PARP inhibitors have shown promising activity in patients with BRCA1/2 mutation-associated ovarian and breast cancers. Accumulating evidence suggests that PARPi may have a wider application in the cancers defective in DNA repair pathways. Understanding more about the molecular abnormalities, exploring novel therapeutic trial strategies and defining potential predictive biomarkers, is critical to rapidly advancing the field of PARPi therapy.
Keywords: parp inhibitor, brca-like cancers, brca1/2 mutation, brca1/2 mutation-associated cancers
Abstract
Poly(ADP-ribose)polymerase inhibitors (PARPis) have shown promising activity in patients with BRCA1/2 mutation-associated (BRCA1/2MUT+) ovarian and breast cancers. Accumulating evidence suggests that PARPi may have a wider application in the treatment of sporadic high-grade serous ovarian cancer, and cancers defective in DNA repair pathways, such as prostate, endometrial, and pancreatic cancers. Several PARPis are currently in phase 1/2 clinical investigation, with registration trials now being designed. Olaparib, one of the most studied PARPis, has demonstrated activity in BRCA1/2MUT+ and BRCA-like sporadic ovarian and breast cancers, and looks promising in prostate and pancreatic cancers. Understanding more about the molecular abnormalities involved in BRCA-like tumors, exploring novel therapeutic trial strategies and drug combinations, and defining potential predictive biomarkers, is critical to rapidly advancing the field of PARPi therapy and improve clinical outcomes.
introduction
Progress has been made over the past two decades in the diagnosis, treatment, and prevention of cancer. A key component of progress in women's cancers was the cloning of the BRCA1 and BRCA2 genes [1, 2] and reporting of The Cancer Genome Atlas' (TCGA) comprehensive molecular analyses of high-grade serous ovarian cancer (HGSOC) and breast cancers [3, 4]. This knowledge is being translated into clinical opportunities through application of these new molecular definitions to tailor therapeutics uniquely to the individual patient.
Knowledge of BRCA1/2 mutation status in a patient has gone from a research question to demonstrated clinical utility directly affecting patient care. Dissection of their normal roles, both critical in normal DNA damage and repair, has led to better understanding of how their loss may cause or alter the course of cancer. Interestingly, neither knock-out nor knock-in models have demonstrated BRCA-1 or -2 to be independently causative in cancer development. They are embryonically lethal in knock-out settings, like many other tumor-suppressor genes [5]; selected knock-out is complementary to second genomic hits. The data for causality come from epidemiologic studies that define a tight relationship between deleterious BRCA-1 and -2 mutations (BRCA1/2MUT+) and development of breast and ovarian cancers [6], and increasingly with other cancers [7]. The seminal advance since the cloning and recognition of the relationship between loss-of-function mutations and breast and ovarian cancers is the identification, validation, and application of new biologically important molecular targets, poly-ADP ribose polymerase (PARP)-1 and PARP family members, and other proteins involved in homologous recombination (HR) repair of DNA damage.
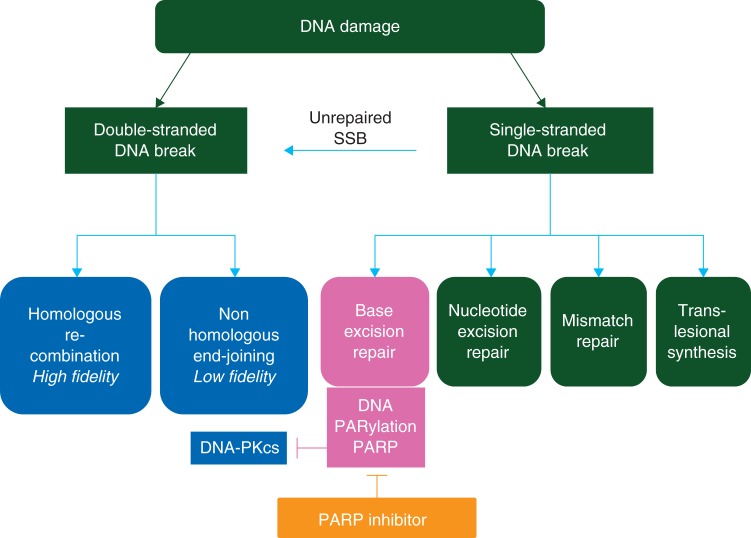
DNA damage repair pathways
Six primary pathways of DNA repair have been identified [8]. They are variably used to address single- and double-stranded DNA break damage (SSB; DSB) from a variety of mechanisms of injury (Figure 1); current results suggest pathway interaction and interdependence. Normal functions, such as cellular metabolism with associated generation of free oxygen radicals and reactive intermediates, ultraviolet light, therapeutic and ambient radiation, chemicals, and day-to-day replication errors, are common factors in the generation of DNA errors [9]. The function of the primary DNA repair pathways begins with sensing DNA damage, followed by recruitment of proteins involved in building the repair complexes [9]. Absence, reduction, or dysfunction of proteins in these pathways can be associated with loss of function of proper DNA repair. Four of the six repair pathways sense single-strand damage. HR, a high fidelity system, and nonhomologous end-joining (NHEJ), lower fidelity, are the two DSB repair programs [8]. BRCA1/2 mediate potentially rate-limiting events in HR [10]. It is now estimated that at least 15% of HGSOC occur in women with germline BRCA1/2MUT+, and another nearly 35% may have acquired defects in the HR pathway, including silencing by methylation, mutation in other repair genes, and activation of pathway inhibitors [3, 11].
Figure 1.
Double-strand break repair and single-strand break repair with poly(ADP-ribose)polymerase inhibitors (PARPis).
Multiple studies suggest that the loss of p53 function cooperates with the loss of BRCA1/2 in tumorigenesis [12, 13]. The normal function of p53 is to recognize DNA damage and arrest cell cycle to either allow repair or to shut the cell down [14]. Incomplete or inadequate DNA repair thus triggers cell death in normal cells. TCGA [4] describes molecular similarities between HGSOC and triple-negative breast cancers (TNBCs), including dysregulation of the p53 and Rb checkpoints, leading to alterations in the expression of cell proliferation genes, DNA synthesis, DNA damage repair, cell cycle regulation, and apoptosis. p53 mutations are found in nearly 90% of HGSOC and in 80% of TNBC, both cancers with BRCA1/2 loss-of-function cohorts [3, 4, 15]. Chromosome breaks caused by loss of BRCA1/2 function activate p53-dependent checkpoint controls and/or apoptosis to prevent tumor formation. Selective pressure favors loss of p53 function to allow cell proliferation [16]. Mutant p53 facilitates G2/M transition, and cells acquire and propagate unrepaired DNA damage.
Loss of HR repair caused by loss of BRCA1/2 function leaves the cell needing alternative methods for DNA damage repair. SSB base excision repair (BER) is a primary back-up system for HR loss in response to BRCA1/2MUT+ [10]. The rate-limiting enzyme in BER, PARP-1, identifies the site of DNA injury and recruits repair complexes [17]. Recently, PARP-1 has been shown also to regulate NHEJ activity by holding this poor fidelity pathway in check [18], and to guide repair by forming PARP/DNA adducts [19]. These varied actions of PARP-1 form the increasingly strong basis for development of the PARP inhibitor class of agents (PARPi).
biology and beyond: parp inhibition
PARP-1 is a highly conserved enzyme focused to assist in the maintenance of genomic integrity [20]. It collaborates with PARG, polyADPribose glycohydrolase, required for hydrolysis and release of single-ADP-ribose moieties [20]. It has numerous other functions, including its cleavage and involvement in apoptosis, gene regulation through histone modification, and DNA decondensation for higher order chromatin function [21] and DNA repair [22]. The PARP-1 enzyme has been implicated in signaling DNA damage through its ability to recognize and rapidly bind to DNA SSB [23]; it also has been shown to participate in controlling the telomere length and chromosome stability [17, 24].
PARP-1 mediates BER by recruiting the scaffolding proteins XRCC1, DNA ligase III, and DNA polymerase ß [22]. The importance of PARP-1 in HR was shown in knock-out studies by a spontaneous increase in nuclear RAD51 focus formation [25], an event that signals active DSB repair. DNA-bound activated PARP-1 uses nicotinamide adenine dinucleotide (NAD+) to polyADPribosylate nuclear target proteins, the site of DNA damage, including topoisomerases, histones, and PARP-1 itself, to signal the need for both DNA SSB and DSB repair [26]. This observation suggests loss of PARP-1 activity where HR is compromised would lead to adverse consequences for the tumor cell.
New findings implicate PARP-1 as a negative regulator of NHEJ. Patel et al. [18] reported that PARP inhibition induces phosphorylation of DNA-dependent protein kinase cs (DNA-PKcs), a rate-limiting step in NHEJ activation. PARP-1-directed NHEJ may occur more selectively in HR-deficient cells where there is a default to secondary pathways. Implications of this include reversal of the genomic instability reported in HR-deficient cells after PARP inhibition. Murai et al. [19] showed PARP inhibitors trap PARP-1 and -2 at damaged DNA where the PARP–DNA complexes were more cytotoxic than unrepaired SSB, implicating PARPi as direct DNA poisons.
BRCA-like behavior and HR dysfunction
Understanding DNA repair biology has allowed us to identify patient subsets with high potential for response PARPi treatment. The marked susceptibility of patients with BRCA1/2MUT+-associated cancers has validated BRCA1/2MUT+ as a predictive biomarker for PARPi response [27]. Tumors in patients with germline BRCA1/2MUT+ contain a second, somatic loss of BRCA1/2, following the Knudson Hypothesis [28]; this occurs as a result of genomic injury and generally incorporates part or all of the second BRCA allele. This leaves the tumor tissue homozygous null for functional BRCA1/2, with impaired HR function. Fong et al. [27] were the first to confirm this link clinically, demonstrating that BRCA1/2MUT+-associated breast, ovarian, and prostate cancer patients receiving the olaparib had a 63% likelihood of clinical benefit. This led to the broad recognition of HR dysfunction (HRD) as a functional biomarker, and opened the door to examine phenocopy susceptibility. Phenocopy patients, those with HRD not caused by BRCA1/2MUT+, are those described as having BRCA-like behavior [29].
BRCA-like behavior has both molecular and clinical characteristics. Many mechanisms reducing BRCA1/2 function and resulting in BRCA-like behavior have been identified. Examples include BRCA1 promoter methylation [11–35% of epithelial ovarian cancers (EOCs)], Fanconi F (FANCF) methylation (5∼20%), and loss or reduction in FANCD2 [30], or other proteins necessary for HR [31, 32]. Nearly always associated with this level of HRD is an obligate mutation in p53 and frequent c-myc amplification. Loss of function of the suppressor gene, PTEN, has been shown to yield BRCA-like behavior, more common in breast and prostate cancers [33, 34]. Coexpression of BRCA1MUT+ and loss of PTEN protein expression were reported to occur in 82.4% of 34 breast tumor biopsies, suggesting that PTEN loss may be a common contributing event causing HRD [33]. Increased PARPi susceptibility was shown in a series of cell lines with PTEN mutation or haploinsufficiency, confirmed in xenograft experiments using the PARPi, olaparib. There is also clinical evidence that olaparib may have a therapeutic utility in PTEN-deficient endometrioid endometrial cancer [35]. These studies provide evidence that PTEN loss of function is a potential predictive biomarker of PARPi responsiveness.
Common clinical manifestations complement the molecular characteristics of BRCA-like behavior. The first BRCA-like behavior identified is susceptibility to platinum and other DNA damaging agents. This was initially inferred from studies demonstrating improved long-term survival of women with BRCA1/2MUT+-associated EOC receiving platinum-based combination chemotherapy [36]. Intra- and inter-strand platinum-DNA crosslinks can create torsion on the double helix and lead to DSBs [31], requiring HR for proper and successful correction. Without repair, further genomic injury is sustained, leading to cell death. Reports also describe increased overall survival and progression-free survival (PFS) for mutation carriers receiving other DNA-damaging agents, such as pegylated liposomal doxorubicin (PLD) [37, 38]. Overall survival with PLD alone was nearly double that expected from large trials in a non-selected (general) population (median PFS 7.1 months; 95% CI 3.7–10.7), and similar findings were reported in a retrospective analysis of outcome following PLD in women who were BRCA1/2 germline mutation carriers and those considered not to harbor a germline mutation [38]. Subsequently, these characterizations have led to population evaluations, now suggesting that HRD occurs in up to 50% of HGSOC [11, 39, 40] and 20% of TNBC [41]. Dissection of these clinical and molecular data will inform further study design and improve therapeutic application of PARPi.
updating clinical applications of PARP inhibitors
Multiple PARPis are in clinical development as single agents and/or in combination therapy (Table 1). The most common PARPi chemistry is that of reversible NAD mimetics, with differences in bioavailability and molar equivalence of PARP enzyme inhibition. There are at least six agents under study in this class; iniparib (BSI-201) is another compound that is not a true PARPi [42]. The loss of BER capacity produced by PARPi has prompted evaluation of these drugs as potential enhancers of DNA damaging cytotoxic agents, such as alkylating agents or radiation therapy, leading to new directions for combination therapies [18, 19].
Table1.
Active PARP is under development
| PARPi | Treatment | Cancer types | Phase |
|---|---|---|---|
| Olaparib (AstraZeneca) | -Monotherapy -Combinations with cytotoxic chemotherapy -Combinations with targeted agents -Combinations with RT |
BRCA1/2MUT+ associated BrCa/OvCa, BRCA-like tumors, Advanced hematologic malignancies and solid tumors, Maintenance study following remission in platinum sensitive OvCa (pending) |
I/II/III |
| Veliparib (Abbott) | -Monotherapy -Combinations with cytotoxic chemotherapy -Combinations with targeted agents -Combinations with RT |
BRCA1/2MUT+ associated BrCa/OvCa, BRCA-like tumors, Advanced hematologic malignancies and solid tumors |
I/II |
| BMN 673 (BioMarin) | - Monotherapy | Advanced hematologic malignancies and solid tumors | I |
| Rucaparib (Clovis) | -Monotherapy -Combinations (carboplatin) |
Advanced solid tumors, Recurrent OvCa, BRCA1/2MUT+ associated BrCa/OvCa |
I/II |
| CEP-9722 (Cephalon) | -Monotherapy -Combinations with cytotoxic chemotherapy |
Advanced solid tumors | I |
| Niraparib (MK-4827) (TesaroBio) | -Monotherapy -Combinations (temazolomide) |
Advanced hematologic malignancies and solid tumors, BRCA1/2MUT+ associated and HER2 negative BrCa, Maintenance study following remission in platinum sensitive OvCa (pending) |
I/III |
*OvCa, ovarian cancer; BrCa, breast cancer; RT, radiation therapy.
Initial dose-finding trials have demonstrated significant clinical activity of PARPi especially in BRCA1/2MUT+ breast and ovarian cancers [43–46]. This suggests that BRCA 1/2MUT+ is a genetic marker for targeted therapy, similar to other therapies targeted against loss-of-suppressor function mutations that have been shown to have clinical benefit. Angiogenesis inhibition provided benefit in germline Von Hippel Landau mutation-related renal clear cell cancer, shown to have a VHL-mediated hypoxia-inducing factor 1α-VEGF drive [47]. Similarly, activating mutations of RET are associated with the pathogenesis and vandetanib-sensitivity of medullary thyroid cancer [48]. Current clinical development for PARPi builds upon these observations. The patient populations targeted in PARPi clinical trials include patients with BRCA1/2MUT+ cancers, BRCA-like cancers, and those with recognized susceptibility to DNA-damaging agents, but without BRCA-like association, such as lung or pancreas cancers (Table 2).
Table 2.
Ongoing clinical trials of PARPis for other malignancies, except breast and ovarian cancers
| Cancer type | Subtypes | PARP inhibitor | Phase |
|---|---|---|---|
| GI malignancies | Colorectal cancer | Veliparib + TMZ Olaparib + irinotecan |
I/II |
| Pretreated colorectal cancer stratified by Microsatellite Instability (MSI) | Olaparib monotherapy | I/II | |
| Gastric cancer | Veliparib + FOLFIRI | I/II | |
| Gastric cancer with low ATM protein level | Paclitaxel +/− olaparib | II | |
| Esophageal cancer | Olaparib + RT | I | |
| Metastatic pancreatic cancer | Olaparib + Gemcitabine Veliparib + modified FOLFOX6 + gemcitabine + gemcitabine/IMRT Veliparib for BRCA or PALB2 mutated pancreatic cancer Gem/cis +/− veliparib |
I/II | |
| Advanced liver cancer | Veliparib + TMZ | II | |
| Lung cancer | Small cell lung cancer | TMZ +/− veliparib Cisplatin/etoposide +/− veliparib |
I/II |
| Stage III surgically unresectable NSCLC | Veliparib + RT + carbo/taxol, + cis/gem Olaparib + RT +/− cisplatin |
I/II | |
| EGFR mutation positive advanced NSCLC | Gefitinib +/− olaparib | I/II | |
| Advanced NSCLC | Olaparib Carbo/taxol +/− veliparib |
II | |
| Head and Neck (H&N) cancer | Locally advanced H&N cancer | Olaparib and cetuximab/RT | I |
| Gynecologic cancer | Recurrent or persistent cervix cancer | Veliparib + cisplatin/paclitaxel + topotecan |
I/II |
| Hematologic malignancies | Refractory multiple myeloma | Veliparib + bortezomib/dexamethasone | I |
| Acute leukemia | Veliparib + TMZ Veliparib + topotecan +/− carboplatin |
I | |
| Refractory lymphoma | Veliparib + topotecan | I | |
| Advanced MCL | CEP-9722 + cis/gem | I | |
| Advanced hematologic malignancies | BMN673 monotherapy E7449 versus E7449 + TMZ versus E7449+ carbo/taxol |
I II |
|
| Prostate cancer | Metastatic castration resistant prostate cancer | Veliparib + TMZ Abiraterone +/− veliparib Olaparib |
I/II |
| Glioblastoma multiforme | Relapsed Glioblastoma | Olaparib + TMZ | I |
| Melanoma | Metastatic melanoma | Veliparib + TMZ Olaparib + dacarbazine E7449 versus E7449 + TMZ versus E7449+ carboplatin/paclitaxel |
I II |
| Sarcoma | Recurrent Ewing's sarcoma | Olaparib | II |
| Advanced solid tumors | Veliparib + low dose cyclophosphamide + capecitabine/oxaliplatin + mitomycin C + carbo/taxol + gemcitabine + carbo/gem Olaparib + topotecan + cis/gem + PLD Niriparib BMN 673 CEP9722 E7449 versus E7449 + TMZ versus E7449+ carbo/taxol |
I/II |
*TMZ, temozolomide; carbo/taxol, carboplatin/paclitaxel; cis/gem, cisplatin/gemcitabine.
Initial phase I/II clinical trials demonstrated single-agent activity of olaparib in BRCA1/2MUT+ breast, ovarian, and prostate cancers, and recurrent HGSOC; [27, 44, 49], no single agent response data have yet been reported for CEP-9722 (Table 3). The study by Gelmon et al. [48] clearly showed that patients with platinum-sensitive HGSOC responded to olaparib without a BRCA1/2 germline mutation. Ledermann et al. [50] recently reported maintenance olaparib significantly improved PFS in a randomized, placebo-controlled, phase II trial in platinum-sensitive HGSOC following a response to two or more lines of platinum-based therapy [50]. They demonstrated a nearly doubling of median PFS post chemotherapy (8.4 versus 4.8 months) and a 65% reduction in risk of disease progression. An interim survival analysis [51] with 58% maturity showed difference between olaparib and placebo, notably in the BRCA1/2MUT+ with a hazard ratio (HR) of 0.18 (95% CI 0.11–0.31) and with a median PFS of 11.2 versus 4.3 months, respectively. Overall survival did not show difference in this group, (HR = 0.74; median: 34.9 versus 31.9 months) probably due to 22.6% of patients on placebo switched to olaparib. As a result of these findings registration trials are being developed with olaparib and other PARPi as maintenance therapy following treatment of platinum-sensitive relapsed ovarian cancer. These types of maintenance study may even be taken into front-line therapy for selected patients.
Table 3.
Single-agent activity with PARPi in phase I/II studiesa
| Phase | Patients population (number) | Dose and schedule | Objective Response (OR) rate | Survival |
|---|---|---|---|---|
| II [50] | Platinum-sensitive-relapsed OvCa (265 patients) |
Olaparib 400 mg bid versus placebo | 12 versus 4% | PFS: 8.4 versus 4.8 months OS: (interim analysis) 29.7 versus 29.9months |
| II [37] | Recurrent OvCa/ BRCA1/2mut (97 patients) | Olaparib 200 mg bid versus 400 mg bid versus PLD 50 mg/m2 IV every 28 days | 31 versus 25% versus 18% | PFS: 6.5 months versus 8.8 months versus 7.1 months |
| II [49] | OvCa (63 patients; 17/63 BRCA1/2mut+) | Olaparib 400 mg bd | Overall 29% (18/63); 41% in BRCAmut (7/17)/24% in non-BRCAmut (11/46) |
NAa |
| II [45] | Recurrent OvCa/BRCA1/2mut(57 patients) | Olaparib 400 mg bd (33 patients) versus 100 mg bd (24 patients) | 33% versus 13% | PFS: 5.8 months versus 1.9 months |
| II [44] | Advanced BrCa/BRCA1/2mut (54 patients) | Olaparib 400 mg bd (27pts) versus 100 mg bd (27pts) | 41% versus 22% | PFS: 5.7 months versus 3.8 months |
| I [43] | Recurrent OvCa/BRCA1/2mut (50 patients) | Olaparib 40–600 mg bd (dose escalation) and 200 mg bd (dose expansion) |
Overall 40%; CBR 46%; CBR 69% in platinum-sensitive (13 patients), CBR 45% in platinum resistant (24 patients), CBR 23% in platinum refractory (13 patients) |
Response duration: 28 weeks |
| II [52] | Recurrent OvCa/BRCA1/2mut (51 patients) | Veliparib 400 mg bd |
20% | |
| I [46] | Recurrent solid tumors (100 patients; 29/100 patients BRCA1/2mut+) | Niraparib 30 mg–400 mg qd (dose escalation) | 40% in BRCAmut+ OvCa (8/20 patients) 50% in BRCAmut+ BrCa (2/4 patients) CBR 43% in CRPC (9/21 patients: 5PTEN loss and 1BRCAmut+) |
Response duration: 387 days (range 159–518) in BRCAmut+ OvCa; 132 and 133 days in BRCAmut+ BrCa; 254 days(range 124–375) in CRPC |
| I [53] | Recurrent solid tumors (29 patients; 11/29 patients BRCA1/2mut+)) | Rucaparib 40 mg–500 mg qd (dose escalation) |
2 PR (2 patients with BRCAmut+) 10 SD (9/10 patients with BRCAmut+) |
NA |
| I [54] | Advanced solid tumors (39 patients; 25/39 patients BRCA1/2mut+) | BMN673 25 µg–1100 µg qd (dose escalation) |
RECIST and/or CA-125 responses in 11/17 patients with BRCA1/2mut+ OvCa; ORR: 2/6 patients with BRCA1/2mut+ BrCa |
NA |
| I [55] | Advanced solid tumors (27 patients) | CEP-9722 150–1000 mg qd (dose escalation) |
Only safety data reported | NA |
aNA, not applicable; CBR, clinical benefit rate; CRPC, castrate-resistant prostate cancer.
The greatest clinical experience to date is with olaparib monotherapy. It generally well tolerated at doses of 400 mg twice daily in capsule formulation with many patients able to take the drug for several years. A new tablet formulation [56 ], reducing the number of pills that need to be taken is being assessed. PK data including AUC0−T and Cmin from 300 mg and 400 mg tablet doses matched or exceeded the 400 mg capsule dose, and 300 mg tablet is expected to be incorporated into further studies in mid-2013. PARPis have been tested in combination with various DNA damaging agents. Studies have shown clinical benefit and interactive adverse events, including bone marrow toxicity and fatigue [27, 43, 57]. Class-based adverse events also include fatigue, headache, nausea, and reflux in 25–40% of patients. Early reports also suggest a possible increased clinical benefit in combination therapy, that may out balance the toxicities [57, 58]. Continued follow-up and diligence are needed to define the risk of long term PARPi therapy.
Current therapeutic directions for PARPi are focused at designing combinations, determining optimal timing of therapy and breadth of application of this key class of agents to and beyond mutation carriers. Agents selected for the combination study include those likely to cause replication fork injury or further DNA damage, and anti-angiogeneic agents. Hypoxia was shown to cause DNA damage when a second DNA hit was included in a mouse model [59]. We exposed microvascular endothelial cells in vitro to the VEGF receptor antagonist, cediranib (AZD2171), in combination with olaparib, demonstrating a cooperative inhibition of angiogenesis (Kim and Kohn, unpublished data). Surprisingly, interactive anti-invasive activity was observed with this combination against a p53-mutant HGSOC cell line, OVCAR8. A phase I study of olaparib and cediranib showed clinical promise [60], and a multi-institutional randomized phase II study is in progress (NCT01116648). Additionally, a phase I study of continuous daily olaparib with bevacizumab was generally well tolerated in patients with advanced solid tumors [61].
Phase I/II studies are ongoing with PARPi and a variety of agents (Table 1). A phase I study of olaparib with carboplatin (AUC4/5) showed clinical benefit in 85% of 27 women with BRCA1/2MUT+-associated recurrent breast and ovarian cancers [58]. A randomized, phase II study of olaparib with paclitaxel (Taxol) and carboplatin (AUC4) followed by olaparib maintenance resulted in a significant improvement in PFS compared with paclitaxel, Bristol-Myers Squibb (New York) and carboplatin, Bristol-Myers Squibb (New York) (AUC6) alone in women with platinum-sensitive recurrent HGSOC (HR = 0.51; median PFS 12.2 versus 9.6 months) [62]. This suggests that combining olaparib with carboplatin required a dose modification of both drugs, illustrated the potential for toxicity interaction with DNA active agents. There was no difference in PFS during the period of chemotherapy in this trial; differences emerged in the maintenance phase. The optimal dosage, scheduling, and sequencing of PARPis and cytotoxic agents require carefully designed clinical trials linked to preclinical studies that specifically address the above issues.
This promising therapeutic potential has elicited considerable interest in clinical development of the PARPi class. Early clinical data also suggest that a BRCA-like gene expression profile may correlate with clinical responses to the platinum drugs in patients with sporadic EOC [63, 64]. Prospective validation and optimization of these signatures in a broad array of cancers, and appropriate selection of a patient population are imperative to achieve the full potential of PARPis.
challenges to PARP inhibitor development
The incorporation of targeted agents into therapy of BRCA1/2MUT+ and BRCA-like cancers presents challenges. First is development of a mechanism with which to identify patients who are most likely to benefit. Discovery and validation of predictive biomarkers is an active area of ongoing research. Biomarkers for patient selection or stratification are recommended by the US Food and Drug Administration for approval of new targeted drugs. Loss of BRCA1/2 expression, generally by demonstration of a deleterious germline mutation, is a validated predictive biomarker. Routine testing of patients is being increasingly adopted as up to 17% of patients with HGSOC, the most common form of ovarian cancer, have germline mutations [65]. However, BRCA1/2 mutation testing does not identify the full range of potentially susceptible patients, and it requires a validated predictive BRCA1/2 mutational testing tool. BRCA1/2 loss in the tumor by mutation or methylation may also be inferable by loss of BRCA1/2 protein expression demonstrated by immunohistochemical staining, leaving reduction in BRCA1/2 protein expression as a potential predictive tool [39].
The histone protein H2AX becomes rapidly phosphorylated and concatemerizes at nascent DNA DSBs [66]. This creates a focus for accumulation of DNA repair and chromatin remodeling proteins. DSBs can be labeled with an antibody to the phosphorylated form, γH2AX, and extent of DSB estimated from the number of labeled foci (Figure 2) [66]. RAD51 is instrumental in initiation of assembly of HR repair proteins at the site of DNA injury [67]. Formation of nuclear RAD51 foci can be assessed by immunofluorescence and is a marker of HR competence. Formation of γH2AX and/or RAD51 foci after DNA damage has been suggested as pharmacodynamic biomarkers of PARPi activity; demonstrating that a change in these parameters early in treatment may be examined as potential predictive biomarkers. A phase 1 study of veliparib and topotecan showed an increase in γH2AX focus formation by immunofluorescence in circulating tumor cells from seven of nine patients [68], with no correlation to clinical outcomes. Inhibition of RAD51 focus formation by PARPi was shown in vitro in EOC ascites primary cultures and correlated with response to PARPi [69]. This suggests that the lack of RAD51 foci may indicate potential drug response [70].
Figure 2.

γH2AX binds to DNA DSBs and RAD51 initiates repair protein assembly in the homologous recombination (HR) pathway.
Predictive biomarkers applied to readily available bioresources, such as archival tissue or non-tumor tissue, have been proposed. Changes in PAR (poly ADP Ribose) incorporation into peripheral blood mononuclear cell DNA were evaluated as a putative early on-treatment pharmacodynamic measure; while present, there was no relationship to clinical outcomes [57]. Basal levels of PAR vary in different cells, reflecting their relative capacity for DNA repair, and requiring demonstration of change in PAR concentrations over time. Hence, identifying an accurate measure of HR potential for application as a predictive biomarker remains necessary to guide administration of PARPi.
Dissecting and defining mechanisms of development of resistance to PARPis, and whether this portends potential collateral resistance to other DNA damaging agents is the second challenge. Acquisition of a secondary mutation in BRCA1/2 that allows BRCA1/2 gene read-through and yields a functional protein has been demonstrated in cell lines and some patients; this was correlated with loss of susceptibility to PARPi treatment [71]. A second, preclinically defined method of resistance is loss of function of 53bp1 [72], a key protein in the NHEJ pathway. Whether or not 53bp1 expression can be used as a selective or predictive biomarker is yet to be determined. Understanding the mechanism(s) of resistance to PARPi will lead to optimal application and sequencing of PARPi and platinum compounds. Studies are needed to evaluate outcomes to subsequent chemotherapies in patients who have received PARPis [73].
conclusion
Several PARPis are under investigation and it is anticipated that this novel and exciting new class of compounds will ultimately receive regulatory approval in select subsets of cancers. This class of agents has tolerable toxicity profiles and has been given to patients for long periods. Clinical benefit has been observed in patients with BRCA1/2MUT+-associated cancers and BRCA-like phenotypes in germline mutation-negative patients. It is for these patients, in particular, that predictive markers for HR deficiency and response to PARPi are needed, so that patients can be selected for therapy. Understanding more about the molecular abnormalities involved in BRCA-like tumors will be critical to advance the field of PARP inhibition therapy and in improving patient selection and consequent clinical outcomes.
funding
This work was supported by the Intramural Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health, USA. No external funding was provided for this study.
disclosure
The authors have declared no conflicts of interest.
acknowledgements
We thank S. Prindiville for critically reviewing the manuscript.
references
- 1.Miki Y, Swensen J, Shattuck-Eidens D, et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994;266:66–71. doi: 10.1126/science.7545954. [DOI] [PubMed] [Google Scholar]
- 2.Wooster R, Bignell G, Lancaster J, et al. Identification of the breast cancer susceptibility gene BRCA2. Nature. 1995;378:789–792. doi: 10.1038/378789a0. [DOI] [PubMed] [Google Scholar]
- 3.The Cancer Genome Atlas Network. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–615. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evers B, Jonkers J. Mouse models of BRCA1 and BRCA2 deficiency: past lessons, current understanding and future prospects. Oncogene. 2006;25:5885–5897. doi: 10.1038/sj.onc.1209871. [DOI] [PubMed] [Google Scholar]
- 6.Antoniou A, Pharoah PD, Narod S, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet. 2003;72:1117–1130. doi: 10.1086/375033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levy-Lahad E, Friedman E. Cancer risks among BRCA1 and BRCA2 mutation carriers. Br J Cancer. 2007;96:11–15. doi: 10.1038/sj.bjc.6603535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Plummer R. Perspective on the pipeline of drugs being developed with modulation of DNA damage as a target. Clin Cancer Res. 2010;16:4527–4531. doi: 10.1158/1078-0432.CCR-10-0984. [DOI] [PubMed] [Google Scholar]
- 9.Gudmundsdottir K, Ashworth A. The roles of BRCA1 and BRCA2 and associated proteins in the maintenance of genomic stability. Oncogene. 2006;25:5864–5874. doi: 10.1038/sj.onc.1209874. [DOI] [PubMed] [Google Scholar]
- 10.Farmer H, McCabe N, Lord CJ, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–921. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- 11.Press JZ, De Luca A, Boyd N, et al. Ovarian carcinomas with genetic and epigenetic BRCA1 loss have distinct molecular abnormalities. BMC Cancer. 2008;8:17. doi: 10.1186/1471-2407-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jonkers J, Meuwissen R, van der Gulden H, et al. Synergistic tumor suppressor activity of BRCA2 and p53 in a conditional mouse model for breast cancer. Nat Genet. 2001;29:418–425. doi: 10.1038/ng747. [DOI] [PubMed] [Google Scholar]
- 13.Liu X, Holstege H, van der Gulden H, et al. Somatic loss of BRCA1 and p53 in mice induces mammary tumors with features of human BRCA1-mutated basal-like breast cancer. Proc Natl Acad Sci USA. 2007;104:12111–12116. doi: 10.1073/pnas.0702969104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meek DW. Tumour suppression by p53: a role for the DNA damage response? Nat Rev Cancer. 2009;9:714–723. doi: 10.1038/nrc2716. [DOI] [PubMed] [Google Scholar]
- 15.Schuyer M, Berns EM. Is TP53 dysfunction required for BRCA1-associated carcinogenesis? Mol Cell Endocrinol. 1999;155:143–152. doi: 10.1016/s0303-7207(99)00117-3. [DOI] [PubMed] [Google Scholar]
- 16.Roy R, Chun J, Powell SN. BRCA1 and BRCA2: different roles in a common pathway of genome protection. Nat Rev Cancer. 2012;12:68–78. doi: 10.1038/nrc3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Virag L, Szabo C. The therapeutic potential of poly(ADP-ribose) polymerase inhibitors. Pharmacol Rev. 2002;54:375–429. doi: 10.1124/pr.54.3.375. [DOI] [PubMed] [Google Scholar]
- 18.Patel AG, Sarkaria JN, Kaufmann SH. Nonhomologous end joining drives poly(ADP-ribose) polymerase (PARP) inhibitor lethality in homologous recombination-deficient cells. Proc Natl Acad Sci USA. 2011;108:3406–3411. doi: 10.1073/pnas.1013715108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murai J, Huang SY, Das BB, et al. Trapping of PARP1 and PARP2 by Clinical PARP Inhibitors. Cancer Res. 2012;72:5588–5599. doi: 10.1158/0008-5472.CAN-12-2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tong WM, Cortes U, Wang ZQ. Poly(ADP-ribose) polymerase: a guardian angel protecting the genome and suppressing tumorigenesis. Biochim Biophys Acta. 2001;1552:27–37. doi: 10.1016/s0304-419x(01)00035-x. [DOI] [PubMed] [Google Scholar]
- 21.Kraus M, Alimzhanov MB, Rajewsky N, et al. Survival of resting mature B lymphocytes depends on BCR signaling via the Ig alpha/beta heterodimer. Cell. 2004;117:787–800. doi: 10.1016/j.cell.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 22.Helleday T, Petermann E, Lundin C, et al. DNA repair pathways as targets for cancer therapy. Nat Rev Cancer. 2008;8:193–204. doi: 10.1038/nrc2342. [DOI] [PubMed] [Google Scholar]
- 23.D'Amours D, Desnoyers S, D'Silva I, et al. Poly(ADP-ribosyl)ation reactions in the regulation of nuclear functions. Biochem J. 1999;342(Pt 2):249–268. [PMC free article] [PubMed] [Google Scholar]
- 24.d'Adda di Fagagna F, Hande MP, Tong WM, et al. Functions of poly(ADP-ribose) polymerase in controlling telomere length and chromosomal stability. Nat Genet. 1999;23:76–80. doi: 10.1038/12680. [DOI] [PubMed] [Google Scholar]
- 25.Schultz N, Lopez E, Saleh-Gohari N, et al. Poly(ADP-ribose) polymerase (PARP-1) has a controlling role in homologous recombination. Nucleic Acids Res. 2003;31:4959–4964. doi: 10.1093/nar/gkg703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hay T, Matthews JR, Pietzka L, et al. Poly(ADP-ribose) polymerase-1 inhibitor treatment regresses autochthonous Brca2/p53-mutant mammary tumors in vivo and delays tumor relapse in combination with carboplatin. Cancer Res. 2009;69:3850–3855. doi: 10.1158/0008-5472.CAN-08-2388. [DOI] [PubMed] [Google Scholar]
- 27.Fong PC, Boss DS, Yap TA, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361:123–134. doi: 10.1056/NEJMoa0900212. [DOI] [PubMed] [Google Scholar]
- 28.Knudson AG., Jr Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci USA. 1971;68:820–823. doi: 10.1073/pnas.68.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turner N, Tutt A, Ashworth A. Hallmarks of ‘BRCAness’ in sporadic cancers. Nat Rev Cancer. 2004;4:814–819. doi: 10.1038/nrc1457. [DOI] [PubMed] [Google Scholar]
- 30.Pejovic T, Yates JE, Liu HY, et al. Cytogenetic instability in ovarian epithelial cells from women at risk of ovarian cancer. Cancer Res. 2006;66:9017–9025. doi: 10.1158/0008-5472.CAN-06-0222. [DOI] [PubMed] [Google Scholar]
- 31.Long KC, Kauff ND. Hereditary ovarian cancer: recent molecular insights and their impact on screening strategies. Curr Opin Oncol. 2011;23:526–530. doi: 10.1097/CCO.0b013e3283499da9. [DOI] [PubMed] [Google Scholar]
- 32.Walsh T, Casadei S, Lee MK, et al. Mutations in 12 genes for inherited ovarian, fallopian tube, and peritoneal carcinoma identified by massively parallel sequencing. Proc Natl Acad Sci USA. 2011;108:18032–18037. doi: 10.1073/pnas.1115052108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saal LH, Gruvberger-Saal SK, Persson C, et al. Recurrent gross mutations of the PTEN tumor suppressor gene in breast cancers with deficient DSB repair. Nat Genet. 2008;40:102–107. doi: 10.1038/ng.2007.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fraser M, Zhao H, Luoto KR, et al. PTEN deletion in prostate cancer cells does not associate with loss of RAD51 function: implications for radiotherapy and chemotherapy. Clin Cancer Res. 2012;18:1015–1027. doi: 10.1158/1078-0432.CCR-11-2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Forster MD, Dedes KJ, Sandhu S, et al. Treatment with olaparib in a patient with PTEN-deficient endometrioid endometrial cancer. Nat Rev Clin Oncol. 2011;8:302–306. doi: 10.1038/nrclinonc.2011.42. [DOI] [PubMed] [Google Scholar]
- 36.Gallagher DJ, Konner JA, Bell-McGuinn KM, et al. Survival in epithelial ovarian cancer: a multivariate analysis incorporating BRCA mutation status and platinum sensitivity. Ann Oncol. 2011;22:1127–1132. doi: 10.1093/annonc/mdq577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaye SB, Lubinski J, Matulonis U, et al. Phase II, open-label, randomized, multicenter study comparing the efficacy and safety of olaparib, a poly (ADP-ribose) polymerase inhibitor, and pegylated liposomal doxorubicin in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer. J Clin Oncol. 2012;30:372–379. doi: 10.1200/JCO.2011.36.9215. [DOI] [PubMed] [Google Scholar]
- 38.Safra T, Borgato L, Nicoletto MO, et al. BRCA mutation status and determinant of outcome in women with recurrent epithelial ovarian cancer treated with pegylated liposomal doxorubicin. Mol Cancer Ther. 2011;10:2000–2007. doi: 10.1158/1535-7163.MCT-11-0272. [DOI] [PubMed] [Google Scholar]
- 39.Hyman DM, Garg K, Grisham RN, et al. BRCA1 immunohistochemistry in high-grade serous ovarian cancers (HGS-OC) characterized for BRCA1 germ-line mutations. J Clin Oncol. 2012;30 suppl; abstr 5043. [Google Scholar]
- 40.Dann RB, DeLola JA, Timms KM, et al. BRCA1/2 mutations and expression: response to platinum chemotherapy in patients with advanced stage epithelial ovarian cancer. Gynecol Oncol. 2012;125:677–682. doi: 10.1016/j.ygyno.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 41.Turner NC, Reis-Filho JS, Russell AM, et al. BRCA1 dysfunction in sporadic basal-like breast cancer. Oncogene. 2007;26:2126–2132. doi: 10.1038/sj.onc.1210014. [DOI] [PubMed] [Google Scholar]
- 42.Liu X, Shi Y, Maag DX, et al. Iniparib nonselectively modifies cysteine-containing proteins in tumor cells and is not a bona fide PARP inhibitor. Clin Cancer Res. 2012;18:510–523. doi: 10.1158/1078-0432.CCR-11-1973. [DOI] [PubMed] [Google Scholar]
- 43.Fong PC, Yap TA, Boss DS, et al. Poly(ADP)-ribose polymerase inhibition: frequent durable responses in BRCA carrier ovarian cancer correlating with platinum-free interval. J Clin Oncol. 2010;28:2512–2519. doi: 10.1200/JCO.2009.26.9589. [DOI] [PubMed] [Google Scholar]
- 44.Tutt A, Robson M, Garber JE, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet. 2010;376:235–244. doi: 10.1016/S0140-6736(10)60892-6. [DOI] [PubMed] [Google Scholar]
- 45.Audeh MW, Carmichael J, Penson RT, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial. Lancet. 2010;376:245–251. doi: 10.1016/S0140-6736(10)60893-8. [DOI] [PubMed] [Google Scholar]
- 46.Sandhu SK, Schelman WR, Wilding G, et al. The poly(ADP-ribose) polymerase inhibitor niraparib (MK4827) in BRCA mutation carriers and patients with sporadic cancer: a phase 1 dose-escalation trial. Lancet Oncol. 2013;14:882–892. doi: 10.1016/S1470-2045(13)70240-7. [DOI] [PubMed] [Google Scholar]
- 47.Patel PH, Chadalavada RS, Chaganti RS, et al. Targeting von Hippel–Lindau pathway in renal cell carcinoma. Clin Cancer Res. 2006;12:7215–7220. doi: 10.1158/1078-0432.CCR-06-2254. [DOI] [PubMed] [Google Scholar]
- 48.Wells SA, Jr, Robinson BG, Gagel RF, et al. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J Clin Oncol. 2012;30:134–141. doi: 10.1200/JCO.2011.35.5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gelmon KA, Tischkowitz M, Mackay H, et al. Olaparib in patients with recurrent high-grade serous or poorly differentiated ovarian carcinoma or triple-negative breast cancer: a phase 2, multicentre, open-label, non-randomised study. Lancet Oncol. 2011;12:852–861. doi: 10.1016/S1470-2045(11)70214-5. [DOI] [PubMed] [Google Scholar]
- 50.Ledermann JA, Harter P, Gourley C, et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N Engl J Med. 2012;366:1382–1392. doi: 10.1056/NEJMoa1105535. [DOI] [PubMed] [Google Scholar]
- 51.Ledermann JA, Gourley C, Friedlander M, et al. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer (SOC) and a BRCA mutation (BRCAm) J Clin Oncol. 2013;31 suppl; abstr 5505. [Google Scholar]
- 52.Coleman R, Sill M, Aghajanian C, et al. A phase II evaluation of the potent, highly selective PARP inhibitor veliparib in the treatment of persistent or recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer in patients who carry a germline BRCA1 or BRCA2 mutation—a Gynecologic Oncology Group Study. Gynecol Oncol. 2013 doi: 10.1016/j.ygyno.2015.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kristeleit R, Shapiro G, LoRusso P, et al. A phase I dose-escalation and PK study of continuous oral rucaparib in patients with advanced solid tumors. J Clin Oncol. 2013;31 suppl; abstr 2585. [Google Scholar]
- 54.De Bono JS, Mina LA, Gonzalez M, et al. First-in-human trial of novel oral PARP inhibitor BMN 673 in patients with solid tumors. J Clin Oncol. 2013;31 suppl; abstr 2580. [Google Scholar]
- 55.Campone M, Plummer R, Stephens P, et al. Phase I dose-escalation study to evaluate the safety, pharmacokinetics, and pharmacodynamics of CEP-9722 (a PARP1–2 inhibitor) as single agent and in combination with temozolomide in patients with advanced solid tumors ( NCT00920595) J Clin Oncol. 2012;30 suppl; abstr 3052. [Google Scholar]
- 56.Gupta A, Dean EJ, Drew Y, et al. Phase I study to determine the bioavailability and tolerability of a tablet formulation of the PARP inhibitor olaparib in patients with advanced solid tumors: Dose-escalation phase. J Clin Oncol. 2012;30 suppl; abstr 3051. [Google Scholar]
- 57.Kummar S, Ji J, Morgan R, et al. A phase I study of veliparib in combination with metronomic cyclophosphamide in adults with refractory solid tumors and lymphomas. Clin Cancer Res. 2012;18:1726–1734. doi: 10.1158/1078-0432.CCR-11-2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee J, Annunziata C, Minasian L, et al. Phase I study of the PARP inhibitor olaparib (O) in combination with carboplatin (C) in BRCA1/2 mutation carriers with breast (Br) or ovarian (Ov) cancer (Ca) J Clin Oncol. 2011;29 suppl; abstr2520. [Google Scholar]
- 59.Economopoulou M, Langer HF, Celeste A, et al. Histone H2AX is integral to hypoxia-driven neovascularization. Nat Med. 2009;15:553–558. doi: 10.1038/nm.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu J, Fleming GF, Tolaney SM, et al. A phase I trial of the PARP inhibitor olaparib (AZD2281) in combination with the antiangiogenic cediranib (AZD2171) in recurrent ovarian or triple-negative breast cancer. J Clin Oncol. 2011;29 doi: 10.1016/j.ejca.2013.05.020. suppl; abstr 5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dean E, Middleton MR, Pwint T, et al. Phase I study to assess the safety and tolerability of olaparib in combination with bevacizumab in patients with advanced solid tumours. Br J Cancer. 2012;106:468–474. doi: 10.1038/bjc.2011.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Oza AM, Cibula D, Oaknin A, et al. Olaparib plus paclitaxel plus carboplatin (P/C) followed by olaparib maintenance treatment in patients (pts) with platinum-sensitive recurrent serous ovarian cancer (PSR SOC): a randomized, open-label phase II study. J Clin Oncol. 2012;30 suppl; abstr 5001. [Google Scholar]
- 63.Konstantinopoulos PA, Spentzos D, Karlan BY, et al. Gene expression profile of BRCAness that correlates with responsiveness to chemotherapy and with outcome in patients with epithelial ovarian cancer. J Clin Oncol. 2010;28:3555–3561. doi: 10.1200/JCO.2009.27.5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kang J, D'Andrea AD, Kozono D. A DNA repair pathway-focused score for prediction of outcomes in ovarian cancer treated with platinum-based chemotherapy. J Natl Cancer Inst. 2012;104:670–681. doi: 10.1093/jnci/djs177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Alsop K, Fereday S, Meldrum C, et al. BRCA mutation frequency and patterns of treatment response in BRCA mutation-positive women with ovarian cancer: a report from the Australian Ovarian Cancer Study Group. J Clin Oncol. 2012;30:2654–2663. doi: 10.1200/JCO.2011.39.8545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bonner WM, Redon CE, Dickey JS, et al. GammaH2AX and cancer. Nat Rev Cancer. 2008;8:957–967. doi: 10.1038/nrc2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee SA, Roques C, Magwood AC, et al. Recovery of deficient homologous recombination in Brca2-depleted mouse cells by wild-type Rad51 expression. DNA Repair (Amst) 2009;8:170–181. doi: 10.1016/j.dnarep.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 68.Kummar S, Chen A, Ji J, et al. Phase I study of PARP inhibitor ABT-888 in combination with topotecan in adults with refractory solid tumors and lymphomas. Cancer Res. 2011;71:5626–5634. doi: 10.1158/0008-5472.CAN-11-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mukhopadhyay A, Elattar A, Cerbinskaite A, et al. Development of a functional assay for homologous recombination status in primary cultures of epithelial ovarian tumor and correlation with sensitivity to poly(ADP-ribose) polymerase inhibitors. Clin Cancer Res. 2010;16:2344–2351. doi: 10.1158/1078-0432.CCR-09-2758. [DOI] [PubMed] [Google Scholar]
- 70.Banerjee S, Kaye S. The role of targeted therapy in ovarian cancer. Eur J Cancer. 2011;47(Suppl 3):S116–S130. doi: 10.1016/S0959-8049(11)70155-1. [DOI] [PubMed] [Google Scholar]
- 71.Swisher EM, Sakai W, Karlan BY, et al. Secondary BRCA1 mutations in BRCA1-mutated ovarian carcinomas with platinum resistance. Cancer Res. 2008;68:2581–2586. doi: 10.1158/0008-5472.CAN-08-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bunting SF, Callen E, Wong N, et al. 53BP1 inhibits homologous recombination in Brca1-deficient cells by blocking resection of DNA breaks. Cell. 2010;141:243–254. doi: 10.1016/j.cell.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ang J, Yap TA, Fong P, et al. Preliminary experience with the use of chemotherapy (CT) following treatment with olaparib, a poly(ADP-ribose) polymerase inhibitor (PARPi), in patients with BRCA1/2-deficient ovarian cancer (BDOC) J Clin Oncol. 2010;28 Suppl:abstract 5041. [Google Scholar]