Abstract
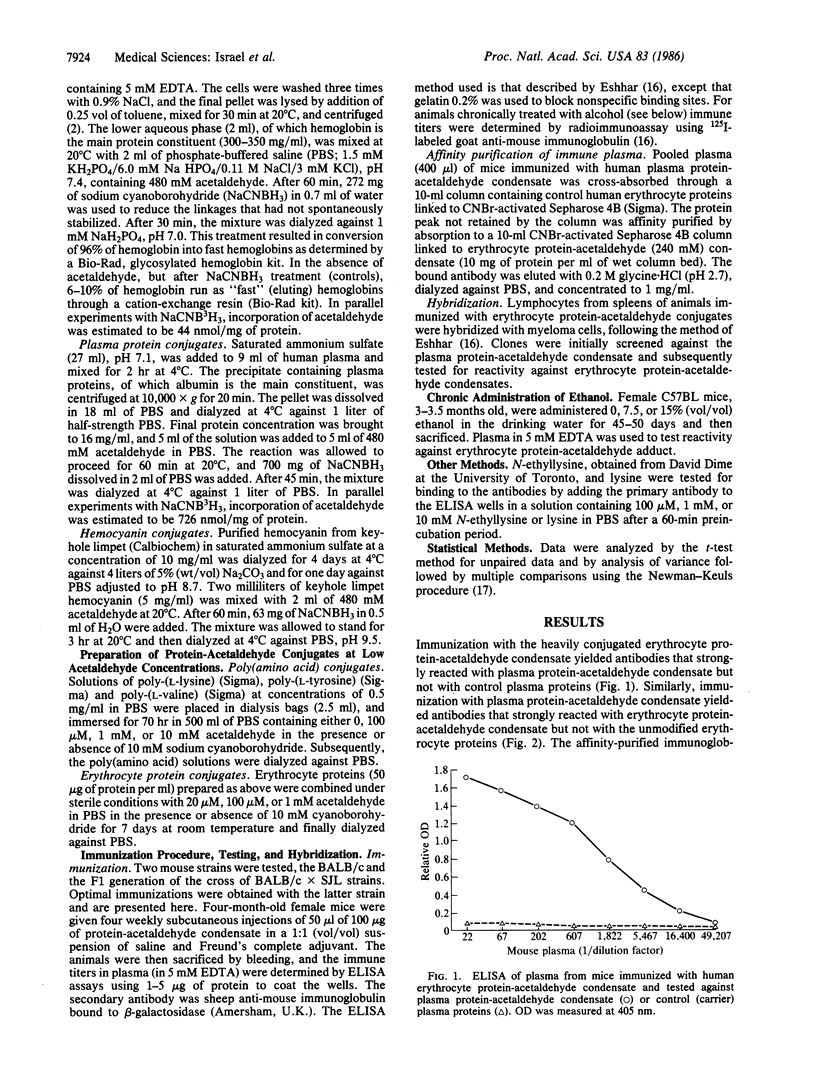
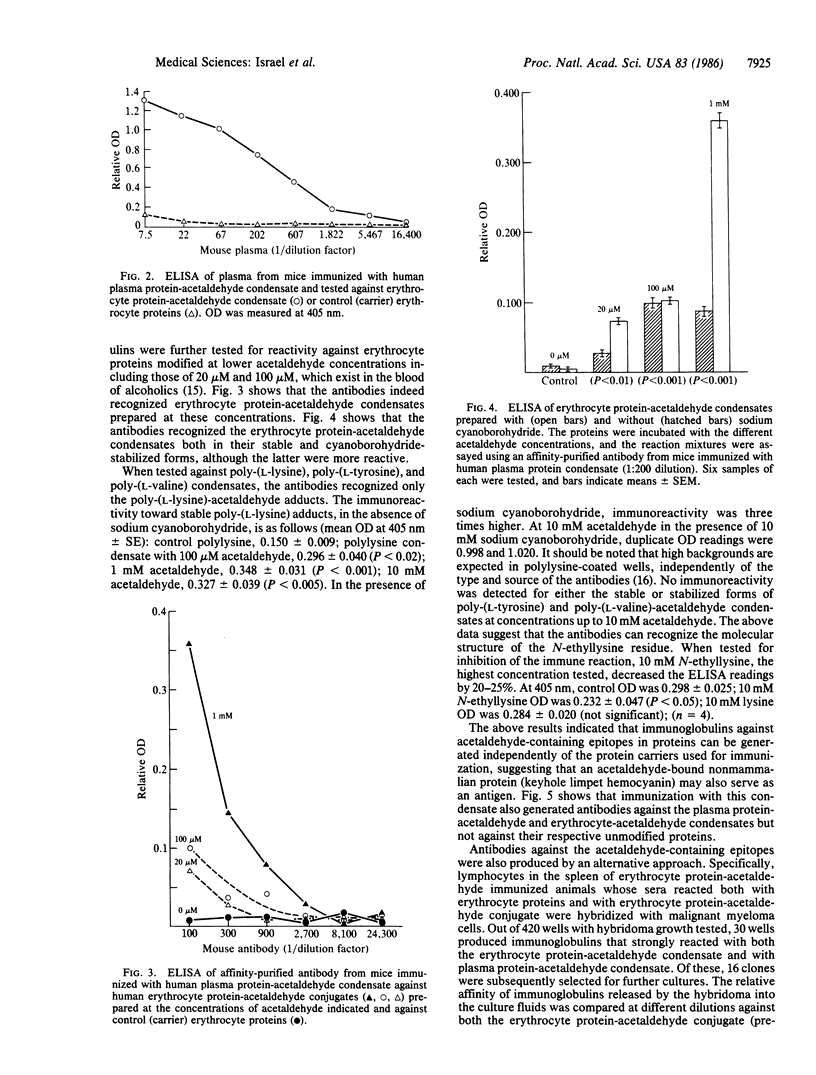
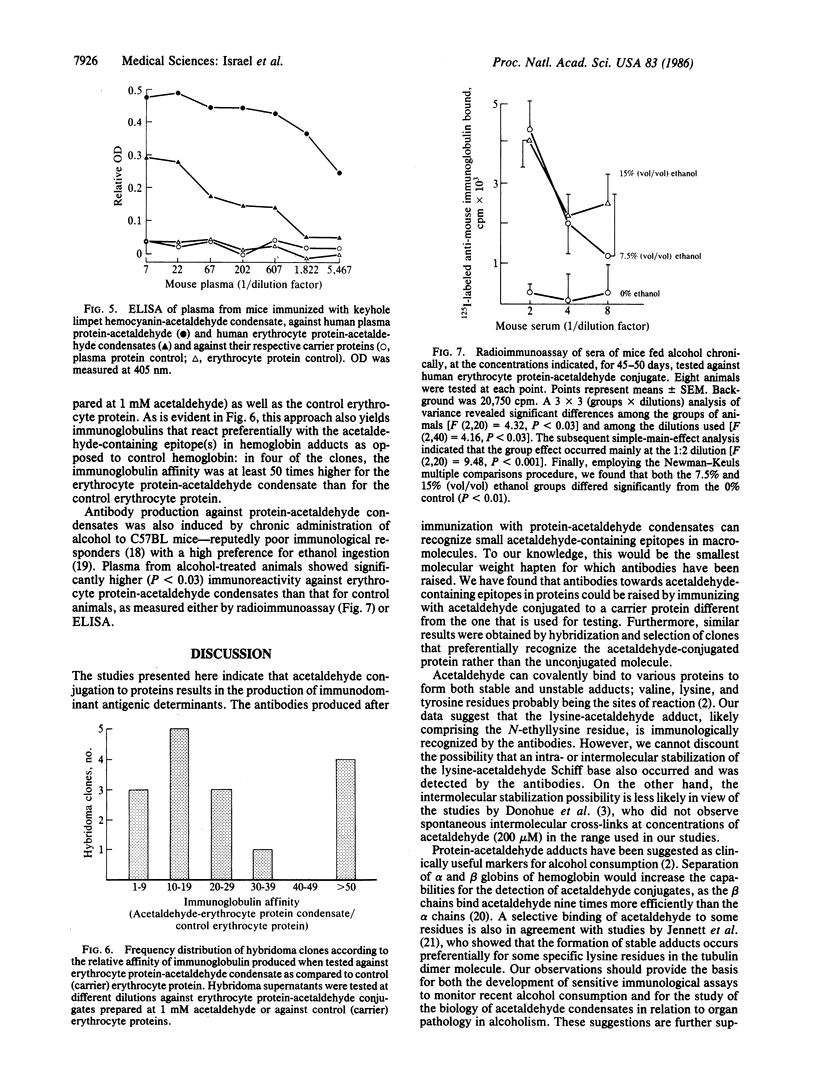
Immunization of mice with acetaldehyde conjugated to human plasma proteins resulted in the production of polyclonal antibodies that reacted with erythrocyte protein-acetaldehyde conjugates, but not with control erythrocyte proteins. Such antibodies recognized erythrocyte protein-acetaldehyde conjugates prepared with 20-100 microM acetaldehyde, concentrations that exist in the blood of alcoholics. The antibodies also recognized acetaldehyde condensation products with synthetic poly-(L-lysine). Immunization with keyhole limpet hemocyanin-acetaldehyde conjugates resulted in antibodies against both plasma protein-acetaldehyde and erythrocyte protein-acetaldehyde conjugates, which did not cross-react with the respective unmodified carrier proteins. Immunization with human erythrocyte protein-acetaldehyde condensates led to the production of antibodies against both the protein moiety as well as the condensate. Monoclonal antibodies with affinities 50 times greater for the condensate than for the carrier protein were produced by hybridization of spleen cells from the immunized mice. Chronic alcohol administration to mice for 45-50 days led to the generation of antibodies that reacted against protein-acetaldehyde conjugates, suggesting that such adducts are formed in vivo and can act as neoantigens. Antibodies against acetaldehyde adducts should be of value in the identification of alcohol consumption and in the study of the biology of the adducts in relation to organ pathology.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Actis G. C., Ponzetto A., Rizzetto M., Verme G. Cell-mediated immunity to acetaldehyde in alcoholic liver disease demonstrated by leukocyte migration test. Am J Dig Dis. 1978 Oct;23(10):883–886. doi: 10.1007/BF01072460. [DOI] [PubMed] [Google Scholar]
- Barry R. E., McGivan J. D. Acetaldehyde alone may initiate hepatocellular damage in acute alcoholic liver disease. Gut. 1985 Oct;26(10):1065–1069. doi: 10.1136/gut.26.10.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochrane A. M., Moussouros A., Portmann B., McFarlane I. G., Thomson A. D., Eddleston, Williams R. Lymphocyte cytotoxicity for isolated hepatocytes in alcoholic liver disease. Gastroenterology. 1977 May;72(5 Pt 1):918–923. [PubMed] [Google Scholar]
- Crossley I. R., Neuberger J., Davis M., Williams R., Eddleston A. L. Ethanol metabolism in the generation of new antigenic determinants on liver cells. Gut. 1986 Feb;27(2):186–189. doi: 10.1136/gut.27.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohue T. M., Jr, Tuma D. J., Sorrell M. F. Acetaldehyde adducts with proteins: binding of [14C]acetaldehyde to serum albumin. Arch Biochem Biophys. 1983 Jan;220(1):239–246. doi: 10.1016/0003-9861(83)90406-x. [DOI] [PubMed] [Google Scholar]
- Gonen B., Rubenstein A., Rochman H., Tanega S. P., Horwitz D. L. Haemoglobin A1: An indicator of the metabolic control of diabetic patients. Lancet. 1977 Oct 8;2(8041):734–737. doi: 10.1016/s0140-6736(77)90237-9. [DOI] [PubMed] [Google Scholar]
- Kakumu S., Leevy C. M. Lymphocyte cytotoxicity in alcoholic hepatitis. Gastroenterology. 1977 Apr;72(4 Pt 1):594–597. [PubMed] [Google Scholar]
- Koenig R. J., Peterson C. M., Jones R. L., Saudek C., Lehrman M., Cerami A. Correlation of glucose regulation and hemoglobin AIc in diabetes mellitus. N Engl J Med. 1976 Aug 19;295(8):417–420. doi: 10.1056/NEJM197608192950804. [DOI] [PubMed] [Google Scholar]
- MOHAMMAD A., OLCOTT H. S., FRAENKEL-CONRAT H. The reaction of proteins with acetaldehyde. Arch Biochem. 1949 Dec;24(2):270–280. [PubMed] [Google Scholar]
- MacSween R. N. Alcohol and liver injury: genetic and immunologic factors. Acta Med Scand Suppl. 1985;703:57–65. doi: 10.1111/j.0954-6820.1985.tb08904.x. [DOI] [PubMed] [Google Scholar]
- Neuberger J., Crossley I. R., Saunders J. B., Davis M., Portmann B., Eddleston A. L., Williams R. Antibodies to alcohol altered liver cell determinants in patients with alcoholic liver disease. Gut. 1984 Mar;25(3):300–304. doi: 10.1136/gut.25.3.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen L. B., Peterson C. M. Differential modification of hemoglobin chains by acetaldehyde. Proc Soc Exp Biol Med. 1986 Jan;181(1):151–156. doi: 10.3181/00379727-181-42237. [DOI] [PubMed] [Google Scholar]
- Nguyen L. B., Peterson C. M. The effect of acetaldehyde concentrations on the relative rates of formation of acetaldehyde-modified hemoglobins. Proc Soc Exp Biol Med. 1984 Nov;177(2):226–233. doi: 10.3181/00379727-177-41935. [DOI] [PubMed] [Google Scholar]
- Nomura F., Lieber C. S. Binding of acetaldehyde to rat liver microsomes: enhancement after chronic alcohol consumption. Biochem Biophys Res Commun. 1981 May 15;100(1):131–137. doi: 10.1016/s0006-291x(81)80073-3. [DOI] [PubMed] [Google Scholar]
- Nuutinen H., Lindros K. O., Salaspuro M. Determinants of blood acetaldehyde level during ethanol oxidation in chronic alcoholics. Alcohol Clin Exp Res. 1983 Spring;7(2):163–168. doi: 10.1111/j.1530-0277.1983.tb05432.x. [DOI] [PubMed] [Google Scholar]
- Sorrell M. F., Leevy C. M. Lymphocyte transformation and alcoholic liver injury. Gastroenterology. 1972 Dec;63(6):1020–1025. [PubMed] [Google Scholar]
- Sorrell M. F., Tuma D. J. Hypothesis: alcoholic liver injury and the covalent binding of acetaldehyde. Alcohol Clin Exp Res. 1985 Jul-Aug;9(4):306–309. doi: 10.1111/j.1530-0277.1985.tb05549.x. [DOI] [PubMed] [Google Scholar]
- Stevens V. J., Fantl W. J., Newman C. B., Sims R. V., Cerami A., Peterson C. M. Acetaldehyde adducts with hemoglobin. J Clin Invest. 1981 Feb;67(2):361–369. doi: 10.1172/JCI110043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivelli L. A., Ranney H. M., Lai H. T. Hemoglobin components in patients with diabetes mellitus. N Engl J Med. 1971 Feb 18;284(7):353–357. doi: 10.1056/NEJM197102182840703. [DOI] [PubMed] [Google Scholar]
- Tuma D. J., Donohue T. M., Jr, Medina V. A., Sorrell M. F. Enhancement of acetaldehyde-protein adduct formation by L-ascorbate. Arch Biochem Biophys. 1984 Nov 1;234(2):377–381. doi: 10.1016/0003-9861(84)90283-2. [DOI] [PubMed] [Google Scholar]



