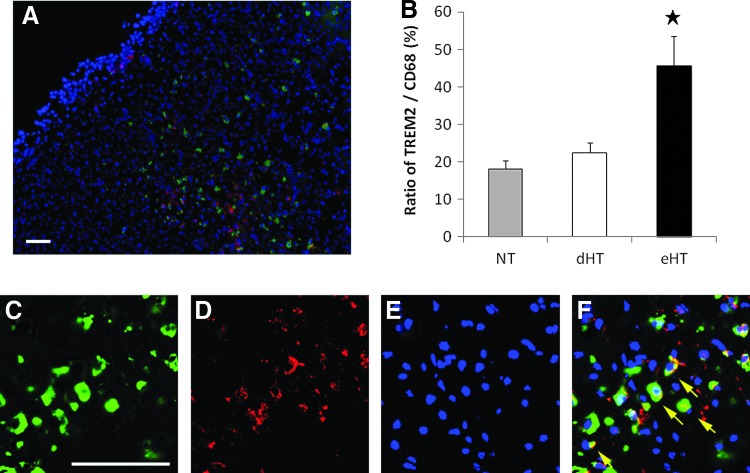
To validate these observations, we then double labeled for CD68 and TREM2 at day 7. The proportion of double-labeled cells to all CD68-positive phagocytes were increased for the eHT group, but not for dHT compared with NT (Fig. 7).
FIG. 7.
Early, but not dHT increased the proportion of TREM2-positive macrophages. Representative micrographs of double-labeled TREM2 and CD68-positive phagocytes. A brain from the eHT group at day 7 shows that TREM2 is expressed in CD68-positive cells at the infarct border (red; TREM2, green; CD68, blue; DAPI) (A). The proportion of the TREM2-positive CD68-positive phagocytes was significantly increased in the eHT group (B). High-power views of the staining is shown in (C–F). TREM2 (D) is highly colocalized with CD68-positive cells (C). DAPI (E). Merged image (F) (★p<0.01). Scale bar=80 μm. Color images available online at www.liebertpub.com/ther
Hypothermia failed to influence HSP60 expression
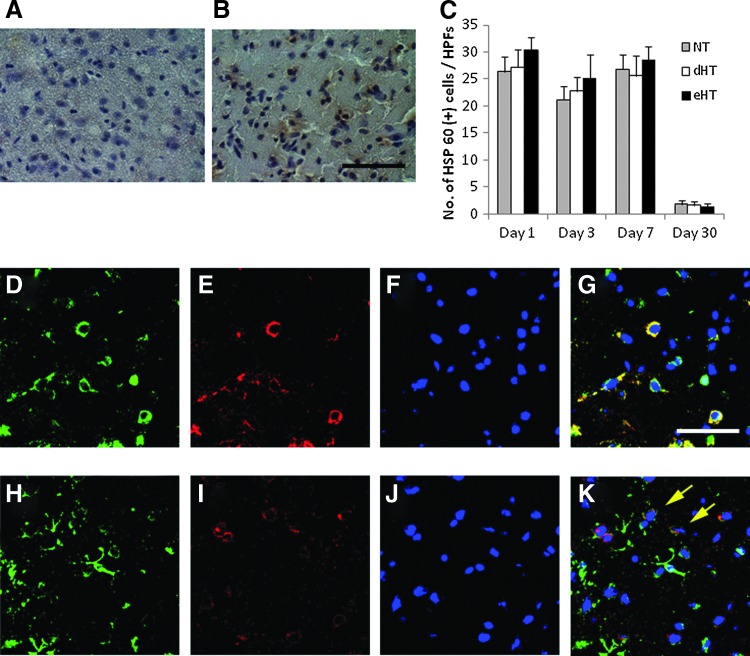
Whereas the precise ligand of TREM2 in the brain has defied investigators in the field for quite some time, one study implicated the HSP60 (Stefano et al., 2009). Thus, we carried out immunostains to determine the extent of HSP60 staining in our model. Consistent with the earlier report, HSP60 was present in both neurons and astrocytes (Fig. 8A–F). A number of HSP60-positive cells increased 1–7 days postischemia, but decreased by day 30. However, neither cooling paradigm affected the number of positive cells compared with NT (Fig. 8G).
FIG. 8.
Immunostaining for 60 kD heat shock protein (HSP60), the proposed ligand for TREM2 is unaffected by hypothermia. DAB staining for HSP60 revealed that ischemic insult upregulates its expression (A; sham, B; ischemic penumbra at day 7). However, neither cooling paradigm changed the numbers of HSP60 positive at any time point (C). Brains were double labeled for HSP60 (red, E, I) plus a neuron (MAP2, D) or astrocyte (GFAP, H) marker, and counterstained with DAPI (F, J). HSP60 was present in both neurons (G) and astrocytes (K). Stains from day 7 are shown. Color images available online at www.liebertpub.com/ther
Discussion
Studies of the inflammatory response to brain infarction contribute to the development of effective neuroprotective therapies. One approach to such investigations is to delineate mechanisms rendering the effects of the already established neuroprotective strategies, such as hypothermia. In this study, we first established a model of hypothermic neuroprotection (eHT) following permanent MCAO where the effects persisted out to 30 days. We then compared this to a hypothermic paradigm that was not protective (dHT). We found that cooling, whether protective or not, led to an attenuated inflammatory response as detected by microglial staining. However, by using a marker specific to macrophages and/or phagocytic microglia (CD68), we found decreased CD68-positive cells only when hypothermia was protective. We further showed that TREM2-positive cells are present as early as a day after MCAO, but peak at about 7 days. Both the hypothermic paradigms led to decreases in TREM2-positive cells at 3 and 14 days, but when these counts were normalized to the total number of microglia/macrophages, we found that protective hypothermia, but not nonprotective hypothermia or NT, actually increased the proportion of TREM2-positive inflammatory cells. Interestingly, while HSP60 has been suggested as an endogenous ligand for TREM2 (Stefano et al., 2009), we failed to see any changes in HSP60 expression following MCAO or hypothermia.
It is now well known that the inflammatory response contributes significantly to injury after ischemia (Yenari et al., 1998; Barone and Feuerstein, 1999). We and others have shown that intraischemic and postischemic hypothermia can be neuroprotective partially by inhibiting the acute inflammatory response (Toyoda et al., 1996; Maier et al., 1998; Han et al., 2002; Deng et al., 2003; Kim et al., 2011). Nevertheless, the mechanisms underlying this protective effect are still not fully elucidated.
Consistent reports from numerous laboratories have shown that cooling is remarkably neuroprotective when applied especially during ischemia (van der Worp et al., 2010; Yenari and Hemmen, 2010). However, any mechanistic observations reported as a result of cooling have been criticized because hypothermia is thought to affect multiple pathological processes that no one mechanism could explain the robust beneficial effect. In spite of its robust neuroprotective effect, there are still conditions where hypothermia fails to protect. In this study, we established that a relatively brief period of cooling delayed by an hour actually fails to protect in a model of permanent MCAO. Thus, we could use these paradigms to isolate specific factors that may lead to protection, rather than factors that may simply be epiphenomenon of cooling.
Whereas delayed cooling would have obvious clinical implications, the purpose of this study was not to test whether delayed cooling is protective or not. In fact, earlier studies from other laboratories have shown that delayed cooling, provided the cooling is maintained for durations of 24 hours or more, led to durable and robust protection (Colbourne et al., 2000; Yanamoto et al., 2001). Rather, we hoped to develop a platform from which potential pathomechanisms could be compared. In this study, we showed that while a nonspecific inflammatory response (microglial/macrophage activation) is likely an epiphenomenon of cooling itself, one neuroprotective benefit of cooling might be the relative upregulation of TREM2 on microglia/macrophages.
Microglia are the resident myeloid-derived cells in the central nervous system that provide constant surveillance of the brain and spinal cord (Thomas, 1992; Kreutzberg, 1996; El Khoury et al., 1998). Studies in brain ischemia models indicate that microglia participate in the progression of ischemic injury and are activated in response to brain ischemia and many other stressors in the brain as early as 6 hours after MCAO, and generate a variety of damaging substances (Deng et al., 2003). Although many studies have shown that microglia exacerbate ischemic injury (Giulian et al., 1993; Lehnardt et al., 2003; Chou et al., 2004; Yenari et al., 2006; Huang et al., 2010), there is also evidence that some aspects of the inflammatory response are important for tissue repair, including phagocytosis of cellular debris, remodeling of the extracellular matrix, and the release of cytokines and trophic factors (Watanabe et al., 2000; Kriz, 2006; Zhao et al., 2006). The challenge will be to find ways to selectively suppress the deleterious effects of microglial activation after a stroke without compromising neurovascular repair and remodeling.
Loss-of-function mutations, in either TREM2, cause Nasu-Hakola disease, a rare and fatal neurodegenerative disease also known as polycystic lipomembranous osteodysplasia with sclerosing leukoencephalopathy (Satoh et al., 2011). Further, recent studies now implicate TREM2 variants as a risk factor for Alzheimer's disease (Guerreiro et al., 2013; Jonsson et al., 2013). The mechanisms of neurodegeneration in these disorders are still unknown, but one hypothesis is that lack of either TREM2 impairs the clearance of apoptotic neurons or beta-amyloid deposition by microglia, leading to the accumulation of necrotic debris and toxic build up of Alzheimer's related plaques. Previous work identified microglia as the brain cells expressing the highest levels of TREM2 (Schmid et al., 2002; Sessa et al., 2004), and it has been experimentally shown that TREM2 mediates microglial phagocytosis of apoptotic neurons (Neumann and Takahashi, 2007; Hsieh et al., 2009; Neumann et al., 2009). In vitro, TREM2 was shown to promote phagocytosis of apoptotic neurons without upregulation of antigen presentation molecules, tumor necrosis factor-α transcripts, or the release of reactive oxygen species (Takahashi et al., 2005). Conversely, loss of TREM2 impairs phagocytosis and promotes inflammation. In an in vivo model of experimental autoimmune encephalomyelitis, blockade of TREM2 using a monoclonal antibody led to the exacerbation of immune responses with increased demyelination and worsened neurological function (Piccio et al., 2007). Very little has been studied about TREM2 in acute neurological insults. However, Sieber et al. (2013) recently documented the temporal profile of TREM2 mRNA expression in a stroke model. They found that TREM2 mRNA peaked 7 days after MCAO and persisted at 28 days, observations in line with our immunohistochemical data presented here. They also documented decreased in mRNA levels in several proinflammatory cytokines and decreased microglial activation in TREM2 knockout mice. However, they failed to see any effect on the ischemic lesion size. Since behavioral testing was not carried out, it remains to be seen whether TREM2 plays any role in ultimate neurological outcome. Existing data would predict that TREM2 augmentation might facilitate brain repair, and Takahashi et al. (2007) showed that intravenous application of TREM2-rich stem cells facilitate brain repair in experimental autoimmune encephalomyelitis.
In this report, we found that TREM2 is upregulated on myeloid cells of the ischemic brain at about day 7 and persists. Previous work identified the microglia as the major brain cell expressing the highest levels of TREM2, with up to 57% of cortical microglia expressing TREM2 (Schmid et al., 2002). We should note that we do not attempt to identify the precise myeloid cell on which TREM2 was observed. We used two different commonly used markers for all myeloid cells (IB4) and phagocytes (CD68). Since circulating monocytes/macrophages are known to enter the ischemic brain, our tools do not allow us to differentiate these cells from resident brain microglia. However, we do not feel that this limitation detracts from the novel observations regarding TREM2 in brain ischemia and therapeutic hypothermia.
A limitation in TREM biology is the difficulty in identifying its ligand, particularly its endogenous ligand in the brain (Klesney-Tait et al., 2006; Turnbull et al., 2006; Hsieh et al., 2009; Stefano et al., 2009). Initial work using a fusion protein consisting of the TREM2 molecule covalently bound to the Fc receptor identified anionic ligands belonging to various microbial moieties, but curiously, TREM2 also bound brain cells (Daws et al., 2003). By using the TREM2 fusion protein plus a reporter cell line, we were previously able to show that the TREM2 ligand was upregulated on and activated by apoptotic neurons, and microglial phagocytosis involved direct recognition of TREM2 (Hsieh et al., 2009). One study suggested that one potential ligand might be HSP60 (Stefano et al., 2009). Stimulation of HSP60 stimulated the phagocytic activity of TREM2-expressing microglia, but not TREM2-deficient microglia (Stefano et al., 2009). However, in the current study, we failed to see any changes in HSP60 expression due to hypothermia. Further investigations are clearly needed to determine the ligand for TREM2 in brain ischemia. However, this is beyond the scope of the present report.
In total, whereas many inflammatory responses are damaging to the ischemic brain, others may be necessary for recovery and repair. Our data here suggest that cooling, whether neuroprotective or not, suppresses microglial and macrophage activation, and this is consistent with our earlier observations (Han et al., 2002). However, it is only in the setting of therapeutic cooling that there is a proportionate increase in TREM2 expression, which suggests that TREM2 may confer beneficial effects in the poststroke period. Strategies to augment TREM2 might be predicted to improve stroke outcome. A limitation of this work is that our observations are correlative, and we do not directly demonstrate the beneficial role of TREM2. Future studies might employ gene knockdown or pharmacological manipulation of TREM2 to more precisely elucidate the role of TREM2 in brain ischemia.
Acknowledgments
This work was supported by grants from National Institutes of Health (NIH) (NS40516 to M.Y. and AR0038 to M.N.) and the Veteran's Merit Awards to M.Y. and M.C.N., the Uehara Foundation Research Fellowship to M.K. and a Veterans Affairs Career Development Award to C.H. Grants to M.Y. and M.N. were administered by the Northern California Institute for Research and Education, and supported by resources of the Veterans Affairs Medical Center, San Francisco, California. M.N. also received support from the Rosalind Russell Arthritis Center.
Disclosure Statement
The authors declare that no competing financial interests exist.
References
- Barone FC, Feuerstein GZ. Inflammatory mediators and stroke: new opportunities for novel therapeutics. J Cereb Blood Flow Metab 1999;19:819–834 [DOI] [PubMed] [Google Scholar]
- Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion: Evaluation of the model and development of a neurologic examination. Stroke 1986;17:472–476 [DOI] [PubMed] [Google Scholar]
- Borlongan CV, Cahill DW, Sanberg PR. Locomotor and passive avoidance deficits following occlusion of the middle cerebral artery. Physiol Behav 1995;58:909–917 [DOI] [PubMed] [Google Scholar]
- Busto R, Globus MY, Dietrich WD, Martinez E, Valdes I, Ginsberg MD. Effect of mild hypothermia on ischemia-induced release of neurotransmitters and free fatty acids in rat brain. Stroke 1989;20:904–910 [DOI] [PubMed] [Google Scholar]
- Chou WH, Choi DS, Zhang H, Mu D, McMahon T, Kharazia VN, Lowell CA, Ferriero DM, Messing RO. Neutrophil protein kinase cdelta as a mediator of stroke-reperfusion injury. J Clin Invest 2004;114:49–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbourne F, Corbett D, Zhao Z, Yang J, Buchan AM. Prolonged but delayed postischemic hypothermia: A long-term outcome study in the rat middle cerebral artery occlusion model. J Cereb Blood Flow Metab 2000;20:1702–1708 [DOI] [PubMed] [Google Scholar]
- Daws MR, Lanier LL, Seaman WE, Ryan JC. Cloning and characterization of a novel mouse myeloid dap12-associated receptor family. Eur J Immunol 2001;31:783–791 [DOI] [PubMed] [Google Scholar]
- Daws MR, Sullam PM, Niemi EC, Chen TT, Tchao NK, Seaman WE. Pattern recognition by trem-2: binding of anionic ligands. J Immunol 2003;171:594–599 [DOI] [PubMed] [Google Scholar]
- Deng H, Han HS, Cheng D, Sun GH, Yenari MA. Mild hypothermia inhibits inflammation after experimental stroke and brain inflammation. Stroke 2003;34:2495–2501 [DOI] [PubMed] [Google Scholar]
- El Khoury J, Hickman SE, Thomas CA, Loike JD, Silverstein SC. Microglia, scavenger receptors, and the pathogenesis of Alzheimer's disease. Neurobiol Aging 1998;19:S81–S84 [DOI] [PubMed] [Google Scholar]
- Feuerstein GZ, Wang X, Barone FC. Inflammatory gene expression in cerebral ischemia and trauma. Potential new therapeutic targets. Ann N Y Acad Sci 1997;825:179–193 [DOI] [PubMed] [Google Scholar]
- Giulian D, Corpuz M, Chapman S, Mansouri M, Robertson C. Reactive mononuclear phagocytes release neurotoxins after ischemic and traumatic injury to the central nervous system. J Neurosci Res 1993;36:681–693 [DOI] [PubMed] [Google Scholar]
- Guerreiro R, Wojtas A, Bras J, Carrasquillo M, Rogaeva E, Majounie E, Cruchaga C, Sassi C, Kauwe JS, Younkin S, Hazrati L, Collinge J, Pocock J, Lashley T, Williams J, Lambert JC, Amouyel P, Goate A, Rademakers R, Morgan K, Powell J, St George-Hyslop P, Singleton A, Hardy J. Trem2 variants in Alzheimer's disease. N Engl J Med 2013;368:117–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han HS, Qiao Y, Karabiyikoglu M, Giffard RG, Yenari MA. Influence of mild hypothermia on inducible nitric oxide synthase expression and reactive nitrogen production in experimental stroke and inflammation. J Neurosci 2002;22:3921–3928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh CL, Koike M, Spusta SC, Niemi EC, Yenari M, Nakamura MC, Seaman WE. A role for trem2 ligands in the phagocytosis of apoptotic neuronal cells by microglia. J Neurochem 2009;109:1144–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang WC, Qiao Y, Xu L, Kacimi R, Sun X, Giffard RG, Yenari MA. Direct protection of cultured neurons from ischemia-like injury by minocycline. Anat Cell Biol 2010;43:325–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson T, Stefansson H, Steinberg S, Jonsdottir I, Jonsson PV, Snaedal J, Bjornsson S, Huttenlocher J, Levey AI, Lah JJ, Rujescu D, Hampel H, Giegling I, Andreassen OA, Engedal K, Ulstein I, Djurovic S, Ibrahim-Verbaas C, Hofman A, Ikram MA, van Duijn CM, Thorsteinsdottir U, Kong A, Stefansson K. Variant of trem2 associated with the risk of Alzheimer's disease. N Engl J Med 2013;368:107–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabori M, Kuroda S, Ito M, Shichinohe H, Houkin K, Kuge Y, Tamaki N. Timing and cell dose determine therapeutic effects of bone marrow stromal cell transplantation in rat model of cerebral infarct. Neuropathology 2012a;33:140–148 [DOI] [PubMed] [Google Scholar]
- Kawabori M, Kuroda S, Sugiyama T, Ito M, Shichinohe H, Houkin K, Kuge Y, Tamaki N. Intracerebral, but not intravenous, transplantation of bone marrow stromal cells enhances functional recovery in rat cerebral infarct: an optical imaging study. Neuropathology 2012b;32:217–226 [DOI] [PubMed] [Google Scholar]
- Kim JY, Kim N, Yenari MA, Chang W. Mild hypothermia suppresses calcium-sensing receptor (casr) induction following forebrain ischemia while increasing gaba-b receptor 1 (gaba-b-r1) expression. Transl Stroke Res 2011;2:195–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klesney-Tait J, Turnbull IR, Colonna M. The trem receptor family and signal integration. Nat Immunol 2006;7:1266–1273 [DOI] [PubMed] [Google Scholar]
- Kreutzberg GW. Microglia: A sensor for pathological events in the cns. Trends Neurosci 1996;19:312–318 [DOI] [PubMed] [Google Scholar]
- Kriz J. Inflammation in ischemic brain injury: Timing is important. Crit Rev Neurobiol 2006;18:145–157 [DOI] [PubMed] [Google Scholar]
- Lehnardt S, Massillon L, Follett P, Jensen FE, Ratan R, Rosenberg PA, Volpe JJ, Vartanian T. Activation of innate immunity in the cns triggers neurodegeneration through a toll-like receptor 4-dependent pathway. Proc Natl Acad Sci USA 2003;100:8514–8519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier CM, Ahern K, Cheng ML, Lee JE, Yenari MA, Steinberg GK. Optimal depth and duration of mild hypothermia in a focal model of transient cerebral ischemia: Effects on neurologic outcome, infarct size, apoptosis, and inflammation. Stroke 1998;29:2171–2180 [DOI] [PubMed] [Google Scholar]
- Neumann H, Kotter MR, Franklin RJ. Debris clearance by microglia: an essential link between degeneration and regeneration. Brain 2009;132:288–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann H, Takahashi K. Essential role of the microglial triggering receptor expressed on myeloid cells-2 (trem2) for central nervous tissue immune homeostasis. J Neuroimmunol 2007;184:92–99 [DOI] [PubMed] [Google Scholar]
- Piccio L, Buonsanti C, Mariani M, Cella M, Gilfillan S, Cross AH, Colonna M, Panina-Bordignon P. Blockade of trem-2 exacerbates experimental autoimmune encephalomyelitis. Eur J Immunol 2007;37:1290–1301 [DOI] [PubMed] [Google Scholar]
- Satoh J, Tabunoki H, Ishida T, Yagishita S, Jinnai K, Futamura N, Kobayashi M, Toyoshima I, Yoshioka T, Enomoto K, Arai N, Saito Y, Arima K. Phosphorylated syk expression is enhanced in nasu-hakola disease brains. Neuropathology 2011;32:149–157 [DOI] [PubMed] [Google Scholar]
- Schmid CD, Sautkulis LN, Danielson PE, Cooper J, Hasel KW, Hilbush BS, Sutcliffe JG, Carson MJ. Heterogeneous expression of the triggering receptor expressed on myeloid cells-2 on adult murine microglia. J Neurochem 2002;83:1309–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessa G, Podini P, Mariani M, Meroni A, Spreafico R, Sinigaglia F, Colonna M, Panina P, Meldolesi J. Distribution and signaling of trem2/dap12, the receptor system mutated in human polycystic lipomembraneous osteodysplasia with sclerosing leukoencephalopathy dementia. Eur J Neurosci 2004;20:2617–2628 [DOI] [PubMed] [Google Scholar]
- Sieber MW, Jaenisch N, Brehm M, Guenther M, Linnartz-Gerlach B, Neumann H, Witte OW, Frahm C. Attenuated inflammatory response in triggering receptor expressed on myeloid cells 2 (trem2) knock-out mice following stroke. PLoS One 2013;8:e52982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefano L, Racchetti G, Bianco F, Passini N, Gupta RS, Panina Bordignon P, Meldolesi J. The surface-exposed chaperone, hsp60, is an agonist of the microglial trem2 receptor. J Neurochem 2009;110:284–294 [DOI] [PubMed] [Google Scholar]
- Takahashi K, Prinz M, Stagi M, Chechneva O, Neumann H. Trem2-transduced myeloid precursors mediate nervous tissue debris clearance and facilitate recovery in an animal model of multiple sclerosis. PLoS Med 2007;4:e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Rochford CD, Neumann H. Clearance of apoptotic neurons without inflammation by microglial triggering receptor expressed on myeloid cells-2. J Exp Med 2005;201:647–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang XN, Cairns B, Cairns N, Yenari MA. Apocynin improves outcome in experimental stroke with a narrow dose range. Neuroscience 2008;154:556–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang XN, Wang Q, Koike MA, Cheng D, Goris ML, Blankenberg FG, Yenari MA. Monitoring the protective effects of minocycline treatment with radiolabeled annexin v in an experimental model of focal cerebral ischemia. J Nucl Med 2007;48:1822–1828 [DOI] [PubMed] [Google Scholar]
- Tang XN, Zheng Z, Giffard RG, Yenari MA. Significance of marrow-derived nicotinamide adenine dinucleotide phosphate oxidase in experimental ischemic stroke. Ann Neurol 2011;70:606–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas WE. Brain macrophages: Evaluation of microglia and their functions. Brain Res Brain Res Rev 1992;17:61–74 [DOI] [PubMed] [Google Scholar]
- Toyoda T, Suzuki S, Kassell NF, Lee KS. Intraischemic hypothermia attenuates neutrophil infiltration in the rat neocortex after focal ischemia-reperfusion injury. Neurosurgery 1996;39:1200–1205 [DOI] [PubMed] [Google Scholar]
- Turnbull IR, Gilfillan S, Cella M, Aoshi T, Miller M, Piccio L, Hernandez M, Colonna M. Cutting edge: Trem-2 attenuates macrophage activation. J Immunol 2006;177:3520–3524 [DOI] [PubMed] [Google Scholar]
- van der Worp HB, Macleod MR, Kollmar R. Therapeutic hypothermia for acute ischemic stroke: Ready to start large randomized trials? J Cereb Blood Flow Metab 2010;30:1079–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H, Abe H, Takeuchi S, Tanaka R. Protective effect of microglial conditioning medium on neuronal damage induced by glutamate. Neurosci Lett 2000;289:53–56 [DOI] [PubMed] [Google Scholar]
- Yanamoto H, Nagata I, Niitsu Y, Zhang Z, Xue JH, Sakai N, Kikuchi H. Prolonged mild hypothermia therapy protects the brain against permanent focal ischemia. Stroke 2001;32:232–239 [DOI] [PubMed] [Google Scholar]
- Yenari MA, Han HS. Neuroprotective mechanisms of hypothermia in brain ischaemia. Nat Rev Neurosci 2012;13:267–278 [DOI] [PubMed] [Google Scholar]
- Yenari MA, Hemmen TM. Therapeutic hypothermia for brain ischemia: where have we come and where do we go? Stroke 2010;41:S72–S74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yenari MA, Kunis D, Sun GH, Onley D, Watson L, Turner S, Whitaker S, Steinberg GK. Hu23f2g, an antibody recognizing the leukocyte cd11/cd18 integrin, reduces injury in a rabbit model of transient focal cerebral ischemia. Exp Neurol 1998;153:223–233 [DOI] [PubMed] [Google Scholar]
- Yenari MA, Onley D, Hedehus M, deCrespigny A, Sun GH, Moseley ME, Steinberg GK. Diffusion- and perfusion-weighted magnetic resonance imaging of focal cerebral ischemia and cortical spreading depression under conditions of mild hypothermia. Brain Res 2000;885:208–219 [DOI] [PubMed] [Google Scholar]
- Yenari MA, Xu L, Tang XN, Qiao Y, Giffard RG. Microglia potentiate damage to blood-brain barrier constituents: Improvement by minocycline in vivo and in vitro. Stroke 2006;37:1087–1093 [DOI] [PubMed] [Google Scholar]
- Zhao BQ, Wang S, Kim HY, Storrie H, Rosen BR, Mooney DJ, Wang X, Lo EH. Role of matrix metalloproteinases in delayed cortical responses after stroke. Nat Med 2006;12:441–445 [DOI] [PubMed] [Google Scholar]
- Zheng Z, Kim JY, Ma H, Lee JE, Yenari MA. Anti-inflammatory effects of the 70 kda heat shock protein in experimental stroke. J Cereb Blood Flow Metab 2008;28:53–63 [DOI] [PubMed] [Google Scholar]