Abstract
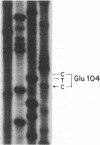
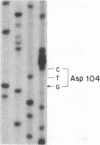
Triose-phosphate isomerase (TPI; D-glyceraldehyde-3-phosphate ketol-isomerase, EC 5.3.1.1) deficiency is a recessive disorder that results in hemolytic anemia and neuromuscular dysfunction. To determine the molecular basis of this disorder, a TPI allele from two unrelated patients homozygous for TPI deficiency was compared with an allele from a normal individual. Each disease-associated sequence harbors a G X C----C X G transversion in the codon for amino acid-104 and specifies a structurally altered protein in which a glutamate residue is replaced by an aspartate residue. The importance of glutamate-104 to enzyme structure and function is implicated by its conservation in the TPI protein of all species that have been characterized to date. The glutamate-to-aspartate substitution results in a thermolabile enzyme as demonstrated by assays of TPI activity in cultured fibroblasts of each patient and cultured Chinese hamster ovary (CHO) cells that were stably transformed with the mutant alleles. Although this substitution conserves the overall charge of amino acid-104, the x-ray crystal structure of chicken TPI indicates that the loss of a side-chain methylene group (-CH2CH2COO- ---- -CH2COO-) is sufficient to disrupt the counterbalancing of charges that normally exists within a hydrophobic pocket of the native enzyme.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alber T., Banner D. W., Bloomer A. C., Petsko G. A., Phillips D., Rivers P. S., Wilson I. A. On the three-dimensional structure and catalytic mechanism of triose phosphate isomerase. Philos Trans R Soc Lond B Biol Sci. 1981 Jun 26;293(1063):159–171. doi: 10.1098/rstb.1981.0069. [DOI] [PubMed] [Google Scholar]
- Alber T., Hartman F. C., Johnson R. M., Petsko G. A., Tsernoglou D. Crystallization of yeast triose phosphate isomerase from polyethylene glycol. Protein crystal formation following phase separation. J Biol Chem. 1981 Feb 10;256(3):1356–1361. [PubMed] [Google Scholar]
- Alber T., Kawasaki G. Nucleotide sequence of the triose phosphate isomerase gene of Saccharomyces cerevisiae. J Mol Appl Genet. 1982;1(5):419–434. [PubMed] [Google Scholar]
- Albery W. J., Knowles J. R. Evolution of enzyme function and the development of catalytic efficiency. Biochemistry. 1976 Dec 14;15(25):5631–5640. doi: 10.1021/bi00670a032. [DOI] [PubMed] [Google Scholar]
- Artavanis-Tsakonas S., Harris J. I. Primary structure of triosephosphate isomerase from Bacillus stearothermophilus. Eur J Biochem. 1980 Jul;108(2):599–611. doi: 10.1111/j.1432-1033.1980.tb04755.x. [DOI] [PubMed] [Google Scholar]
- Banner D. W., Bloomer A. C., Petsko G. A., Phillips D. C., Pogson C. I., Wilson I. A., Corran P. H., Furth A. J., Milman J. D., Offord R. E. Structure of chicken muscle triose phosphate isomerase determined crystallographically at 2.5 angstrom resolution using amino acid sequence data. Nature. 1975 Jun 19;255(5510):609–614. doi: 10.1038/255609a0. [DOI] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Brown J. R., Daar I. O., Krug J. R., Maquat L. E. Characterization of the functional gene and several processed pseudogenes in the human triosephosphate isomerase gene family. Mol Cell Biol. 1985 Jul;5(7):1694–1706. doi: 10.1128/mcb.5.7.1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corran P. H., Waley S. G. The amino acid sequence of rabbit muscle triose phosphate isomerase. Biochem J. 1975 Feb;145(2):335–344. doi: 10.1042/bj1450335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker R. S., Mohrenweiser H. W. Cell proliferation-associated expression of a recently evolved isozyme of triosephosphate isomerase. Biochem Genet. 1985 Apr;23(3-4):267–280. doi: 10.1007/BF00504324. [DOI] [PubMed] [Google Scholar]
- Decker R. S., Mohrenweiser H. W. Origin of the triosephosphate isomerase isozymes in humans: genetic evidence for the expression of a single structural locus. Am J Hum Genet. 1981 Sep;33(5):683–691. [PMC free article] [PubMed] [Google Scholar]
- Eber S. W., Dünnwald M., Belohradsky B. H., Bidlingmaier F., Schievelbein H., Weinmann H. M., Krietsch K. G. Hereditary deficiency of triosephosphate isomerase in four unrelated families. Eur J Clin Invest. 1979 Jun;9(3):195–202. doi: 10.1111/j.1365-2362.1979.tb00923.x. [DOI] [PubMed] [Google Scholar]
- Frischauf A. M., Lehrach H., Poustka A., Murray N. Lambda replacement vectors carrying polylinker sequences. J Mol Biol. 1983 Nov 15;170(4):827–842. doi: 10.1016/s0022-2836(83)80190-9. [DOI] [PubMed] [Google Scholar]
- Furth A. J., Milman J. D., Priddle J. D., Offord R. E. Studies on the subunit structure and amino acid sequence of trisoe phosphate isomerase from chicken breast muscle. Biochem J. 1974 Apr;139(1):11–22. doi: 10.1042/bj1390011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gritz L., Davies J. Plasmid-encoded hygromycin B resistance: the sequence of hygromycin B phosphotransferase gene and its expression in Escherichia coli and Saccharomyces cerevisiae. Gene. 1983 Nov;25(2-3):179–188. doi: 10.1016/0378-1119(83)90223-8. [DOI] [PubMed] [Google Scholar]
- Grütter M. G., Hawkes R. B., Matthews B. W. Molecular basis of thermostability in the lysozyme from bacteriophage T4. Nature. 1979 Feb 22;277(5698):667–669. doi: 10.1038/277667a0. [DOI] [PubMed] [Google Scholar]
- Hawkes R., Grutter M. G., Schellman J. Thermodynamic stability and point mutations of bacteriophage T4 lysozyme. J Mol Biol. 1984 May 15;175(2):195–212. doi: 10.1016/0022-2836(84)90474-1. [DOI] [PubMed] [Google Scholar]
- Jongsma A. P., Hagemeijer A., Meera Khan P. Regional mapping of TPI, LDH-B, Pep-B on chromosome 12 of man. Birth Defects Orig Artic Ser. 1975;11(3):189–191. [PubMed] [Google Scholar]
- Kester M. V., Jacobson E. L., Gracy R. W. The synthesis of a labile triosephosphate isomerase isozyme in human lymphoblasts and fibroblasts. Arch Biochem Biophys. 1977 Apr 30;180(2):562–569. doi: 10.1016/0003-9861(77)90074-1. [DOI] [PubMed] [Google Scholar]
- Kolb E., Harris J. I., Bridgen J. Triose phosphate isomerase from the coelacanth. An approach to the rapid determination of an amino acid sequence with small amounts of material. Biochem J. 1974 Feb;137(2):185–197. doi: 10.1042/bj1370185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H. S., Yuan P. M., Gracy R. W. Primary structure of human triosephosphate isomerase. J Biol Chem. 1984 Oct 10;259(19):11958–11968. [PubMed] [Google Scholar]
- Maquat L. E., Chilcote R., Ryan P. M. Human triosephosphate isomerase cDNA and protein structure. Studies of triosephosphate isomerase deficiency in man. J Biol Chem. 1985 Mar 25;260(6):3748–3753. [PubMed] [Google Scholar]
- Matthews B. W., Weaver L. H., Kester W. R. The conformation of thermolysin. J Biol Chem. 1974 Dec 25;249(24):8030–8044. [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Mohrenweiser H. W., Fielek S. Elevated frequency of carriers for triosephosphate isomerase deficiency in newborn infants. Pediatr Res. 1982 Nov;16(11):960–963. doi: 10.1203/00006450-198211000-00012. [DOI] [PubMed] [Google Scholar]
- Mohrenweiser H. W., Fielek S., Wurzinger K. H. Characteristics of enzymes of erythrocytes from newborn infants and adults: activity, thermostability, and electrophoretic profile as a function of cell age. Am J Hematol. 1981 Sep;11(2):125–136. doi: 10.1002/ajh.2830110203. [DOI] [PubMed] [Google Scholar]
- Mohrenweiser H. W. Frequency of enzyme deficiency variants in erythrocytes of newborn infants. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5046–5050. doi: 10.1073/pnas.78.8.5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perutz M. F., Raidt H. Stereochemical basis of heat stability in bacterial ferredoxins and in haemoglobin A2. Nature. 1975 May 15;255(5505):256–259. doi: 10.1038/255256a0. [DOI] [PubMed] [Google Scholar]
- Peters J., Hopkinson D. A., Harris H. Genetic and non-genetic variation of triose phosphate isomerase isozymes in human tissues. Ann Hum Genet. 1973 Jan;36(3):297–312. doi: 10.1111/j.1469-1809.1973.tb00592.x. [DOI] [PubMed] [Google Scholar]
- Petsko G. A., Davenport R. C., Jr, Frankel D., RaiBhandary U. L. Probing the catalytic mechanism of yeast triose phosphate isomerase by site-specific mutagenesis. Biochem Soc Trans. 1984 Apr;12(2):229–232. doi: 10.1042/bst0120229. [DOI] [PubMed] [Google Scholar]
- Phillips D. C., Sternberg M. J., Thornton J. M., Wilson I. A. An analysis of the structure of triose phosphate isomerase and its comparison with lactate dehydrogenase. J Mol Biol. 1978 Feb 25;119(2):329–351. doi: 10.1016/0022-2836(78)90440-0. [DOI] [PubMed] [Google Scholar]
- Pichersky E., Gottlieb L. D., Hess J. F. Nucleotide sequence of the triose phosphate isomerase gene of Escherichia coli. Mol Gen Genet. 1984;195(1-2):314–320. doi: 10.1007/BF00332765. [DOI] [PubMed] [Google Scholar]
- RIEDER S. V., ROSE I. A. The mechanism of the triosephosphate isomerase reaction. J Biol Chem. 1959 May;234(5):1007–1010. [PubMed] [Google Scholar]
- Rogers P. A., Brenton D. P., Hopkinson D. A. Changes in the activity and isozyme patterns of glycolytic enzymes during stimulation of normal human lymphocytes with phytohaemagglutinin. Ann Hum Genet. 1980 Jan;43(3):213–226. doi: 10.1111/j.1469-1809.1980.tb01555.x. [DOI] [PubMed] [Google Scholar]
- Ross J. A precursor of globin messenger RNA. J Mol Biol. 1976 Sep 15;106(2):403–420. doi: 10.1016/0022-2836(76)90093-0. [DOI] [PubMed] [Google Scholar]
- SCHNEIDER A. S., VALENTINE W. N., HATTORI M., HEINS H. L., Jr HEREDITARY HEMOLYTIC ANEMIA WITH TRIOSEPHOSPHATE ISOMERASE DEFICIENCY. N Engl J Med. 1965 Feb 4;272:229–235. doi: 10.1056/NEJM196502042720503. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh C., Neel J. V., Yamashita A., Goriki K., Fujita M., Hamilton H. B. The frequency among Japanese of heterozygotes for deficiency variants of 11 enzymes. Am J Hum Genet. 1983 Jul;35(4):656–674. [PMC free article] [PubMed] [Google Scholar]
- Scalenghe F., Turco E., Edström J. E., Pirrotta V., Melli M. Microdissection and cloning of DNA from a specific region of Drosophila melanogaster polytene chromosomes. Chromosoma. 1981;82(2):205–216. doi: 10.1007/BF00286105. [DOI] [PubMed] [Google Scholar]
- Straus D., Gilbert W. Chicken triosephosphate isomerase complements an Escherichia coli deficiency. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2014–2018. doi: 10.1073/pnas.82.7.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus D., Raines R., Kawashima E., Knowles J. R., Gilbert W. Active site of triosephosphate isomerase: in vitro mutagenesis and characterization of an altered enzyme. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2272–2276. doi: 10.1073/pnas.82.8.2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vives-Corrons J. L., Rubinson-Skala H., Mateo M., Estella J., Feliu E., Dreyfus J. C. Triosephosphate isomerase deficiency with hemolytic anemia and severe neuromuscular disease: familial and biochemical studies of a case found in Spain. Hum Genet. 1978 Jun 9;42(2):171–180. doi: 10.1007/BF00283637. [DOI] [PubMed] [Google Scholar]
- Weaver L. H., Kester W. R., Ten Eyck L. F., Matthews B. W. The structure and stability of thermolysin. Experientia Suppl. 1976;26:31–39. doi: 10.1007/978-3-0348-7675-9_2. [DOI] [PubMed] [Google Scholar]
- Wigler M., Silverstein S., Lee L. S., Pellicer A., Cheng Y. c., Axel R. Transfer of purified herpes virus thymidine kinase gene to cultured mouse cells. Cell. 1977 May;11(1):223–232. doi: 10.1016/0092-8674(77)90333-6. [DOI] [PubMed] [Google Scholar]