Abstract
Aims
Nitrite (NO2–), a dietary constituent and nitric oxide (NO) oxidation product, mediates cardioprotection after ischaemia/reperfusion (I/R) in a number of animal models when administered during ischaemia or as a pre-conditioning agent hours to days prior to the ischaemic episode. When present during ischaemia, the reduction of nitrite to bioactive NO by deoxygenated haem proteins accounts for its protective effects. However, the mechanism of nitrite-induced pre-conditioning, a normoxic response which does not appear to require reduction of nitrite to NO, remains unexplored.
Methods and results
Using a model of hypoxia/reoxygenation (H/R) in cultured rat H9c2 cardiomyocytes, we demonstrate that a transient (30 min) normoxic nitrite treatment significantly attenuates cell death after a hypoxic episode initiated 1 h later. Mechanistically, this protection depends on the activation of protein kinase A, which phosphorylates and inhibits dynamin-related protein 1, the predominant regulator of mitochondrial fission. This results morphologically, in the promotion of mitochondrial fusion and functionally in the augmentation of mitochondrial membrane potential and superoxide production. We identify AMP kinase (AMPK) as a downstream target of the mitochondrial reactive oxygen species (ROS) generated and show that its oxidation and subsequent phosphorylation are essential for cytoprotection, as scavenging of ROS prevents AMPK activation and inhibits nitrite-mediated protection after H/R. The protein kinase A-dependent protection mediated by nitrite is reproduced in an intact isolated rat heart model of I/R.
Conclusions
These data are the first to demonstrate nitrite-dependent normoxic modulation of both mitochondrial morphology and function and reveal a novel signalling pathway responsible for nitrite-mediated cardioprotection.
Keywords: Ischaemia, Protein kinase A, Pre-conditioning, Nitrite/nitrate, Mitochondria
1. Introduction
Nitrite, a dietary constituent and nitric oxide (NO) oxidation product, has emerged as an intrinsic signalling molecule that mediates cytoprotection during ischaemia/reperfusion (I/R) in a number of organ systems.1–7 In the heart, nanomolar increases in the circulating nitrite concentration significantly decrease infarct size in murine and canine models of myocardial infarction.8–10 This protection, thought to be dependent on the reduction of nitrite to bioactive NO by myoglobin and xanthine oxidoreductase in the heart, is optimized in the ischaemic conditions of anoxia and low pH.5,10–13 Notably, we and others have observed that, in a manner mimicking ischaemic pre-conditioning (IPC), nitrite not only confers cardioprotection when administered during ischaemia, but also when transiently present hours prior to the onset of the ischaemic episode.1,9,14,15 While this phenomenon suggests that nitrite is able to mediate signalling even in non-ischaemic conditions, the mechanisms underlying this normoxic nitrite-dependent pre-conditioning are unknown.
Modulation of mitochondrial function contributes to the cardioprotection conferred by a number of pre-conditioning agents including sub-lethal ischaemia,16,17 volatile anaesthetics,18 and adenosine.19 For example, the inhibition of ATP production and the incremental augmentation of mitochondrial reactive oxygen species (ROS) production have been linked to cardioprotection induced by IPC.20 These alterations in mitochondrial function induce protective downstream adaptive responses including the activation of the metabolic sensor AMPK.21,22 More recently, accumulating evidence suggests that changes in mitochondrial dynamics (fission and fusion), resulting in altered mitochondrial tubular networks within the cell, can modulate the cellular response to I/R.16 Pharmacological inhibition of the fission regulatory protein, dynamin-related protein-1 (Drp1), or overexpression of the fusion promoting mitofusins (Mfn1 and 2), resulting in the formation of elongated cellular mitochondrial networks, has been shown to decrease infarct size in rodent models of myocardial infarction.16,23 While the activity of Drp1 is modulated by post-translational modifications such as protein kinase A (PKA)-dependent phosphorylation,24 the signalling mechanisms that underlie PKA-mediated phosphorylation of Drp1 in the cardiomyocyte remain unknown. While we have previously shown that nitrite modulates mitochondrial function during I/R by reversibly inhibiting complex I activity, concomitantly decreasing reperfusion ROS generation and preventing permeability pore opening,14 the effect of nitrite on mitochondrial dynamics, particularly during non-ischaemic conditions, has not been explored.
Herein, we investigate the mechanism by which normoxic nitrite confers pre-conditioning. We demonstrate for the first time in H9c2 cardiomyocytes that nitrite modulates mitochondrial dynamics by activating protein kinase A to phosphorylate and inhibit Drp1, leading to the enhancement of mitochondrial fusion in normoxia. Functionally, this augments mitochondrial superoxide production, which oxidizes and activates AMPK, an essential step in nitrite-mediated pre-conditioning. These data are the first to show that nitrite regulates mitochondrial morphology and function in normoxia and that this modulation is important for cardioprotection. The implications of these results will be discussed in the contexts of mitochondrial physiology as well as the role of nitrite not only as a potential therapeutic agent, but also as a natural component of cardioprotective diets.
2. Experimental procedures
2.1. Materials
All reagents were purchased from Sigma-Aldrich (St Louis, MO, USA) except where indicated. H9c2 cardiomyocytes were purchased from ATCC (Rockville, MD, USA). All antibodies were commercially available and detailed information as well as western blot conditions are supplied in Supplementary material online, Methods.
2.2. Animals
Twelve-week-old-male Sprague–Dawley rats (Harlan Sprague Dawley, Inc. Indianapolis, IN, USA) were anaesthetized by intraperitoneal injection of 50 mg/kg pentobarbital sodium, and anaesthesia was monitored by pinching of the toe.
2.3. Hypoxia/reoxygenation (H/R)
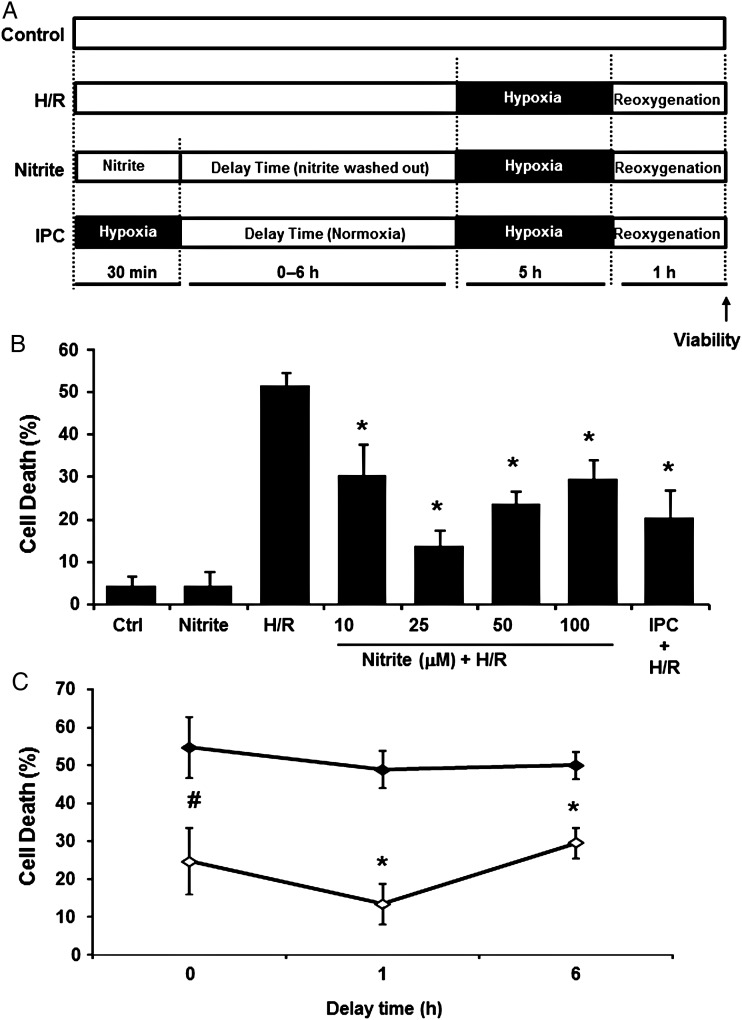
Control cells were maintained in normoxia (21% O2, 5% CO2) throughout. Ischaemia was simulated by subjecting cells to hypoxia, (1% O2, 5% CO2, 94% N2; 5 h), in modified Esumi buffer [137 mmol/L NaCl, 12 mmol/L KCl, 0.5 mmol/L MgCl2, 0.9 mmol/L CaCl2, 20 mmol/L HEPES; 20 mmol/L 2-deoxy-d-glucose (2-DG), pH 6.2]25 as described by previous publications.26–28 Cells were then reoxygenated in the same buffer in normoxia (21% O2, 5% CO2) for 1 h. IPC was achieved by incubating cells in Esumi buffer without 2-deoxy-d-glucose (2-DG) in hypoxia (1% O2, 5% CO2, 94% N2; 30 min), followed by a delay time (1 h) in normoxia (in DMEM–FBS cell growth medium) before H/R (as described above) was initiated. Nitrite treatment (0–100 µM) was for 30 min in normoxia in growth medium, followed by washing of the cells with PBS once and a delay time (0–6 h in growth medium) prior to the onset of H/R (Figure 1A).
Figure 1.
Nitrite mediates cytoprotection after in vitro H/R. (A) Experimental model to simulate I/R. H9c2 cells were subjected to hypoxia (1% O2, 5% CO2; 20 mmol/L 2-DG; pH 6.2; 5 h), followed by reoxygenation (21% O2, 1 h). Nitrite was administered at 21% O2 for 30 min, after which nitrite was washed out and cells kept in normoxia for a delay time of 0–6 h prior to H/R. IPC treatment was 1% O2 (30 min). (B) Percentage of dead cells (measured by cellular LDH release) after H/R initiated 1 h after nitrite (0–100 µmol/L) treatment. n = 5; n = 3 for normoxic nitrite, 100 µmol/L and IPC. (C) Percentage of H/R-induced death in cells treated with (open symbols) or without (filled symbols) nitrite (25 µmol/L) 0–6 h prior to H/R. Asterisks indicate P < 0.01 and hash indicates P < 0.05 vs. H/R; n = 4 per group.
2.4. Lactate dehydrogenase activity
Lactate dehydrogenase (LDH) activity in the media was measured spectrophotometrically by measuring the decrease in NADH at 340 nm and was expressed as a per cent of total LDH in the media and lysed cells.
2.5. Oxygen consumption
Oxygen consumption rate was measured using the Seahorse XF24 Extracellular Flux analyser (Seahorse Bioscience, Billerica MA) as described in Mo et al.29 and Supplementary material online, Methods.
2.6. Membrane potential
Membrane potential was measured using TMRM (Molecular Probes, Eugene, CA, USA), according to manufacturer's instructions. Cells (in PBS buffer) were incubated with 1 µmol/L TMRM and fluorescence monitored at 544/574 nm prior to and after the addition of nitrite, oligomycin, and FCCP. Membrane potential was quantified as a per cent of the total range (determined by the difference between Oligomycin and FCCP signals).
2.7. Superoxide measurement
Intra-mitochondrial superoxide levels were quantified using MitoSOX™ Red (Invitrogen, Carlsbad, CA, USA), according to manufacturer's instructions. Briefly, cells were pre-loaded with MitoSOX Red (5 µmol/L; 37°C; 15 min). After three washes, fluorescence (510/580 nm) was read using a Synergy plate reader (BioTek Instrument, Inc., Winooski, VT, USA) prior to and after the injection of nitrite into the well. Alternatively, cells were pre-loaded with MitoSOX Red, washed three times and confocal microscopy images were taken over time.
2.8. Aconitase activity
Aconitase activity was measured by spectrophotometrically monitoring the conversion of citrate to isocitrate as previously described.30
2.9. Generation of Rho 0 cells
Mitochondrial DNA-depleted (Rho 0; ρ0) cells were created by growing H9c2 cells in media supplemented with ethidium bromide (0.634 µmol/L), uridine (0.205 mmol/L), and sodium pyruvate (1 mmol/L) for 7–21 days.
2.10. Adenoviral transfection
The constructs for the Adenoviruses AdCMVCatalase (AdCat) and Adempty (Vector) were manufactured by inserting catalase and LacZ genes into the E1 region of an Ad5 E1/partial E3-deleted replication-deficient adenoviral vector.31
2.11. Silencing of AMPKα
Cells were transfected with either rat AMPKα1 siRNA (sc-270142; Santa Cruz Biotechnology, Santa Cruz, CA, USA) or control siRNA (sc-37007), according to manufacturer's instructions. Knockdown of AMPKα1 expression was verified by western blot using anti-AMPKα1 (C-20) (sc-19128) antibody.
2.12. GFP-Drp1 plasmid transfection
Drp1 mutant plasmids containing either wild-type Drp1 or the S656A mutant Drp1 (with shRNA to silence wild-type Drp1) were a kind gift from Dr Stefan Strack and were used as characterized and described in Cribbs and Strack.32
2.13. AMPK oxidation
Oxidation was assessed by a modified biotin switch method adopted from Wang et al.,33 and described in Supplementary material online, Methods.
2.14. PKA activity
PKA activity was measured by determining the level of PKA-substrate phosphorylation by ELISA using the PKA activity kit (Enzo Life Sciences; ADI-EKS-390A).
2.15. Immunofluorescence microscopy
Cell imaging was performed as described in Supplementary material online, Methods. Briefly, cells were treated with nitrite (25 μM) and, following nitrite removal, were fixed using 1% paraformaldehyde, and then stained with rabbit anti-TOM20 (SantaCruz, clone sc-11415, 1:1000) and imaged for mitochondrial morphology using a Provis AX70 research microscope (Olympus, Center Valley, PA, USA) using a ×60 objective lens, and processed with MagnaFire (Optronics, Goleta, CA, USA). In parallel experiments, cells were transfected with the CellLight® Mitochondria-GFP reagent (Molecular Probes, Invitrogen) according to manufacturer's instructions, and the next day, treated with or without nitrite, and then imaged by microscopy.
2.16. Mitochondrial isolation
For measurement of Drp1 translocation, cells were lysed and subjected to differential centrifugation as described previously. LDH activity was measured in the mitochondrial fraction to determine purity of the preparation and found to be undetectable.
2.17. Isolated perfused heart
Hearts were isolated from male Sprague–Dawley rats (250 g) and subjected to retrograde perfusion with Krebs–Henseleit (KH) buffer as previously described in Curtis et al.34 and in Supplementary material online, Methods. Infarct size was determined at 2 h by TTC staining and left ventricular developed pressure monitored throughout as previously described.14,34
2.18. Mitochondrial permeability transition pore assay
Mitochondrial permeability transition pore (MPTP) opening was assessed by measuring quenching of calcein-AM fluorescence as described in Hausenloy et al.35 H9c2 cells were treated with or without nitrite, in the presence or absence of compound C, and then after 1 h of washout, were subjected to H/R. Cells were co-loaded with calcein-AM (1 µmol/L; Molecular Probe) and cobalt-chloride (CoCl2 1 mmol/L) at 37°C for 20 min, resulting in mitochondrial localization of calcein fluorescence. MPTP opening was measured as the decrease in mitochondrial calcein signal (expressed as the percentage of the initial value) using a microplate reader (emitting at 488 nm and detecting at 505 nm).
2.19. Image analysis of mitochondrial morphology and density measurements
To quantify mitochondrial morphology, custom-written macros were developed for the NIH Image J software (1.44) (http://www.imagejdocu.tudor.lu), as previously described in Dagda et al.36 and Supplementary material online, Methods.
2.20. Statistics
All values are means ± SEM of at least three independent experiments. N values for each experiment are listed in the figure legend. Single comparisons were tested for significance by two-tailed Student's t-test. Multiple comparisons were made using ANOVA followed by Bonferroni correction. Significance is noted when P < 0.05.
3. 3. Results
3.1. Nitrite mediates cytoprotection in an in vitro model of hypoxia/reoxygenation
To determine whether normoxic nitrite-mediated protection occurs at the level of the myocyte, an in vitro model of hypoxia/reoxygenation (H/R) using H9c2 cells was established to simulate I/R (Figure 1A). After subjecting cells to hypoxia (1% O2; 5 h), then reoxygenation (21% O2; 1 h), significant cell death (51.3 ± 3.4%), measured by LDH release, was observed compared with normoxic controls (4.3 ± 2.4%) (Figure 1B). To determine whether nitrite could prevent death in this model, cells were treated with nitrite (10–100 µmol/L) in normoxia for 30 min, after which they were washed to remove nitrite. Measurement of nitrite levels (372 ± 8.4 nmol/L in nitrite-treated cells vs. 382 ± 9.3 nmol/L in control cells) after washout demonstrated that all nitrite was removed for the washout period. The cells were then incubated in normoxia for a delay time of 1 h before being subjected to H/R. Concentrations of nitrite 10 µmol/L and above significantly attenuated cell death when administered 1 h before the onset of hypoxia (Figure 1B), demonstrating that nitrite mediates pre-conditioning in this model. Nitrite-mediated protection showed a biphasic concentration response, in which the maximal effect was observed at 25 μmol/L and was similar in magnitude to the protection mediated by IPC, a well-characterized classical cytoprotective program37,38 (Figure 1B). The inclusion of sodium bicarbonate (25 mM) to maintain a stable pH during H/R did not affect the ability of nitrite to mediate protection, suggesting that nitrite-dependent pre-conditioning was not pH dependent (Supplementary material online, Figure S1). A time course, in which the delay time between the end of nitrite treatment and the initiation of hypoxia, was varied (0–6 h), revealed that nitrite could effectively prevent cell death when transiently present up to 6 h prior to the hypoxic episode (Figure 1C). Collectively, these data demonstrate that nitrite mediates tolerance to H/R in myocytes over a wide range of concentrations and times.
3.2. Nitrite-dependent PKA activation and mitochondrial fusion are required for cytoprotection
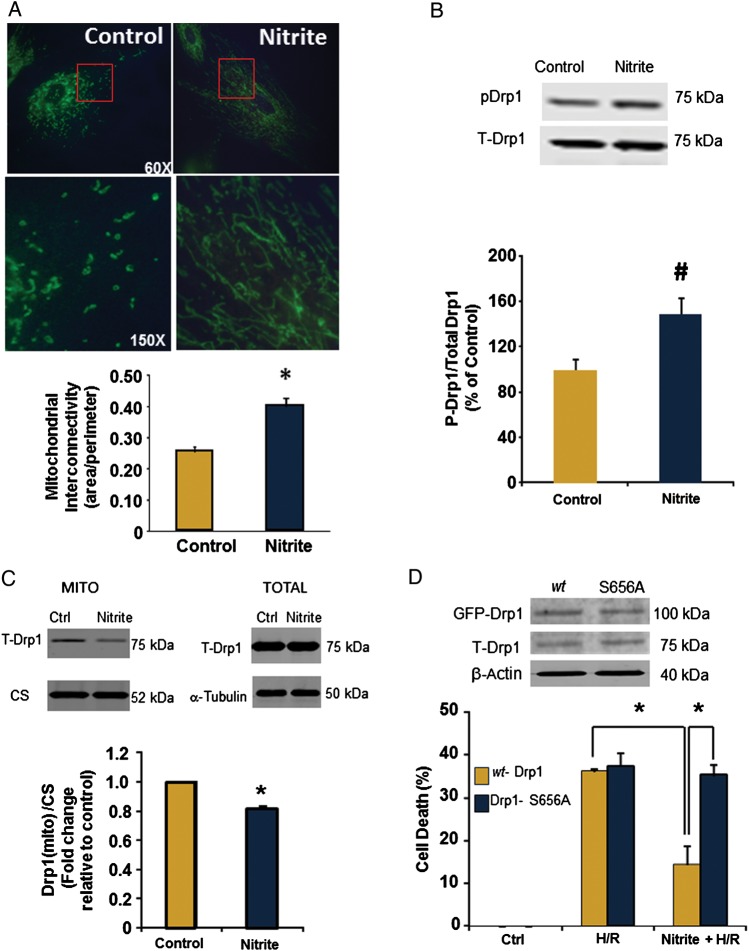
We next sought to determine the mechanism of nitrite-mediated pre-conditioning. Modulation of mitochondrial function has been implicated in ischaemic and pharmacological pre-conditioning and changes in mitochondrial dynamics, particularly increased mitochondrial fusion, confer cardioprotection.16,39 Thus, we first examined the effect of nitrite on mitochondrial morphology. Cells were treated with or without nitrite and then stained with antibodies specific for the translocase of the outer mitochondrial membrane of 20 kDa (TOM20) (Figure 2A) or mitochondrially targeted GFP (Supplementary material online, Figure S2A) to visualize mitochondrial networks. Nitrite significantly increased mitochondrial interconnectivity (0.37 ± 0.03 vs. 0.26 ± 0.01 in untreated cells), consistent with increased mitochondrial fusion (Figure 2A).
Figure 2.
Nitrite promotes mitochondrial fusion by increasing Drp1 phosphorylation. Cells were treated with or without nitrite (25 µmol/L; 21% O2, 30 min) and collected 1 h after nitrite removal. (A) Cells stained with antibodies to TOM20. Top panel is ×60 magnification and bottom panel is ×150 magnification of image section in red box. Quantification of interconnectivity using n = 20–23 fields/group. (B) Representative immunoblot for phosphorylated and total Drp1 and quantification of Phospho-Drp1 levels (normalized to total Drp1). n = 4. (C) Representative immunoblot and quantification of Drp1 expression (and citrate synthase; CS) in whole cells and the mitochondrial fraction of cells treated with or without nitrite (25 µmol/L). (D) Protein expression of exogenous (GFP-Drp1) and endogenous (T-Drp1) in transfected cells. Cell death after H/R in wild-type (yellow) or cells expressing the S656A mutant (blue) treated with or without nitrite. Asterisks indicate P < 0.01 and hash indicates P < 0.05 vs. control; n = 5 per group.
Mitochondrial networks are altered in response to changes in the balance between mitochondrial fusion and fission. To determine whether nitrite-mediated fusion was a result of increased fusion or the inhibited fission, the effect of nitrite on Drp1 and Mfn1, the major catalytic enzymes that mediate fission and fusion, respectively, was assessed. There was no significant change in Mfn1 expression 1 h after nitrite treatment (data not shown). Drp1 is regulated predominantly by post-translational modification, with phosphorylation of the protein at serine 656 (S656) leading to the inhibition of its activity and an attenuation of fission.24,32. While there was no change in total Drp1 protein expression, phosphorylation of S656 was significantly increased 1 h after normoxic nitrite treatment (Figure 2B and Supplementary material online, Figure S2B). Nitrite treatment also decreased the expression of Drp1 in the mitochondrial fraction of the cells, consistent with nitrite-mediated inhibition of Drp1 translocation from the cytosol to the mitochondrion (Figure 2C).
To test whether the phosphorylation of Drp1 at S656 was required to mediate nitrite-dependent cytoprotection after H/R, cells stably transfected with GFP-tagged plasmids encoding wildtype Drp1 or a non-phosphorylatable mutant of Drp1 (S656A) (Figure 2D) were treated with nitrite and subjected to H/R. Importantly, the plasmid containing the S656A plasmid also included shRNA to silence wild-type Drp1 protein such that only S656A was expressed.32 Consistent with the necessity for Drp1 phosphorylation at S656 to mediate cytoprotection, nitrite-attenuated cell death after H/R in cells expressing wtDrp1 (14.5 ± 4.5% vs. 36.2 ± 0.5 H/R alone), but did not mediate protection in cells transfected with the non-phosphorylatable mutant S656A (35.2 ± 2.3% vs. 37.3 ± 3.2 in H/R alone) (Figure 2D).
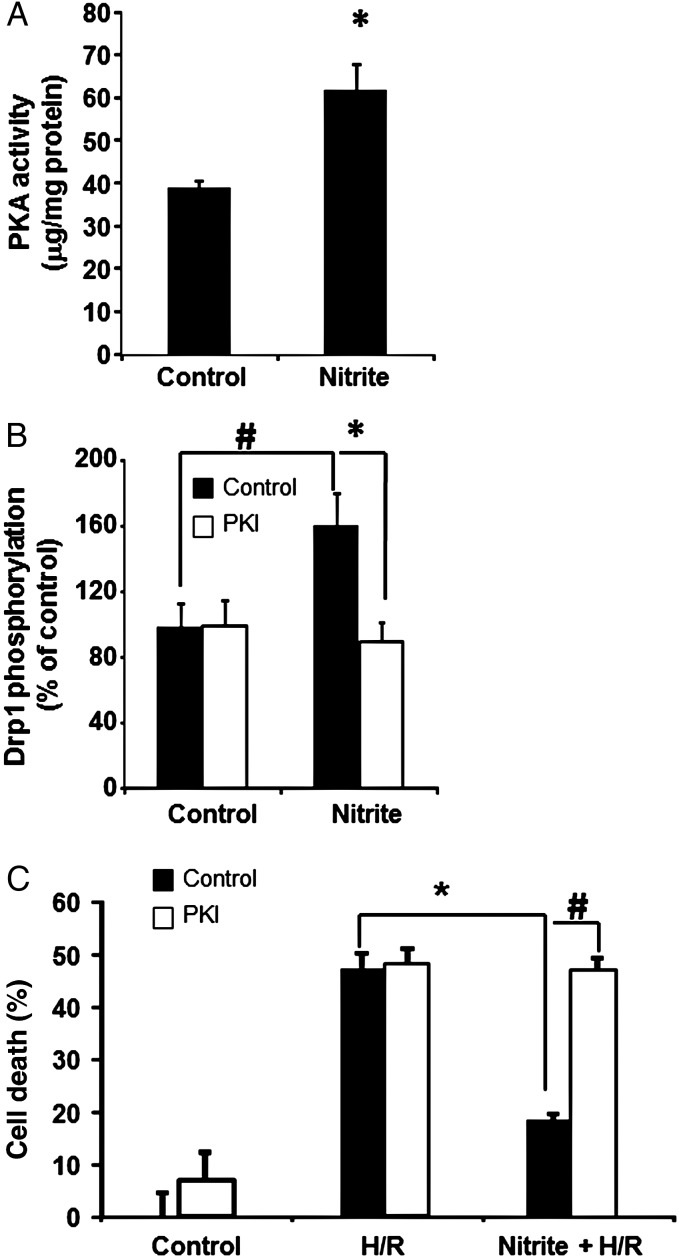
Drp1 phosphorylation at S656 is mediated by PKA.24 PKA activity levels were significantly greater in cells collected 1 h after a normoxic nitrite treatment (Figure 3A). Furthermore, pre-treatment of cells with PKI (5 µmol/L), a pharmacological inhibitor of PKA, decreased PKA activity by ∼80% and inhibited the nitrite-mediated phosphorylation of Drp1 (Figure 3B). Additionally, the inhibition of PKA with PKI abolished nitrite-dependent cytoprotection, when H/R was initiated 1 h after nitrite treatment (Figure 3C). Collectively, these findings demonstrate that nitrite-induced activation of PKA and subsequent phosphorylation of Drp1 at S656 are essential for nitrite-mediated normoxic pre-conditioning of cells against H/R injury.
Figure 3.
Nitrite-induced Drp1 phosphorylation is dependent on increased PKA activity. (A) PKA activity in control and nitrite-treated cells 1 h after nitrite removal. (B) Phospho-Drp1 levels (normalized to total Drp1) measured 1 h after cells were treated with saline or nitrite (25 µmol/L), with and without PKI (5 µmol/L) n = 4. (C) Cell death 1 h after nitrite treatment in untreated cells or those treated with PKI. Asterisks indicate P < 0.01 and hashes indicate P < 0.05 vs. control; n = 3 per group.
3.3. Nitrite-mediated Drp1 phosphorylation augments mitochondrial superoxide production
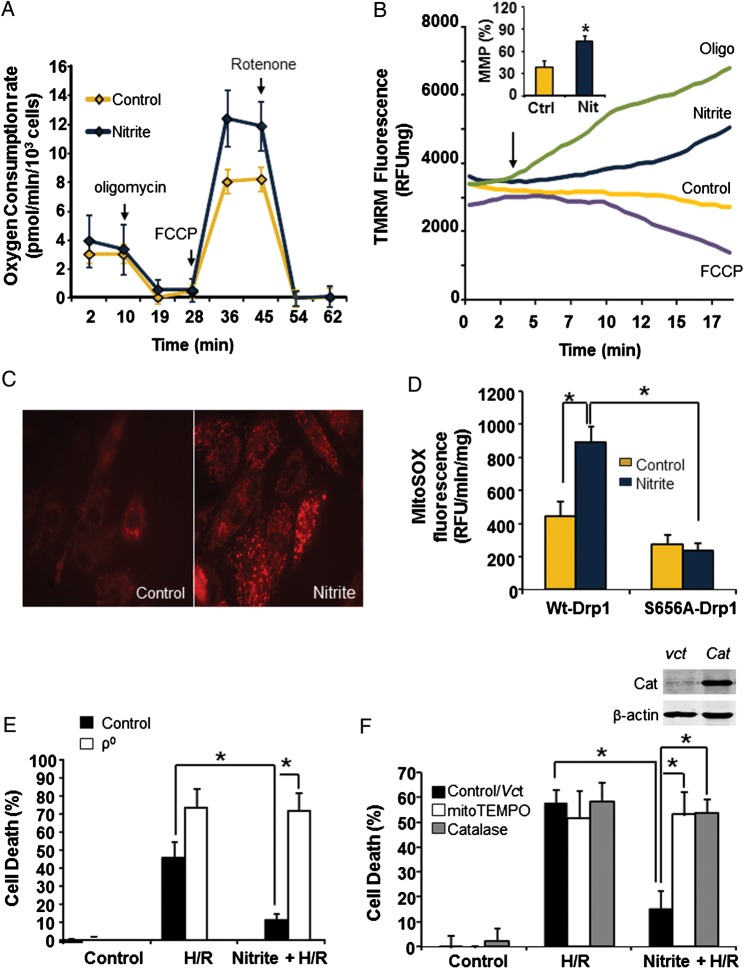
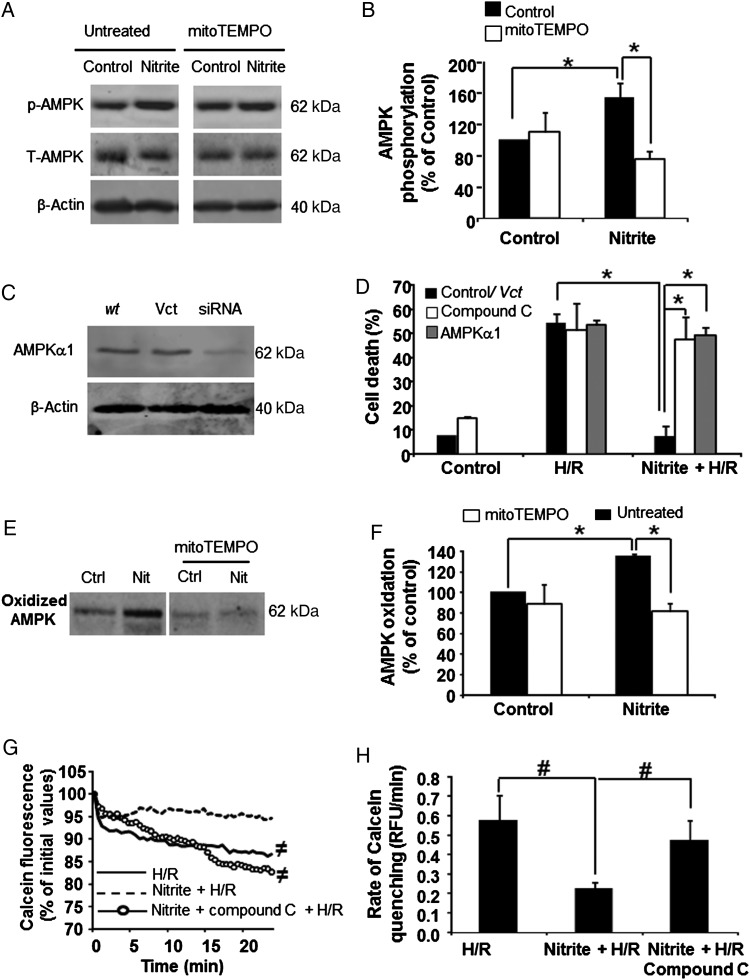
To determine whether nitrite-mediated Drp1 phosphorylation alters mitochondrial function, cells were treated with nitrite (normoxia, 30 min), and then mitochondrial respiration, membrane potential (MMP), and superoxide production were assessed. Respiration was measured basally and in the presence of the ATPase inhibitor oligomycin (2 µmol/L), the uncoupler FCCP (7.5 µmol/L), and the complex I inhibitor rotenone (2 µmol/L) to determine the rates of basal, non-ATP linked, maximal, and non-mitochondrial oxygen consumption, respectively, in the intact cells. Nitrite treatment significantly increased the maximal respiratory rate (12.1 ± 0.4 vs. 8.1 ± 0.1 pmol O2/min/103 cells), but had no significant effect on basal respiration or proton leak (Figure 4A). Consistent with prior studies demonstrating that mitochondrial fusion increases membrane potential,40–42 nitrite treatment also resulted in increased MMP (73.5 ± 7.7% vs. 38.6 ± 8.8% in untreated) as measured by the potentiometric dye TMRM (Figure 4B). Not surprisingly, this increase in MMP was associated with augmented mitochondrial superoxide production in nitrite-treated cells (893.4 ± 95.5 vs. 447 ± 84.7 RFU/min/mg in control) as measured by MitoSOX Red (Figure 4C and D; Supplementary material online, Figure S3A). This nitrite-induced increase was confirmed by electron paramagnetic spin resonance (EPR) using the spin trap CMH (1-hydroxy-3-methoxy-carbonyl-2,2,5,5-tetramethylpyrrolidine) (Supplementary material online, Figure S3B). Further, the activity of the redox-sensitive protein aconitase was significantly decreased after nitrite treatment, also indicative of increased oxidant production (Supplementary material online, Figure S3C). This oxidation of aconitase was abrogated by inhibition of PKA or scavenging of ROS by the mitochondrially targeted scavenger mitoTEMPO (Supplementary material online, Figure S3C). Furthermore, nitrite-induced increased superoxide generation was dependent on the phosphorylation of Drp1, as it was not observed in cells expressing the non-phosphorylatable mutant Drp1 protein (Figure 4D).
Figure 4.
Nitrite-mediated Drp1 phosphorylation augments mitochondrial superoxide that is essential for cytoprotection. Cells were treated with or without nitrite (25 µmol/L) at 21% O2 (30 min). (A) Representative traces of oxygen consumption rates in control (yellow) and nitrite-treated (blue) cells 1 h after nitrite removal. (B) Representative TMRM traces for cells treated with nothing (yellow), nitrite (blue), Oligomycin (5 µmol/L; green), and FCCP (10 µmol/L; purple). Arrow notes the addition of each treatment. Inset: quantification of the membrane potential as a per cent of the total TMRM range (determined by FCCP and oligomycin), n = 4. Asterisks indicate P < 0.01. (C) Representative confocal microscopy images of control (left panel) and nitrite-treated (right panel) cells stained with MitoSox Red. (D) Quantitation of the rate of mitochondrial superoxide production in wt-Drp1 or S656A-transfected cells treated with or without nitrite. n = 4. Asterisks indicate P < 0.01. (E) Cell death after H/R initiated 1 h after nitrite treatment in control or ρ0 cells. n = 4. Asterisks indicate P < 0.0001. (F) Cell death after H/R initiated 1 h after saline or nitrite treatment in untreated cells, those treated with MitoTEMPO (2 µmol/L) or those overexpressing catalase (Cat). Representative western blot of catalase expression in cells transfected with adenovirus containing vector (Vct) or catalase (inset). Asterisks indicate P < 0.01 vs. control. n = 4
3.4. Nitrite-mediated cytoprotection is dependent on mitochondrial superoxide generation
Given that nitrite-mediated cytoprotection was dependent on Drp1 phosphorylation and Drp1 phosphorylation led to mitochondrial superoxide production, we next tested whether mitochondrial reactive oxygen species generation was required for nitrite-dependent cytoprotection after H/R. The requirement for global mitochondrial signalling in nitrite-mediated cytoprotection was first tested by generating ρ0 H9c2 cells lacking a functional electron transport chain. The lack of a respiratory chain was confirmed by a significant decrease in the activity of complex IV in these cells (4.9 ± 3.0 µmol cytochrome c/min/mg vs. 21.8 ± 2.0 wild-type). Nitrite was unable to protect ρ0 cells from H/R initiated 1 h after nitrite treatment (72.0 ± 6.7% cell death). Although ρ0 cells have altered metabolism compared with wild-type cells, these data suggested that a functional mitochondrial respiratory chain was involved in the mechanism of protection (Figure 4E).
To more specifically test the role of mitochondrial ROS generation in nitrite-mediated protection, cells were co-treated with the mitochondrially targeted ROS scavenger mitoTEMPO (2 µmol/L), which had no significant effect on cell death mediated by H/R at this concentration, but abrogated the protective effect of nitrite after H/R (Figure 4F). Mitochondrial superoxide is rapidly converted to the more stable oxidant hydrogen peroxide (H2O2), which can diffuse out of the mitochondrion. To determine whether H2O2 was responsible for the nitrite-dependent cytoprotection, adenovirus-mediated overexpression of catalase was used to scavenge H2O2 (Figure 4F, inset). While cells transfected with the empty vector were protected by nitrite after H/R (15.1 ± 7.6% cell death vs. 57.9 ± 5.3 in H/R alone), nitrite-induced cytoprotection was abolished in catalase-overexpressing cells (53.8 ± 5.6% cell death vs. 58.4 ± 7.8 in H/R alone) (Figure 4F). Taken together, these data reveal that the production of mitochondrial ROS is required for nitrite-mediated cytoprotection after H/R.
3.5. Nitrite-mediated protection is dependent on the oxidation and phosphorylation of AMPK
We next sought to determine the downstream cytoprotective target of nitrite-induced mitochondrial ROS. AMPK is an integral metabolic sensor whose activation is implicated in cytoprotection after I/R.43 Furthermore, recent studies have shown that oxidation of AMPK by H2O2 can lead to its auto-phosphorylation and subsequent activation.21 Thus, we next tested whether nitrite could activate AMPK and whether this activation was required for nitrite-mediated cytoprotection. Treatment of cells with nitrite for 30 min in normoxia showed a significant increase in the phosphorylation of AMPK at Thr172 compared with untreated cells (Figure 5A). Additionally, this AMPK activation was required for nitrite-mediated cytoprotection as treatment with compound C (5 µmol/L), a pharmacological inhibitor of AMPK, abolished nitrite-mediated cytoprotection after H/R (53.8 ± 5.6 vs. 53.3 ± 2.0% cell death in H/R alone) (Figure 5B). The requirement of AMPK activation for nitrite-induced cytoprotection was further confirmed in cells transfected with siRNA against the α1 catalytic subunit of AMPK, in which nitrite did not prevent cell death after subsequent H/R (Figure 5B).
Figure 5.
Nitrite-mediated cytoprotection is dependent on AMPK activation. Cells were treated with or without nitrite (25 µmol/L; 21% O2) in the presence or absence of mitoTEMPO (2 µmol/L; 30 min). (A) Western blot of phospho-AMPK (p-AMPK), total AMPK (t-AMPK) and β-actin, and quantitation of p-AMPK/t-AMPK/β-actin 1 h after nitrite removal. n = 4 for untreated and n = 3 for mitoTEMPO. (B) Cells were transfected with an empty vector (Vct) or siRNA against AMPKα1. Representative immunoblot of AMPKα1 expression 48 h after transfection. Cell death after H/R initiated 1 h (grey) after treatment with nitrite in cells transfected with siRNA to AMPKα1. Cell death measured after H/R initiated 1 h following nitrite treatment is also shown for control cells and those pre-treated with compound C (5 µmol/L; open bars) n = 4. (C) Western blot representing oxidized AMPK and blot quantitation after nitrite treatment in untreated cells and those co-treated with mitoTEMPO. Asterisks indicate P < 0.01, n = 4. (D) Representative calcein fluorescence traces and quantification of permeability transition pore opening in cells after H/R in the absence and presence of nitrite (50 µmol/L) as well as in the presence of compound C and nitrite. n = 4. Hashes indicate P < 0.05.
To determine whether nitrite-induced mitochondrial ROS could oxidize AMPK leading to its auto-phosphorylation, we first determined whether AMPK was oxidized by nitrite. Cells were treated with nitrite (25–50 µmol/L; 21% O2) and subjected to a modified biotin switch assay in which oxidized proteins were labelled with maleimide conjugated biotin. As shown in Figure 5C, significant AMPK oxidation was observed in cells treated with nitrite compared with untreated cells (Figure 5C). To determine whether nitrite-induced mitochondrial ROS were responsible for the AMPK oxidation observed, cells were co-treated with nitrite and mitoTEMPO. Scavenging of mitochondrial ROS significantly attenuated both nitrite-dependent AMPK oxidation (Figure 5C) and phosphorylation (Figure 5A). Taken together, these results indicate that nitrite-mediated cytoprotection after H/R is dependent on the oxidation and subsequent activation of AMPK.
Opening of the mitochondrial permeability pore is a major event in the propagation of apoptotic cell death after I/R. Since the permeability pore is a known downstream target of AMPK,44,45 we next determined whether nitrite-mediated activation of AMPK inhibited pore opening. Measurement of the quenching of the fluorescent dye calcein by cobalt chloride loaded into the cells at the time of reoxygenation demonstrated that H/R stimulated permeability pore opening and that nitrite significantly inhibited this. Furthermore, inhibition of AMPK by compound C attenuated the ability of nitrite to prevent permeability transition (Figure 5D and Supplementary material online, Figure S4), demonstrating that inhibition of the permeability transition pore is a downstream mechanism by which AMPK mediates nitrite-induced protection.
3.6. Nitrite mediates cytoprotection through the PKA-Drp1-AMPK pathway in the perfused heart
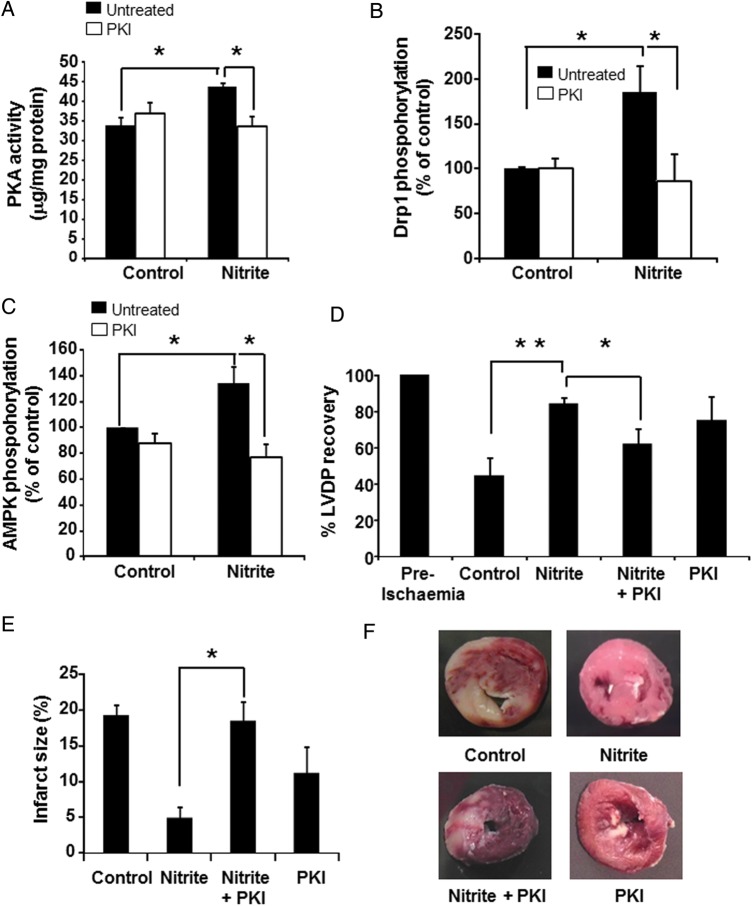
We next sought to determine whether nitrite elicited the PKA-dependent cytoprotective pathway in the intact heart. Adult rat hearts were isolated and perfused with nitrite (10 µmol/L) for 10 min, followed by a washout period (10 min) during which the heart was perfused with nitrite-free buffer. After this washout period, nitrite-treated hearts showed a significant increase in PKA activity (Figure 6A) as well as phosphorylation of Drp1 and AMPK (Figure 6B and C), consistent with the nitrite-dependent activation of the PKA-Drp1-AMPK pathway. The hearts were then subjected to global no-flow ischaemia (20 min) and reperfusion (2 hours) during which left ventricular developed pressure (LVDP) was measured. In these hearts, I/R induced a 55.4 ± 10.1% decrease in LVDP consistent with I/R damage and nitrite significantly protected against this decrease (Figure 6C and Supplementary material online, Table S1). Notably, perfusion with a higher concentration of nitrite (25 µmol/L) also significantly increased PKA activity, phosphorylation of Drp1, and AMPK as well as protected against I/R-induced decrease in LVDP (data not shown). To determine whether this nitrite-mediated protection was dependent on the activation of PKA, heart were perfused with nitrite (10 µmol/L) in the presence or absence of the PKA inhibitor PKI (0.1 mmol/L) for 10 min and then perfused with nitrite-free buffer for a washout period of 10 min before being subjected to I/R. Inhibition of PKA abolished the nitrite-induced increase in PKA activity (Figure 6A) and phosphorylation of AMPK (Figure 6B) and Drp1 (Figure 6C) as well as attenuated nitrite-mediated protection (Figure 6D). Inhibition of PKA also significantly abrogated the ability of nitrite to decrease infarct size (measured at the end of reperfusion) in the heart (Figure 6E and F). These data demonstrate that nitrite activates the PKA-Drp1-AMPK pathway in the isolated heart and that nitrite-mediated protection is dependent on the activation of PKA.
Figure 6.
Nitrite activates the PKA-Drp1-AMPK pathway and mediates delayed cardioprotection in perfused hearts. (A) PKA activity, (B) phosphorylation of Drp1, and (C) phosphorylation of AMPK in isolated rat hearts treated with or without nitrite (10 µmol/L) in the presence (white bars) or absence (black bars) of PKI (5 µmol/L). (D and F) Recovery of LVDP (D) and infarct size (E and F) of perfused hearts subjected to global I/R with and without nitrite pre-treatment (10 min of nitrite perfusion followed by a washout period), in the presence or absence of the PKA inhibitor PKI. n = 4 per group. Asterisks indicate P < 0.01 and double asterisk indicates P < 0.001. (F) Representative images of TTC stained heart sections used to determine infarct size.
4. Discussion
Nitrite was initially tested as a potential I/R therapeutic based on its ability to preferentially mediate NO-based signalling in ischaemic hypoxic conditions.1,5 Almost a decade later, it is clear that nitrite is equally protective when administered prior to ischaemia, as a pharmacological pre-conditioning agent.9,14,15 However, the normoxic signalling resulting in this delayed protection remains virtually unexplored. The current study demonstrates that nitrite-mediated cardiomyocyte protection is dependent on the modulation of mitochondrial morphology and function, leading to the downstream activation of AMPK. Specifically, nitrite enhances the PKA-dependent phosphorylation of Drp1, resulting structurally in increased mitochondrial fusion and functionally in augmented mitochondrial membrane potential and mitochondrial-derived ROS generation. This production of mitochondrial ROS is essential for the oxidation and subsequent activation of the protective metabolic sensor AMPK (Supplementary material online, Figure S5). We show that this pathway is activated by nitrite in the intact adult heart. Furthermore, this study is the first demonstration of protective nitrite-dependent normoxic signalling in the mitochondrion of cardiomyocytes.
Prior work from our group suggests that the S-nitrosation of complex I resulting in the inhibition of mitochondrial ROS production at the time of reperfusion is essential in nitrite-mediated cardioprotection after I/R.14,46 Notably, this significant inhibition of complex I was present only after mitochondria were subjected to I/R. This previously described mechanism likely complements the results presented in the current study as nitrite appears to mediate differing modes of cardioprotection in two temporally distinct windows. Moreover, the initiation of these two mechanisms is likely governed in tissue by oxygen and pH levels. When administered prior to ischaemia/hypoxia at physiological oxygen tensions, nitrite activates PKA to modulate mitochondrial dynamics resulting in augmented ROS generation with no inhibition of complex I (demonstrated by the lack of inhibited respiration; Figure 4A). However, during ischaemia, as tissue becomes anoxic and acidotic, nitrite mediates S-nitrosation to significantly inhibit complex I and decrease reperfusion ROS generation. Hence, future studies are required to determine whether cross-talk exists between these two mechanisms.
As mentioned above, we and others have previously described ischaemic hypoxic nitrite signalling, which is dependent on the reduction of nitrite to bioactive NO by proteins such as myoglobin and xanthine oxidoreductase in the heart.5,10 However, the current study demonstrates that nitrite increases PKA activity in normoxia, a condition in which it is unlikely that nitrite generates NO. While the chemistry behind normoxic signalling in this system remains unclear, nitrite can be oxidized to nitrogen dioxide (NO2·) through a haem catalysed peroxidase reaction in the presence of H2O2.47 Indeed, Wang et al. have recently shown that NO2· generation potentially underlies the mechanism of nitrite-mediated wound healing in normoxic airway epithelial cells.48 It is possible that identical chemistry leads to the nitration of adenylate cyclase or phosphodiesterases to alter their activity and increase cellular cAMP levels, leading to activation of PKA in myocytes. Alternatively, nitrite may react in vivo to form electrophilic fatty acids, some of which have been observed to increase adenylate cyclase activity.49 Notably, prior proteomic studies have associated the cardioprotective effects of nitrite with increased PKA protein expression.50 Additionally, PKA has been shown to increase nitrite levels through the induction of eNOS activity.51 However, this study is the first to report direct evidence of PKA activation by nitrite, particularly in normoxia.
Our data confirm prior studies demonstrating the cardioprotective effect of mitochondrial fusion. Mitochondrial fission, particularly mediated by shear stress-dependent NO, has been associated with reoxygenation injury in endothelial cells.52 Moreover, the elongation of mitochondrial networks as a result of either the pharmacological inhibition of Drp1 or overexpression of Mfn2 has previously been shown to decrease infarct size in murine models of myocardial infarction.16 Notably, the cytoprotective effects mediated by mitochondrial fusion have been attributed predominantly to the inhibition of the translocation of the pro-apoptotic proteins Bax and Bak to the mitochondrion which ultimately prevents cytochrome c release.53 While nitrite prevents cell death and pore opening in our model, we show that this is dependent on the production of mitochondrial ROS and activation of AMPK. This is consistent with prior studies, demonstrating that the permeability transition pore is a downstream target for AMPK.44,45 Though the current study shows an effect of AMPK on pore opening, this may not be the only mechanism by which AMPK mediates cytoprotection in this system. Activation of this metabolic sensor is also known to mediate cardioprotection through the opening of ATP-sensitive potassium channels,54 the modulation of autophagy,55,56 and the stabilization of hypoxia-inducible factor-1α.57,58 Interestingly, a number of pre-conditioning agents, including IPC, are known to augment mitochondrial ROS production18,20 in a manner similar to that observed with nitrite in this study. Additionally, ROS-induced activation of AMPK has been previously observed with sevoflurane-induced pre-conditioning in isolated Langendorff-perfused rat hearts.59 However, this report is the first to link the modulation of mitochondrial dynamics to the activation of AMPK in the context of pre-conditioning and begs the question of whether the induction of mitochondrial fusion is a central mechanism common to all preconditioning agents.
We and others have previously established that administration of oral nitrite from 24 h to 1 week prior to ischaemia mediates tissue protection after I/R.9,15 It has been speculated that given the abundance of nitrate (reduced to nitrite by oral bacteria) in the Mediterranean Diet, nitrite may be the active cardioprotective agent in this diet.15 The presence of mitochondrial fusion has not been compared in subjects on this diet vs. other diets. We show here that nitrite mediates increased PKA activity and phosphorylation of Drp1 in the rat heart. However, the effect of a nitrite-rich diet is potentially an interesting avenue for further future investigations. In addition to enhancing myocardial tissue viability after I/R injury, activation of AMPK has been shown to improve cardiac function in pathologies such as type II diabetes60 and drug-related cardiomyopathy.61 Notably, oral nitrite also mediates protection in these conditions,62,63 suggesting that nitrite-mediated mitochondrial fusion and subsequent AMPK activation may play a role in these protective effects.
In conclusion, the results presented here describe a novel mechanism by which nitrite mediates delayed cytoprotection after I/R. These data for the first time demonstrate nitrite-dependent regulation of mitochondrial dynamics as well as normoxic modulation of mitochondrial function. Together, these data greatly expand the role of nitrite in the regulation of cellular bioenergetics as well as in the adaptation to the response.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Funding
This work was supported by the Institute of Transfusion Medicine and the Hemophilia Center of Western Pennsylvania, the National Institutes of Health (1R01HL096973 to S.S.; R01NS065789 to C.T.C.) and by the American Heart Association (09SDG2150066 to S.S.).
Supplementary Material
Acknowledgements
We thank Dr Stefan Strack for the generous gift of Drp1 mutants.
Conflict of interest: none declared.
References
- 1.Duranski MR, Greer JJ, Dejam A, Jaganmohan S, Hogg N, Langston W, et al. Cytoprotective effects of nitrite during in vivo ischemia-reperfusion of the heart and liver. J Clin Invest. 2005;115:1232–1240. doi: 10.1172/JCI22493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tripatara P, Patel NS, Webb A, Rathod K, Lecomte FM, Mazzon E, et al. Nitrite-derived nitric oxide protects the rat kidney against ischemia/reperfusion injury in vivo: role for xanthine oxidoreductase. J Am Soc Nephrol. 2007;18:570–580. doi: 10.1681/ASN.2006050450. [DOI] [PubMed] [Google Scholar]
- 3.Jung KH, Chu K, Ko SY, Lee ST, Sinn DI, Park DK, et al. Early intravenous infusion of sodium nitrite protects brain against in vivo ischemia–reperfusion injury. Stroke. 2006;37:2744–2750. doi: 10.1161/01.STR.0000245116.40163.1c. [DOI] [PubMed] [Google Scholar]
- 4.Dezfulian C, Raat N, Shiva S, Gladwin MT. Role of the anion nitrite in ischemia–reperfusion cytoprotection and therapeutics. Cardiovasc Res. 2007;75:327–338. doi: 10.1016/j.cardiores.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Webb A, Bond R, McLean P, Uppal R, Benjamin N, Ahluwalia A. Reduction of nitrite to nitric oxide during ischemia protects against myocardial ischemia–reperfusion damage. Proc Natl Acad Sci USA. 2004;101:13683–13688. doi: 10.1073/pnas.0402927101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar D, Branch BG, Pattillo CB, Hood J, Thoma S, Simpson S, et al. Chronic sodium nitrite therapy augments ischemia-induced angiogenesis and arteriogenesis. Proc Natl Acad Sci USA. 2008;105:7540–7545. doi: 10.1073/pnas.0711480105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov. 2008;7:156–167. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez FM, Shiva S, Vincent PS, Ringwood LA, Hsu LY, Hon YY, et al. Nitrite anion provides potent cytoprotective and antiapoptotic effects as adjunctive therapy to reperfusion for acute myocardial infarction. Circulation. 2008;117:2986–2994. doi: 10.1161/CIRCULATIONAHA.107.748814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bryan NS, Calvert JW, Elrod JW, Gundewar S, Ji SY, Lefer DJ. Dietary nitrite supplementation protects against myocardial ischemia–reperfusion injury. Proc Natl Acad Sci USA. 2007;104:19144–19149. doi: 10.1073/pnas.0706579104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hendgen-Cotta UB, Merx MW, Shiva S, Schmitz J, Becher S, Klare JP, et al. Nitrite reductase activity of myoglobin regulates respiration and cellular viability in myocardial ischemia-reperfusion injury. Proc Natl Acad Sci USA. 2008;105:10256–10261. doi: 10.1073/pnas.0801336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shiva S, Huang Z, Grubina R, Sun J, Ringwood LA, MacArthur PH, et al. Deoxymyoglobin is a nitrite reductase that generates nitric oxide and regulates mitochondrial respiration. Circ Res. 2007;100:654–661. doi: 10.1161/01.RES.0000260171.52224.6b. [DOI] [PubMed] [Google Scholar]
- 12.Rassaf T, Flogel U, Drexhage C, Hendgen-Cotta U, Kelm M, Schrader J. Nitrite reductase function of deoxymyoglobin: oxygen sensor and regulator of cardiac energetics and function. Circ Res. 2007;100:1749–1754. doi: 10.1161/CIRCRESAHA.107.152488. [DOI] [PubMed] [Google Scholar]
- 13.Li H, Samouilov A, Liu X, Zweier JL. Characterization of the magnitude and kinetics of xanthine oxidase-catalyzed nitrite reduction. Evaluation of its role in nitric oxide generation in anoxic tissues. J Biol Chem. 2001;276:24482–24489. doi: 10.1074/jbc.M011648200. [DOI] [PubMed] [Google Scholar]
- 14.Shiva S, Sack MN, Greer JJ, Duranski M, Ringwood LA, Burwell L, et al. Nitrite augments tolerance to ischemia/reperfusion injury via the modulation of mitochondrial electron transfer. J Exp Med. 2007;204:2089–2102. doi: 10.1084/jem.20070198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raat NJ, Noguchi AC, Liu VB, Raghavachari N, Liu D, Xu X, et al. Dietary nitrate and nitrite modulate blood and organ nitrite and the cellular ischemic stress response. Free Radic Biol Med. 2009;47:510–517. doi: 10.1016/j.freeradbiomed.2009.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ong SB, Subrayan S, Lim SY, Yellon DM, Davidson SM, Hausenloy DJ. Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation. 2010;121:2012–2022. doi: 10.1161/CIRCULATIONAHA.109.906610. [DOI] [PubMed] [Google Scholar]
- 17.Steenbergen C, Das S, Su J, Wong R, Murphy E. Cardioprotection and altered mitochondrial adenine nucleotide transport. Basic Res Cardiol. 2009;104:149–156. doi: 10.1007/s00395-009-0002-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stowe DF, Kevin LG. Cardiac preconditioning by volatile anesthetic agents: a defining role for altered mitochondrial bioenergetics. Antioxid Redox Signal. 2004;6:439–448. doi: 10.1089/152308604322899512. [DOI] [PubMed] [Google Scholar]
- 19.Donato M, Gelpi RJ. Adenosine and cardioprotection during reperfusion—an overview. Mol Cell Biochem. 2003;251:153–159. [PubMed] [Google Scholar]
- 20.Vanden Hoek TL, Becker LB, Shao Z, Li C, Schumacker PT. Reactive oxygen species released from mitochondria during brief hypoxia induce preconditioning in cardiomyocytes. J Biol Chem. 1998;273:18092–18098. doi: 10.1074/jbc.273.29.18092. [DOI] [PubMed] [Google Scholar]
- 21.Zmijewski JW, Banerjee S, Bae H, Friggeri A, Lazarowski ER, Abraham E. Exposure to hydrogen peroxide induces oxidation and activation of AMP-activated protein kinase. J Biol Chem. 2010;285:33154–33164. doi: 10.1074/jbc.M110.143685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schumacker PT. Lung cell hypoxia: role of mitochondrial reactive oxygen species signaling in triggering responses. Proc Am Thorac Soc. 2011;8:477–484. doi: 10.1513/pats.201103-032MW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim H, Scimia MC, Wilkinson D, Trelles RD, Wood MR, Bowtell D, et al. Fine-tuning of Drp1/Fis1 availability by AKAP121/Siah2 regulates mitochondrial adaptation to hypoxia. Mol Cell. 2011;44:532–544. doi: 10.1016/j.molcel.2011.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dickey AS, Strack S. PKA/AKAP1 and PP2A/Bbeta2 regulate neuronal morphogenesis via Drp1 phosphorylation and mitochondrial bioenergetics. J Neurosci. 2011;31:15716–15726. doi: 10.1523/JNEUROSCI.3159-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Esumi K, Nishida M, Shaw D, Smith TW, Marsh JD. NADH measurements in adult rat myocytes during simulated ischemia. Am J Physiol. 1991;260:H1743–H1752. doi: 10.1152/ajpheart.1991.260.6.H1743. [DOI] [PubMed] [Google Scholar]
- 26.Lecour S, Suleman N, Deuchar GA, Somers S, Lacerda L, Huisamen B, et al. Pharmacological preconditioning with tumor necrosis factor-alpha activates signal transducer and activator of transcription-3 at reperfusion without involving classic prosurvival kinases (Akt and extracellular signal-regulated kinase) Circulation. 2005;112:3911–3918. doi: 10.1161/CIRCULATIONAHA.105.581058. [DOI] [PubMed] [Google Scholar]
- 27.Stephanou A, Brar BK, Scarabelli TM, Jonassen AK, Yellon DM, Marber MS, et al. Ischemia-induced STAT-1 expression and activation play a critical role in cardiomyocyte apoptosis. J Biol Chem. 2000;275:10002–10008. doi: 10.1074/jbc.275.14.10002. [DOI] [PubMed] [Google Scholar]
- 28.Suleman N, Somers S, Smith R, Opie LH, Lecour SC. Dual activation of STAT-3 and Akt is required during the trigger phase of ischaemic preconditioning. Cardiovasc Res. 2008;79:127–133. doi: 10.1093/cvr/cvn067. [DOI] [PubMed] [Google Scholar]
- 29.Mo L, Wang Y, Geary L, Corey C, Alef MJ, Beer-Stolz D, et al. Nitrite activates AMP kinase to stimulate mitochondrial biogenesis independent of soluble guanylate cyclase. Free Radic Biol Med. 2012;53:1440–1450. doi: 10.1016/j.freeradbiomed.2012.07.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gardner PR, Nguyen DD, White CW. Aconitase is a sensitive and critical target of oxygen poisoning in cultured mammalian cells and in rat lungs. Proc Natl Acad Sci USA. 1994;91:12248–12252. doi: 10.1073/pnas.91.25.12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khoo NK, White CR, Pozzo-Miller L, Zhou F, Constance C, Inoue T, et al. Dietary flavonoid quercetin stimulates vasorelaxation in aortic vessels. Free Radic Biol Med. 2010;49:339–347. doi: 10.1016/j.freeradbiomed.2010.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cribbs JT, Strack S. Reversible phosphorylation of Drp1 by cyclic AMP-dependent protein kinase and calcineurin regulates mitochondrial fission and cell death. EMBO Rep. 2007;8:939–944. doi: 10.1038/sj.embor.7401062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang X, Kettenhofen NJ, Shiva S, Hogg N, Gladwin MT. Copper dependence of the biotin switch assay: modified assay for measuring cellular and blood nitrosated proteins. Free Radic Biol Med. 2008;44:1362–1372. doi: 10.1016/j.freeradbiomed.2007.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Curtis E, Hsu LL, Noguchi AC, Geary L, Shiva S. Oxygen regulates tissue nitrite metabolism. Antioxid Redox Signal. 2012;17:951–961. doi: 10.1089/ars.2011.4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hausenloy D, Wynne A, Duchen M, Yellon D. Transient mitochondrial permeability transition pore opening mediates preconditioning-induced protection. Circulation. 2004;109:1714–1717. doi: 10.1161/01.CIR.0000126294.81407.7D. [DOI] [PubMed] [Google Scholar]
- 36.Dagda RK, Gusdon AM, Pien I, Strack S, Green S, Li C, et al. Mitochondrially localized PKA reverses mitochondrial pathology and dysfunction in a cellular model of Parkinson’s disease. Cell Death Differ. 2011;18:1914–1923. doi: 10.1038/cdd.2011.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bolli R. The late phase of preconditioning. Circ Res. 2000;87:972–983. doi: 10.1161/01.res.87.11.972. [DOI] [PubMed] [Google Scholar]
- 38.Jennings RB, Murry CE, Reimer KA. Preconditioning myocardium with ischemia. Cardiovasc Drugs Ther. 1991;5:933–938. doi: 10.1007/BF00053555. [DOI] [PubMed] [Google Scholar]
- 39.Ong SB, Hausenloy DJ. Mitochondrial morphology and cardiovascular disease. Cardiovasc Res. 2010;88:16–29. doi: 10.1093/cvr/cvq237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Olichon A, Baricault L, Gas N, Guillou E, Valette A, Belenguer P, et al. Loss of OPA1 perturbates the mitochondrial inner membrane structure and integrity, leading to cytochrome c release and apoptosis. J Biol Chem. 2003;278:7743–7746. doi: 10.1074/jbc.C200677200. [DOI] [PubMed] [Google Scholar]
- 41.Bach D, Pich S, Soriano FX, Vega N, Baumgartner B, Oriola J, et al. Mitofusin-2 determines mitochondrial network architecture and mitochondrial metabolism. A novel regulatory mechanism altered in obesity. J Biol Chem. 2003;278:17190–17197. doi: 10.1074/jbc.M212754200. [DOI] [PubMed] [Google Scholar]
- 42.Pich S, Bach D, Briones P, Liesa M, Camps M, Testar X, et al. The Charcot-Marie-Tooth type 2A gene product, Mfn2, up-regulates fuel oxidation through expression of OXPHOS system. Hum Mol Genet. 2005;14:1405–1415. doi: 10.1093/hmg/ddi149. [DOI] [PubMed] [Google Scholar]
- 43.Young LH. AMP-activated protein kinase conducts the ischemic stress response orchestra. Circulation. 2008;117:832–840. doi: 10.1161/CIRCULATIONAHA.107.713115. [DOI] [PubMed] [Google Scholar]
- 44.Paiva MA, Rutter-Locher Z, Goncalves LM, Providencia LA, Davidson SM, Yellon DM, et al. Enhancing AMPK activation during ischemia protects the diabetic heart against reperfusion injury. Am J Physiol Heart Circ Physiol. 2011;300:H2123–2134. doi: 10.1152/ajpheart.00707.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pauly M, Daussin F, Burelle Y, Li T, Godin R, Fauconnier J, et al. AMPK activation stimulates autophagy and ameliorates muscular dystrophy in the mdx mouse diaphragm. Am J Pathol. 2012;181:583–592. doi: 10.1016/j.ajpath.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 46.Raat NJ, Shiva S, Gladwin MT. Effects of nitrite on modulating ROS generation following ischemia and reperfusion. Adv Drug Deliv Rev. 2009;61:339–350. doi: 10.1016/j.addr.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 47.van der Vliet A, Eiserich JP, Halliwell B, Cross CE. Formation of reactive nitrogen species during peroxidase-catalyzed oxidation of nitrite. A potential additional mechanism of nitric oxide-dependent toxicity. J Biol Chem. 1997;272:7617–7625. doi: 10.1074/jbc.272.12.7617. [DOI] [PubMed] [Google Scholar]
- 48.Wang L, Frizzell SA, Zhao X, Gladwin MT. Normoxic cyclic GMP-independent oxidative signaling by nitrite enhances airway epithelial cell proliferation and wound healing. Nitric Oxide. 2012;26:203–210. doi: 10.1016/j.niox.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Coles B, Bloodsworth A, Eiserich JP, Coffey MJ, McLoughlin RM, Giddings JC, et al. Nitrolinoleate inhibits platelet activation by attenuating calcium mobilization and inducing phosphorylation of vasodilator-stimulated phosphoprotein through elevation of cAMP. J Biol Chem. 2002;277:5832–5840. doi: 10.1074/jbc.M105209200. [DOI] [PubMed] [Google Scholar]
- 50.Perlman DH, Bauer SM, Ashrafian H, Bryan NS, Garcia-Saura MF, Lim CC, et al. Mechanistic insights into nitrite-induced cardioprotection using an integrated metabolomic/proteomic approach. Circ Res. 2009;104:796–804. doi: 10.1161/CIRCRESAHA.108.187005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Venkatesh PK, Pattillo CB, Branch B, Hood J, Thoma S, Illum S, et al. Dipyridamole enhances ischaemia-induced arteriogenesis through an endocrine nitrite/nitric oxide-dependent pathway. Cardiovasc Res. 2010;85:661–670. doi: 10.1093/cvr/cvq002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Giedt RJ, Yang C, Zweier JL, Matzavinos A, Alevriadou BR. Mitochondrial fission in endothelial cells after simulated ischemia/reperfusion: role of nitric oxide and reactive oxygen species. Free Radic Biol Med. 2012;52:348–356. doi: 10.1016/j.freeradbiomed.2011.10.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Neuspiel M, Zunino R, Gangaraju S, Rippstein P, McBride H. Activated mitofusin 2 signals mitochondrial fusion, interferes with Bax activation, and reduces susceptibility to radical induced depolarization. J Biol Chem. 2005;280:25060–25070. doi: 10.1074/jbc.M501599200. [DOI] [PubMed] [Google Scholar]
- 54.Sukhodub A, Jovanovic S, Du Q, Budas G, Clelland AK, Shen M, et al. AMP-activated protein kinase mediates preconditioning in cardiomyocytes by regulating activity and trafficking of sarcolemmal ATP-sensitive K(+) channels. J Cell Physiol. 2007;210:224–236. doi: 10.1002/jcp.20862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Matsui Y, Kyoi S, Takagi H, Hsu CP, Hariharan N, Ago T, et al. Molecular mechanisms and physiological significance of autophagy during myocardial ischemia and reperfusion. Autophagy. 2008;4:409–415. doi: 10.4161/auto.5638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Matsui Y, Takagi H, Qu X, Abdellatif M, Sakoda H, Asano T, et al. Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circulation Res. 2007;100:914–922. doi: 10.1161/01.RES.0000261924.76669.36. [DOI] [PubMed] [Google Scholar]
- 57.Lee M, Hwang JT, Lee HJ, Jung SN, Kang I, Chi SG, et al. AMP-activated protein kinase activity is critical for hypoxia-inducible factor-1 transcriptional activity and its target gene expression under hypoxic conditions in DU145 cells. J Biol Chem. 2003;278:39653–39661. doi: 10.1074/jbc.M306104200. [DOI] [PubMed] [Google Scholar]
- 58.Jung SN, Yang WK, Kim J, Kim HS, Kim EJ, Yun H, et al. Reactive oxygen species stabilize hypoxia-inducible factor-1 alpha protein and stimulate transcriptional activity via AMP-activated protein kinase in DU145 human prostate cancer cells. Carcinogenesis. 2008;29:713–721. doi: 10.1093/carcin/bgn032. [DOI] [PubMed] [Google Scholar]
- 59.Lamberts RR, Onderwater G, Hamdani N, Vreden MJ, Steenhuisen J, Eringa EC, et al. Reactive oxygen species-induced stimulation of 5′AMP-activated protein kinase mediates sevoflurane-induced cardioprotection. Circulation. 2009;120:S10–15. doi: 10.1161/CIRCULATIONAHA.108.828426. [DOI] [PubMed] [Google Scholar]
- 60.Cool B, Zinker B, Chiou W, Kifle L, Cao N, Perham M, et al. Identification and characterization of a small molecule AMPK activator that treats key components of type 2 diabetes and the metabolic syndrome. Cell Metab. 2006;3:403–416. doi: 10.1016/j.cmet.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 61.Konishi M, Haraguchi G, Ohigashi H, Ishihara T, Saito K, Nakano Y, et al. Adiponectin protects against doxorubicin-induced cardiomyopathy by anti-apoptotic effects through AMPK up-regulation. Cardiovasc Res. 2011;89:309–319. doi: 10.1093/cvr/cvq335. [DOI] [PubMed] [Google Scholar]
- 62.Zhu SG, Kukreja RC, Das A, Chen Q, Lesnefsky EJ, Xi L. Dietary nitrate supplementation protects against Doxorubicin-induced cardiomyopathy by improving mitochondrial function. J Am Coll Cardiol. 2011;57:2181–2189. doi: 10.1016/j.jacc.2011.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Carlstrom M, Larsen FJ, Nystrom T, Hezel M, Borniquel S, Weitzberg E, et al. Dietary inorganic nitrate reverses features of metabolic syndrome in endothelial nitric oxide synthase-deficient mice. Proc Natl Acad Sci USA. 2010;107:17716–17720. doi: 10.1073/pnas.1008872107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.