Abstract
Neuronal damage in HIV-associated Neurocognitive Disorders (HAND) has been linked to inflammation induced by soluble factors released by HIV-infected, and non-infected, activated macrophages/microglia (HIV M/M) in the brain. It has been suggested that aberrant neuronal cell cycle activation determines cell fate in response to these toxic factors. We have previously shown increased expression of cell cycle proteins such as E2F1 and phosphorylated pRb in HAND midfrontal cortex in vivo and in primary neurons exposed to HIV M/M supernatants in vitro. In addition, we also demonstrated have previously shown that MDMx (also referred to as MDM4), a negative regulator of E2F1, was decreased in the brain in a primate model of HIV-induced CNS neurodegeneration. Thus, we hypothesized that MDMx provides indirect neuroprotection from HIV-induced neurodegeneration in our in vitro model. In this report, we found significant reductions in MDMx protein levels in the mid-frontal cortex of patients with HAND. In addition, treatment of primary rat neuroglial cultures with HIV M/M led to NMDA receptor- and calpain-dependent degradation of MDMx and decreased neuronal survival, while overexpression of MDMx conferred partial protection from HIV M/M toxicity in vitro. Further, our results demonstrate that MDMx is a novel and direct calpain substrate. Finally, blocking MDMx activity led to neuronal death in vitro in the absence of toxic stimulus, which was reversed by calpain inhibition. Overall, our results indicate that MDMx plays a pro-survival role in neurons, and that strategies to stabilize and/or induce MDMx can provide neuroprotection in HAND and in other neurodegenerative diseases where calpain activation contributes to neuropathogenesis.
Keywords: Calpain, caspase, HIV-associated neurocognitive disorder, MDMx, neuron, neuroprotection
Introduction
Approximately 50% of HIV (+) patients suffer from a spectrum of deficits in cognition, motor function, behavior and emotion, collectively termed HIV-associated Neurocognitive Disorders (HAND) (Masliah, DeTeresa, Mallory, & Hansen, 2000; Justin C. McArthur et al., 2004). The areas of cognition affected in HAND patients include concentration, executive functioning, memory, and attention; and usually are accompanied by depression, psychosis, and anxiety of varying severity (J. C. McArthur, 2004). Extensive in vitro and in vivo studies indicate that HIV-infected monocytes and macrophages (M/Ms) serve as viral reservoirs during the chronic course of disease. These cells act as a source for viral proteins and other secreted mediators of neuronal damage and toxicity, as supported by the presence of infected macrophages and microglia along with pathological indicators of neuronal damage and death, dendritic simplification, axonal damage, and synaptic loss observed in the CNS of HAND patients (Ellis, 2010; Gonzalez-Scarano & Martin-Garcia, 2005; Kaul, Zheng, Okamoto, Gendelman, & Lipton, 2005; Masliah et al., 1997). Additionally, the levels of macrophage activation markers, neopterin and β2-microglobulin, are elevated in the cerebrospinal fluid (CSF) of HIV-infected patients (Edén et al., 2010; Hagberg et al., 2010; Yilmaz et al., 2006).
CNS viral reservoirs, established primarily in HIV-infected macrophages, are an important source of a wide variety of toxic factors which ultimately contribute to the neuropathological changes in the HAND brain(Gonzalez-Scarano & Martin-Garcia, 2005). Extensive studies have shown that infected and/or activated macrophages can release viral proteins such as gp120 and Tat, as well as soluble factors including glutamate, TNF-α, quinolinic acid, reactive oxygen species (ROS), and cytokines such as CCL2 (monocyte chemotactic protein-1, MCP-1) and interleukin-6 (IL-6), all of which have been shown to adversely affect neurons. Additionally, these viral and soluble factors also induce secretion of more of these and other neurotoxic factors, such as excitatory amino acids, from macrophages/microglia as well as neighboring astrocytes (Giulian et al., 1996; Gonzalez-Scarano & Martin-Garcia, 2005; Gorry et al., 2003; Lindl, Marks, Kolson, & Jordan-Sciutto, 2010; Price et al., 1988; Soontornniyomkij et al., 1998). Consistent with the role of macrophages in HAND neuropathogenesis, levels of neuroinflammatory factors (e.g. TNF-α, CCL2, and IL-6) are increased in the cerebrospinal fluid (CSF) of HIV-infected patients (C. L. Achim et al., 1996; C.L. Achim & Wiley, 1996; Conant et al., 1998; Gisolf et al., 2000; Sippy, Hofman, Wallach, & Hinton, 1995). These findings underscore the central role macrophages play in HIV-associated neuropathogenesis.
There are several non-mutually exclusive mechanisms by which neuroinflammation can lead to synaptic damage and neuronal death in HAND, including oxidative stress (Hu, Sheng, Lokensgard, Peterson, & Rock, 2009; Reynolds, Laurie, Mosley, & Gendelman, 2007), N-methyl-D-aspartate (NMDA) receptor (NMDAR) activation (Giulian et al., 1996; O’Donnell et al., 2006), caspase and/or calpain activation (O’Donnell et al., 2006; Y. Wang et al., 2007), and aberrant cell cycle regulation (Akay, Lindl, Wang, et al., 2011; K. L. Jordan-Sciutto, Wang, Murphey-Corb, & Wiley, 2002; K. L. Jordan-Sciutto, Wang, Murphy-Corb, & Wiley, 2000; Y. Wang et al., 2010; Y. Wang et al., 2007). Interestingly, many reports have suggested altered expression of several cell cycle proteins, including E2F1, pRb, p53, and cyclin-dependent kinase 5 (cdk5), in several neurodegenerative diseases including Alzheimer Disease, Parkinson Disease, Amyotrophic lateral sclerosis and HAND (K. Jordan-Sciutto, Rhodes, & Bowser, 2001; K. L. Jordan-Sciutto, Dorsey, Chalovich, Hammond, & Achim, 2003; K. L. Jordan-Sciutto, Malaiyandi, & Bowser, 2002; K. L. Jordan-Sciutto, Morgan, & Bowser, 1999; K. L. Jordan-Sciutto, Wang, et al., 2002; K. L. Jordan-Sciutto et al., 2000; Ranganathan & Bowser, 2003; Y. Wang et al., 2010). We have previously shown increased expression of E2F1 and phosphorylated pRb (ppRb) in several brain regions in macaques with simian immunodeficiency virus encephalitis (SIVE) in an in vivo model of HIV-associated CNS disease (K. L. Jordan-Sciutto, Wang, et al., 2002; K. L. Jordan-Sciutto et al., 2000). We have also observed decreased expression of a negative regulator of E2F1, murine double minute x/4 (MDMx), in the SIVE brain (Strachan, Koike, Siman, Hall, & Jordan-Sciutto, 2005).
MDMx is a homologue of the E3 ligase murine double minute 2 (MDM2), which has been originally identified and studied as a p53-regulating protein (Shvarts et al., 1996). In dividing cells, MDMx acts with MDM2 to inhibit the pro-apoptotic functions of p53 by directly binding p53, and by enhancing the E3 ligase activity of MDM2, while lacking intrinsic ligase activity itself(Sharp, Kratowicz, Sank, & George, 1999; X. Wang, Wang, & Jiang, 2011). Additionally, MDM2 and MDMx inhibit the pro-apoptotic activity of E2F1 in mitotic cells (Loughran & La Thangue, 2000; Strachan, Jordan-Sciutto, Rallapalli, Tuan, & Hall, 2003). Within the mouse CNS, MDMx is necessary for normal development, as mdmx −/− mice display significant neuronal apoptosis and are embryonically lethal (Migliorini et al., 2002). Additionally, MDMx knockdown in vitro damages neurons in the absence of toxic stress (Benosman et al., 2007). Finally, DNA-damaging agents, glutamate and extracellular potassium depletion lead to decreased levels of MDMx protein in cultured neurons (Benosman et al., 2007).
We previously demonstrated decreased MDMx expression and increased cytoplasmic E2F1 expression in the frontal cortex, basal ganglia and hippocampus of SIV-infected macaques with encephalitis (SIVE), and increased cytoplasmic E2F1 expression in HIVE (Strachan et al., 2005). We further showed that cytoplasmic E2F1 in HEK293 cells is associated with calpain activation and transcription-independent degradation of MDMx (Strachan et al., 2005). Using an in vitro model of HIV-associated neurodegeneration in which soluble factors released by HIV-infected monocyte-derived macrophages induce neuronal damage, we investigated the role of MDMx as a determinant of neuronal survival in HAND (Y. Wang et al., 2007). Our results show that MDMx expression is decreased in brain tissue of HAND patients. Further, we show, for the first time, that MDMx is a direct calpain substrate, and that calpain-mediated MDMx degradation following NMDA receptor activation partially contributes to neuronal death in vitro. Lastly, we demonstrate that overexpression of MDMx partially protects neurons in an in vitro model of HAND, and that pharmacological inhibition of MDMx induces calpain-mediated neuronal damage.
Materials and Methods
Chemicals and Reagents
N-methyl-D-aspartic acid (NMDA), (+)-5-methyl-10,11- dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine maleate (MK801) and SJ-172550 were purchased from Tocris Bioscience (Ellisville, MO). MDL-28170 and protease inhibitor cocktail were from Sigma Aldrich (St. Louis, MO) and native calpain-1 from porcine erythrocytes was from EMD Chemicals (Gibbstown, NJ). Q-VD-OPh (quinolyl-valyl-O-methylaspartyl-[-2, 6-difluorophenoxy]-methyl ketone) was purchased from R&D Systems, Inc (Minneapolis, MN). Rabbit polyclonal antibody to MDMx, donkey anti-goat HRP, and propidium iodide were from Santa Cruz Biotechnology (Santa Cruz, CA), and goat anti-rabbit HRP antibody was purchased from Thermo Scientific (Waltham, MA). Poly-L-lysine was from Peninsula Laboratories (San Carlos, CA). DAPI was from Molecular Probes (Carlsbad, CA). Neurobasal media, B27 supplement, goat anti-mouse β-lactamase and fluoricillin green substrate were from Invitrogen (Carlsbad, CA). The antibody that recognizes calpain-cleaved spectrin was a generous gift from Dr. Robert Siman at the University of Pennsylvania.
HIV-1 Infection of Macrophages and Collection of Supernatants
The supernatants from primary human monocyte-derived macrophages were prepared as described previously (O’Donnell et al., 2006). For the generation of supernatants from HIV-infected monocyte-derived macrophages (MDM), primary monocytes were isolated from the peripheral blood of HIV (−) healthy volunteers in accordance with protocols approved by the University of Pennsylvania Committee on Studies Involving Human Beings. Monocytes were differentiated into macrophages for 7 days at a density of 1×106 per well in 6-well Cellbind plates (Corning, Lowell, MA), as described previously (Akay, Lindl, Wang, et al., 2011). On 7 days in vitro (DIV), the monocyte-derived macrophages were incubated with a specific primary HIV isolate, Jago (100 ng of p24 per well), for 24 h. This primary isolate was acquired from cell-free cerebrospinal fluid (CSF) harvested by lumbar puncture from a patient undergoing diagnostic testing at the University of Pennsylvania, who was confirmed to have HIV-associated dementia (HAD) by established clinical and neuropsychological criteria at the time of virus isolation, as defined by the Dana Consortium (Chen et al., 2002; “Clinical confirmation of the American Academy of Neurology algorithm for HIV-1-associated cognitive/motor disorder. The Dana Consortium on Therapy for HIV Dementia and Related Cognitive Disorders,” 1996). At the end of incubation, the virus was removed from the media by two washes with Dulbecco’s modified eagle medium (DMEM). The cultures were incubated in fresh macrophage media for up to 15–17 days post-infection. HIV infection was monitored by reverse transcriptase activity in the supernatants (HIV M/M) and the supernatants with peak reverse transcriptase activity that consistently induced ~40–50% neuronal damage at a dilution of 1:20 were used for all experiments. The supernatants from monocyte-derived macrophages of the same donors that were cultured in the absence of HIV-1 infection served as mock controls (Mock).
Primary Neuroglial Cultures
Rat pups were harvested at embryonic day 17 from timed-pregnant Sprague-Dawley adults euthanized by CO2 inhalation and cervical dislocation, in accordance with the Institutional Animal Care and Use Committee (IACUC) protocols. Primary cortical neuroglial cultures were prepared, as described previously (Akay, Lindl, Wang, et al., 2011). The cells were plated at a density of 2 × 106 cells in 60 mm petri dishes or at a density of 1 × 105 cells per well in 96well tissue culture plates coated with poly-L-lysine. The cultures were maintained in neurobasal media with B27 supplement at 37°C with 5% CO2. Half of the media was replaced with fresh media every 7 days and the experiments were performed at 21 days in vitro (DIV). HIV-1-infected (HIV M/M) or mock-infected (Mock) monocyte-derived macrophage supernatants were added to cultures at the indicated dilutions.
Assessment of Neuronal Damage
Primary rat neuroglial cultures in 96-well plates were assessed for MAP2 fluorescence using a MAP2 cell-based ELISA, as described previously (Akay, Lindl, Wang, et al., 2011; Y. Wang et al., 2007; White et al., 2011). In parallel, cultures grown on coverslips were confirmed for neuronal viability under the same experimental conditions, by hand counting using a propidium iodide (PI) exclusion assay. Briefly, PI is a red fluorescent stain that binds nucleic acids by intercalating between bases for use as a label for nucleic acids. PI is also membrane impermeable, and is excluded from viable cells while it accumulates in the nuclei of cells with damaged plasma and nuclear membranes, indicative of irreversible cell damage. In our experiments, 15 μM PI was added to tissue culture media 15 minutes before the fixation step followed by subsequent staining for MAP2 and DAPI (nuclear stain) using standard immunofluorescence techniques for the identification of dead cells in culture via fluorescent microscopy (White et al., 2011). Dead cells are identified as those which are negative for MAP2, positive for PI, and showing a fragmented and/or dense nuclear morphology as detected by DAPI, whereas live cells were identified as those which are negative for MAP2, positive for PI, and showing intact nuclei by DAPI. 1000 cells on average were counted for each condition.
Immunoblotting for Primary Neuroglial Cultures
Whole cell protein extracts were prepared by scraping cells in lysis buffer (50 mM Tris pH 7.5, 120 mM NaCl, 0.5 % NP-40, 5 mM NaF and 0.5 mM Na Orthovanadate supplemented with a protease inhibitor cocktail). The lysates were incubated on ice for 10 minutes and cleared by centrifugation at 14,000 g for 10 minutes. The protein concentrations of supernatants were determined by the Bradford method. 4–12% SDS-polyacrylamide gradient gels (Invitrogen) and Immun-Blot polyvinylidene fluoride (PVDF) membranes (Biorad, Hercules, CA) were used for electrophoresis and transfer, respectively. Following standard protocols for immunoblotting (Akay, Lindl, Wang, et al., 2011), the membranes were visualized using enhanced chemiluminescence (ECL) reagent (Thermo Scientific, Rockford, IL) and autoradiography.
Subcellular Fractionation
Cytoplasmic protein extractions were prepared by scraping the cultures in ice-cold cytoplasmic lysis buffer (10 mM (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 10 mM KCl, 10 mM Ethylenediaminetetraacetic acid (EDTA), 4% Nonidet P-40 and 10 mM dithiothreitol (DTT), supplemented with a protease inhibitor cocktail). The cellular debris was cleared by centrifugation at 14,000 g for 3 min. The supernatants were saved as cytoplasmic fractions. The remaining pellets were resuspended in the nuclear lysis buffer (20 mM HEPES, 0.4 M NaCl, 1 mM EDTA, 10% glycerol and 10 mM DTT, supplemented with a protease inhibitor cocktail) and were incubated on a rocking platform at 4°C for 2 h, followed by centrifugation at 14,000 g for 5 min. The collected supernatants were the nuclear extracts. These fractions were analyzed by routine immunoblotting techniques.
Human brain tissue
Tissue samples from the mid-frontal cortices of HIV (−) control and HIV (+) human autopsy cases used for this study were obtained from the tissue banks of National NeuroAIDS Tissue Consortium (NNTC) (Table 1). For western immunoblotting, whole cell lysates were prepared as described previously, and 50 μg protein was loaded to each lane of 10 % Bis-Tris gels and processed further (Akay, Lindl, Shyam, et al., 2011; Lindl, Akay, Wang, White, & Jordan-Sciutto, 2007).
Table 1.
The cases used for western immunoblotting in Figure 1A.
| HIV Status | Neurocog | Sex | Age | PMI | NNTC ID |
|---|---|---|---|---|---|
| Control 1 | Normal | M | 53 | 22.5 | MHBB584 |
| Control 2 | Normal | M | 61 | 6.5 | MHBB589 |
| Control 3 | Normal | M | 48 | 9 | MHBB604 |
| Control 4 | Normal | M | 50 | 16 | MHBB606 |
| HIV 1 | Normal | M | 45 | N/A | 1119 |
| HIV 2 | Normal | M | 49 | N/A | 2012 |
| HIV 3 | Normal | M | 46 | N/A | 2029 |
| HIV 4 | Normal | N/A | N/A | N/A | CC128 |
| HIV 5 | Normal | N/A | N/A | N/A | CE125 |
| HIV 6 | HAND | N/A | 47 | N/A | 1052 |
| HIV 7 | HAND | N/A | 46 | N/A | 4028 |
| HIV 8 | HAND | N/A | 41 | N/A | 6037 |
| HIV 9 | HAND | M | 57 | 5.5 | 7480 |
| HIV 10 | HAND | F | 54 | 7 | 100066 |
| HIV 11 | HAND | M | 36 | 2.5 | 6883 |
| HIV 12 | HAND | M | 49 | 67.33 | 8270 |
| HIV 13 | HAND | M | 43 | N/A | 8483 |
| HIV 14 | Normal | N/A | 39 | N/A | 6052 |
| HIV 15 | Normal | M | 50 | 18 | 8087 |
| HIV 16 | Normal | N/A | 46 | 2.75 | 6771 |
| HIV 17 | Normal | N/A | N/A | N/A | CE166 |
| HIV 18 | HAND | N/A | 34 | N/A | 4041 |
| HIV 19 | HAND | N/A | 34 | N/A | 6007 |
| HIV 20 | HAND | M | 35 | 9.5 | 6866 |
| HIV 21 | HAND | F | 34 | 5 | 7680 |
Neurocog: neurocognitive status, PMI: postmortem interval, N/A: not available, NNTC: National NeuroAIDS Tissue Consortium.
In vitro Calpain Cleavage Assay
pcDNA3.1-MDMx (human) was transcribed and translated using TNT quick coupled transcription kit (Promega, Madison, WI). 1 μg of MDMx was incubated in the assay buffer which contained 50 mM Tris–HCl pH 7.4, 100 mM KCl, 2 mM DTT with 2.5 U/ml of recombinant calpain I in the presence or absence of the indicated amounts of CaCl2 for 90 min at 37°C. Incubation was stopped by the addition of the SDS loading buffer to the reaction tubes. The samples were then boiled for 10 min at 95°C, separated on 4–12% SDS-polyacrylamide gradient gels and transferred to PVDF membranes. The membranes were immunoblotted and visualized, as described above.
Construction of Viral Vectors
pcDNA3.1-MDMx (human) was cloned into the recombinant adeno-associated virus (AAV) cloning plasmid, pZac2.1 (University of Pennsylvania Vector Core), using BamHI-BamHI restriction enzyme sites. The AAV were packaged into recombinant AAV particles using pPACK-H plasmid that encodes adenovirus glycoprotein and helper plasmid (University of Pennsylvania Vector Core).
Infection of Primary Neuroglial Cultures with Viral Vectors
Fifty percent of culture media of 21DIV primary rat neuroglial cultures in 60-mm dishes or 96-well plates was changed one day prior to infection. The infections were carried out by addition of thawed virus directly into the culture media. The amount of AAV particles was empirically titrated in 21DIV primary neuronal cultures at dilutions not exceeding 1:40. Experiments were carried out three days post-infection.
Statistical Analysis
One-way ANOVA with post-hoc Newman-Keuls multiple comparison test was used for statistical analysis of in vitro immunoblot quantifications, MAP2 ELISA and neuronal viability assays. For immunoblot studies of human tissue, Student’s t-test was performed. In experiments using human tissue samples, additional statistical analyses were performed between the post-mortem interval (PMI) and age, and the relative MDMx protein levels detected by immunoblotting, to investigate possible correlations between these parameters. For all statistical analyses performed, p values of < 0.05 were considered significant.
Results
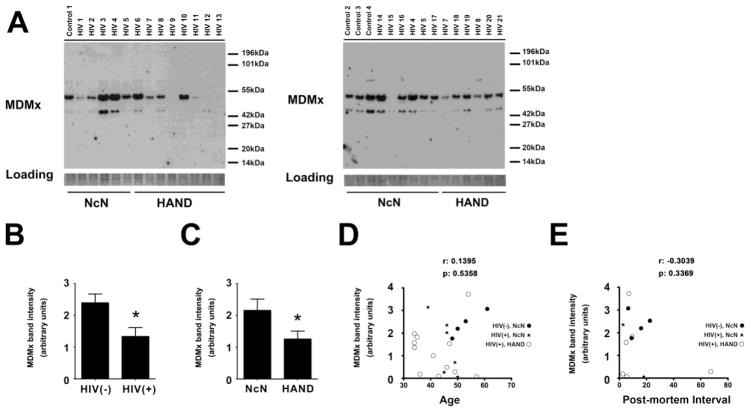
MDMx levels are decreased in the mid-frontal cortices of neurocognitively impaired HIV (+) patients
In this study, we examined MDMx protein expression in brain tissue from HIV-infected patients by immunoblotting. MDMx migrated as a doublet in immunoblots of whole cell lysates from human mid-frontal cortical samples, as shown in Figure 1A (See Table 1 for a summary of cases). The quantification of the MDMx immunoblotting data, as shown in Figure 1B, revealed that total MDMx protein levels were significantly decreased in the mid-frontal cortices of HIV-infected patients, as compared with those in uninfected controls (*p<0.05). Additionally, when we compared MDMx levels according to the neurocognitive status of the cases, we observed statistically significant decreases in MDMx levels in HAND patients, as compared with those in HIV-infected, neurocognitively normal (NcN) patients (Figure 1C, *p<0.05). These findings suggest that MDMx protein is decreased in the HAND cortex, in agreement with our previous findings in the macaque model of HIV-induced neurodegeneration (Strachan et al., 2005). Of note, analysis of immunoblots for correlations between MDMx protein levels and age (Figure 1D) or post-mortem interval (PMI, Figure 1E) showed no significance.
Figure 1. Decreased MDMx expression in the HAND cortex.
A, B and C. Whole cell tissue lysates from the midfrontal cortex of a cohort of NcN (n=13) and HAND (n=12) cases (Table 1) were used for immunoblotting. The membranes were blotted for MDMx, which were later stained with coomassie to control for loading (A). Statistically significant decreases in MDMx expression is observed in HIV(+) cases compared with those observed in HIV(−) group (B, Student’s t-test, Mann-Whitney U post-hoc, * p<0.05); as well as in HAND cases compared with those observed in NcN group (C, Student’s t-test, Mann-Whitney U post-hoc, * p<0.05)
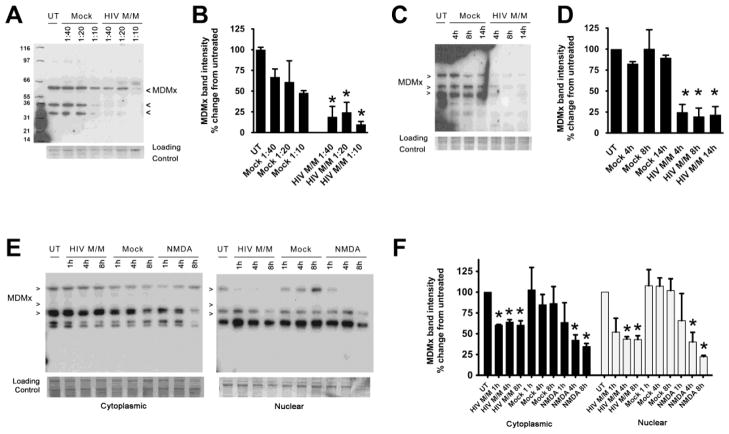
MDMx is decreased in a time- and dose-dependent manner in an in vitro model of HIV-associated neurodegeneration
We have previously utilized supernatants secreted from primary human monocyte-derived macrophages infected with a primary HIV isolate (Jago) to treat primary rat neuroglial cultures, and have shown that these supernatants (HIV M/M) consistently lead to dose- and time-dependent neuronal damage in this in vitro model of HIV-induced neuronal damage (Akay, Lindl, Wang, et al., 2011; O’Donnell et al., 2006; Y. Wang et al., 2007; White et al., 2011). To begin to investigate the mechanism(s) underlying reduced MDMx levels detected in HAND brain tissue, we assessed MDMx protein levels in HIV M/M-treated primary neuroglial cultures. As seen in Figure 2A, we observed multiple MDMx isoforms in these cultures. Importantly, HIV M/M that was diluted 1:40 or 1:20 in culture media led to significant decreases in all MDMx isoforms after 20 hours, whereas the treatment of cultures with supernatants from mock-treated monocyte-derived macrophages (Mock) at similar dilutions did not cause significant loss of MDMx relative to untreated cultures (Figure 2B, * p<0.05). Additionally, significant decreases in MDMx protein levels were detectable as early as 4 hours after exposure to HIV M/M at a dilution of 1:20 (Figures 2C and 2D, * p<0.05). Further, the analysis of the cytoplasmic and nuclear extracts of cultures exposed to Mock or HIV M/M revealed that HIV M/M-induced, significant losses of MDMx isoforms were induced by HIV M/M in both compartments (Figures 2E and 2F, * p<0.05). NMDA was used as a positive control for neurotoxicity. These results suggest that MDMx is decreased in a dose- and time-dependent manner in our in vitro model of HIV-associated neurotoxicity.
Figure 2. HIV M/M leads to decreases in MDMx in a time- and dose-dependent manner.
A – D. Primary neuroglial cultures that were 21 days in vitro (DIV) were treated with mock-infected macrophage supernatants (Mock) or HIV-infected macrophage supernatants (HIV M/M) at the indicated doses for 20 hours. Western immunoblotting of whole cell protein lysates shows a dose-dependent (A, B) and a time-dependent (C, D) decrease in MDMx levels (n=4, One-way ANOVA, post-hoc Newman-Keuls, * p<0.05 vs. untreated). E–F. Cytoplasmic and nuclear extracts from primary neuroglial cultures treated with either Mock (1:20 dilution), HIV M/M (1:20 dilution) or NMDA (10 μM) for 1, 4, or 8 hours were immunoblotted for MDMx. HIV M/M treatment led to statistically significant decreases in MDMx protein levels in both cytoplasmic and nuclear fractions. Likewise, NMDA treatments led to significant decreases in nuclear MDMx levels starting at 4 hour following treatment (n=3, One-way ANOVA, post-hoc Newman-Keuls, * p<0.05 vs. untreated). Mock did not cause a change in MDMx levels in either cytoplasmic or nuclear fractions. Representative immunoblots of at least three biological replicates are shown. Note that the antibody used against MDMx detects at least three isoforms in protein lysates.
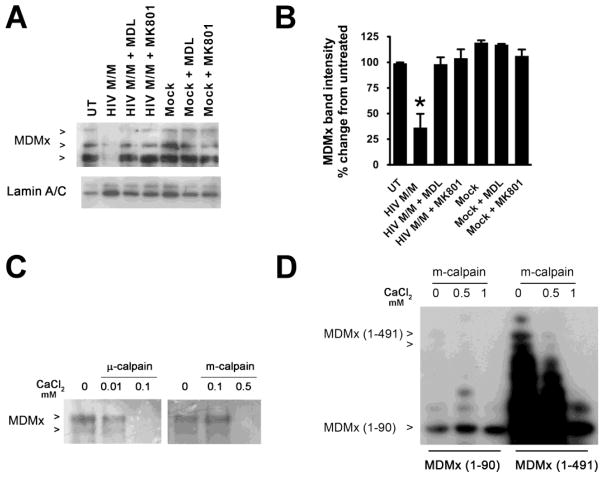
Degradation of MDMx is calpain-dependent
We have previously shown that HIV M/M-induced neurotoxicity occurs through the activation of N-methyl-D-aspartic acid (NMDA) receptor (NMDAR) and subsequent calpain activation in our in vitro model (O’Donnell et al., 2006; Y. Wang et al., 2007). In this study, treatment of cultures with NMDA decreased MDMx protein, at levels comparable to those observed in HIV M/M-treated cultures (Figures 2E and 2F). Additionally, pre-incubation of neuroglial cultures with the NMDAR inhibitor, MK801, prevented HIV M/M-induced MDMx degradation (Figures 3A and 3B, * p<0.05). Further, pre-treatment of cultures with a calpain inhibitor, MDL28170, prevented MDMx loss, suggesting a role for calpains in MDMx loss (Figures 3A and 3B, * p<0.05). Next, we performed an in vitro calpain cleavage assay to determine if MDMx is a direct calpain substrate. As seen in Figure 3C, in vitro transcribed and translated full-length MDMx (1–491) was a substrate for two types of calpain: μ-calpain, which is activated by nanomolar concentrations of CaCl2, and m-calpain, which is activated by micromolar concentrations of CaCl2. However, a truncated form of MDMx, MDMx (1–90) is not degraded by m-calpain under the same conditions (Figure 3D). These data collectively suggest that activated calpains induced by HIV M/M directly degrade MDMx at a site/ (or sites) located in the internal region and/or carboxyl terminus.
Figure 3. HIV M/M-induced decrease in MDMx is dependent on NMDA receptor and calpain activation.
A and B. Primary neuroglial cultures were treated with either Mock (1:20 dilution) or HIV M/M (1:20 dilution) for 20 hours in the presence or absence of the calpain inhibitor, MDL 28170 (20 μM) or an NMDA receptor antagonist, MK801 (10 μM). The inhibitors were added to the culture media 30 minutes prior to Mock or HIV M/M treatments. The nuclear lysates were immunoblotted for MDMx. HIV M/M exposure lead to a decrease in MDMx, which was blocked by pre-treatment with MDL 28170 or MK801 ((n=5, One-way ANOVA, post-hoc Newman-Keuls, * p<0.05 vs. untreated, vs. Mock, vs. HIV M/M + MDL, and vs. HIV M/M + MK801). Lamin A/C was used to show successful nuclear protein extraction. C. In vitro transcribed and translated human MDMx was incubated with native calpain-1 in the presence of different concentrations of CaCl2 and the lysates were immunoblotted for MDMx. μ-calpain activation by lower mM doses of CaCl2, or m-calpain activation by higher mM concentrations of CaCl2 led to degradation of MDMx. D. In vitro transcribed and translated human full–length MDMx (1–491) (lanes 1–3) or truncated MDMx (1–90) (lanes 4–6) were incubated with native calpain-1 in the presence of increasing concentrations of CaCl2 and the lysates were immunoblotted for MDMx. Calpain activation led to degradation of MDMx (1–491), while MDMx (1–90) was not degraded by calpain under the same conditions.
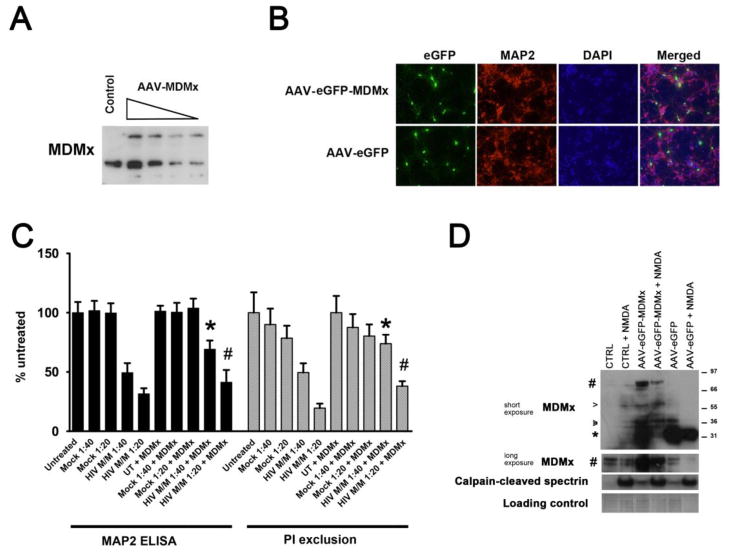
Overexpression of MDMx attenuates HIV M/M-induced neurotoxicity
Based on our findings which suggest that excitotoxic injury led to calpain-mediated MDMx degradation, we determined whether overexpression of MDMx would provide neuroprotection from HIV M/M in vitro. We overexpressed full-length MDMx in neuroglial cultures via an adeno-associated virus (AAV) expression system. Using this system, we achieved a viral titer-dependent overexpression of MDMx in neuroglial cultures (Figure 4A). Figure 4B shows representative immunofluorescence images of cultures overexpressing MDMx. Neuronal viability as measured by cell-based MAP2 ELISA and PI exclusion assays showed that HIV M/M-induced neurotoxicity in our in vitro model was attenuated in cultures that overexpressed MDMx (Figure 4C, *p< 0.05 vs. HIV M/M 1:40, # p<0.05 vs. HIV M/M 1:20). Importantly, overexpressed MDMx protein was still degraded in cultures exposed to NMDA (Figure 4D). These results suggest a neuroprotective function for MDMx in our in vitro HIV-associated neurotoxicity model, and that suggest that additional strategies to stabilize MDMx protein or block MDMx degradation may promote neuroprotection.
Figure 4. Overexpression of MDMx in neurons provides partial protection from HIV M/M-induced neuronal damage.
An Adeno-associated virus expression system was utilized for overexpression of human MDMx in primary neurons in vitro. A. Infection with AAV-MDMx led to increased MDMx expression in cultures in a dose-dependent manner, as assessed by immunoblotting for MDMx. B. Following three days after infection with AAV-eGFP-MDMx or AAV-eGFP, primary rat cortical cultures on coverslips were immunostained for MAP2 and DAPI. Images were captured by epifluorescent microscopy. Representative images are shown. Magnification 40X. C. Attenuation of HIV M/M-induced MAP2 loss in neurons overexpressing MDMx, as compared with those observed in HIV M/M-treated cultures, as assessed by cell-based MAP2 ELISA (black bars), and PI exclusion (gray bars) (* p< 0.05 vs. HIV M/M 1:40, # p<0.05 vs. HIV M/M 1:20, One way-ANOVA, Newman-Keuls post-hoc). D. Three days after infection with AAV- eGFP-MDMx or AAV-eGFP, neuroglial cultures were treated with either 20 μM of NMDA, or were left untreated. The lysates were immunoblotted for MDMx, and calpain activation was assessed by immunoblotting for the calpain-cleaved spectrin. Pound sign denotes the bands for overexpressed MDMx, arrowheads mark the bands for endogenous MDMx, and asterisk denotes the band for GFP.
Inhibition of MDMx activity leads to calpain-dependent neuronal death
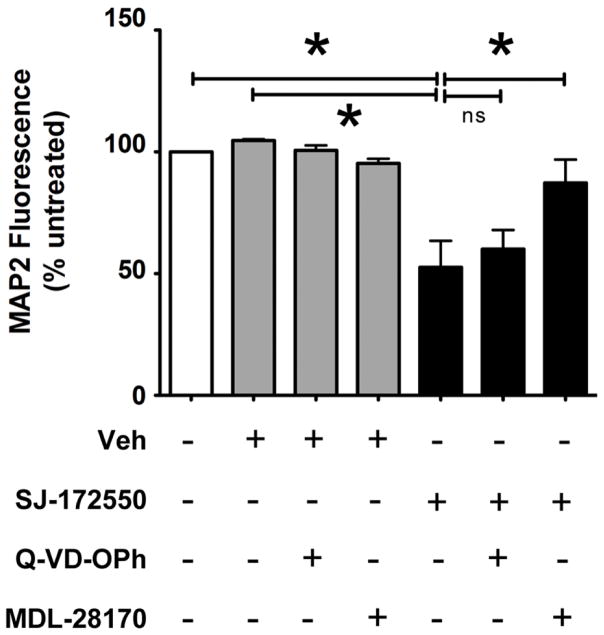
Finally, we questioned whether pharmacological inhibition of MDMx activity would impact neuronal viability in vitro. We used a cell-permeable and MDMx-specific inhibitor, SJ-172550, which binds to the p53-binding pocket at the N terminus of MDMx and blocks p53 docking (Reed et al., 2010). We first determined that the exposure of primary neuroglial cultures to SJ-172550 at 20 μM for 20 hours caused 50% neuronal death in the absence of external stimuli (data not shown). Next, we determined whether blocking caspases or calpains would prevent neuronal death induced by MDMx inhibition. As seen in Figure 5, the pre-treatment of cultures with a pan-caspase inhibitor, Q-VD-OPh before the addition of SJ-172550 did not prevent neuronal loss. However, the pre-treatment of cultures with MDL28170 prevented neuronal loss induced by SJ-172550 (n=3, *p<0.05). Taken together, our findings suggest that blocking MDMx activity causes caspase-independent, calpain-mediated death in primary neurons in vitro, and further, suggest an important pro-survival role for MDMx in post-mitotic neurons.
Figure 5. Inhibition of MDMx activity leads to calpain-dependent neuronal death.
21DIV primary neuroglial cultures were treated with SJ-172550 (20 μM) for 20 hours in the presence or absence of either the calpain inhibitor MDL 28170 (20 μM) or the pan-caspase inhibitor Q-VD-OPh (50 μM). The inhibitors were added to the culture media 30 minutes prior to SJ-172550 treatments. Neuronal viability was assessed by cell-based MAP2 ELISA (Vehicle: 0.04% DMSO, n=3, *p<0.05, ns= not significant, Student’s t-test).
Discussion
Multiple studies have demonstrated that altered/aberrant expression or modification of cell cycle proteins and cell cycle-related transcription and regulatory factors play important roles in neuronal viability in various neurodegenerative diseases, including HIV-associated neurocognitive disorders (HAND) (K. Jordan-Sciutto et al., 2001; K. L. Jordan-Sciutto et al., 2003; K. L. Jordan-Sciutto, Malaiyandi, et al., 2002; K. L. Jordan-Sciutto et al., 1999; K. L. Jordan-Sciutto, Wang, et al., 2002; K. L. Jordan-Sciutto et al., 2000; Ranganathan & Bowser, 2003; Y. Wang et al., 2010). In the current study, we demonstrate that MDMx, an important regulator of a major cell cycle-related transcription factor, E2F1, is decreased significantly in the mid-frontal cortex of HAND patients compared to that in neurocognitively normal, HIV-infected patients. Further, we show that these changes in MDMx protein are not correlated with either age or postmortem interval. We have previously shown that E2F1 levels were increased in the neuronal cytoplasm of the HAND brain as well as in the basal ganglia and the cortical neurons of SIV encephalitic macaques, whereas we have detected reciprocal decreases in MDMx levels in the neurons of SIVE brain (K. L. Jordan-Sciutto, Wang, et al., 2002). While E2F1 overexpression has been shown to be pro-apoptotic in post-mitotic neurons (O’Hare et al., 2000), E2F1 does not induce the expression of classical E2F1 transcriptional targets in the HAND cortex, as we have recently reported (Y. Wang et al., 2010). These differences in subcellular localization of E2F1, as well as the non-classical roles of E2F1 in post-mitotic neurons, suggest that the regulatory mechanisms that govern E2F1:MDMx interactions described in dividing cells might not apply to post-mitotic neurons.
In the current study, we demonstrate that all MDMx isoforms were decreased in our in vitro model of HIV-associated neurodegeneration. Several mechanisms have been shown to contribute to the presence of multiple MDMx isoforms, including pre-mRNA alternative splicing, phosphorylation and ubiquitination, as determined in human fibroblasts and cancer cells (de Graaf et al., 2003; Jeyaraj, O’Brien, & Chandler, 2009; Markey, 2011; Pereg et al., 2005; R. Rallapalli, Strachan, Cho, Mercer, & Hall, 1999; Ravikumar Rallapalli, Strachan, Tuan, & Hall, 2003). However, the mechanism(s) underlying the presence of MDMx isoforms in primary neurons are not known. Future studies investigating these pathways will be instrumental towards delineating the function of specific MDMx isoforms in post-mitotic neurons.
In our in vitro model of disease, MDMx protein levels are decreased in response to calpain activation in neurons (O’Donnell et al., 2006; Y. Wang et al., 2007; White et al., 2011). Our findings regarding calpain activation are in agreement with our previous findings, in which we have shown that calpains, but not caspases, are the major death proteases activated in neurons exposed to HIV M/M or NMDA (O’Donnell et al., 2006; Y. Wang et al., 2007; White et al., 2011). Additionally, we have recently reported that calpain levels in untreated neuroglial cultures increased with age while caspase-3 levels decreased (Y. Wang et al., 2012). Calpains are intracellular Ca2+-dependent cysteine proteases that degrade many proteins (Sorimachi, Hata, & Ono, 2011). While we had previously shown that overexpression of an endogenous calpain inhibitor, calpastatin, stabilized MDMx in HEK293 cells, to date there is no report suggesting MDMx as a direct calpain substrate in dividing cell types, or in neurons(Strachan et al., 2005). In this report, we demonstrate that MDMx is a direct calpain substrate. Although previous studies in dividing cell types demonstrated a caspase cleavage site within the internal region of MDMx by which it can be degraded and subsequently be targeted for proteasomal degradation(Gentiletti et al., 2002); to date, a calpain cleavage site within MDMx has not been identified. The examination of calpain cleavage sites of a number of known calpain target proteins reveals certain characteristics for these preferred cleavage sites, such as a proline-rich region (Tompa et al., 2004). Based on these shared characteristics, several algorithms are used to more accurately predict such sites (duVerle, Takigawa, Ono, Sorimachi, & Mamitsuka, 2010). The analysis of the primary amino acid sequence of MDMx using “Calpain for Modulatory Proteolysis Database” (calpain.org) predicts the region surrounding threonine at position 347 as a calpain cleavage site. Although a substantial undertaking, it will be important to identify the calpain cleavage site(s) on MDMx in future investigations for several reasons. First, the overexpression of MDMx provided partial protection from neuronal death in our in vitro model, as well as in other in vitro models of neurotoxicity (Benosman et al., 2007). The full-length MDMx used in our overexpression studies is still a calpain substrate; and as degradation is likely to be more efficient than protein synthesis, MDMx overexpression in our studies attenuates damage and death, but does not completely prevent it, as demonstrated by our results showing NMDA-mediated degradation in MDMx still occurring in cultures overexpressing MDMx. Future studies will be instrumental towards determining whether overexpression of a calpain-resistant MDMx mutant will provide further neuroprotection in our in vitro model. Secondly, the majority of MDMx’s functions are determined by protein:protein interactions(Lenos & Jochemsen, 2011). While we did not detect any accumulation of distinct MDMx cleavage products in vitro or in vivo, transient MDMx cleavage products may still form, altering downstream pathways by disrupting specific protein:protein interactions or potentially acting as a dominant-negative MDMx. Thus, by identifying the domain(s) on MDMx that are targeted by calpain for cleavage, we can begin to understand the specific pathways downstream from MDMx that may be disrupted in neurons in response to neurotoxic stimuli. Finally, strategies that can stabilize MDMx and promote its accumulation within neurons will be useful in treatment approaches for HAND, during which decreased MDMx and increased calpain activation contribute to neuronal damage.
The in vitro calpain cleavage assay demonstrated that MDMx is a direct target of calpain in the absence of additional cellular factors. In addition to calpain, there is evidence that other factors may still be involved in MDMx degradation, in parallel with, or subsequent to, calpain activation. For example, cathepsins, downstream effectors of calpains, have been demonstrated to cause MDMx degradation in a previous study (Strachan et al., 2001). Another possible mechanism of MDMx loss is dysregulation at the mRNA level. Previous studies have shown that MDMx regulation occurs primarily at the protein level, suggesting that changes at the transcriptional level are not as likely(Benosman et al., 2007). However, future studies using human macrophages and HIV M/M-exposed neurons our in vitro model will be contributory to address both of these possibilities.
Studies exploring the specific roles of MDMx in post-mitotic neurons are limited. The phenotype of mdmx knockout mice reveals that MDMx is necessary for CNS development (Xiong, Van Pelt, Elizondo-Fraire, Liu, & Lozano, 2006). Interestingly, the severe cell death observed in the brains of these embryos is rescued by concurrent silencing of p53. Multiple studies demonstrate that MDMx:MDM2 heterodimers bind and ubiquitinate p53, targeting p53 for degradation by the proteasome, and thus, allowing cells to maintain a low basal level of this pro-apoptotic protein under normal conditions (Wade, Wang, & Wahl, 2010). Additionally, MDMx silencing in cerebellar granule neurons caused caspase-3 activation and cell death in the absence of a toxic insult (Benosman et al., 2007). Thus, it is highly likely that the massive cell loss observed in mdmx−/− mice during development is due to p53-driven and caspase-mediated apoptosis. In our studies, we demonstrate that treatment of neurons with SJ-172550, which has been shown to inhibit binding of p53 to MDMx in other cell types, leads to death in post-mitotic neurons in the absence of toxic stimuli. However, our findings differ from the abovementioned studies in that neither the inhibition of p53 transcriptional activity by pre-treatment of neurons with Pifithrin-α (data not shown), nor the inhibition of caspase activation via a pan-caspase inhibitor, Q-VD-OPh, conferred protection from SJ-172550-mediated neurotoxicity, while SJ-172550-induced neuronal death was reversed by calpain inhibition. These results suggest that neuronal death observed in our cultures involves a p53- and caspase-independent mechanism, contrary to findings from previous studies. There are several factors that need to be considered for these differences. First, we utilized SJ-172550 to determine the implications of the disruption of MDMx:p53 binding on neuronal viability, while previous studies have examined the effect of silencing of MDMx (Benosman et al., 2007). Secondly, SJ-172550 binding to MDMx at the amino terminus may disrupt its binding to a protein other than/in addition to p53, triggering the activation of a downstream pro-death pathway involving calpains. The effect of SJ-172550 on MDMx association with other proteins in post-mitotic differentiating neurons using co-immunoprecipitation approaches will begin to address this possibility, and will provide additional information on pathways downstream from MDMx in neurons. Finally, while SJ-172550 has been shown to specifically block MDMx:p53 interaction upon binding to MDMx without affecting its stability, those findings were not verified in cells. In vitro, p53 binding may be masking a calpain cleavage site on MDMx, which may become accessible by calpains in SJ-172550-treated neurons, leading to increased calpain-mediated degradation. The identification of calpain cleavage site(s) on MDMx, as mentioned above, will be instrumental to assess this possibility.
In summary, our findings show that MDMx stability and activity are crucial for the survival of post-mitotic neurons, and support current literature involving other tissue and cell types which suggests that MDMx is regulated mainly at the protein level by post-translational modifications that impact its stability and protein:protein interactions. Further, MDMx overexpression provided partial protection from HIV-associated neurodegeneration in our in vitro model. Finally, we identify MDMx as a novel calpain substrate. These findings suggest that strategies to stabilize MDMx will have therapeutic implications which are applicable, not only in HAND, but also in other neurodegenerative diseases in which calpain activation is an underlying mechanism of neuronal death and dysfunction.
Acknowledgments
Grants: This work was supported by the following grants: NS41202 (KJS) and MH095671 (DLK).
We would like to thank Dr. Kathryn A. Lindl and Jacob Zyskind for their contributions to the discussions during the preparation of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achim CL, Masliah E, Heyes MP, Sarnacki P, Hilty C, Baldwin M, Wiley CA. Macrophage Activation Factors in the Brains of AIDS Patients. J NeuroAIDS. 1996;1(2):1–16. doi: 10.1300/J128v01n02_01. [DOI] [PubMed] [Google Scholar]
- Achim CL, Wiley CA. Inflammation in AIDS and the role of the macrophage in brain pathology. Current Opinion in Neurology. 1996;9(3):221–225. doi: 10.1097/00019052-199606000-00013. [DOI] [PubMed] [Google Scholar]
- Akay C, Lindl KA, Shyam N, Nabet B, Goenaga-Vazquez Y, Ruzbarsky J, Jordan-Sciutto KL. Activation status of integrated stress response pathways in neurons and astrocytes of HAND cortex. Neuropathol Appl Neurobiol. 2011 doi: 10.1111/j.1365-2990.2011.01215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akay C, Lindl KA, Wang Y, White MG, Isaacman-Beck J, Kolson DL, Jordan-Sciutto KL. Site-specific hyperphosphorylation of pRb in HIV-induced neurotoxicity. Mol Cell Neurosci. 2011;47(2):154–165. doi: 10.1016/j.mcn.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benosman S, Gross I, Clarke N, Jochemsen AG, Okamoto K, Loeffler JP, Gaiddon C. Multiple neurotoxic stresses converge on MDMX proteolysis to cause neuronal apoptosis. Cell Death Differ. 2007;14(12):2047–2057. doi: 10.1038/sj.cdd.4402216. [DOI] [PubMed] [Google Scholar]
- Chen W, Sulcove J, Frank I, Jaffer S, Ozdener H, Kolson DL. Development of a human neuronal cell model for human immunodeficiency virus (HIV)-infected macrophage-induced neurotoxicity: apoptosis induced by HIV type 1 primary isolates and evidence for involvement of the Bcl-2/Bcl-xL-sensitive intrinsic apoptosis pathway. J Virol. 2002;76(18):9407–9419. doi: 10.1128/JVI.76.18.9407-9419.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinical confirmation of the American Academy of Neurology algorithm for HIV-1-associated cognitive/motor disorder. The Dana Consortium on Therapy for HIV Dementia and Related Cognitive Disorders. Neurology. 1996;47(5):1247–1253. doi: 10.1212/wnl.47.5.1247. [DOI] [PubMed] [Google Scholar]
- Conant K, Garzino-Demo A, Nath A, McArthur JC, Halliday W, Power C, Major EO. Induction of monocyte chemoattractant protein-1 in HIV-1 Tat-stimulated astrocytes and elevation in AIDS dementia. Proc Natl Acad Sci U S A. 1998;95(6):3117–3121. doi: 10.1073/pnas.95.6.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Graaf P, Little NA, Ramos YFM, Meulmeester E, Letteboer SJF, Jochemsen AG. Hdmx Protein Stability Is Regulated by the Ubiquitin Ligase Activity of Mdm2. Journal of Biological Chemistry. 2003;278(40):38315–38324. doi: 10.1074/jbc.M213034200. [DOI] [PubMed] [Google Scholar]
- duVerle D, Takigawa I, Ono Y, Sorimachi H, Mamitsuka H. CaMPDB: a resource for calpain and modulatory proteolysis. Genome Inform. 2010;22:202–213. [PubMed] [Google Scholar]
- Edén A, Fuchs D, Hagberg L, Nilsson S, Spudich S, Svennerholm B, Gisslén M. HIV-1 Viral Escape in Cerebrospinal Fluid of Subjects on Suppressive Antiretroviral Treatment. Journal of Infectious Diseases. 2010;202(12):1819–1825. doi: 10.1086/657342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis R. HIV and antiretroviral therapy: impact on the central nervous system. Prog Neurobiol. 2010;91(2):185–187. doi: 10.1016/j.pneurobio.2009.10.016. S0301-0082(09)00167-1 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentiletti F, Mancini F, D’Angelo M, Sacchi A, Pontecorvi A, Jochemsen AG, Moretti F. MDMX stability is regulated by p53-induced caspase cleavage in NIH3T3 mouse fibroblasts. Oncogene. 2002;21(6):867–877. doi: 10.1038/sj.onc.1205137. [DOI] [PubMed] [Google Scholar]
- Gisolf EH, van Praag RM, Jurriaans S, Portegies P, Goudsmit J, Danner SA, Prins JM. Increasing cerebrospinal fluid chemokine concentrations despite undetectable cerebrospinal fluid HIV RNA in HIV-1-infected patients receiving antiretroviral therapy. J Acquir Immune Defic Syndr. 2000;25(5):426–433. doi: 10.1097/00042560-200012150-00007. [DOI] [PubMed] [Google Scholar]
- Giulian D, Yu J, Li X, Tom D, Li J, Wendt E, Noonan C. Study of receptor-mediated neurotoxins released by HIV-1-infected mononuclear phagocytes found in human brain. J Neurosci. 1996;16(10):3139–3153. doi: 10.1523/JNEUROSCI.16-10-03139.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. 2005 doi: 10.1038/nri1527. [Review] [173 refs] [DOI] [PubMed] [Google Scholar]
- Gorry PR, Ong C, Thorpe J, Bannwarth S, Thompson KA, Gatignol A, Purcell DF. Astrocyte infection by HIV-1: mechanisms of restricted virus replication, and role in the pathogenesis of HIV-1-associated dementia. Curr HIV Res. 2003;1(4):463–473. doi: 10.2174/1570162033485122. [DOI] [PubMed] [Google Scholar]
- Hagberg L, Cinque P, Gisslen M, Brew B, Spudich S, Bestetti A, Fuchs D. Cerebrospinal fluid neopterin: an informative biomarker of central nervous system immune activation in HIV-1 infection. AIDS Research and Therapy. 2010;7(1):15. doi: 10.1186/1742-6405-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S, Sheng WS, Lokensgard JR, Peterson PK, Rock RB. Preferential sensitivity of human dopaminergic neurons to gp120-induced oxidative damage. J Neurovirol. 2009;15(5–6):401–410. doi: 10.3109/13550280903296346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeyaraj S, O’Brien DM, Chandler DS. MDM2 and MDM4 splicing: an integral part of the cancer spliceome. Front Biosci. 2009;14:2647–2656. doi: 10.2741/3402. [DOI] [PubMed] [Google Scholar]
- Jordan-Sciutto K, Rhodes J, Bowser R. Altered subcellular distribution of transcriptional regulators in response to Abeta peptide and during Alzheimer’s disease. Mech Ageing Dev. 2001;123(1):11–20. doi: 10.1016/s0047-6374(01)00334-7. S0047-6374(01)00334-7 [pii] [DOI] [PubMed] [Google Scholar]
- Jordan-Sciutto KL, Dorsey R, Chalovich EM, Hammond RR, Achim CL. Expression patterns of retinoblastoma protein in Parkinson disease. J Neuropathol Exp Neurol. 2003;62(1):68–74. doi: 10.1093/jnen/62.1.68. [DOI] [PubMed] [Google Scholar]
- Jordan-Sciutto KL, Malaiyandi LM, Bowser R. Altered distribution of cell cycle transcriptional regulators during Alzheimer disease. J Neuropathol Exp Neurol. 2002;61(4):358–367. doi: 10.1093/jnen/61.4.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan-Sciutto KL, Morgan K, Bowser R. Increased Cyclin G1 Immunoreactivity During Alzheimer’s Disease. J Alzheimers Dis. 1999;1(6):409–417. doi: 10.3233/jad-1999-1605. [DOI] [PubMed] [Google Scholar]
- Jordan-Sciutto KL, Wang G, Murphey-Corb M, Wiley CA. Cell cycle proteins exhibit altered expression patterns in lentiviral-associated encephalitis. J Neurosci. 2002;22(6):2185–2195. doi: 10.1523/JNEUROSCI.22-06-02185.2002. 22/6/2185 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan-Sciutto KL, Wang G, Murphy-Corb M, Wiley CA. Induction of cell-cycle regulators in simian immunodeficiency virus encephalitis. Am J Pathol. 2000;157(2):497–507. doi: 10.1016/S0002-9440(10)64561-0. S0002-9440(10)64561-0 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul M, Zheng J, Okamoto S, Gendelman HE, Lipton SA. HIV-1 infection and AIDS: consequences for the central nervous system. 2005 doi: 10.1038/sj.cdd.4401623. [Review] [199 refs] [DOI] [PubMed] [Google Scholar]
- Lenos K, Jochemsen AG. Functions of MDMX in the modulation of the p53-response. J Biomed Biotechnol. 2011;2011:876173. doi: 10.1155/2011/876173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindl KA, Akay C, Wang Y, White MG, Jordan-Sciutto KL. Expression of the endoplasmic reticulum stress response marker, BiP, in the central nervous system of HIV-positive individuals. Neuropathol Appl Neurobiol. 2007;33(6):658–669. doi: 10.1111/j.1365-2990.2007.00866.x. NAN866 [pii] [DOI] [PubMed] [Google Scholar]
- Lindl KA, Marks DR, Kolson DL, Jordan-Sciutto KL. HIV-associated neurocognitive disorder: pathogenesis and therapeutic opportunities. J Neuroimmune Pharmacol. 2010;5(3):294–309. doi: 10.1007/s11481-010-9205-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughran O, La Thangue NB. Apoptotic and growth-promoting activity of E2F modulated by MDM2. Mol Cell Biol. 2000;20(6):2186–2197. doi: 10.1128/mcb.20.6.2186-2197.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markey MP. Regulation of MDM4. Front Biosci. 2011;16:1144–1156. doi: 10.2741/3780. [DOI] [PubMed] [Google Scholar]
- Masliah E, DeTeresa RM, Mallory ME, Hansen LA. Changes in pathological findings at autopsy in AIDS cases for the last 15 years. Aids. 2000;14(1):69–74. doi: 10.1097/00002030-200001070-00008. [DOI] [PubMed] [Google Scholar]
- Masliah E, Heaton RK, Marcotte TD, Ellis RJ, Wiley CA, Mallory M, Grant I. Dendritic injury is a pathological substrate for human immunodeficiency virus-related cognitive disorders. HNRC Group. The HIV Neurobehavioral Research Center. Ann Neurol. 1997;42(6):963–972. doi: 10.1002/ana.410420618. [DOI] [PubMed] [Google Scholar]
- McArthur JC. HIV dementia: an evolving disease. J Neuroimmunol. 2004;157(1–2):3–10. doi: 10.1016/j.jneuroim.2004.08.042. [DOI] [PubMed] [Google Scholar]
- McArthur JC, McDermott MP, McClernon D, St Hillaire C, Conant K, Marder K, Epstein LG. Attenuated Central Nervous System Infection in Advanced HIV/AIDS With Combination Antiretroviral Therapy. Arch Neurol. 2004;61(11):1687–1696. doi: 10.1001/archneur.61.11.1687. [DOI] [PubMed] [Google Scholar]
- Migliorini D, Lazzerini Denchi E, Danovi D, Jochemsen A, Capillo M, Gobbi A, Marine JC. Mdm4 (Mdmx) regulates p53-induced growth arrest and neuronal cell death during early embryonic mouse development. Mol Cell Biol. 2002;22(15):5527–5538. doi: 10.1128/MCB.22.15.5527-5538.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell LA, Agrawal A, Jordan-Sciutto KL, Dichter MA, Lynch DR, Kolson DL. Human immunodeficiency virus (HIV)-induced neurotoxicity: roles for the NMDA receptor subtypes. J Neurosci. 2006;26(3):981–990. doi: 10.1523/JNEUROSCI.4617-05.2006. 26/3/981 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hare MJ, Hou ST, Morris EJ, Cregan SP, Xu Q, Slack RS, Park DS. Induction and modulation of cerebellar granule neuron death by E2F-1. J Biol Chem. 2000;275(33):25358–25364. doi: 10.1074/jbc.M001725200. [DOI] [PubMed] [Google Scholar]
- Pereg Y, Shkedy D, de Graaf P, Meulmeester E, Edelson-Averbukh M, Salek M, Shiloh Y. Phosphorylation of Hdmx mediates its Hdm2- and ATM-dependent degradation in response to DNA damage. Proc Natl Acad Sci U S A. 2005;102(14):5056–5061. doi: 10.1073/pnas.0408595102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price RW, Brew B, Sidtis J, Rosenblum M, Scheck AC, Cleary P. The brain in AIDS: central nervous system HIV-1 infection and AIDS dementia complex. Science. 1988;239:586–592. doi: 10.1126/science.3277272. [DOI] [PubMed] [Google Scholar]
- Rallapalli R, Strachan G, Cho B, Mercer WE, Hall DJ. A novel MDMX transcript expressed in a variety of transformed cell lines encodes a truncated protein with potent p53 repressive activity. J Biol Chem. 1999;274(12):8299–8308. doi: 10.1074/jbc.274.12.8299. [DOI] [PubMed] [Google Scholar]
- Rallapalli R, Strachan G, Tuan RS, Hall DJ. Identification of a domain within MDMX-S that is responsible for its high affinity interaction with p53 and high-level expression in mammalian cells. Journal of Cellular Biochemistry. 2003;89(3):563–575. doi: 10.1002/jcb.10535. [DOI] [PubMed] [Google Scholar]
- Ranganathan S, Bowser R. Alterations in G(1) to S phase cell-cycle regulators during amyotrophic lateral sclerosis. Am J Pathol. 2003;162(3):823–835. doi: 10.1016/S0002-9440(10)63879-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed D, Shen Y, Shelat AA, Arnold LA, Ferreira AM, Zhu F, Dyer MA. Identification and characterization of the first small molecule inhibitor of MDMX. J Biol Chem. 2010;285(14):10786–10796. doi: 10.1074/jbc.M109.056747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds A, Laurie C, Mosley RL, Gendelman HE. Oxidative stress and the pathogenesis of neurodegenerative disorders. Int Rev Neurobiol. 2007;82:297–325. doi: 10.1016/S0074-7742(07)82016-2. S0074-7742(07)82016-2 [pii] [DOI] [PubMed] [Google Scholar]
- Sharp DA, Kratowicz SA, Sank MJ, George DL. Stabilization of the MDM2 oncoprotein by interaction with the structurally related MDMX protein. J Biol Chem. 1999;274(53):38189–38196. doi: 10.1074/jbc.274.53.38189. [DOI] [PubMed] [Google Scholar]
- Shvarts A, Steegenga WT, Riteco N, van Laar T, Dekker P, Bazuine M, Jochemsen AG. MDMX: a novel p53-binding protein with some functional properties of MDM2. EMBO J. 1996;15(19):5349–5357. [PMC free article] [PubMed] [Google Scholar]
- Sippy BD, Hofman FM, Wallach D, Hinton DR. Increased expression of tumor necrosis factor-alpha receptors in the brains of patients with AIDS. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;10(5):511–521. [PubMed] [Google Scholar]
- Soontornniyomkij V, Nieto-Rodriguez JA, Martinez AJ, Kingsley LA, Achim CL, Wiley CA. Brain HIV burden and length of survival after AIDS diagnosis. Clin Neuropathol. 1998;17(2):95–99. [PubMed] [Google Scholar]
- Sorimachi H, Hata S, Ono Y. Impact of genetic insights into calpain biology. Journal of Biochemistry. 2011;150(1):23–37. doi: 10.1093/jb/mvr070. [DOI] [PubMed] [Google Scholar]
- Strachan GD, Jordan-Sciutto KL, Rallapalli R, Tuan RS, Hall DJ. The E2F-1 transcription factor is negatively regulated by its interaction with the MDMX protein. J Cell Biochem. 2003;88(3):557–568. doi: 10.1002/jcb.10318. [DOI] [PubMed] [Google Scholar]
- Strachan GD, Koike MA, Siman R, Hall DJ, Jordan-Sciutto KL. E2F1 induces cell death, calpain activation, and MDMX degradation in a transcription independent manner implicating a novel role for E2F1 in neuronal loss in SIV encephalitis. J Cell Biochem. 2005;96(4):728–740. doi: 10.1002/jcb.20574. [DOI] [PubMed] [Google Scholar]
- Tompa P, Buzder-Lantos P, Tantos A, Farkas A, Szilagyi A, Banoczi Z, Friedrich P. On the sequential determinants of calpain cleavage. J Biol Chem. 2004;279(20):20775–20785. doi: 10.1074/jbc.M313873200. [DOI] [PubMed] [Google Scholar]
- Wade M, Wang YV, Wahl GM. The p53 orchestra: Mdm2 and Mdmx set the tone. Trends Cell Biol. 2010;20(5):299–309. doi: 10.1016/j.tcb.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Wang J, Jiang X. MdmX Protein Is Essential for Mdm2 Protein-mediated p53 Polyubiquitination. Journal of Biological Chemistry. 2011;286(27):23725–23734. doi: 10.1074/jbc.M110.213868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Shyam N, Ting JH, Akay C, Lindl KA, Jordan-Sciutto KL. E2F1 localizes predominantly to neuronal cytoplasm and fails to induce expression of its transcriptional targets in human immunodeficiency virus-induced neuronal damage. Neurosci Lett. 2010;479(2):97–101. doi: 10.1016/j.neulet.2010.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, White MG, Akay C, Chodroff RA, Robinson J, Lindl KA, Jordan-Sciutto KL. Activation of cyclin-dependent kinase 5 by calpains contributes to human immunodeficiency virus-induced neurotoxicity. J Neurochem. 2007;103(2):439–455. doi: 10.1111/j.1471-4159.2007.04746.x. JNC4746 [pii] [DOI] [PubMed] [Google Scholar]
- Wang Y, Zyskind JW, Colacurcio DJ, Lindl KA, Ting JH, Grigoriev G, Jordan-Sciutto KL. Differential roles for caspase-mediated and calpain-mediated cell death in 1- and 3-week-old rat cortical cultures. Neuroreport. 2012;23(18):1052–1058. doi: 10.1097/WNR.0b013e32835ad25d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White MG, Wang Y, Akay C, Lindl KA, Kolson DL, Jordan-Sciutto KL. Parallel high throughput neuronal toxicity assays demonstrate uncoupling between loss of mitochondrial membrane potential and neuronal damage in a model of HIV-induced neurodegeneration. Neurosci Res. 2011;70(2):220–229. doi: 10.1016/j.neures.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong S, Van Pelt CS, Elizondo-Fraire AC, Liu G, Lozano G. Synergistic roles of Mdm2 and Mdm4 for p53 inhibition in central nervous system development. Proc Natl Acad Sci U S A. 2006;103(9):3226–3231. doi: 10.1073/pnas.0508500103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz A, Fuchs D, Hagberg L, Nillroth U, Stahle L, Svensson JO, Gisslen M. Cerebrospinal fluid HIV-1 RNA, intrathecal immunoactivation, and drug concentrations after treatment with a combination of saquinavir, nelfinavir, and two nucleoside analogues: the M61022 study. BMC Infectious Diseases. 2006;6(1):63. doi: 10.1186/1471-2334-6-63. [DOI] [PMC free article] [PubMed] [Google Scholar]