Abstract
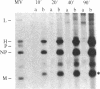
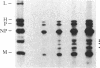
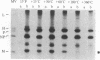
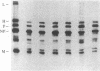
Measles virus matrix protein expression is restricted in the persistently infected brain cells of patients with the chronic neurological disease subacute sclerosing panencephalitis (SSPE). Prior studies of the nature of this restriction have identified polyadenylylated matrix gene-encoded RNA transcripts unable to direct effective translation. The defective nature of these mRNAs readily accounted for the inability to detect matrix protein in these persistently infected cells and suggested that in SSPE the restriction of matrix protein expression is achieved by preventing its synthesis. Recently, however, we reported evidence that matrix protein is synthesized in at least one example of this persistent infection, the SSPE cell line IP-3-Ca. In this case, failure of matrix protein to accumulate normally accounted for its restricted expression [Sheppard, R. D., Raine, C. S., Bornstein, M. B. & Udem, S. A. (1985) Science 228, 1219-1221]. To clarify the nature of the restriction displayed by IP-3-Ca cells, the synthesis and fate of the matrix protein of this SSPE cell line were examined in detail. No evidence of constraints on the efficiency of matrix protein mRNA transcription or translation was found. Instead, the restricted expression proved to be the result of rapid posttranslational degradation of matrix protein. We suggest that matrix protein gene mutations incurred in the course of genome replication are likely to be responsible for the diversity of observed mechanisms restricting matrix protein expression. In that event, the nature and position of the nucleotide substitution(s) would be the determinants of the level at which restricted expression is achieved.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baczko K., Carter M. J., Billeter M., ter Meulen V. Measles virus gene expression in subacute sclerosing panencephalitis. Virus Res. 1984 Oct;1(7):585–595. doi: 10.1016/0168-1702(84)90015-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birrer M. J., Bloom B. R., Udem S. Characterization of measles polypeptides by monoclonal antibodies. Virology. 1981 Jan 30;108(2):381–390. doi: 10.1016/0042-6822(81)90446-3. [DOI] [PubMed] [Google Scholar]
- Blumberg B. M., Rose K., Simona M. G., Roux L., Giorgi C., Kolakofsky D. Analysis of the Sendai virus M gene and protein. J Virol. 1984 Nov;52(2):656–663. doi: 10.1128/jvi.52.2.656-663.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstein T., Jacobsen L. B., Zeman W., Chen T. T. Persistent infection of BSC-1 cells by defective measles virus derived from subacute sclerosing panencephalitis. Infect Immun. 1974 Dec;10(6):1378–1382. doi: 10.1128/iai.10.6.1378-1382.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bächi T. Intramembrane structural differentiation in Sendai virus maturation. Virology. 1980 Oct 15;106(1):41–49. doi: 10.1016/0042-6822(80)90219-6. [DOI] [PubMed] [Google Scholar]
- Büechi M., Bächi T. Microscopy of internal structures of Sendai virus associated with the cytoplasmic surface of host membranes. Virology. 1982 Jul 30;120(2):349–359. doi: 10.1016/0042-6822(82)90036-8. [DOI] [PubMed] [Google Scholar]
- Carter M. J., Willcocks M. M., ter Meulen V. Defective translation of measles virus matrix protein in a subacute sclerosing panencephalitis cell line. Nature. 1983 Sep 8;305(5930):153–155. doi: 10.1038/305153a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase A. T., Gantz D., Eble B., Walker D., Stowring L., Ventura P., Blum H., Wietgrefe S., Zupancic M., Tourtellotte W. Natural history of restricted synthesis and expression of measles virus genes in subacute sclerosing panencephalitis. Proc Natl Acad Sci U S A. 1985 May;82(9):3020–3024. doi: 10.1073/pnas.82.9.3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall W. W., Choppin P. W. Evidence for lack of synthesis of the M polypeptide of measles virus in brain cells in subacute sclerosing panencephalitis. Virology. 1979 Dec;99(2):443–447. doi: 10.1016/0042-6822(79)90026-6. [DOI] [PubMed] [Google Scholar]
- Hall W. W., Choppin P. W. Measles-virus proteins in the brain tissue of patients with subacute sclerosing panencephalitis: absence of the M protein. N Engl J Med. 1981 May 7;304(19):1152–1155. doi: 10.1056/NEJM198105073041906. [DOI] [PubMed] [Google Scholar]
- Hall W. W., Lamb R. A., Choppin P. W. Measles and subacute sclerosing panencephalitis virus proteins: lack of antibodies to the M protein in patients with subacute sclerosing panencephalitis. Proc Natl Acad Sci U S A. 1979 Apr;76(4):2047–2051. doi: 10.1073/pnas.76.4.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland J., Spindler K., Horodyski F., Grabau E., Nichol S., VandePol S. Rapid evolution of RNA genomes. Science. 1982 Mar 26;215(4540):1577–1585. doi: 10.1126/science.7041255. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lin F. H., Thormar H. Absence of M protein in a cell-associated subacute sclerosing panencephalitis virus. Nature. 1980 Jun 12;285(5765):490–492. doi: 10.1038/285490a0. [DOI] [PubMed] [Google Scholar]
- Machamer C. E., Hayes E. C., Zweerink H. J. Cells infected with a cell-associated subacute sclerosing panencephalitis virus do not express M protein. Virology. 1981 Jan 30;108(2):515–520. doi: 10.1016/0042-6822(81)90460-8. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Fox C. F. Protein-protein interactions within paramyxoviruses identified by native disulfide bonding or reversible chemical cross-linking. J Virol. 1980 Jan;33(1):152–166. doi: 10.1128/jvi.33.1.152-166.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrby E., Kristensson K., Brzosko W. J., Kapsenberg J. G. Measles virus matrix protein detected by immune fluorescence with monoclonal antibodies in the brain of patients with subacute sclerosing panencephalitis. J Virol. 1985 Oct;56(1):337–340. doi: 10.1128/jvi.56.1.337-340.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux L., Waldvogel F. A. Instability of the viral M protein in BHK-21 cells persistently infected with Sendai virus. Cell. 1982 Feb;28(2):293–302. doi: 10.1016/0092-8674(82)90347-6. [DOI] [PubMed] [Google Scholar]
- Sheppard R. D., Raine C. S., Bornstein M. B., Udem S. A. Measles virus matrix protein synthesized in a subacute sclerosing panencephalitis cell line. Science. 1985 Jun 7;228(4704):1219–1221. doi: 10.1126/science.4001938. [DOI] [PubMed] [Google Scholar]
- Shimizu K., Isida N. The smallest protein of Sendi virus: its candidate function of binding nucleocaspsid to envelope. Virology. 1975 Oct;67(2):427–437. [PubMed] [Google Scholar]
- Udem S. A. Measles virus: conditions for the propagation and purification of infectious virus in high yield. J Virol Methods. 1984 Feb;8(1-2):123–136. doi: 10.1016/0166-0934(84)90046-6. [DOI] [PubMed] [Google Scholar]
- Wechsler S. L., Weiner H. L., Fields B. N. Immune response in subacute sclerosing panencephalitis: reduced antibody response to the matrix protein of measles virus. J Immunol. 1979 Aug;123(2):884–889. [PubMed] [Google Scholar]
- Yoshida T., Nagai Y'Yoshii S., Maeno K., Matsumoto T. Membrane (M) protein of HVJ (Sendai virus): its role in virus assembly. Virology. 1976 May;71(1):143–161. doi: 10.1016/0042-6822(76)90101-x. [DOI] [PubMed] [Google Scholar]
- Young K. K., Heineke B. E., Wechsler S. L. M protein instability and lack of H protein processing associated with nonproductive persistent infection of HeLa cells by measles virus. Virology. 1985 Jun;143(2):536–545. doi: 10.1016/0042-6822(85)90392-7. [DOI] [PubMed] [Google Scholar]
- ter Meulen V., Carter M. J. Measles virus persistency and disease. Prog Med Virol. 1984;30:44–61. [PubMed] [Google Scholar]