Parameningeal (PM) site is a well-known adverse prognostic factor in children with rhabdomyosarcoma (RMS). A pooled analysis of data from 1105 patients with PM RMS differentiates those with good prognosis (36% patients with 0-1 risk factor: 10-yr OS 80.9%) from high risk PM patients (28% with 3-4 factors: 10-yr OS 51.1%). Furthermore, this analysis reinforces the necessity for radiotherapy in PM RMS.
Keywords: meningeal involvement, parameningeal, radiotherapy, rhabdomyosarcoma
Abstract
Background
Parameningeal (PM) site is a well-known adverse prognostic factor in children with localized rhabdomyosarcoma (RMS). To identify risk factors associated with outcome at this site, we pooled data from 1105 patients treated in 10 studies conducted by European and North American cooperative groups between 1984 and 2004.
Patients and methods
Clinical factors including age, histology, size, invasiveness, nodal involvement, Intergroup Rhabdomyosarcoma Study (IRS) clinical group, site, risk factors for meningeal involvement (MI), study group, and application of radiotherapy (RT) were studied for their impact on event-free and overall survival (EFS and OS).
Results
Ten-year EFS and OS were 62.6 and 66.1% for the whole group. Patients without initial RT showed worse survival (10-year OS 40.8% versus 68.5% for RT treated patients). Multivariate analysis focusing on 862 patients who received RT as part of their initial treatment revealed four unfavorable prognostic factors: age <3 or >10 years, signs of MI, unfavorable site, and tumor size. Utilizing these prognostic factors, patients could be classified into different risk groups with 10-year OS ranging between 51.1 and 80.9%.
Conclusions
While, in general, PM localization is regarded as an adverse prognostic factor, the current analysis differentiates those with good prognosis (36% patients with 0–1 risk factor: 10-year OS 80.9%) from high-risk PM patients (28% with 3–4 factors: 10-year OS 51.1%). Furthermore, this analysis reinforces the necessity for RT in PM RMS.
introduction
Rhabdomyosarcoma (RMS) is the most common soft tissue sarcoma in childhood [1]. Twenty percent of RMS occurs in the parameningeal (PM) area, either arising from a PM site (nasopharynx, nasal cavity, parapharyngeal area, paranasal sinuses, infratemporal and pterygopalatine fossa, middle ear, or mastoid) or from another site with extension into a PM site.
Although the PM site has proven an unfavorable prognostic factor in recent prospective clinical trials [2–4], several studies have suggested that survival for children with PM RMS is not uniformly poor [5–7]. Pooling the results of consecutive contemporary RMS trials of different cooperative groups has increased our knowledge about RMS at other sites [8, 9] and patients with metastatic disease [10]. The first international RMS workshop, reporting on 230 PM RMS treated from 1979 to 1989, showed that the only prognostic factor of significance was the size of the tumor [7]. To update this earlier analysis, we report here the largest series on PM RMS patients to date, treated in the past two decades, in order to define prognostic factors for children treated according to contemporary European and North American cooperative group protocols.
patients and methods
patient population
Analyses were carried out on data derived from 10 studies from three international cooperative groups conducted along a period of 20 years [2, 4, 11–16]. Details about patient population, study design, and treatment delivered have been already reported elsewhere and in brief in a supplementary file (available at Annals of Oncology online) and supplementary Tables S1–S3, available at Annals of Oncology online. In total, 1105 patients with localized PM RMS diagnosed from January 1984 to December 2004, aged 0–18 years and previously untreated were considered for this analysis. Cerebrospinal fluid positive patients were excluded.
Histological confirmation by central pathology review had already been carried out as part of each trial.
Tumor sites were classified into seven more specific anatomic sites: (i) middle ear–mastoid, (ii) nasal cavity–nasopharynx, (iii) parapharyngeal space, (iv) paranasal sinuses, (v) infratemporal and pterygoid palatine fossa, (vi) orbital primary with PM extension [i.e. with bone erosion of the orbital roof, or intracranial extension (ICE) or cranial nerve palsy (CNP), or with extension to a PM area], and (vii) other (all other sites).
statistical analysis
Data were pooled in a single master database at the Istituto Oncologico Veneto (Padova, Italy), where all statistical analyses were carried out.
The survival probability was computed by means of the Kaplan–Meier method, and 5- and 10-year estimates were reported for descriptive purpose. Heterogeneity in survival among strata of considered variables was assessed through the log-rank test. Survival time was calculated from the date of the start of treatment to the time of last follow-up or event. Tumor progression, relapse, occurrence of second malignancy, or death from any cause were considered for event-free survival (EFS).
Gender, age category (≤1, 1–3, 3–10, >10), histology (embryonal, alveolar, RMS not otherwise specified), size (≤5 cm, >5 cm), invasiveness, nodal involvement, IRS post-surgical group, site of primary, risk factors for meningeal involvement (MI), study group (IRSG/COG, AIEOP-STSC, SIOP), study period (early studies: IRS-III, IRS-IVp, IRS-IV, RMS79, RMS88, SIOP-MMT84, SIOP-MMT89; more recent studies: D9803, RMS96, SIOP-MMT95), and use of radiation therapy (RT) during initial therapy were studied for their impact on EFS and overall survival (OS).
Multivariate analysis was conducted using the Cox proportional hazards regression method to determine the independent prognostic significance of the clinical factors considered. A backward variable selection procedure was applied to the covariates with a P-value of at least 0.2 at univariate analysis. Multiplicative interaction among covariates was tested including interaction terms in the model and examining the P-value of that term. Hazard ratios (HRs) with the 95% confidence intervals (95% CIs), calculated according to the Wald method, were reported for significant variables.
A prognostic score was devised using the factors identified as prognostically significant for OS by multivariate analysis.
All data analyses were carried out using the SAS statistical package (SAS, release 9.2; SAS Institute Inc., Cary, NC).
results
patient characteristics
The characteristics of 1105 patients are listed in supplementary Table S4, available at Annals of Oncology online. The median age at diagnosis was 6.1 years (0.1–17.9 years). The median follow-up of survivors was 8.2 years (interquartile range 5.7–10.9 years). Estimated 5- and 10-year OS and EFS for all patients were 69.5% (95% CI: 66.7–72.2%) and 66.1% (95% CI: 63.0–68.9%) for OS and 64.9% (95% CI: 62.0–67.7%) and 62.6% (95% CI: 59.6–65.5%) for EFS, respectively (supplementary Figure S1, available at Annals of Oncology online). Relapse was localized in 68%, metastatic in 23.7%, and combined in 8.3%.
At univariate analysis (supplementary Table S5, available at Annals of Oncology online), patients ≤1 year of age had the worst prognosis, whereas patients between 3 and 10 years had the best prognosis. Site of the tumor was significantly correlated with outcome; infratemporal and pterygopalatine fossa and paranasal sinus sites showed the worst prognosis, whereas patients with tumors at other PM sites showed better outcomes. According to these results, sites were recoded as unfavorable and favorable PM sites with 5-year OS of 60.5% and 74.2%. Patients with ICE, cranial base bone erosion (CBBE), or CNP showed worse survival compared with those without. Signs of MI were recorded as a single variable according to the presence of ICE with or without CNP and/or CBBE. Patients without any sign of MI did best (5-year OS 79.4%), but the presence of CNP and/or CBBE decreased OS to 70.9%, whereas those with ICE fared even worse with a 5-year OS of 61.1%. Tumors confined to tissue or organ of origin (T1) did better than tumors with invasion beyond the tissue or organ of origin (T2). Small tumor size and embryonal histology had a positive impact on survival. Ten-year OS figures for recent studies were similar to OS for the earlier studies.
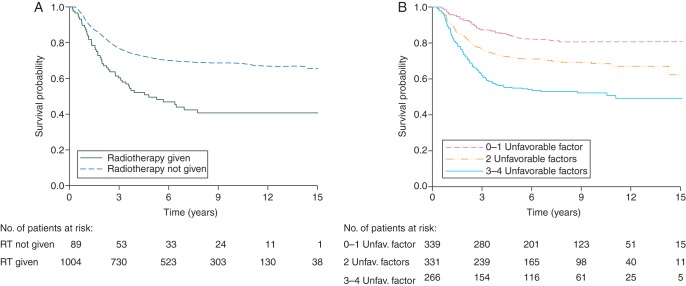
Patients who received RT as part of their initial treatment had a much better 5-year OS than those where RT was omitted: 71.4 versus 49.6% (P < 0.0001; Figure 1A). Most patients who did not receive RT were from International Society of Pediatric Oncology (SIOP) studies where 14.8% (60 of 406) did not receive RT as part of initial treatment. In Intergroup Rhabdomyosarcoma Study Group (IRSG)/Children's Oncology Group (COG) and Associazione Italiana Ematologia Oncologia Pediatrica-Soft Tissue Sarcoma Committee (AIEOP-STSC) studies, RT was omitted in only 4.2 and 4% of patients, respectively. The impact of the omission of RT was similar across age categories.
Figure 1.
(A) Dotted line, OS by RT given; solid line, RT not given. (B) OS by prognostic score; adverse prognostic factors considered were: age <3 or >10 years; presence of MI; tumor size >5 cm; unfavorable primary PM site.
Cox regression analysis could be carried out in 932 patients with complete data for all prognostic variables. The effect of RT appeared strongly related to site; patients with tumors at a favorable PM site that received RT did better than those with tumors at unfavorable PM site, whereas, for patients that did not receive RT, tumor site did not show a significant prognostic effect. Furthermore, larger tumor size, histology other than embryonal, age <1 year, and MI all had an independent and worsening impact on OS (Table 1).
Table 1.
Cox regression analysis for OS (multivariate analysis)—analysis performed on 932 patients (304 events) with all data available
| Characteristics | HR (95% CI) | P-value |
|---|---|---|
| Age (years) | ||
| ≤1 | 2.07 (1.04–4.12) | 0.0005 |
| 2–3 | 0.86 (0.58–1.26) | |
| 4–10 | 0.65 (0.50–0.86) | |
| >10 | 1 | |
| Signs of MI | ||
| None | 1 | <0.0001 |
| CNP and/or CBBE | 1.77 (1.28–2.45) | |
| ICE±CNP±CBBE | 2.14 (1.58–2.91) | |
| Size of tumor (cm) | ||
| ≤5 | 1 | 0.004 |
| >5 | 1.42 (1.11–1.80) | |
| Histology | ||
| Embryonal RMS | 1 | 0.030 |
| Alveolar RMS | 1.40 (1.06–1.83) | |
| RMS NOS | 1.44 (0.91–2.28) | |
| Site (if RT delivered) | ||
| Favorable | 1 | 0.005 |
| Unfavorable | 1.44 (1.12–1.85) | |
| Site (if no RT delivered) | ||
| Favorable | 1 | 0.286 |
| Unfavorable | 0.68 (0.33–1.38) | |
| RT given (favorable site) | ||
| No | 1 | <0.0001 |
| Yes | 0.35 (0.23–0.54) | |
| RT given (unfavorable site) | ||
| No | 1 | 0.362 |
| Yes | 0.74 (0.39–1.40) | |
CI, confidence interval; CNP, cranial nerve palsy; CBBE, cranial base bone erosion; ICE, intracranial extension; RMS, rhabdomyosarcoma; NOS, not otherwise specified; RT, radiation therapy.
Since RT was such a strong prognostic factor and the routine use of RT for RMS patients with PM localizations is now standard in all of the current cooperative groups treatment protocols, we performed a separate multivariate analysis on the 862 patients who received RT as part of their initial treatment (Table 2). This analysis confirmed the same independent prognostic factors, except histology.
Table 2.
Cox regression analysis for OS (multivariate analysis)—analysis performed on 862 patients (265 events) treated with RT, with all data available
| Characteristics | HR (95% CI) | P-value | Characteristics | HR (95% CI) | P-value |
|---|---|---|---|---|---|
| Age (years) | Age (years) | ||||
| ≤1 | 2.47 (1.06–5.73) | <0.0001 | Favorable | 1 | <0.0001 |
| 2–3 | 0.91 (0.60–1.37) | Unfavorable | 1.81 (1.41–2.31) | ||
| 4–10 | 0.56 (0.42–0.74) | ||||
| >10 | 1 | ||||
| Signs of MI | Signs of MI | ||||
| None | 1 | <0.0001 | None | 1 | <0.0001 |
| CNP and/or CBBE | 1.74 (1.22–2.48) | Any | 1.91 (1.40–2.60) | ||
| ICE±CNP±CBBE | 2.05 (1.48–2.85) | ||||
| Size of tumor (cm) | Size of tumor (cm) | ||||
| ≤5 | 1 | 0.022 | ≤5 | 1 | 0.0163 |
| >5 | 1.35 (1.04–1.74) | >5 | 1.37 (1.06–1.76) | ||
| Site | Site | ||||
| Favorable PM | 1 | 0.001 | Favorable PM | 1 | 0.0009 |
| Unfavorable PM | 1.52 (1.18–1.96) | Unfavorable PM | 1.52 (1.19–1.94) | ||
PM, parameningeal; for other abbreviations, see Table 1.
In order to define a possible prognostic score, the four covariates were categorized as a dummy variable identifying four adverse prognostic features (age <3 or >10 years, presence of MI, tumor size >5 cm, unfavorable primary PM site) whose effect on risk of death was qualitatively similar. Omission of RT was excluded from the model. Thus, we created a numerical score from 0 to 4 by adding up the number of adverse factors recorded for each patient. Ten-year OS was significantly different for three groups: 80.7% (95% CI: 75.3–85.0) for patients with zero or one factor, 68.4% (95% CI: 62.5–73.6) for patients with two factors, and 52.2% (95% CI: 45.6–58.3) for those with three or four factors (P < 0.0001, Figure 1B). Only 6.5% of patients had no adverse factor (score 0), 28% had 1 adverse factor (score 1), 36% had 2, 22.5% had 3, and 7% had 4 adverse factors.
discussion
This study has shown that the PM site should not uniformly be regarded as an unfavorable prognostic factor in children with RMS. Multivariate analysis in patients who received RT as part of their initial therapy revealed four independent adverse prognostic factors: unfavorable age, MI, large tumor size, and unfavorable primary subsite. Only a minority (22%) of patients (those with 3–4 adverse factors) had a poor prognosis (10-year OS 51.1%), whereas 29% with 0–1 adverse factor had a relatively good prognosis (10-year OS 80.9%). It is possible, therefore, that patients with high-risk PM RMS could be selected for innovative systemic or local therapies, while those with good prognosis should receive standard regimens.
In current cooperative group treatment protocols, most PM patients are eligible for randomized study questions: in the COG ARST0531 protocol, all PM patients with group III disease and embryonal histology and all patients with alveolar histology are eligible for a randomized study. In the EpSSG-RMS-2005 study, most PM RMS patients are eligible for the randomized, investigational arm and only young PM patients (<10 years) with small (≤5 cm) tumors of embryonal histology receive standard treatment. Implementation of a treatment stratification based on the prognostic score proposed by this study will adequately select the minority of patients with higher risk PM disease for innovative therapies.
This study also underlines the absolute need for RT for children with RMS located at a PM site. This is in contrast with a similar pooled analysis for orbital RMS which, although showing 10-year EFS to be significantly better for patients receiving RT as part of their initial treatment compared with those who did not (82 versus 53%), confirmed no statistical difference in OS (87 versus 86%), taking the advantage of a favorable ‘salvage gap’ with rescue utilizing further treatment [9]. SIOP group data from that analysis showed that up to 40% of patients with an orbital localization could be treated successfully without the use of RT and without disadvantage to the survival of the whole group. However, the total burden of therapy must be taken into account as those who relapsed not only received RT as part of their second therapy but also needed additional chemotherapy [9]. While the majority of late sequelae in PM RMS survivors are attributable to RT [17]; in this analysis, 10-year EFS for those who did not receive RT as part of initial treatment was only 25.1% (versus 66.0% for those who did). Moreover, although relatively few of those who relapsed after initial RT could be salvaged (OS 71.4%), the OS for those treated with delayed RT remained inferior at 49.6%. This is in line with a previous study from the SIOP group reporting an unsuccessful attempt to avoid RT in PM RMS in the very young [18]. In the current study, almost 15% of SIOP patients did not receive RT, compared with 4% in IRSG/COG or AIEOP-STSC cohorts. This likely explains the inferior survival figures for SIOP patients seen in univariate analysis. However, when corrected for the use of RT in multivariate analysis, the survival difference among cooperative groups disappeared, underlining the importance of RT as an essential component of primary treatment. In contrast to the different philosophies that remain for the treatment of orbital RMS, all cooperative groups now uniformly advocate the use of RT for the PM site in all age categories, although deviations are permitted, and utilized, in the very young.
Survival at this site has not improved over time. Several new drugs and drug combinations were tested without improving outcome and Vincristine, Actinomycin D, Cyclophosphamide or Vincristine, Acinomycin D, Ifosfamide remaining standard chemotherapy combinations for localized RMS [2, 4]. Although cross-sectional imaging techniques (Magnetic Resonance Imaging and Computed Tomography) have shown continuous improvement over the years, leading to better recognition of ICE [19] and the potential for more appropriate local therapy, this has not resulted in improved outcome. Multivariate retrospective analysis of the influence of RT parameters on the outcome of children with PM RMS treated in IRSG protocols II–IV demonstrated that a radiation dose >47.5 Gy was associated with lower rates of local failure. However, hyperfractionation strategies with a consequently higher radiation dose have not been shown to improve results when studied in a randomized trial [20]. A recent analysis comparing intensity-modulated RT (IMRT) and three-dimensional conformal RT (3D-CRT), applied in a non-randomized way in D9803, could not demonstrate an improvement in EFS or local control rate for IMRT, although the target dose coverage was better in IMRT compared with 3D-CRT [21].
Inherent to the PM site is the frequent inability to achieve oncologically effective margins at surgical resection. Although a minority of COG patients underwent an initial macroscopic radical resection with microscopic residue before systemic therapy, the lower IRS group was not associated with better outcome in univariate analysis (supplementary Table S5, available at Annals of Oncology online). Adding brachytherapy to macroscopic radical surgery, to take care of microscopic residual disease, did not improve outcome in a single-center series [22], although reduced late effects are a potential benefit of this novel local strategy.
Differences in treatment components among protocols were studied for their impact on outcome. However, most variables studied were highly correlated with the cooperative group and consequently with differences in components of treatment. For instance, the timing of RT differed among the various cooperative groups and protocols and might have an impact on outcome. However, as timing is highly associated with the cooperative group, it is also linked to other factors such as chemotherapy regimen and duration, use of surgery, and RT dose. In addition, delayed RT may be related to toxicity encountered early in treatment. The power of these subanalyses was also limited as, for several items, data were not available for all three cooperative groups.
In conclusion, data from this pooled analysis of international experience suggest that all patients with PM RMS, regardless of age, require adequate RT. The development of a prognostic scoring system allows the identification of patients with the most unfavorable prognosis to be identified at the outset and considered for future innovative therapies. These should focus on techniques to optimize local control (e.g. IMRT or proton beam RT), with or without radiosensitization or incorporating newer agents into standard treatment approaches.
funding
COG clinical trials were supported by the National Cancer Institute/National Institutes of Health (U10 CA98543-08, U10 CA98413-08). AIEOP-STSC studies were supported by Fondazione Città della Speranza.
disclosure
The authors have declared no conflicts of interest.
Supplementary Material
acknowledgements
The authors would like to thank Beverly Raney for sharing his experience as pioneer and expert in the treatment of parameningeal rhabdomyosarcoma.
references
- 1.Miller RW, Young JL, Jr, Novakovic B. Childhood cancer. Cancer. 1995;75:395–405. doi: 10.1002/1097-0142(19950101)75:1+<395::aid-cncr2820751321>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 2.Arndt CA, Stoner JA, Hawkins DS, et al. Vincristine, actinomycin, and cyclophosphamide compared with vincristine, actinomycin, and cyclophosphamide alternating with vincristine, topotecan, and cyclophosphamide for intermediate-risk rhabdomyosarcoma: Children's Oncology Group study D9803. J Clin Oncol. 2009;27:5182–5188. doi: 10.1200/JCO.2009.22.3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dantonello TM, Int-Veen C, Harms D, et al. Cooperative trial CWS-91 for localized soft tissue sarcoma in children, adolescents, and young adults. J Clin Oncol. 2009;27:1446–1455. doi: 10.1200/JCO.2007.15.0466. [DOI] [PubMed] [Google Scholar]
- 4.Oberlin O, Rey A, Sanchez de Toledo J, et al. Randomized comparison of intensified six-drug versus standard three-drug chemotherapy for high-risk nonmetastatic rhabdomyosarcoma and other chemotherapy-sensitive childhood soft tissue sarcomas: long-term results from the International Society of Pediatric Oncology MMT95 study. J Clin Oncol. 2012;30:2457–2465. doi: 10.1200/JCO.2011.40.3287. [DOI] [PubMed] [Google Scholar]
- 5.Raney RB, Meza J, Anderson JR, et al. Treatment of children and adolescents with localized parameningeal sarcoma: experience of the Intergroup Rhabdomyosarcoma Study Group protocols IRS-II through -IV, 1978–1997. Med Pediatr Oncol. 2002;38:22–32. doi: 10.1002/mpo.1259. [DOI] [PubMed] [Google Scholar]
- 6.Hawkins DS, Anderson JR, Paidas CN, et al. Improved outcome for patients with middle ear rhabdomyosarcoma: a Children's Oncology Group study. J Clin Oncol. 2001;19:3073–3079. doi: 10.1200/JCO.2001.19.12.3073. [DOI] [PubMed] [Google Scholar]
- 7.Benk V, Rodary C, Donaldson SS, et al. Parameningeal rhabdomyosarcoma: results of an international workshop. Int J Radiat Oncol Biol Phys. 1996;36:533–540. doi: 10.1016/s0360-3016(96)00362-8. [DOI] [PubMed] [Google Scholar]
- 8.Raney B, Anderson J, Jenney M, et al. Late effects in 164 patients with rhabdomyosarcoma of the bladder/prostate region: a report from the international workshop. J Urol. 2006;176:2190–2194. doi: 10.1016/j.juro.2006.07.064. [DOI] [PubMed] [Google Scholar]
- 9.Oberlin O, Rey A, Anderson J, et al. Treatment of orbital rhabdomyosarcoma: survival and late effects of treatment–results of an international workshop. J Clin Oncol. 2001;19:197–204. doi: 10.1200/JCO.2001.19.1.197. [DOI] [PubMed] [Google Scholar]
- 10.Oberlin O, Rey A, Lyden E, et al. Prognostic factors in metastatic rhabdomyosarcomas: results of a pooled analysis from United States and European cooperative groups. J Clin Oncol. 2008;26:2384–2389. doi: 10.1200/JCO.2007.14.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arndt C, Tefft M, Gehan E, et al. A feasibility, toxicity, and early response study of etoposide, ifosfamide, and vincristine for the treatment of children with rhabdomyosarcoma: a report from the Intergroup Rhabdomyosarcoma Study (IRS) IV pilot study. J Pediatr Hematol Oncol. 1997;19:124–129. doi: 10.1097/00043426-199703000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Crist W, Gehan EA, Ragab AH, et al. The third Intergroup Rhabdomyosarcoma Study. J Clin Oncol. 1995;13:610–630. doi: 10.1200/JCO.1995.13.3.610. [DOI] [PubMed] [Google Scholar]
- 13.Crist WM, Anderson JR, Meza JL, et al. Intergroup Rhabdomyosarcoma Study-IV: results for patients with nonmetastatic disease. J Clin Oncol. 2001;19:3091–3102. doi: 10.1200/JCO.2001.19.12.3091. [DOI] [PubMed] [Google Scholar]
- 14.Bisogno G, De RC, Gamboa Y, et al. Improved survival for children with parameningeal rhabdomyosarcoma: results from the AIEOP soft tissue sarcoma committee. Pediatr Blood Cancer. 2008;50:1154–1158. doi: 10.1002/pbc.21527. [DOI] [PubMed] [Google Scholar]
- 15.Flamant F, Rodary C, Rey A, et al. Treatment of non-metastatic rhabdomyosarcomas in childhood and adolescence. Results of the second study of the International Society of Paediatric Oncology: MMT84. Eur J Cancer. 1998;34:1050–1062. doi: 10.1016/s0959-8049(98)00024-0. [DOI] [PubMed] [Google Scholar]
- 16.Stevens MC, Rey A, Bouvet N, et al. Treatment of nonmetastatic rhabdomyosarcoma in childhood and adolescence: third study of the International Society of Paediatric Oncology–SIOP Malignant Mesenchymal Tumor 89. J Clin Oncol. 2005;23:2618–2628. doi: 10.1200/JCO.2005.08.130. [DOI] [PubMed] [Google Scholar]
- 17.Raney RB, Asmar L, Vassilopoulou-Sellin R, et al. Late complications of therapy in 213 children with localized, nonorbital soft-tissue sarcoma of the head and neck: a descriptive report from the Intergroup Rhabdomyosarcoma Studies (IRS)-II and - III. IRS Group of the Children's Cancer Group and the Pediatric Oncology Group. Med Pediatr Oncol. 1999;33:362–371. doi: 10.1002/(sici)1096-911x(199910)33:4<362::aid-mpo4>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 18.Defachelles AS, Rey A, Oberlin O, et al. Treatment of nonmetastatic cranial parameningeal rhabdomyosarcoma in children younger than 3 years old: results from International Society of Pediatric Oncology studies MMT 89 and 95. J Clin Oncol. 2009;27:1310–1315. doi: 10.1200/JCO.2008.19.5701. [DOI] [PubMed] [Google Scholar]
- 19.Michalski JM, Meza J, Breneman JC, et al. Influence of radiation therapy parameters on outcome in children treated with radiation therapy for localized parameningeal rhabdomyosarcoma in Intergroup Rhabdomyosarcoma Study Group trials II through IV. Int J Radiat Oncol Biol Phys. 2004;59:1027–1038. doi: 10.1016/j.ijrobp.2004.02.064. [DOI] [PubMed] [Google Scholar]
- 20.Donaldson SS, Meza J, Breneman JC, et al. Results from the IRS-IV randomized trial of hyperfractionated radiotherapy in children with rhabdomyosarcoma–a report from the IRSG. Int J Radiat Oncol Biol Phys. 2001;51:718–728. doi: 10.1016/s0360-3016(01)01709-6. [DOI] [PubMed] [Google Scholar]
- 21.Lin C, Donaldson SS, Meza JL, et al. Effect of radiotherapy techniques (IMRT vs. 3D-CRT) on outcome in patients with intermediate-risk rhabdomyosarcoma enrolled in COG D9803-a report from the Children's Oncology Group. Int J Radiat Oncol Biol Phys. 2012;82:1764–1770. doi: 10.1016/j.ijrobp.2011.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buwalda J, Schouwenburg PF, Blank LE, et al. A novel local treatment strategy for advanced stage head and neck rhabdomyosarcomas in children: results of the AMORE protocol. Eur J Cancer. 2003;39:1594–1602. doi: 10.1016/s0959-8049(03)00363-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.