Abstract
To find an effective mucosal adjuvant for influenza virus-like particles (VLPs), we compared the effects of known adjuvants Alum, CpG DNA, monophosphoryl lipid A (MPL), poly IC, gardiquimod, and cholera toxin (CT). Mice that were intranasally immunized with Alum, CpG, MPL, and CT adjuvanted VLPs showed higher levels of antibodies in both sera and mucosa. Hemagglutination inhibition and virus neutralizing activities were enhanced in groups adjuvanted with Alum, MPL, or CT. Influenza virus specific long-lived cells secreting IgG and IgA antibodies were found at high levels both in bone marrow and spleen in the Alum, CpG and CT adjuvanted groups. A similar level of protection was observed among different adjuvanted groups, except the CT adjuvant that showed a higher efficacy in lowering lung viral loads after challenge. Alum and CT adjuvants differentially increased influenza VLP-mediated activation of dendritic cells and splenocytes in vitro, supporting the in vivo pattern of antibody isotypes and cytokine production. These results suggest that Alum, MPL, or CpG adjuvants, which have been tested clinically, can be developed as an effective mucosal adjuvant for influenza VLP vaccines.
Introduction
Influenza virus infection remains a serious respiratory disease and vaccination is known to be the most effective measure for preventing infectious diseases (24). Licensed influenza vaccination provides approximately 30%–40% protection for the elderly, suggesting the need to improve the vaccine efficacy (39). Development of effective adjuvants can be an approach to improve the efficacy of vaccines against influenza as well as other infectious agents.
Aluminium hydroxide (Alum) is licensed and is the most well-accepted adjuvant for human vaccines. Alum is known to act on monocytes, macrophages, or granulocytes to induce cytokines that generate a local immunostimulatory environment, eventually leading to activation of dendritic cells (4). Toll-like receptors (TLRs), a family of receptors for recognizing pathogen-associated molecular patterns on cells of the innate immune system, play a critical role in detecting and responding to microbial infections. Thus, mimicking the immune stimulating responses of microorganisms, molecular adjuvants based on TLR ligands or agonists have been demonstrated to enhance the immunogenicity of vaccines and are increasingly recognized as key adjuvant targets. As examples, TLR ligands such as monophosphoryl lipid A (MPL), CpG, poly IC, Flagellin, and gardiquimod have been demonstrated to be effective adjuvants when co-administered with vaccine antigens (9,15,18,33,35–37). MPL, a TLR4 agonist, has been used extensively in clinical trials as a component in prophylactic and therapeutic vaccines targeting infectious disease (20,23). The TLR9 agonist CpG (ODN1826) has been tested as an adjuvant in clinical trials for the hepatitis B virus vaccine and induced increased antibody responses (14). Poly IC (a TLR3 ligand) is also known to exhibit potent adjuvant effects on inducing a T cell helper type 1 (Th1) immune response (36). Gardiquimod (a TLR7 ligand) has been shown to activate antigen-presenting cells, T cells, and natural killer (NK) cells, and was reported to elicit Norwalk virus-like particle-specific serum IgG and mucosal IgA antibody responses at mucosal sites (22,33).
As a promising vaccine candidate, virus-like particles (VLP) represent an attractive vaccine since they are similar to virus in size and structure but noninfectious, highlighting a high safety feature (12,36). Our previous studies demonstrated that influenza VLPs containing hemagglutinin (HA) derived from A/PR/8/34 virus (A/PR8 VLP) were able to induce protective immune responses in the absence of adjuvants (29,32). However, adjuvant studies to improve the efficacy of influenza VLP vaccines are very limited. It is particularly important to compare different adjuvants in the same experimental condition. Here, we focused on comparing immunogenic effects of various adjuvants that are licensed or tested clinically in the context of influenza A/PR8 VLPs (VLP). We used Alum, CpG DNA (CpG), MPL, poly IC, and gardiquimod as mucosal adjuvants in the context of A/PR8 VLP vaccine. Cholera toxin (CT) was used as a positive mucosal adjuvant control. Our comparative adjuvant study suggests that Alum, CT, MPL, and CpG significantly enhanced the immunogenicity of influenza A/PR8 VLP vaccine in different manner.
Materials and Methods
Virus and cells
Influenza virus A/PR/8/1934 (H1N1; abbreviated as A/PR8) was grown in 10-day-old embryonated hen's eggs for 2.5 days at 36°–37°C. Allantoic fluids of infected eggs were harvested after being stored overnight at 4°C and centrifuged to remove cell debris. The virus was purified from allantoic fluid by using a discontinuous sucrose gradient (15%, 30%, and 60% layers) and ultracentrifugation (at 28,000 rpm for 60 min). The purified virus was inactivated by mixing the virus with formalin at a final concentration of 1:4,000 (vol/vol) as described previously (30). Spodoptera frugiperda Sf9 cells were maintained in suspension in serum-free SF900II medium (Gibco-BRL) at 27°C in spinner flasks at a speed of 70–80 rpm. Madin-Darby canine kidney (MDCK) cells were grown and maintained in Dulbecco's modified Eagle's medium (DMEM).
Influenza virus-like particle (VLP) vaccine
Influenza VLPs containing A/PR8 H1 HA and M1 were prepared as described (29,30). For generation of recombinant baculoviruses (rBVs), cDNAs encoding A/PR8 H1 HA, and M1 were cloned into the pFastBac plasmid, a BV transfer vector (Invitrogen). Recombinant Bacmid baculovirus DNAs (rAcNPV) containing PR8 HA were isolated from transformed DH10Bac cells and were used to transfect Sf9 insect cells following the manufacturer's instructions (Invitrogen). To produce VLPs containing influenza M1 and HA, Sf9 cells were co-infected with rBVs expressing HA and M1 at multiplicity of infection (MOI) of 4 and 2, respectively. VLPs were purified from culture supernatants by using low-speed centrifugation (2000 g for 20 min at 4°C) and ultracentrifugation (100,000 g for 60 min). The sedimented particles were resuspended in PBS at 4°C overnight and further purified through a 20%–30%–60% discontinuous sucrose gradient ultracentrifugation at 100,000 g for 1 h at 4°C. The VLP bands were collected and analyzed by Western blot probed with anti-M1 antibody and mouse polyclonal anti-sera to A/PR/8/34 for detecting HA.
Mice and vaccination
Female inbred BALB/c mice (Charles River, Wilmington, MA) aged 6 to 8 weeks were used. Mice (6 mice per group) were intranasally immunized twice with a 4-week interval. Five μg of A/PR8 VLPs (VLP) alone or in the presence of 2 μg of adjuvants such as CpG ODN1826 (CPG) (TLR9 ligand, Invitrogen, San Diego, CA) (V+CPG), monophosphoryl lipid A (MPL) (TLR4 ligand, InvivoGen, San Diego, CA) (V+MPL), poly IC (TLR3 ligand, InvivoGen, San Diego, CA) (V+P), a TLR7 agonist gardiquimod (V+G), and cholera toxin (CT) (V+CT) (Sigma-Aldrich, St. Louis, MO) in 50 μL of PBS were used. Low dose 10 μg [V+A(L)] or high dose 60 μg [V+A(H)] of Alum (Sigma-Aldrich) was used. All mouse studies were followed by approved Institutional Animal Care and Use Committee (IACUC) protocol that operates under the federal Animal Welfare Law and regulations of the Department of Health and Human Services.
Antibody response and virus neutralizing activity
Blood samples were collected at 2 weeks post-immunizations. Influenza virus specific antibodies IgG and IgA were determined in sera by enzyme-linked immunosorbent assay (ELISA) as described previously (28,29,32). As coating antigens to measure virus specific antibodies, egg-grown inactivated influenza virus (A/PR8) was coated onto 96-well microtiter plates (MaxiSorp immunoplate; Nunc Life Technologies, Basel, Switzerland) with 100 μL in coating buffer (0.1 M sodium carbonate, pH 9.5, 4 μg inactivated virus per ml) at 4°C overnight. Th1 (IgG2a and IgG2b) or Th2 (IgG1) polarization was determined by Th1:Th2 index calculation ([IgG2a+IgG2b]/2)/(IgG1) (36). An index value<1 indicates a Th2 polarization, and an index value>1 a Th1 polarization. Serum neutralizing activities from immunized mouse sera were performed using MDCK cells following a procedure previously described (32).
Hemagglutination inhibition activity
For determination of hemagglutination inhibition activity (HAI) in serum, serum was first treated with receptor destroy enzyme (RDE) by incubation overnight at 37°C, and then incubated 30 min at 56°C. Sera were serially diluted twofold in 25 μL PBS and 4 hemagglutination units (HAU) of influenza A/PR8 virus were used in a volume of 25 μL. The contents of each well were gently mixed with a micropipettor, and then the plates were incubated for 30 min at room temperature. Finally, 50 μL of a 0.5% chicken erythrocyte suspension was added to each well. The reciprocal of the highest serum dilution capable of preventing hemagglutination was scored as the HI titer.
Lung IgG and IgA responses
For the measurement of lung IgG and IgA immune responses, 6 mice from each group were sacrificed at week 4 after boost. The whole lung extracts prepared as homogenates using frosted glass slides were centrifuged at 1000 rpm for 10 min to collect supernatants, which were frozen and kept at −80°C until use. IgG and IgA from lung extracts were determined by ELISA using inactivated virus (A/PR8) as a coating antigen (30).
Antibody secreting cells (ASC) responses in spleen or bone marrow
Spleen lymphocytes were collected from sacrificed mice to determine influenza virus-specific antibody-secreting plasma cells (ASC) (29). For ASC detection, inactivated A/PR8 viruses were used to coat 96-well culture plates (Costar). Freshly isolated cells from spleen (1×106 cells) were added to each well and incubated for 6 days at 37°C with 5% CO2. After removing cells from the culture plates, horseradish peroxidase (HRP)-conjugated secondary goat-anti-mouse antibodies were added. As a measure of HRP activity, the substrate o-phenylenediamine (Zymed, San Francisco, CA) was used and the optical density was read at 450 nm.
Viral challenge infection
For viral challenge, groups of mice (12 mice per group) immunized with VLP alone or with adjuvanted VLPs (MPL, CPG, CT, Alum(H)) were intranasally infected with A/PR8 (10×LD50) in 50 μL of PBS per mouse at week 4 after the second immunization. Six mice from each group were sacrificed at day 4 postchallenge to determine lung virus titers. Remaining 6 mice were observed daily to monitor changes in body weight and to record survival rates (25% loss in body weight). We followed an approved IACUC protocol for this study. The whole-lung extracts prepared as homogenates using frosted glass slides were centrifuged at 1000 rpm for 10 min to collect supernatants. Determination of viral titers in lung extracts was performed using MDCK cells, as previously described (32).
Cytokine ELISPOT assay
All antibodies against mouse cytokines used in cytokine ELISPOT assays were purchased from BD/PharMingen (San Diego, Calif.). Anti-mouse gamma interferon (IFN-γ), interleukin-2 (IL-2), IL-4, and IL-5 antibodies were used to coat Multiscreen 96-well filtration plates (Millipore). Spleens were harvested from mice at day 4 postchallenge and singular cells were prepared. Freshly isolated splenocytes (1.5×106 cells) were added to each well and stimulated with inactivated A/PR8 (0.2 μg per well). The plates were incubated for 36 h at 37°C with 5% CO2. Development and counting of cytokine ELISPOTs were performed as described previously (28). Ready-Set-Go IFN-γ, IL-2, IL-4, and IL-5 kits (eBioscience, San Diego, CA) were used for detecting cytokine levels in splenocytes following the manufacturer's procedures.
Bone marrow-derived dendritic cell (BMDC) preparation and treatment
Dendritic cells (BMDCs) were generated from bone marrow of BALB/c mice (3,11). Briefly, the harvested cells from femur and tibia bones were seeded onto 6-well culture plates and incubated in the presence of granulocytes-macrophages colony stimulation factor (10 ng/mL). BMDCs (2.5×105 cells/mL) were treated with 5 μg/mL of A/PR8 VLP plus 60 μg/mL of Alum or 5 μg/mL of CT. To measure cytokine levels, BMDC culture supernatants were collected after 2 days of culture and used for ELISA. Mouse interleukin (IL)-12 p70, IL-6, and tumor necrosis factor (TNF)-α ELISA kits of Ready-Set-Go® (eBioscience) were used as described by manufacturer's manuals.
Flow cytometry
BMDCs (BALB/c mice) after incubation with A/PR8 VLP plus adjuvants or spleen cells after mixed lymphocyte reactions were stained with surface markers. For mixed lymphocyte reactions, the BMDCs were first treated with A/PR8 VLPs and adjuvants for 2 days. Then, 2×105 cells/well of spleen cells and 1×104 cells/well of activated BMDCs were co-cultured in 96-well culture plate for 5 days. The stimulated spleen cells were harvested for intracellular cytokine staining. Antibodies CD16/32 (clone 93, eBioscience), CD40 (clone 1C10, eBioscence), CD86 (clone GL1, eBioscence), CD4 (clone RM4-5, BD Pharmigen), MHCII (M5/114. 15.2 clone, eBioscience), and CD11b (clone M1/70, eBioscience) were used for surface staining of BMDCs or spleen cells. For intracellular cytokine (IFN-γ, IL-4) staining, a Cytofix/Cytoperm™ Fixation/Permeabilization Solution Kit with BD GolgiStop™ was used. Stained cells were analyzed by flow cytometer LSRFortessa (BD Biosciences) and FlowJo program (Tree Star Inc.).
Statistical analysis
All parameters were recorded for individuals within all groups. Statistical comparisons of data were carried out using PC-SAS system. A value of p<0.05 was considered significant.
Results
Effects of adjuvants on antibody responses by VLP vaccination
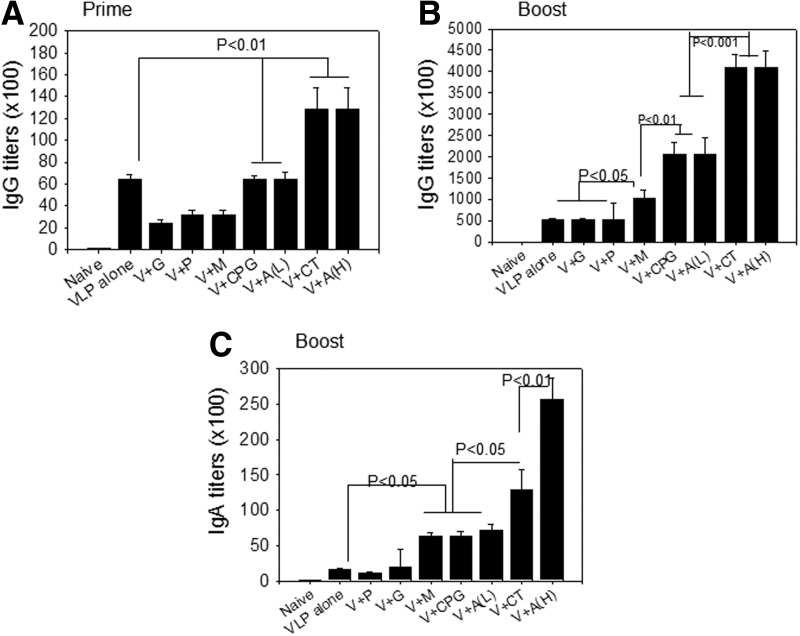
To determine effects of various adjuvants with known mechanisms on VLP vaccination, groups of mice were intranasally immunized with 5 μg (total protein) of A/PR8 VLP alone (VLP) or together with adjuvants (2 μg unless specified): Gardiquimod (V+G), poly IC (V+P), MPL (V+M), CpG (V+CPG), cholera toxin (V+CT), low (10 μg) and high (60 μg) dose of Alum (V+A(L), V+A(H)). To measure humoral immune responses, we determined serum IgG and IgA antibody responses specific for influenza virus (A/PR8). After a priming immunization, mice immunized with VLPs in the presence of Alum (H) or CT showed significantly higher IgG responses compared to VLP vaccine alone (p<0.01) (Fig. 1A). At week 2 after boost, mice immunized with VLPs in the presence of MPL (p<0.05), CpG (p<0.01), Alum (L) (p<0.01), CT (p<0.001), or Alum (H) (p<0.001) showed significantly higher IgG responses compared to VLP vaccine alone (Fig. 1B). Among the adjuvants tested, CT (p<0.001) or Alum (H) (p<0.001) adjuvanted VLP vaccine similarly showed highest IgG responses compared to CPG or Alum (L) adjuvants (p<0.05) (Fig. 1B). Serum IgA responses were determined at 2 weeks after boost immunization, and showed a similar pattern as observed in serum IgG antibody responses. Higher levels of IgA antibody were detected in groups of mice that were adjuvanted with MPL (p<0.05), CpG (p<0.05), Alum (L) (p<0.05), CT (p<0.01), or Alum (H) (p<0.001) compared to the VLP vaccine alone group (Fig. 1C). However, gardiquimod and poly IC did not show any adjuvant effects on enhancing IgG or IgA antibody responses upon VLP immunization. These results indicate that intranasally administered adjuvants such as MPL, CpG, Alum, and CT can significantly enhance serum IgG and IgA antibody responses of A/PR8 VLP vaccine.
FIG. 1.
Influenza A/PR8 virus specific total IgG and IgA response. (A) Prime IgG antibody responses specific to A/PR8 virus. (B) Boost IgG antibody responses specific to A/PR8 virus. (C) Serum IgA antibody responses specific to A/PR8 virus after boost. Mice (n=6) were immunized with A/PR8 (H1N1) VLP vaccine alone or in combination with adjuvants via the intranasal route. Groups are as follows. VLPs alone, V+CpG, V+M, V+P, V+G, V+CT, V+A (L, 10 μg), and V+A (H, 60 μg), as described in methods. Virus-specific antibody responses measured by ELISA are expressed as the reciprocals of serum dilutions that give 2-fold higher of standard deviations compared to naïve serum samples. After prime, groups V+Alum (H) or V+CT showed significantly higher IgG responses compared to VLP vaccine alone (p<0.01). After boost, significant higher responses were found compared to VLP alone: V+MPL (p<0.05), CPG (p<0.01), Alum (L) (p<0.01), CT (p<0.001), or Alum (H) (p<0.001). Significantly higher levels of IgA antibody were also detected in the following adjuvanted groups compared to VLPs alone: MPL (p<0.05), CPG (p<0.05), Alum (L) (p<0.05), CT (p<0.01), or Alum (H) (p<0.001).
Adjuvant effects on IgG subtype responses
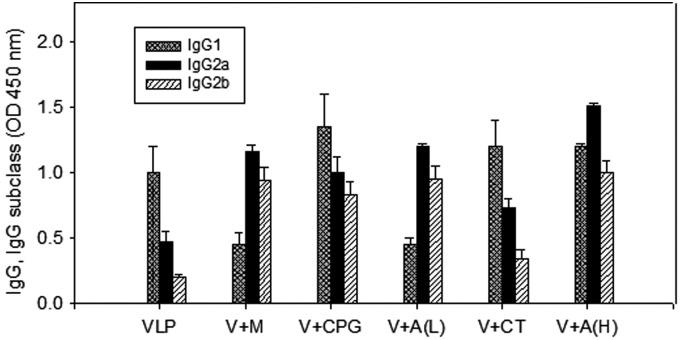
IgG subclass profiles may vary by parameters such as type and quantity of vaccine antigens and routes of vaccine delivery (36). To determine adjuvant effects on the pattern of IgG subclasses obtained by VLP vaccination, we measured IgG1, IgG2a, and IgG2b subclasses specific to influenza virus (Fig. 2 and Table 1). Among different adjuvant groups, we selected MPL, CpG, Alum (H), Alum (L), and CT, since these adjuvants showed higher levels of serum IgG antibody responses. The MPL or Alum adjuvanted VLP groups showed higher IgG2a and IgG2b responses compared to those of the VLP alone group (p<0.05) (Table 1, Fig 2). In contrast, the groups with adjuvants CpG or CT showed higher IgG1 responses (p<0.05) (Table 1). Taken together, the results show that different adjuvants show differential effects on IgG subclass responses induced by A/PR8 VLP intranasal immunization.
FIG. 2.
Influenza A/PR8 specific IgG subclass antibody response. A/PR/8 virus specific IgG subclass levels (IgG1, IgG2a, IgG2b) were determined after boost. Results are expressed as averages of optical density readings at 450 nm (OD450) with 100-fold diluted serum samples in each group of mice (n=6).
Table 1.
Th1:Th2 Index for VLPs Alone and Adjuvanted VLPs
| VLP vaccine | VLP | V+M | V+CpG | V+A(L) | V+A(H) | V+CT |
|---|---|---|---|---|---|---|
| IgG1(Th2)1 |
1.00 |
0.45 |
1.35 |
0.45 |
1.20 |
1.20 |
| IgG2a(Th1)1 |
0.48 |
1.17 |
1.30 |
1.20 |
1.51 |
0.73 |
| IgG2b(Th1)1 |
0.20 |
0.95 |
0.83 |
0.95 |
1.00 |
0.34 |
| Th1:Th2 index2 | 0.34 | 2.36 | 0.79 | 2.39 | 1.04 | 0.46 |
The values are optical density readings at 100 fold serum dilution samples. 2The index has been calculated as described in Material and Methods section. Index<1=Th2 polarization. Index>1=Th1 polarization. Th1 (IgG2a and IgG2b) or Th2 (IgG1) polarization were determined by Th1:Th2 index calculation ([IgG2a+IgG2b]/2)/(IgG1).
Mucosal IgG and IgA antibody responses after adjuvanted VLP intranasal immunization
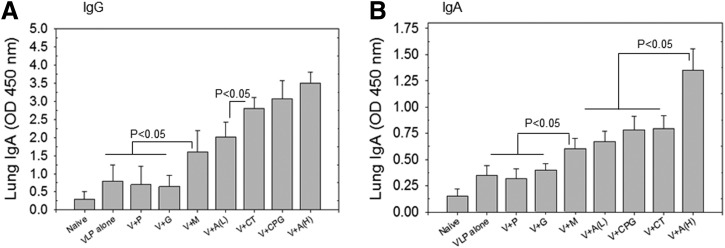
As a respiratory pathogen, influenza virus infects lung tissues as a major site of replication. Therefore, mucosal immunity is important in preventing infection. IgG and IgA antibodies were determined in lung extracts at 2 weeks after boost immunization. As shown in Figure 3A, higher levels of lung IgG antibody responses were detected in the groups of mice that were intranasally immunized with PR8 VLPs and the following adjuvants: Alum (H), CPG, MPL, Alum(L), or CT (p<0.05). Meanwhile, other adjuvants such as poly IC and gardiquimod were not effective in enhancing mucosal IgG antibody responses in the context of the VLP vaccine. A similar pattern of lung IgA antibody responses was found after mucosal immunization with VLPs and adjuvants. That is, significant increases in lung IgA antibody levels were observed with adjuvants such as Alum (H), Alum (L), CPG, MPL, or CT (p<0.05) (Fig. 3B).
FIG. 3.
Lung IgG and IgA response. Lung IgG and IgA were determined at week 4 after boost. (A) Lung IgG antibody responses. (B) Lung IgA antibody responses. Results are expressed as averages of optical density readings at 450 nm (OD 450) with 10-fold diluted lung samples in each group of mice (n=6). For lung IgG responses, p<0.05 between VLP alone or V+G or V+P and V+M or V+A(L); p<0.05 between V+M or V+A(L) and V+CT or V+CpG or V+A(H). For lung IgA responses, p<0.05 between VLP alone or V+G or V+P and V+M or V+A(L) or V+CpG or V+CT; p<0.05 between V+M or V+A(L) or V+CpG or V+CT and V+A(H).
Adjuvant effects on inducing functional antibody responses
The hemagglutination inhibition (HAI) and virus neutralizing titers are important correlates in predicting protection against influenza. Mouse sera after boost were used to determine HAI and neutralizing titers. As shown in Figure 4A, Alum (H) adjuvanted VLPs showed highest HAI titers among the groups (p<0.01). MPL or Alum (L) adjuvanted VLPs also showed higher HAI titers compared to those of VLPs alone (p<0.05). Other adjuvanted VLPs (poly IC, CPG, gardiquimod) did not show increases in HAI titers. For determination of virus neutralizing activities, mouse sera after boost were used from the groups in which higher serum IgG responses were observed. As seen in Figure 4B and 4C, the groups with adjuvants Alum (H), Alum (L), CT, or MPL showed higher neutralizing activities (p<0.05). In particular, MPL or Alum (H) adjuvant showed higher neutralizing titers than other adjuvants such as Alum (L) and CT (p<0.05). The results indicate that Alum and MPL can be developed as effective adjuvants for enhancing functional antibodies by influenza VLP vaccination.
FIG. 4.
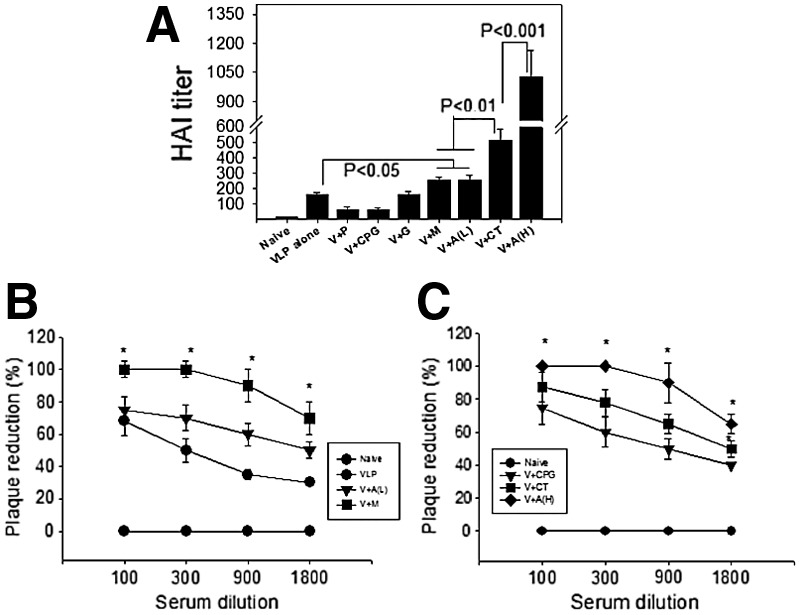
Hemagglutination inhibition activity (HAI) and neutralizing activity. HAI and neutralizing activity were determined at week 2 after boost. (A) HAI activity. The highest serum dilution capable of preventing hemagglutination was scored as the HAI activity. (B, C) Neutralizing activity. Serial dilutions of serum samples were incubated with infectious influenza viruses and percentages of plaque forming units were determined. Neutralizing activities were expressed as the percentage of plaque reduction compared to the medium control. For HAI activity significance: p<0.05 between VLP alone or V+P or V+CpG or V+G and V+M or V+A(L); p<0.01 between V+M or V+A(L) and V+CT; p<0.001 between V+CT and V+A(H). For neutralizing activity significance: (B) At 500X serum dilution: p<0.05 between V+M and VLP alone or V+A(L), at 1500 and 4500 serum dilution: p<0.05 between V+M and V+A(L), between V+M and V+A(L); at 13,500 serum dilution: p<0.05 between V+M and V+A(L). (C) At 500X serum dilution: p<0.05 between A(H) and V+CT or V+CPG, at 1500 and 4500X serum dilution: p<0.05 between V+A(H) and V+CT, between V+CT and V+CPG; at 13,500X serum dilution: p<0.05 between V+A(H) and V+CT.
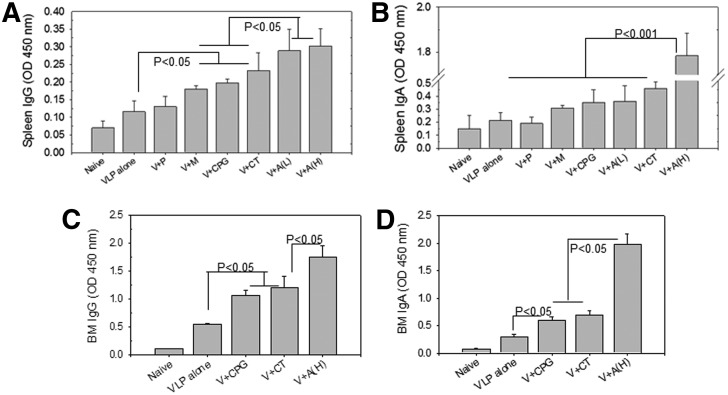
Mucosal adjuvants enhance antibody secreting cell responses
During B cell differentiation and development, a fraction of germinal center B cells traffic to the bone marrow and develop into antibody secreting plasma cells (APC). To investigate the induction of antibody secreting cells, bone marrow and spleen cells were harvested 1 month after boost and stimulated in vitro with A/PR8 virus for 6 days (Fig. 5). VLP vaccination in the presence of adjuvants Alum (H), Alum (L), CpG, MPL, or CT showed higher levels of IgG antibodies bound to the A/PR8 virus coated plates compared to VLPs alone vaccination (Fig. 5A). A similar pattern of adjuvant effects was observed for IgA antibodies produced during in vitro cultures except that the Alum (H) group showed much higher levels of IgA antibodies than other control or adjuvant groups (Fig. 5B).
FIG. 5.
Antibody secreting cell response. Antibody secreting cells were determined at week 4 after boost. (A) Spleen IgG antibody secreting cell responses. (B) Spleen IgA antibody secreting cell responses. (C) Bone marrow IgG antibody secreting cell responses. (D) Bone marrow IgA antibody secreting cell responses. N=6 per group. For IgG antibody secreting cell responses in spleen, p<0.05 between VLP alone and V+M or V+CpG or V+CT; p<0.05 between V+M or V+CpG or V+CT and V+A(L) or V+A(H). For IgA antibody secreting cells in spleen, p<0.001 between VLP alone or V+P or V+M or V+CpG or V+A(L) or V+CT and A(H).
For IgG and IgA antibodies produced from in vitro cultures of bone marrow cells, we selected the Alum (H), CPG, and CT-adjuvanted VLP groups because of their higher adjuvant effects on VLP vaccination. As expected, significantly enhanced levels of IgG (Fig. 5C) and IgA (Fig. 5D) antibodies were produced during the 6 days in vitro cultures of bone marrow cells from these adjuvanted groups compared to the VLP alone group. Importantly, cells from bone marrow were able to produce higher levels of A/PR8 virus specific IgG and IgA antibodies compared to those from spleen cells as evidenced by higher scales of ELISA antibody readings from bone marrow cells (Fig. 5). Overall, mucosally delivered adjuvants (CpG, CT, Alum) were found to increase antibody secreting cell responses after intranasal VLP vaccination.
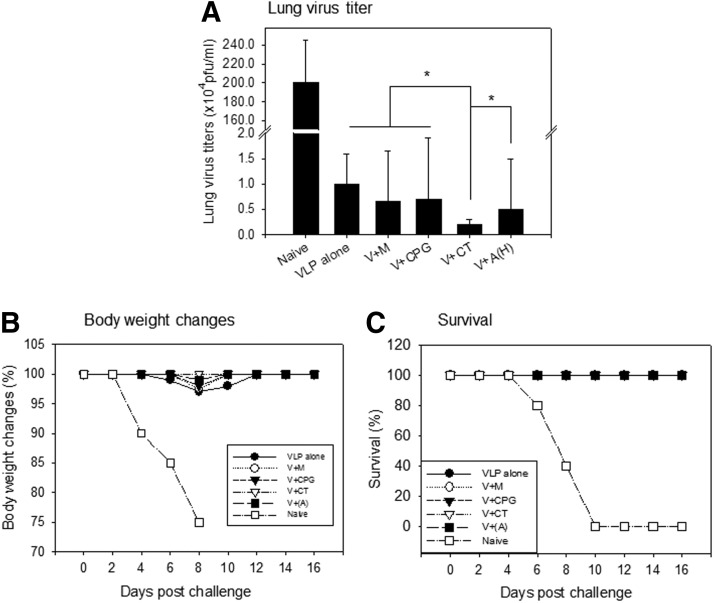
CT adjuvanted VLP vaccine confers enhanced lung viral clearance after challenge
We investigated protective efficacy in mice immunized with VLPs alone or with adjuvanted VLPs described above. All mice immunized were protected against lethal challenge infection with A/PR8 virus and displayed no significant differences on body weight changes and survival rates among vaccinated groups (Fig. 6B, C). Nonetheless, the CT-adjuvanted group showed a significant decrease in lung virus loads compared to other vaccinated groups (Fig. 6A, *p<0.05).
FIG. 6.
Protective efficacy after lethal challenge infection. Lung samples from individual mice in each group (n=6) were collected on day 4 postchallenge with a lethal dose of mouse-adapted A/PR/8/34 (10X LD50). Each lung sample was diluted to 1 mL with Dulbecco's modified Eagle's medium. (A) Lung virus titer; (B) Body weight changes; (C) Survival rates.
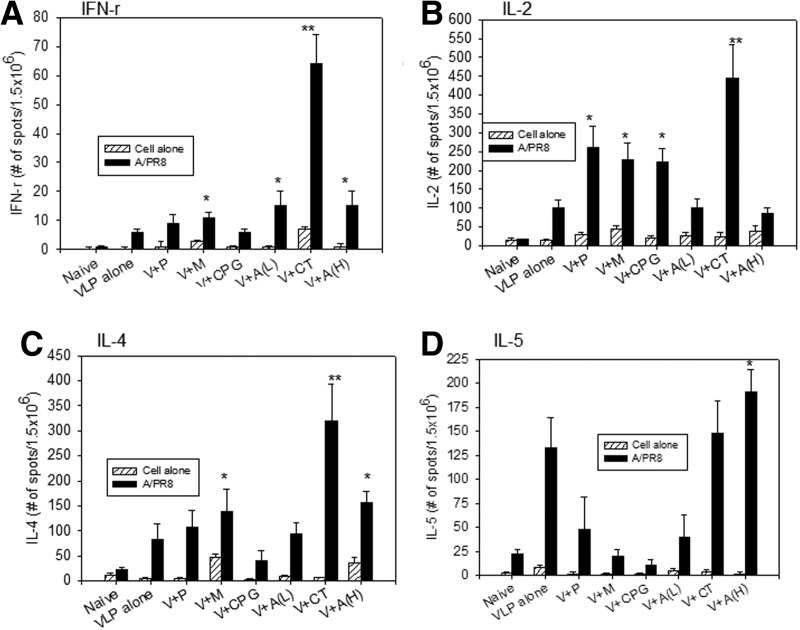
Adjuvanted groups show differential cytokine expressing cellular responses
The induction of cellular immune responses is important for the control of viral replication and inducing a certain pattern of host immune responses. We determined cytokine IFN-γ, IL-2, IL-4, and IL-5 responses in splenocytes from mice immunized with different mucosal adjuvants. Spleens were harvested from mice at day 4 postchallenge and splenocytes were stimulated with inactivated A/PR8 virus to quantify cellular responses secreting Th1-type (IFN-γ and IL-2) and Th2-type (IL-4 and IL-5) cytokines (Fig. 7). In VLPs alone and adjuvanted groups V+M, V+CPG, V+A(L), V+CT, and V+A(H) showed mixed Th1 and Th2-like responses. In particular, the CT adjuvanted group showed higher levels of both Th1 and Th2 type cytokines. It is worth to note that MPL, CpG, and Alum (low) adjuvant groups (V+M, V+CpG, V+A(L), Fig. 7) showed lower levels of IL-5 cytokine response, which appear to be correlated with Th1:Th2 index of antibody isotypes. However, in vitro cytokine expression phenotype responses did not exactly mimic the same expected pattern of serum IgG isotype antibody responses in vivo.
FIG. 7.
Cytokine-secreting splenocytes following challenge infection. Cytokine phenotypes (A) IFN-γ, (B) IL-2, (C) IL-4, and (D) IL-5 ELISPOT assays were performed using mouse splenocytes following challenge infection. Splenocytes were isolated at day 4 postchallenge and cytokine-secreting cells were determined after stimulation with inactivated A/PR8. Splenocytes from naïve or immunized mice in the absence of inactivated A/PR8 virus stimulator were used as background controls (Cell alone). The spots for cytokine-producing cells from the spleen were counted and expressed based on 1.5×106 cells per well. Each column represents the arithmetic mean from six mice. (A) IFN- γ ELISPOT. *p<0.05 compared to VLPs alone, **p<0.01 compared to VLPs alone and other adjuvanted groups. There was significance (p<0.05) between V+CT and V+P or V+M or V+CPG. (B) IL-2: *p<0.05 compared to VLP alone, **p<0.01 compared to VLP alone. (C) IL-4: *p<0.05 compared to VLP alone, **p<0.01 compared to VLP alone. There was significance (p<0.05) between V+CT and V+M or V+A(H). (D) IL-5: *p<0.05 compared to VLP alone or V+Alum.
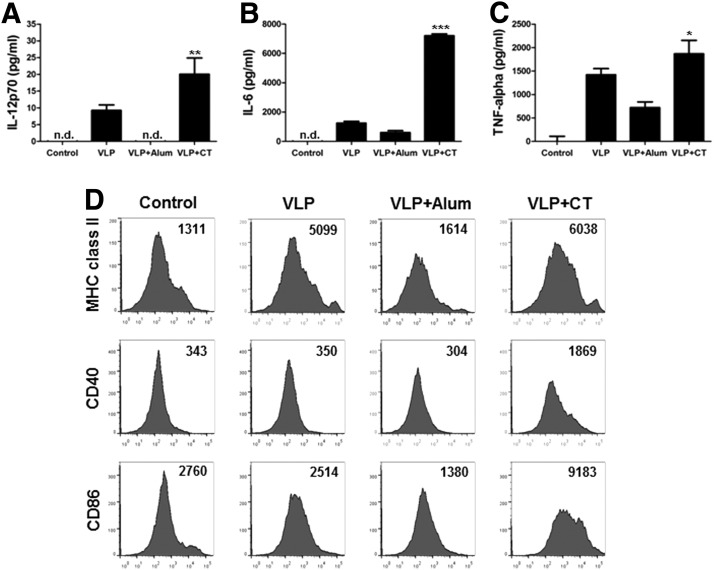
Adjuvants activate immune cells differentially in vitro
The action mechanisms of Alum and CT are not well understood compared to adjuvants based on TLR ligands such as MPL and CPG. We determined whether the Alum and CT adjuvants would activate dendritic cells. A/PR8 VLP vaccine alone activated dendritic cells (BMDC) to secrete IL-12, IL-6, and TNF-α cytokines at low levels (Fig. 8A, B, C). Addition of CT to A/PR8 VLPs significantly increased levels of IL-12 and IL-6 cytokines whereas inclusion of Alum inhibited or did not increase cytokine production (Fig. 8A, B, C). Also, the CT adjuvant enhanced the cell surface expression of activation markers such as MHCII, CD40, and CD86 but Alum-adjuvanted VLPs did not increase cell surface marker expression of BMDC (Fig. 8D).
FIG. 8.
CT adjuvant stimulates BMDCs in combination with A/PR8 VLP vaccine. The amounts of IL-12 (A), IL-6 (B), and TNF-α (C) produced by BMDCs (2.5×105 cells/mL) were measured. The culture supernatants were used for ELISA. Data are presented as the mean±SD from four individual wells and representative of three independent experiments. *, **, *** indicate p<0.05, 0.01, and 0.001, respectively, compared to PR8 VLP only treated DCs. (D) Expression levels of cell surface activation markers of dendritic cells after incubation with A/PR8 VLPs in the presence of adjuvants indicated. The number of histograms indicates mean fluorescence intensity (MFI) of each marker. Representative data are shown from three independent experiments.
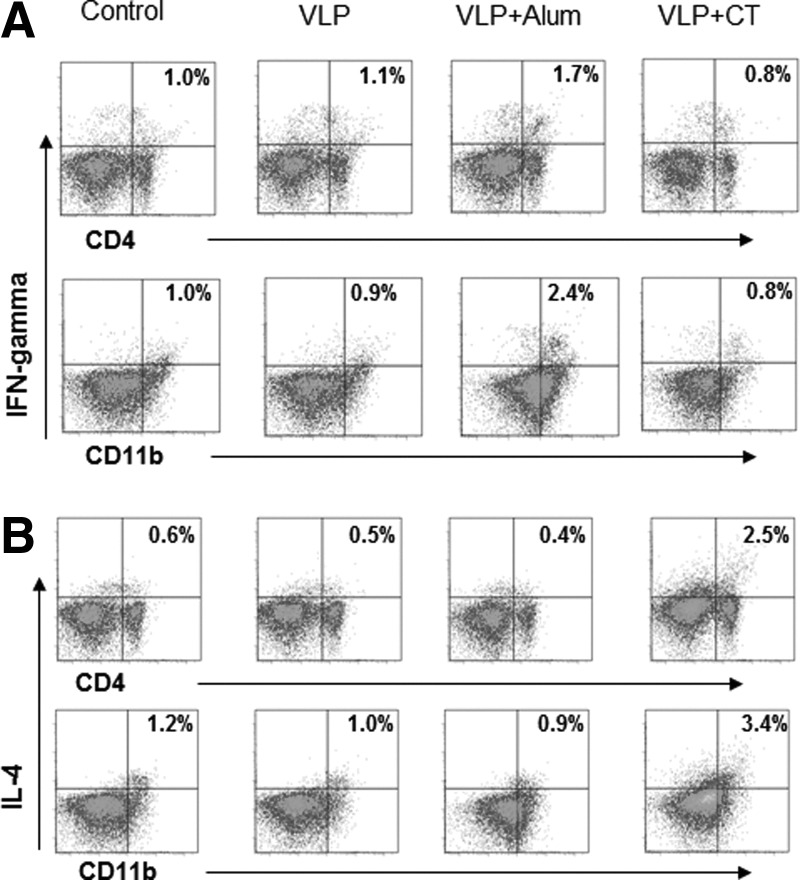
To determine the effects of adjuvant-stimulated BMDC on inducing lymphocytes to express the IFN-γ cytokine, intracellular cytokine staining of splenocytes was performed after incubation with A/PR8 VLP antigen-loaded BMDC in the presence or absence of adjuvants Alum or CT (Fig. 9). BMDC treated with A/PR8 VLPs in the presence of Alum adjuvant increased the numbers of CD4+ and CD11b+ splenic cells expressing IFN-γ (Fig. 9A) but not IL-4 cytokine (Fig. 9B). In contrast, A/PR8 VLP and CT treated BMDC showed an increase in numbers of CD4+ and CD11b+ splenic cells expressing IL-4 but not IFN-γ (Fig. 9A). Therefore, the CT and Alum adjuvants stimulate immune cells in a different manner.
FIG. 9.
Adjuvant mediated VLP antigen-loaded dendritic cells differentially induce splenocytes secreting cytokines. (A) IFN-γ intracellular cytokine staining of spleen cells upon incubation with BMDCs that had been treated A/PR8 VLP plus adjuvants. (B) IL-4 intracellular cytokine staining of spleen cells upon incubation with BMDCs that had been treated A/PR8 VLP plus adjuvants. Spleen cells were cultured for 5 days in the presence of BMDCs treated with A/PR8 VLP plus adjuvants in advance. The numbers indicate percentages of the double positive cell population. Representative data were shown from three independent experiments.
Discussion
Influenza VLP vaccines have been shown to induce protection, suggesting that VLPs can be a promising vaccine candidate (2,7,10,12,29,31,34). Immune responses after mucosal immunization are relatively low (32). Thus, mucosal immunization often requires an effective adjuvant to improve the vaccine efficacy. The present study demonstrates that adjuvants CT, Alum, MPL, or CpG, but not poly IC or gardiquimod significantly enhanced the immunogenicity of influenza A/PR8 VLPs after intranasal immunization. Different adjuvants were found to exhibit differential effects on increasing the immune responses to co-administered influenza VLP vaccines, although their mechanisms for producing different effects remain unknown. Different adjuvanted groups and the VLP alone vaccinated group showed a similar level of protection after lethal challenge, although a moderate efficacy in lowering lung viral loads was observed in the CT adjuvant group. Therefore, enhanced immunogenicity of VLP vaccines by the use of adjuvants might not faithfully translate into increased protective efficacy.
Poly IC (TLR3 ligand) and gardiquimod (TLR7 ligand) adjuvants did not increase immunogenicity following intranasal influenza VLP vaccination in this study. In a previous study, poly IC was shown to increase the immunogenicity of human immunodeficiency virus (HIV) VLPs where a high dose of antigen (20 μg) with a high dose of poly IC (50 μg, 100 μg) were used with three immunizations via the intraperitoneal route (18,36). Gardiquimod has demonstrated its efficacy in dendritic cell-based tumor immunotherapy (33,36) in which a high dose of gardiquimod (20 μg) was used. In our study, low doses of antigen (5 μg) and adjuvants (2 μg) were used because a high dose of 10 μg of adjuvant CpG mixed with influenza VLPs caused adverse symptoms after intranasal administration (data not shown). Meanwhile a high dose of Alum (10 μg or 60 μg per mouse) was used since Alum did not show any sign of toxicity after intranasal administration. Also a low dose of adjuvant was chosen since adjuvants need to be compared with the known mucosal adjuvant CT (32). This suggests that different types of antigens, doses of adjuvants, and routes of administration affect immune responses to a vaccine as also shown in previous studies (4,36).
Results shown in the present study indicate that the pattern of adaptive immune responses is also affected by types and doses of adjuvants. MPL, a TLR4 agonist, showed a trend of inducing a high level of IgG2a antibody, indicating a Th1 type immune response. A low dose of Alum (L) adjuvant showed a higher index of Th1-like IgG2a and IgG2b antibodies, but a high dose of Alum (H) or CpG induced a relatively high level of IgG1 antibody responses (Table 1). Subcutaneous immunization of HIV p24 and gp140 antigens mixed with CpG adjuvant was shown to induce dominant IgG2a antibodies to p24 antigen but IgG1 antibodies to gp120 antigen (36). Intranasal priming immunization with A/PR8 VLPs induced higher levels of IgG2a antibodies and a subsequent boost significantly increased the levels of IgG1 antibodies (data not shown). Further studies are needed for better understanding of the immune mechanisms affecting the pattern of antibody isotypes induced by vaccination.
TLR agonists MPL, CpG, poly IC and gardiquimod target dendritic cells (DCs) directly (4). Dendritic cells are known to be professional antigen presenting cells, highly effective for capturing antigens and presenting processed peptide antigens to naïve T cells in major histocompatibility complexes (25–27). TLR agonist adjuvants are known to activate DCs that are subsequently producing pro-inflammatory cytokines (19). Types of innate immune systems activated by different TLR agonist adjuvants can differentially shape the adaptive immune systems such as cellular and humoral immune responses (4,26). We found that adjuvants MPL (TLR4 agonist) or CpG (TLR9 agonist) significantly enhanced the immunogenicity of influenza VLPs. Meanwhile, poly IC (TLR3 agonist) and gardiquimod (TLR7 agonist) adjuvants did not increase immunogenicity to intranasal VLP vaccination. It was also reported that a TLR9 agonist, but not TLR7 and 8 agonists, enhanced the protective efficacy of inactivated respiratory syncytial virus vaccine (1). Therefore, a careful selection of adjuvant is important depending on specific vaccine antigens and desired types of protective immunity.
In contrast to TLR ligand adjuvants, the action mechanisms of Alum and CT are not well understood yet. Alum is the only adjuvant approved for routine use in humans in the United States. Therefore, it is an important finding that Alum as a mucosal adjuvant enhanced the immunogenicity of VLP vaccines, which will further help in developing effective mucosal vaccines. Addition of CT adjuvant to influenza VLPs enhanced the production of cytokines and cell surface activation markers of dendritic cells (BMDC), but Alum mixed with influenza VLPs did not. Meanwhile, Alum adjuvant and influenza VLP-treated dendritic cells induced higher numbers of spleen cells to secrete IFN-γ cytokine, whereas CT induced more cells to secrete IL-4 cytokine. These results show a correlation with the pattern of antibody isotypes (Fig. 2 and Table 1). That is, Th1 type IgG2a antibodies were induced by Alum adjuvant, but Th2 type IgG1 antibodies were observed in the CT-adjuvanted group. Alum was known to activate inflammasome via the production of uric acid (17). Alternatively, alum can act as an adjuvant in the presence or absence of NALP3 and IL-1β (5,16,38).
AS04 (adjuvant system 04) adjuvant was recently licensed for human use, and successfully used for recombinant human papillomavirus vaccine. (8,13,21). AS04, a mixed adjuvant of aluminum salt and TLR4 agonist MPL triggers innate immune cytokine responses leading to an activation of antigen presenting cells such as dendritic cells and thus enhanced adaptive immunity (6). The present study also demonstrated that MPL inclusion for intranasal immunization showed the adjuvant effects on increasing the immunogenicity of influenza VLP vaccine. It is important to explore the potential effects of combined adjuvants such as alum and TLR ligands in future plans.
Acknowledgments
This work was supported by a grant from the Kyung Hee University in 2012 (KHU-20120459), NIH/NIAID grants, AI093772 (S.M.K.) and AI087782 (S.M.K.).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Brewer JM, Conacher M, Satoskar A, Bluethmann H, and Alexander J. In interleukin-4-deficient mice, alum not only generates T helper 1 responses equivalent to freund's complete adjuvant, but continues to induce T helper 2 cytokine production. Eur J Immunol 1996;26:2062–2066 [DOI] [PubMed] [Google Scholar]
- 2.Bright RA, Carter DM, Daniluk S, et al. Influenza virus-like particles elicit broader immune responses than whole virion inactivated influenza virus or recombinant hemagglutinin. Vaccine 2007;25:3871–3878 [DOI] [PubMed] [Google Scholar]
- 3.Cheong HJ, Song JY, Heo JY., et al. Immunogenicity and safety of the influenza A/H1N1 2009 inactivated split-virus vaccine in young and older adults: MF59-adjuvanted vaccine versus nonadjuvanted vaccine. Clin Vaccine Immunol 2011;18:1358–1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.del Pilar Martin M, Weldon WC, Zarnitsyn VG, et al. Local response to microneedle-based influenza immunization in the skin. MBio 2012;3:e00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eisenbarth SC, Colegio OR, O'Connor W, Sutterwala FS. and Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature 2008;453:1122–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frenck RW, Jr, Belshe R, Brady RC, et al. Comparison of the immunogenicity and safety of a split-virion, inactivated, trivalent influenza vaccine (Fluzone(R)) administered by intradermal and intramuscular route in healthy adults. Vaccine 2011;29:5666–5674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galarza JM, Latham T,and Cupo A. Virus-like particle (VLP) vaccine conferred complete protection against a lethal influenza virus challenge. Viral Immunol 2005;18: 244–251 [DOI] [PubMed] [Google Scholar]
- 8.Garcon N, Morel S, Didierlaurent A, Descamps D, Wettendorff M, and Van Mechelen M. Development of an AS04-adjuvanted HPV vaccine with the adjuvant system approach. BioDrugs 2011;25:217–226 [DOI] [PubMed] [Google Scholar]
- 9.Giannini SL, Hanon E, Moris P, et al Enhanced humoral and memory B cellular immunity using HPV16/18 L1 VLP vaccine formulated with the MPL/aluminium salt combination (AS04) compared to aluminium salt only. Vaccine 2006;24:5937–5949 [DOI] [PubMed] [Google Scholar]
- 10.Haynes JR, Dokken L, Wiley JA, et al. Influenza-pseudotyped Gag virus-like particle vaccines provide broad protection against highly pathogenic avian influenza challenge. Vaccine 2009;27:530–541 [DOI] [PubMed] [Google Scholar]
- 11.Kang JH, Oh CE, Lee J, et al. Safety and immunogenicity of a new trivalent inactivated split-virus influenza vaccine in healthy Korean children: A randomized, double-blinded, active-controlled, phase III study. J Korean Med Sci 2011;26:1421–1427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kang SM, Song JM, Quan FS, and Compans RW. Influenza vaccines based on virus-like particles. Virus Res 2009;143:140–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khatun S, Akram Hussain SM, Chowdhury S, et al. Safety and immunogenicity profile of human papillomavirus-16/18 AS04 adjuvant cervical cancer vaccine: A randomized controlled trial in healthy adolescent girls of Bangladesh. Jpn J Clin Oncol 2012;42:36–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim MC, Song JM, Kwon EOYM, Lee YJ, Compans RW, and Kang SM. Virus-like particles containing multiple M2 extracellular domains confer improved cross-protection against various subtypes of influenza virus. Mol Ther 2013;21:485–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim YC, Park JH, and Prausnitz MR. Microneedles for drug and vaccine delivery. Adv Drug Deliv Rev 2012;64:1547–1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kool M, Petrilli V, De Smedt T, et al. Cutting Edge: Alum adjuvant stimulates inflammatory dendritic cells through activation of the NALP3 inflammasome. J Immunol 2008;181:3755–3759 [DOI] [PubMed] [Google Scholar]
- 17.Kool M, Soullie T, van Nimwegen M, et al. Alum adjuvant boosts adaptive immunity by inducing uric acid and activating inflammatory dendritic cells. J Exp Med 2008;205: 869–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koutsonanos DG, Vassilieva EV, Stavropoulou A, et al. Delivery of subunit influenza vaccine to skin with microneedles improves immunogenicity and long-lived protection. Sci Rep 2012;2: 357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kwissa M., Amara RR, Robinson HL, et al. Adjuvanting a DNA vaccine with a TLR9 ligand plus Flt3 ligand results in enhanced cellular immunity against the simian immunodeficiency virus. J Exp Med 2007;204:2733–2746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin M, Michalek SM, and Katz J. Role of innate immune factors in the adjuvant activity of monophosphoryl lipid A. Infect Immun 2003;71:2498–2507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McKeage K, and Romanowski B. AS04-adjuvanted human papillomavirus (HPV) types 16 and 18 vaccine (Cervarix(R)): A review of its use in the prevention of premalignant cervical lesions and cervical cancer causally related to certain oncogenic HPV types. Drugs 2011;71:465–488 [DOI] [PubMed] [Google Scholar]
- 22.Okamoto S, Matsuoka S, Takenaka N, et al. Intranasal immunization with a formalin-inactivated human influenza A virus whole-virion vaccine alone and intranasal immunization with a split-virion vaccine with mucosal adjuvants show similar levels of cross-protection. Clin Vaccine Immunol 2012;19:979–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Persing DH, Coler RN, Lacy MJ, et al. Taking toll: Lipid A mimetics as adjuvants and immunomodulators. Trends Microbiol 2002;10:S32–37 [DOI] [PubMed] [Google Scholar]
- 24.Plotkin SA. Vaccines: Past, present and future. Nat Med 2005;11: S5–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pulendran B. Modulating vaccine responses with dendritic cells and Toll-like receptors. Immunol Rev 2004;199:227–250 [DOI] [PubMed] [Google Scholar]
- 26.Pulendran B, and Ahmed R. Translating innate immunity into immunological memory: Implications for vaccine development. Cell 2006;124:849–863 [DOI] [PubMed] [Google Scholar]
- 27.Pulendran B, Lingappa J, Kennedy MK, et al. Developmental pathways of dendritic cells in vivo: Distinct function, phenotype, and localization of dendritic cell subsets in FLT3 ligand- treated mice. J Immunol 1997;159:2222–2231 [PubMed] [Google Scholar]
- 28.Quan FS, Compans RW, Nguyen HH, and Kang SM. Induction of heterosubtypic immunity to influenza virus by intranasal immunization. J Virol 2008;82:1350–1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quan FS, Kim MC, Lee BJ, Song JM, Compans RW, and Kang SM. Influenza M1 VLPs containing neuraminidase induce heterosubtypic cross-protection. Virology 2012;430:127–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quan FS, Steinhauer D, Huang C, Ross TM, Compans RW, and Kang SM. A bivalent influenza VLP vaccine confers complete inhibition of virus replication in lungs. Vaccine 2008;26:3352–3361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quan FS, Vunnava A, Compans RW, and Kang SM. Virus-like particle vaccine protects against 2009 H1N1 pandemic influenza virus in mice. PLoS One 2010;5:e9161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quan FS, Yoo DG, Song JM, Clements JD, Compans RW, and Kang SM. Kinetics of immune responses to influenza virus-like particles and dose-dependence of protection with a single vaccination. J Virol 2009;83:4489–4497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ragone G, Bresin A, Piermarini F, et al. The Tcl1 oncogene defines secondary hair germ cells differentiation at catagen-telogen transition and affects stem-cell marker CD34 expression. Oncogene 2009;28:1329–1338 [DOI] [PubMed] [Google Scholar]
- 34.Ross TM, Mahmood K, Crevar CJ, Schneider-Ohrum K, Heaton PM, and Bright RA. A trivalent virus-like particle vaccine elicits protective immune responses against seasonal influenza strains in mice and ferrets. PLoS One 2009;4:e6032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Skountzou I, Martin MD, Wang B, et al. Salmonella flagellins are potent adjuvants for intranasally administered whole inactivated influenza vaccine. Vaccine 2010;28:4103–4112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Visciano ML, Tagliamonte M, Tornesello ML, Buonaguro FM, and Buonaguro L. Effects of adjuvants on IgG subclasses elicited by virus-like particles. J Transl Med 2012;10:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang BZ, Xu R, Quan FS, Kang SM, Wang L, and Compans RW. Intranasal immunization with influenza VLPs incorporating membrane-anchored flagellin induces strong heterosubtypic protection. PLoS One 2010;5:e13972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang XY, Yao X, Wan YM, Wang B, Xu JQ, and Wen YM. Responses to multiple injections with alum alone compared to injections with alum adsorbed to proteins in mice. Immunol Lett 2013;149:88–92 [DOI] [PubMed] [Google Scholar]
- 39.Weldon WC, Zarnitsyn VG, Esser ES, et al. Effect of adjuvants on responses to skin immunization by microneedles coated with influenza subunit vaccine. PLoS One 2012;7:e41501. [DOI] [PMC free article] [PubMed] [Google Scholar]