Abstract
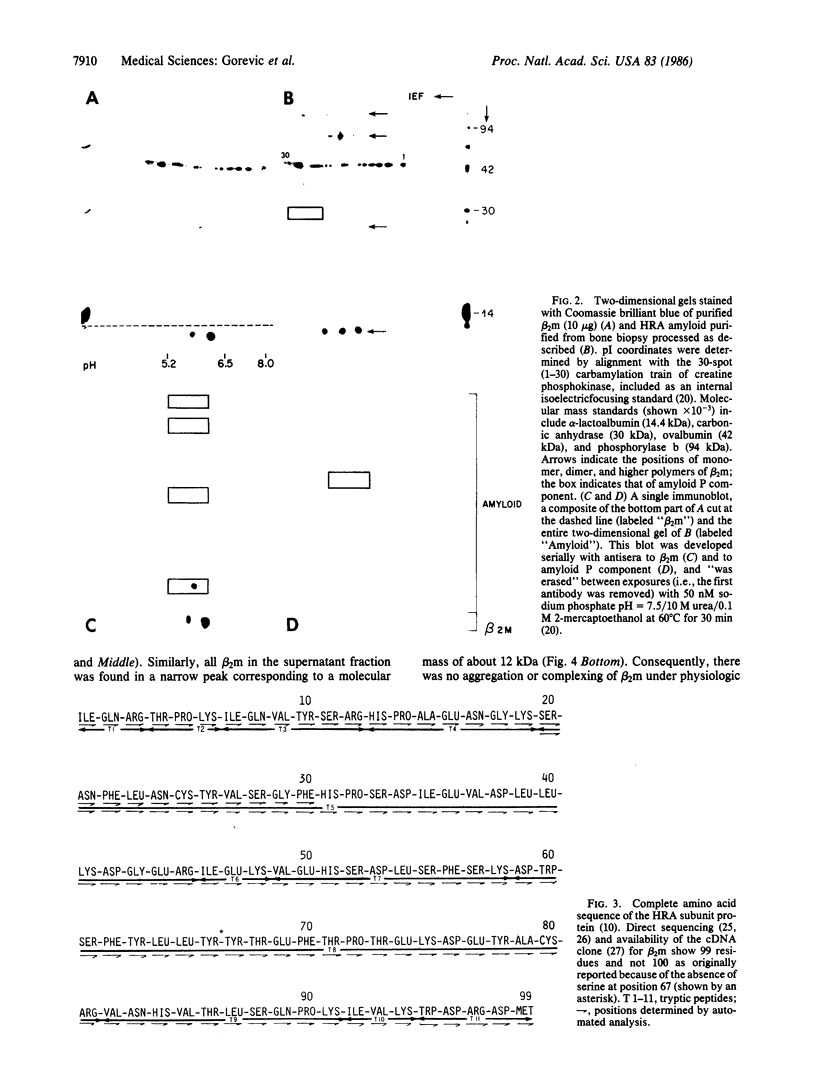
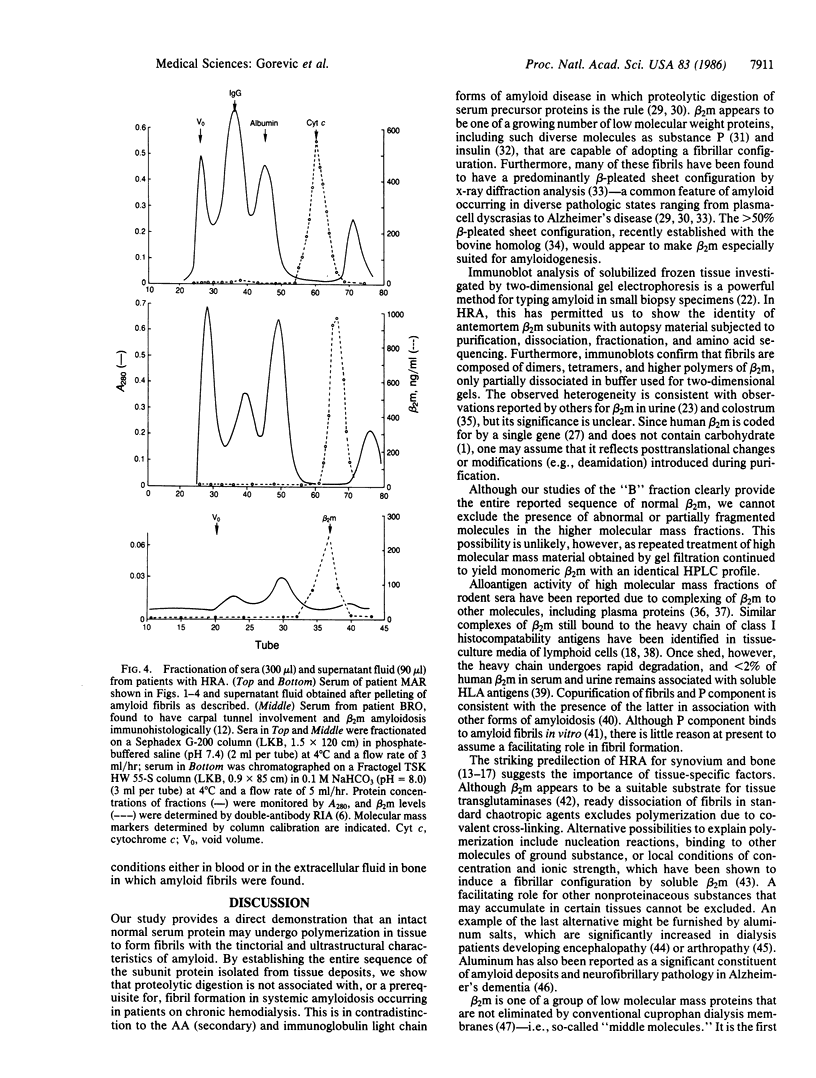
Systemic amyloidosis with a predilection for bone and synovium may complicate the course of patients on long-term hemodialysis. This form of amyloidosis can be typed as distinct from other amyloid diseases by using small tissue samples obtained by bone biopsy and at postmortem. Immunoblot analysis of two-dimensional gels of partially solubilized amyloid fibrils established that tissue deposits are composed of monomers, dimers, and higher polymers of beta 2-microglobulin (beta 2m) and that amyloid P component was also present. Anti-beta 2m antiserum recognized fibrils, as shown by immunoelectron microscopy. Purified monomer isolated from dissociated fibrils yielded peptides corresponding to the entire known sequence of beta 2m. Virtually all serum beta 2m, as well as that present in tissue fluid bathing amyloid fibrils, was monomeric. Hemodialysis-related amyloidosis is an example of a deposition disease occurring in hemodialysis patients. We have shown conclusively that, in this amyloid disease, polymerization of an intact normal serum protein to a fibrillar configuration may occur without proteolysis. We propose the designation A beta 2m for this form of amyloid fibril subunit protein.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson N. L., Anderson N. G. Analytical techniques for cell fractions. XXII. Two-dimensional analysis of serum and tissue proteins: multiple gradient-slab gel electrophoresis. Anal Biochem. 1978 Apr;85(2):341–354. doi: 10.1016/0003-2697(78)90230-0. [DOI] [PubMed] [Google Scholar]
- Bardin T., Kuntz D., Zingraff J., Voisin M. C., Zelmar A., Lansaman J. Synovial amyloidosis in patients undergoing long-term hemodialysis. Arthritis Rheum. 1985 Sep;28(9):1052–1058. doi: 10.1002/art.1780280913. [DOI] [PubMed] [Google Scholar]
- Becker J. W., Reeke G. N., Jr Three-dimensional structure of beta 2-microglobulin. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4225–4229. doi: 10.1073/pnas.82.12.4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berggård I., Bearn A. G. Isolation and properties of a low molecular weight beta-2-globulin occurring in human biological fluids. J Biol Chem. 1968 Aug 10;243(15):4095–4103. [PubMed] [Google Scholar]
- Bernier G. M., Fanger M. W. Synthesis of 2 -microglobulin by stimulated lymphocytes. J Immunol. 1972 Aug;109(2):407–409. [PubMed] [Google Scholar]
- Casey T. T., Stone W. J., DiRaimondo C. R., Brantley B. D., DiRaimondo C. V., Gorevic P. D., Page D. L. Tumoral amyloidosis of bone of beta 2-microglobulin origin in association with long-term hemodialysis: a new type of amyloid disease. Hum Pathol. 1986 Jul;17(7):731–738. doi: 10.1016/s0046-8177(86)80183-6. [DOI] [PubMed] [Google Scholar]
- Connors L. H., Shirahama T., Skinner M., Fenves A., Cohen A. S. In vitro formation of amyloid fibrils from intact beta 2-microglobulin. Biochem Biophys Res Commun. 1985 Sep 30;131(3):1063–1068. doi: 10.1016/0006-291x(85)90198-6. [DOI] [PubMed] [Google Scholar]
- Cunningham B. A., Wang J. L., Berggård I., Peterson P. A. The complete amino acid sequence of beta 2-microglobulin. Biochemistry. 1973 Nov 20;12(24):4811–4822. doi: 10.1021/bi00748a001. [DOI] [PubMed] [Google Scholar]
- Fésüs L., Falus A., Erdei A., Laki K. Human beta 2-microglobulin is a substrate of tissue transglutaminase: polymerization in solution and on the cell surface. J Cell Biol. 1981 Jun;89(3):706–710. doi: 10.1083/jcb.89.3.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gejyo F., Yamada T., Odani S., Nakagawa Y., Arakawa M., Kunitomo T., Kataoka H., Suzuki M., Hirasawa Y., Shirahama T. A new form of amyloid protein associated with chronic hemodialysis was identified as beta 2-microglobulin. Biochem Biophys Res Commun. 1985 Jun 28;129(3):701–706. doi: 10.1016/0006-291x(85)91948-5. [DOI] [PubMed] [Google Scholar]
- Glenner G. G. Amyloid deposits and amyloidosis. The beta-fibrilloses (first of two parts). N Engl J Med. 1980 Jun 5;302(23):1283–1292. doi: 10.1056/NEJM198006053022305. [DOI] [PubMed] [Google Scholar]
- Glenner G. G. Amyloid deposits and amyloidosis: the beta-fibrilloses (second of two parts). N Engl J Med. 1980 Jun 12;302(24):1333–1343. doi: 10.1056/NEJM198006123022403. [DOI] [PubMed] [Google Scholar]
- Glenner G. G., Eanes E. D., Bladen H. A., Linke R. P., Termine J. D. Beta-pleated sheet fibrils. A comparison of native amyloid with synthetic protein fibrils. J Histochem Cytochem. 1974 Dec;22(12):1141–1158. doi: 10.1177/22.12.1141. [DOI] [PubMed] [Google Scholar]
- Gorevic P. D., Casey T. T., Stone W. J., DiRaimondo C. R., Prelli F. C., Frangione B. Beta-2 microglobulin is an amyloidogenic protein in man. J Clin Invest. 1985 Dec;76(6):2425–2429. doi: 10.1172/JCI112257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorevic P. D., Rodrigues M. M., Krachmer J. H., Green C., Fujihara S., Glenner G. G. Lack of evidence for protein AA reactivity in amyloid deposits of lattice corneal dystrophy and amyloid corneal degeneration. Am J Ophthalmol. 1984 Aug 15;98(2):216–224. doi: 10.1016/0002-9394(87)90357-6. [DOI] [PubMed] [Google Scholar]
- Grey H. M., Kubo R. T., Colon S. M., Poulik M. D., Cresswell P., Springer T., Turner M., Strominger J. L. The small subunit of HL-A antigens is beta 2-microglobulin. J Exp Med. 1973 Dec 1;138(6):1608–1612. doi: 10.1084/jem.138.6.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall P. W., Ricanati E. S., Vacca C. V. Characterization of human beta2-microglobulin by isoelectric focusing. Clin Chim Acta. 1977 May 16;77(1):37–42. doi: 10.1016/0009-8981(77)90399-0. [DOI] [PubMed] [Google Scholar]
- Huaux J. P., Noël H., Malghem J., Maldague B., Devogelaer J. P., Nagant de Deuxchaisnes C. Erosive azotemic osteoarthropathy: possible role of amyloidosis. Arthritis Rheum. 1985 Sep;28(9):1075–1076. doi: 10.1002/art.1780280918. [DOI] [PubMed] [Google Scholar]
- Kirschner D. A., Abraham C., Selkoe D. J. X-ray diffraction from intraneuronal paired helical filaments and extraneuronal amyloid fibers in Alzheimer disease indicates cross-beta conformation. Proc Natl Acad Sci U S A. 1986 Jan;83(2):503–507. doi: 10.1073/pnas.83.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters C. L., Multhaup G., Simms G., Pottgiesser J., Martins R. N., Beyreuther K. Neuronal origin of a cerebral amyloid: neurofibrillary tangles of Alzheimer's disease contain the same protein as the amyloid of plaque cores and blood vessels. EMBO J. 1985 Nov;4(11):2757–2763. doi: 10.1002/j.1460-2075.1985.tb04000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyakawa Y., Tanigaki N., Kreiter V. P., Moore G. E., Pressman D. Characterization of soluble substances in plasma carrying HL-A alloantigenic activity and HL-A common antigenic activity. Transplantation. 1973 Mar;15(3):312–319. doi: 10.1097/00007890-197303000-00008. [DOI] [PubMed] [Google Scholar]
- Natori T., Hansen T. Component fragments obtained by acid dissociation of the alpha- glycoprotein associated with beta2-microglobulin in mouse plasma. Transplantation. 1977 Feb;23(2):191–195. [PubMed] [Google Scholar]
- Netter P., Kessler M., Burnel D., Hutin M. F., Delones S., Benoit J., Gaucher A. Aluminum in the joint tissues of chronic renal failure patients treated with regular hemodialysis and aluminum compounds. J Rheumatol. 1984 Feb;11(1):66–70. [PubMed] [Google Scholar]
- Parker K. C., Strominger J. L. Sequence of human beta 2-microglobulin: a correction. Mol Immunol. 1982 Mar;19(3):503–504. doi: 10.1016/0161-5890(82)90217-6. [DOI] [PubMed] [Google Scholar]
- Parkinson I. S., Ward M. K., Feest T. G., Fawcett R. W., Kerr D. N. Fracturing dialysis osteodystrophy and dialysis encephalopathy. An epidemiological survey. Lancet. 1979 Feb 24;1(8113):406–409. doi: 10.1016/s0140-6736(79)90883-3. [DOI] [PubMed] [Google Scholar]
- Pepys M. B., Baltz M. L., de Beer F. C., Dyck R. F., Holford S., Breathnach S. M., Black M. M., Tribe C. R., Evans D. J., Feinstein A. Biology of serum amyloid P component. Ann N Y Acad Sci. 1982;389:286–298. doi: 10.1111/j.1749-6632.1982.tb22144.x. [DOI] [PubMed] [Google Scholar]
- Pepys M. B., Dyck R. F., de Beer F. C., Skinner M., Cohen A. S. Binding of serum amyloid P-component (SAP) by amyloid fibrils. Clin Exp Immunol. 1979 Nov;38(2):284–293. [PMC free article] [PubMed] [Google Scholar]
- Perry E. K., Oakley A. E., Candy J. M., Perry R. H. Properties and possible significance of substance P and insulin fibrils. Neurosci Lett. 1981 Sep 25;25(3):321–325. doi: 10.1016/0304-3940(81)90412-2. [DOI] [PubMed] [Google Scholar]
- Peterson P. A., Cunningham B. A., Berggård I., Edelman G. M. 2 -Microglobulin--a free immunoglobulin domain. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1697–1701. doi: 10.1073/pnas.69.7.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulik M. D., Bloom A. D. Beta 2 -microglobulin production and secretion by lymphocytes in culture. J Immunol. 1973 May;110(5):1430–1433. [PubMed] [Google Scholar]
- Poulik M. D., Perry D. J., Sekine T. Statistical analysis of beta 2-microglobulin levels in sera of lung and GI tract cancer patients. Vox Sang. 1980 Jun;38(6):328–333. doi: 10.1111/j.1423-0410.1980.tb04501.x. [DOI] [PubMed] [Google Scholar]
- Poulik M. D. beta2-Microglobulin: present status. Prog Clin Biol Res. 1976;5:155–177. [PubMed] [Google Scholar]
- Prelli F., Pras M., Frangione B. The primary structure of human tissue amyloid P component from a patient with primary idiopathic amyloidosis. J Biol Chem. 1985 Oct 25;260(24):12895–12898. [PubMed] [Google Scholar]
- Roth J., Bendayan M., Carlemalm E., Villiger W., Garavito M. Enhancement of structural preservation and immunocytochemical staining in low temperature embedded pancreatic tissue. J Histochem Cytochem. 1981 May;29(5):663–671. doi: 10.1177/29.5.6166664. [DOI] [PubMed] [Google Scholar]
- Röckel A., Gilge U., Liewald A., Heidland A. Elimination of low molecular weight proteins during hemofiltration. Artif Organs. 1982 Aug;6(3):307–311. doi: 10.1111/j.1525-1594.1982.tb01678.x. [DOI] [PubMed] [Google Scholar]
- Sebert J. L., Fardellone P., Marie A., Deramond H., Lambrey G., Legars D., Galibert P., Smajda A., Fournier A. Destructive spondylarthropathy in hemodialyzed patients: possible role of amyloidosis. Arthritis Rheum. 1986 Feb;29(2):301–303. doi: 10.1002/art.1780290222. [DOI] [PubMed] [Google Scholar]
- Shirahama T., Skinner M., Cohen A. S., Gejyo F., Arakawa M., Suzuki M., Hirasawa Y. Histochemical and immunohistochemical characterization of amyloid associated with chronic hemodialysis as beta 2-microglobulin. Lab Invest. 1985 Dec;53(6):705–709. [PubMed] [Google Scholar]
- Smithies O., Poulik M. D. Initiation of protein synthesis at an unusual position in an immunoglobulin gene? Science. 1972 Jan 14;175(4018):187–189. doi: 10.1126/science.175.4018.187. [DOI] [PubMed] [Google Scholar]
- Vincent C., Revillard J. P. Characterization of molecules bearing HLA determinants in serum and urine. Transplant Proc. 1979 Jun;11(2):1301–1302. [PubMed] [Google Scholar]
- Vincent C., Revillard J. P., Galland M., Traeger J. Serum beta2-microglobulin in hemodialyzed patients. Nephron. 1978;21(5):260–268. doi: 10.1159/000181402. [DOI] [PubMed] [Google Scholar]
- Wibell L., Evrin P. E., Berggård I. Serum 2 -microglobulin in renal disease. Nephron. 1973;10(5):320–331. doi: 10.1159/000180203. [DOI] [PubMed] [Google Scholar]