Abstract
Aims
Recent studies suggest that proarrhythmic effects of cardiac glycosides (CGs) on cardiomyocyte Ca2+ handling involve generation of reactive oxygen species (ROS). However, the specific pathway(s) of ROS production and the subsequent downstream molecular events that mediate CG-dependent arrhythmogenesis remain to be defined.
Methods and results
We examined the effects of digitoxin (DGT) on Ca2+ handling and ROS production in cardiomyocytes using a combination of pharmacological approaches and genetic mouse models. Myocytes isolated from mice deficient in NADPH oxidase type 2 (NOX2KO) and mice transgenically overexpressing mitochondrial superoxide dismutase displayed markedly increased tolerance to the proarrhythmic action of DGT as manifested by the inhibition of DGT-dependent ROS and spontaneous Ca2+ waves (SCW). Additionally, DGT-induced mitochondrial membrane potential depolarization was abolished in NOX2KO cells. DGT-dependent ROS was suppressed by the inhibition of PI3K, PKC, and the mitochondrial KATP channel, suggesting roles for these proteins, respectively, in activation of NOX2 and in mitochondrial ROS generation. Western blot analysis revealed increased levels of oxidized CaMKII in WT but not in NOX2KO hearts treated with DGT. The DGT-induced increase in SCW frequency was abolished in myocytes isolated from mice in which the Ser 2814 CaMKII phosphorylation site on RyR2 is constitutively inactivated.
Conclusion
These results suggest that the arrhythmogenic adverse effects of CGs on Ca2+ handling involve PI3K- and PKC-mediated stimulation of NOX2 and subsequent NOX2-dependent ROS release from the mitochondria; mitochondria-derived ROS then activate CaMKII with consequent phosphorylation of RyR2 at Ser 2814.
Keywords: Calcium, Reactive oxygen species, NADPH oxidase, Mitochondria, CaMKII
1. Introduction
Cardiac glycosides (CGs) are an important class of naturally occurring drugs whose primary target is the plasma membrane Na+/K+-ATPase (NKA).1 CGs have been traditionally used in the treatment of congestive heart failure (HF) due to their ability to increase cardiac contractility.2 However, adverse side effects of CGs,2 including cardiac arrhythmias,3 remain a serious problem especially in elderly populations.4 Digitoxin (DGT) and digoxin are the two available CGs with equal efficacy and similar toxicities.5 A thorough understanding of the mechanisms that underlie the therapeutic and toxic effects of CGs is essential for improved therapeutic outcomes. Additionally, improved understanding of CG cardiotoxicity is likely to provide insights into myocardial diseases marked by defective intracellular Ca2+ homeostasis.
The beneficial impact of CGs on cardiac performance has been attributed to changes in ionic balance secondary to inhibition of NKA.1 An elevation of intracellular Na+ causes accumulation of intracellular Ca2+ thereby leading to increased myocyte contractility.6,7 The arrhythmic side effects of CGs are generally ascribed to excessive cellular Ca2+ accumulation (Ca2+ overload) that results in the spontaneous release of Ca2+ (Ca2+ waves) from the sarcoplasmic reticulum (SR) through ryanodine receptor 2 (RyR2) channels.1 This ill-timed Ca2+ release causes oscillations of the membrane potential known as delayed afterdepolarizations (DADs) by activation of Ca2+-dependent depolarizing currents. These DADs can then result in ectopic excitation and triggered activity.8,9
Recent studies have suggested an alternative mechanism for proarrhythmic alterations in myocyte Ca2+ handling caused by CGs involving the generation of reactive oxygen species (ROS).10–12 The specific intracellular pathways leading to ROS formation, and the causal links between ROS, abnormal Ca2+ cycling, and arrhythmias remain to be elucidated. In the present study, we examined the mechanisms of ROS production by DGT and the downstream processes behind the proarrhythmic effects of DGT in cardiac myocytes using a combination of pharmacological approaches and genetic mouse models.
2. Methods
Two-to-four-month-old male wild-type (WT), NADPH oxidase type 2 knockout (NOX2KO),13 inducible mitochondrial superoxide dismutase (SOD2) overexpression,14 and RyR2 knock-in (RyR2-S2814A)15 mice were used. All animal procedures were approved by The Ohio State University Institutional Animal Care and Use Committee and conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996). See Supplementary material online for more details.
2.1. Myocyte isolation and confocal microscopy
Mouse ventricular myocytes were isolated using Liberase TH Research Grade enzyme (Roche). Confocal imaging was recorded using an Olympus Fluoview 1000 confocal microscope. See Supplementary material online for more details.
2.2. Western blotting
Samples were prepared from Langendorff-perfused intact mouse hearts, and then instantly frozen in liquid nitrogen. Cardiac homogenates were prepared and analysed by western blot. See Supplementary material online for more details.
2.3. Statistical analysis
Data are presented as mean ± SE. Statistical analyses were performed using ANOVA with Tukey's post hoc test. Values of P < 0.05 were accepted as statistically significant.
3. Results
3.1. DGT-dependent alterations in Ca2+ handling in WT myocytes
Intracellular Ca2+ cycling in isolated, paced (0.3 Hz) wild-type (WT) mouse ventricular myocytes was monitored under baseline conditions (control group) and in the presence of DGT (50–300 nM) (Supplementary material online, Figure S1A and B). Exposure to DGT resulted in a significant increase in the amplitude of Ca2+ transients at drug concentrations >50 nM. However, at DGT concentrations of 150 nM and higher, the potentiation of Ca2+ transients was undermined by the occurrence of proarrhythmic spontaneous Ca2+ waves (SCWs) affiliated with a decrease in the SR Ca2+ content (Supplementary material online, Figure S2). Diphenyliodonium chloride (DPI), and cyclosporin A (CsA), inhibitors of NADPH oxidase (NOX), and mitochondrial permeability transition pore (MPTP), respectively, significantly decreased the frequency of SCWs while preserving the enhanced Ca2+ transient amplitude in the presence of DGT (Supplementary material online, Figure S1A and C). With the caveat that DPI and CsA might act on other targets,16,17 these results are consistent with the notion that NOX- and mitochondria-derived ROS may contribute to the proarrhythmic effects of DGT on Ca2+ handling.
3.2. NOX2 ablation prevents DGT-dependent alterations in Ca2+ handling and ROS
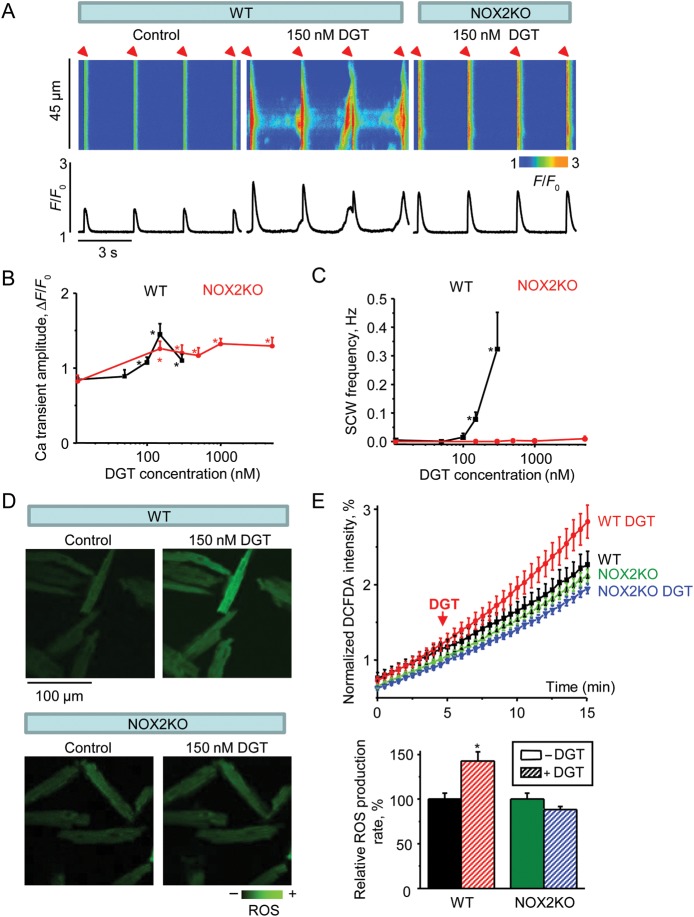
NADPH oxidase type 2 (NOX2) is considered to be a major isoform associated with redox signalling in cardiac cells.18 Therefore, we used NOX2 deficient mice (NOX2KO) to test the potential involvement of this enzyme in DGT-dependent changes in Ca2+ handling and ROS (Figure 1). The frequency of SCWs was markedly decreased in NOX2KO myocytes with DGT failing to induce SCWs at high concentrations (up to 5 µM DGT; see Figure 1C). Of note, the elevation of Ca2+ transient amplitude by DGT was partially preserved in NOX2KO myocytes (Figure 1B). Additionally, NOX2 ablation stabilized the SR Ca2+ content in the face of DGT challenge (Supplementary material online, Figure S2). To determine whether prevention of DGT-dependent Ca2+ waves by NOX2 ablation is attributable to reduced ROS production, we performed ROS measurements in NOX2KO vs. WT myocytes with the ROS indicator CM-H2DCFDA. DGT increased ROS in WT but not in NOX2KO cells (Figure 1D and E). Thus, NOX2 deletion prevented DGT-dependent ROS production and inhibited SCWs.
Figure 1.
NOX2 ablation prevents DGT-dependent alterations in Ca2+ handling and ROS. (A) Representative line-scan images (top) and time-dependent profiles (bottom) of intracellular Ca2+ handling in myocytes in the presence or absence of 150 nM DGT as indicated. The first two panels from the left were from WT cells and the right panel was from a NOX2KO cell. (B and C) Pooled data for the amplitude of Ca2+ transients and frequency of SCWs in the presence of different concentrations of DGT (n = 6–22). (D) Confocal images of ROS generation from WT and NOX2KO myocytes before and after application of DGT. (E) Normalized averaged traces of CM-H2DCFDA fluorescence (top) along with the corresponding pooled data (bottom) for relative ROS accumulation rates calculated from the slopes at 10–15 min. Traces were normalized to the maximal ROS signal obtained by application of 10 mM H2O2 at the end of experiments (n = 16–35). *P < 0.05 vs. data without DGT.
To examine the possibility that ablation of NOX2 indirectly altered responsiveness of NKA to DGT, we performed control experiments with measurements of intracellular Na+ accumulation in NOX2KO vs. WT myocytes using the Na+ indicator sodium green. Exposure to DGT (150 nM) led to similar increases in sodium green fluorescence in NOX2KO and WT myocytes (Supplementary material online, Figure S3) suggesting that NKA-dependent Na+ transport activity was unaltered in NOX2KO myocytes. Thus, the observed resistance of these cells to the proarrhythmic effects of DGT was specifically attributable to the deficiency of NOX2.
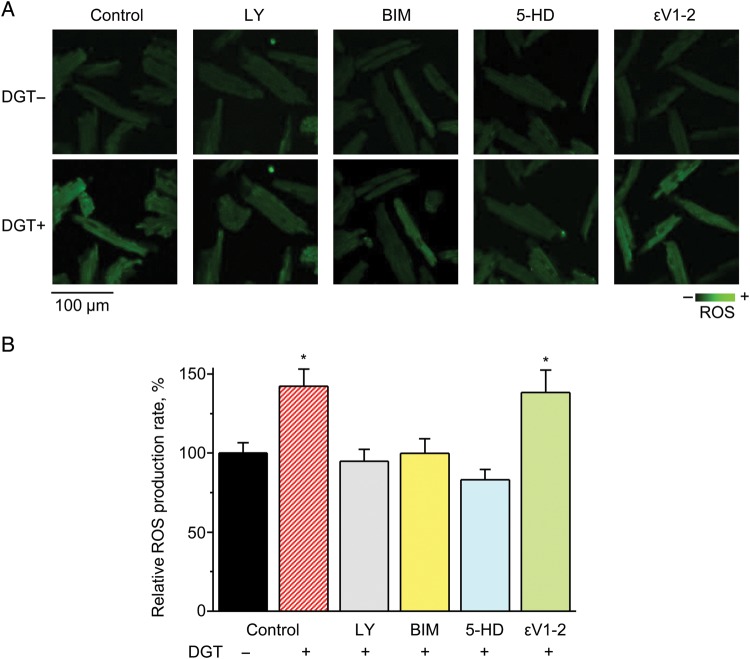
Since PI3K and PKC have been both implicated as components of CG signalling19 and shown to activate NOX2 in various cell systems,20–22 we tested their involvement in the NOX2-dependent ROS release induced by DGT. Pre-treatment with the PI3K or PKC inhibitors, LY294002 (LY) or Bisindolylmaleimide I (BIM), respectively, prevented DGT-induced ROS release in WT myocytes (Figure 2), thus suggesting a specific role for PI3K and PKC signalling in DGT-dependent NOX2 activation. Rather than acting upstream of NOX2, the epsilon isoform of PKC (PKCε) could mediate the effects of DGT downstream of NOX2, possibly via ROS-dependent activation of the mitochondrial KATP channel23 (see below). In order to examine this possibility, we used ɛV1–2, a specific inhibitor for PKCε, as opposed to the pan PKC inhibitor BIM. As shown in Figure 2, ɛV1-2 had no significant effect on DGT-dependent ROS confirming that BIM acted upstream of NOX2.
Figure 2.
PI3K, PKC, and mitoKATP are involved in DGT-induced ROS production. (A) Confocal images of ROS generation from WT myocytes before and after the application of DGT in control or in the presence of the following reagents, as indicated: 10 µM LY294002 (LY), an inhibitor of PI3K; 100 nM Bisindolylmaleimide I (BIM), a pan inhibitor of PKC; 200 µM 5-hydroxydecanoic acid (5-HD), an inhibitor of the mitoKATP channel; or 100 nM ɛV1-2, a selective blocker of PKCɛ. (B) Pooled data on the relative ROS accumulation rates (n = 10–13). *P < 0.05 vs. data without DGT.
3.3. SOD2 overexpression increases resistance to arrhythmic toxicity of DGT
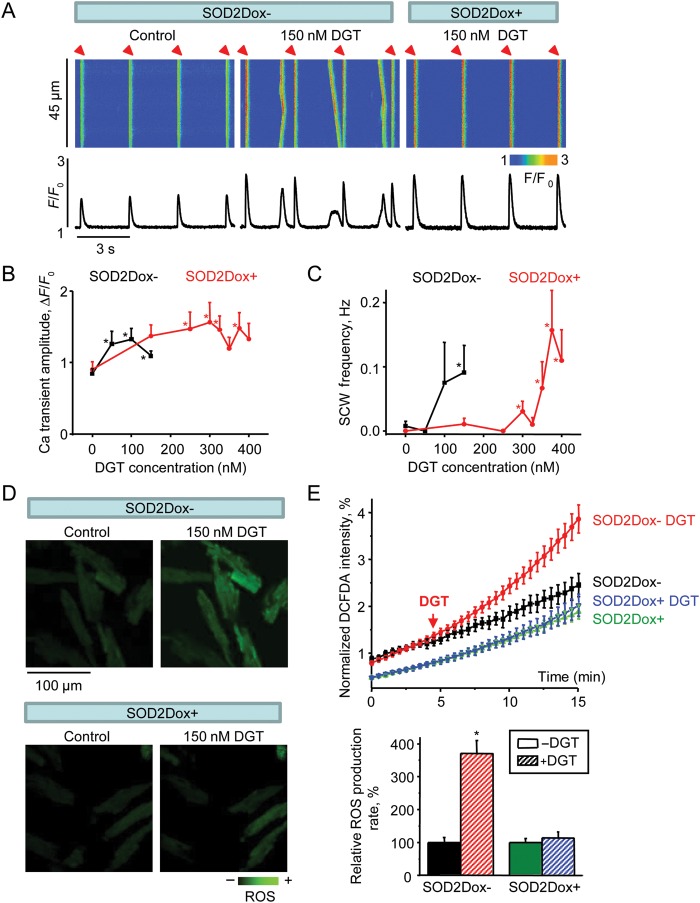
The results of our pharmacological analysis suggested that besides NOX2, mitochondria may play a key role in DGT-dependent alterations in ROS and Ca2+ handling. To verify the role of mitochondria in the observed effects of DGT, we used a doxycycline (Dox)-inducible mouse model of mitochondrial superoxide dismutase (SOD2) overexpression. Similar to WT myocytes, DGT increased the propensity for SCWs in myocytes derived from uninduced mice (SOD2Dox-) (Figure 3). However, induction of SOD2 overexpression (SOD2Dox+) resulted in a reduction of DGT-dependent SCWs. The dose–response of the arrhythmogenic effects of DGT shifted markedly to higher concentrations in SOD2Dox+ myocytes (Figure 3C). Notably, the increase in Ca2+ transient amplitude by DGT was preserved in these cells (Figure 3B). Similar to the results in WT myocytes, DGT reduced the SR Ca2+ content in SOD2Dox- myocytes. However, overexpression of SOD2 restored SR Ca2+ content to the control level (Supplementary material online, Figure S2). Consistent with the effects of SOD2 overexpression in DGT-dependent changes in Ca2+ handling, the rate of DGT-dependent ROS release was inhibited in SOD2Dox+ myocytes as compared to SOD2Dox- (Figure 3D and E). Thus, overexpression of SOD2 decreased proarrhythmic toxicity of DGT by reducing mitochondrial ROS release.
Figure 3.
SOD2 overexpression increases resistance to arrhythmogenic DGT toxicity. (A) Representative line-scan images (top) and time-dependent profiles (bottom) of intracellular Ca2+ handling in myocytes in the presence or absence of 150 nM DGT as indicated. The first two panels from the left were from SOD2Dox- cells and the right panel was from a SOD2Dox+ cell. (B and C) Pooled data for the amplitude of Ca2+ transients and frequency of SCWs in the presence of different concentrations of DGT (n = 6–34). (D) Confocal images of ROS generation from uninduced (SOD2Dox-) and SOD2 overexpression-induced (SOD2Dox+) myocytes before and after the application of DGT. (E) Normalized averaged traces of ROS production (top) along with the corresponding pooled data (bottom) for relative ROS accumulation rates calculated from the slopes at 10–15 min. Traces were normalized to the maximal ROS signal obtained by the application of 10 mM H2O2 at the end of experiments (n = 7–18). *P < 0.05 vs. data without DGT.
3.4. NOX2 is essential for DGT-dependent mitochondrial membrane depolarization
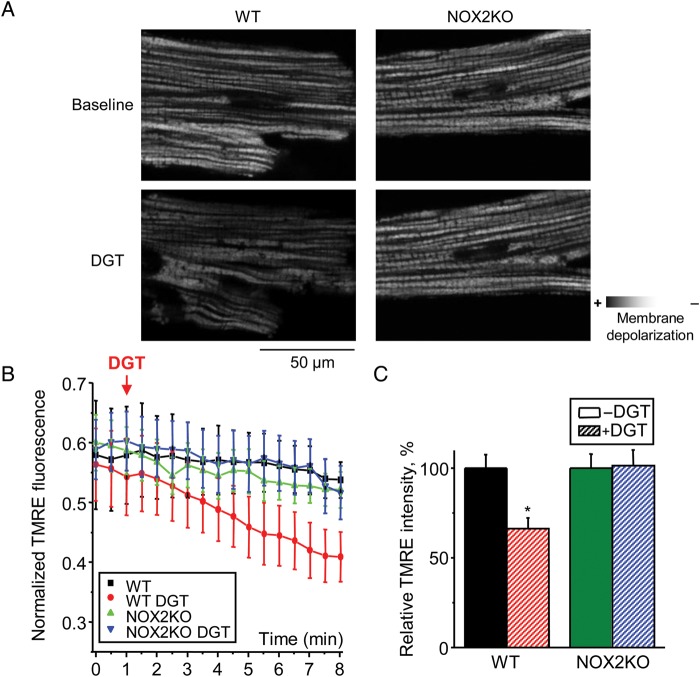
The reliance of the proarrhythmic effects of DGT on both NOX2 and mitochondria suggests that DGT action involves interplay between these ROS generating systems. ROS release by mitochondria is associated with depolarization of the mitochondrial membrane potential (MMP). Therefore, to further elucidate the link between NOX2 and mitochondria, we performed mitochondrial membrane potential measurements by using tetramethylrhodamine ethyl ester (TMRE) in WT and NOX2KO myocytes. Application of DGT caused MMP depolarization in WT myocytes, but it failed to appreciably change MPP in NOX2KO cells (Figure 4). These results confirm that DGT-dependent ROS release by mitochondria requires the involvement of NOX2.
Figure 4.
DGT causes mitochondrial membrane depolarization in WT but not in NOX2KO myocytes. (A) Representative images of mitochondrial membrane potential measured with TMRE in WT and NOX2KO myocytes under control condition and in the presence of 150 nM DGT as indicated. (B) Normalized traces of TMRE fluorescence. Data were normalized to the minimum TMRE fluorescence signal in the presence of 3 µM FCCP. (F (x,y,t) - Fmin)/Fmin, where F(x,y,t) is the average fluorescence at the X–Y scan at time t, Fmin is the minimal fluorescence signal during FCCP application. (C) Bar graphs of mitochondrial membrane potential 7 min after DGT application (n = 3–12). *P < 0.05 vs. without DGT.
It is known that ROS facilitates the mitochondrial KATP channel (mitoKATP) opening,23,24 and that, in turn opening of mitoKATP stimulates mitochondrial ROS generation and depolarizes MMP.25 Thus, mitoKATP is well suited to mediate the action of NOX2-derived ROS on mitochondrial ROS release upon exposure to CGs. Consistent with a role of mitoKATP in DGT-induced ROS release, inhibition of mitoKATP with 5-hydroxydecanoic acid (5-HD) prevented DGT-dependent elevation of ROS in WT myocytes (Figure 2).
3.5. DGT causes NOX2-dependent oxidation of CaMKII and increases RyR2 phosphorylation at Ser 2814
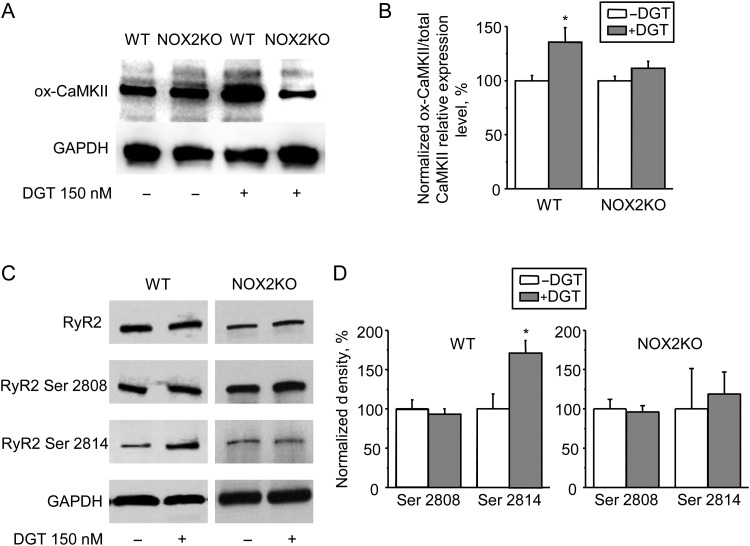
CaMKII has emerged as an important mediator of ROS-dependent signalling in cardiac myocytes.26 We hypothesized that CaMKII mediates the arrhythmogenic effects of DGT by phosphorylation of downstream targets including RyR2 and phospholamban (PLB). To test the hypothesis that CaMKII is involved in ROS-dependent effects of DGT on Ca2+ handling, we first assessed oxidation of CaMKII at methionine 281/282 (ox-CaMKII) using an ox-CaMKII assay. DGT treatment significantly increased ox-CaMKII levels in WT but not in NOX2KO hearts (Figure 5A and B). Of note, the levels of autophosphorylation at Thr 287 were not significantly different between the groups (Supplementary material online, Figure S4). Thus, DGT causes CaMKII oxidation via activation of NOX2.
Figure 5.
CaMKII activation by NOX2 increases Ser 2814 phosphorylation on RyR2 in WT myocytes. (A and B) Representative western blots and summary data of CaMKII oxidation at methionine 281/282 (ox-CaMKII) and GAPDH from ventricular tissue prepared from WT and NOX2KO mice with or without 150 nM DGT (n = 6–7). (C and D) Gel results and normalized pooled data representing phosphorylation of RyR2 s at Ser 2808 (PKA-dependent) and Ser 2814 (CaMKII-dependent) sites with total RyR2 and GAPDH in control and DGT-treated samples (n = 3). Data were normalized to control (-DGT) on the same gel. *P < 0.05 vs. data without DGT.
Next we examined whether DGT results in increased RyR2 phosphorylation by CaMKII. As shown in Figure 5C and D, DGT increased phosphorylation at Ser 2814, the primary CaMKII phosphorylation site on RyR2. Of note, there was no change in phosphorylation at Ser 2808, a site mainly phosphorylated by PKA. Similarly, DGT increased phosphorylation of PLB at Thr 17, also a target for CaMKII phosphorylation in cardiac myocytes (Supplementary material online, Figure S5). In contrast, RyR2 (Ser 2808 and Ser 2814) and PLB (Ser 16 and Thr 17) phosphorylation levels did not differ between control and DGT-treated NOX2KO samples, indicating that the effects of CaMKII on RyR2 and PLB require NOX2.
3.6. Preventing RyR2 phosphorylation at Ser 2814 abolishes DGT-dependent alterations in Ca2+ handling
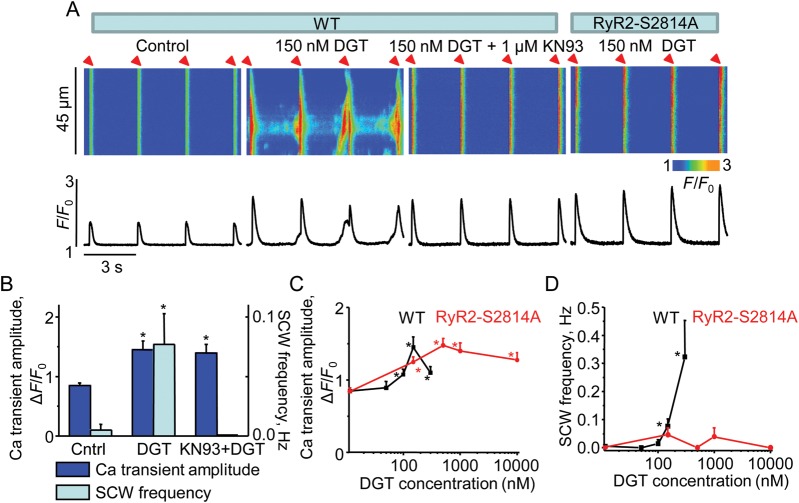
To further examine the effects of DGT-mediated CaMKII activation on Ca2+ cycling, we measured Ca2+ transients and spontaneous waves in myocytes treated with the CaMKII blocker KN93. CaMKII inhibition by KN93 reduced SCWs without changing the Ca2+ transient amplitude (Figure 6A and B). Additionally, to test directly the role of RyR2 phosphorylation by CaMKII in the proarrhythmic effects of DGT, we used RyR2 knock-in mice in which CaMKII phosphorylation of RyR2 is inhibited by replacing serine at 2814 with alanine (RyR2-S2814A). As demonstrated in Figure 6A and D, ablation of the CaMKII phosphorylation site on RyR2 significantly decreased the susceptibility of DGT-treated myocytes to proarrhythmogenic Ca2+ waves. Similar to the results obtained in SOD2Dox+ and NOX2KO myocytes, DGT did not significantly alter the SR Ca2+ content in RyR2-S2814A myocytes (Supplementary material online, Figure S2). These results confirm that the arrhythmogenic effects of DGT involve phosphorylation of RyR2 by CaMKII at Ser 2814.
Figure 6.
Decrease in propensity towards DGT-triggered Ca2+ waves in KN93-treated and RyR2-S2814A myocytes. (A) Representative line-scan images (top) and time-dependent profiles (bottom) of intracellular Ca2+ handling in myocytes in the presence or absence of 150 nM DGT as indicated. The first three panels from the left were from WT cells and the right panel was from a RyR2-S2814A cell. (B) Pooled data for the amplitude of Ca2+ transients and frequency of SCWs from WT myocytes with or without 1 µM KN93. (C and D) Bar graphs for the amplitude of Ca2+ transients and frequency of SCWs in the presence of different concentrations of DGT (n = 6–27). *P < 0.05 vs. data without DGT.
Apart from increasing RyR2 phosphorylation via activation of CaMKII, CG-dependent ROS could modify RyR2 directly via oxidation. Therefore, we measured the effects of DGT on the levels of RyR2 free thiols in WT and RyR2-S2814A hearts. DGT reduced free thiols to a similar level in both WT and RyR2-S2814A RyR2s (Supplementary material online, Figure S6). These results suggest that (i) RyR2 redox modification and phosphorylation occur in parallel in response to CGs; and (ii) enhanced RyR2 redox modification alone is not sufficient to increase myocyte arrhythmogenic propensity.
4. Discussion
In this study, we used a combination of pharmacological tools and genetic mouse models to investigate the mechanisms of generation of ROS as well as of subsequent actions of ROS on Ca2+ handling that are involved in the proarrhythmic adverse effects of CGs. Our major findings are: (i) PI3K- and PKC-dependent activation of NOX2 mediates the effects of CGs, by supplying a critical source of ROS to induce further release of ROS from the mitochondria; (ii) CG-induced ROS activate CaMKII resulting in increased phosphorylation of RyR2 at Ser 2814 and proarrhythmic Ca2+ waves.
4.1. The arrhythmogenic effects of DGT involve NOX2-dependent ROS release from mitochondria
CG-induced arrhythmias are commonly attributed to excessive accumulation of intracellular Ca2+, i.e. Ca2+ overload.27 Recently, we showed that aside from this classical mechanism, the proarrhythmic alterations in Ca2+ handling in the presence of CGs may result from the elevation of ROS.12 However, the specific sources of ROS, including the roles of NOX and mitochondria, as well as the molecular steps leading to their mobilization remain uncertain. The results of the present study indicate that both NOX2 and mitochondria are critical to CG-dependent ROS and arrhythmogenesis. In particular, myocytes isolated from mice deficient in NOX2 and mice overexpressing SOD2 displayed markedly increased tolerance to the proarrhythmic action of DGT as manifested by inhibition of DGT-dependent ROS and diastolic Ca2+ waves (Figures 1 and 3). These results complemented with those on the effects of pharmacological inhibitors of NOX and MPTP (Supplementary material online, Figure S1) provide unequivocal evidence for the role of these ROS generating systems in CG proarrhythmic toxicity.
Our data also gained mechanistic insights into the interaction between NOX2 and mitochondria underlying the proarrhythmic effects of DGT. Ablation of NOX2 prevented the mitochondrial membrane depolarization caused by DGT thus placing NOX2 upstream of mitochondria as a critical mediator of mitochondrial response to DGT. Previous studies suggested the mitoKATP might be involved in the facilitation of mitochondrial ROS release in various settings.25,28 Indeed, activation of mitoKATP is reportedly ROS-dependent.23,24 Moreover, ROS-dependent opening of mitoKATP can lead to the production of more ROS through depolarization of the mitochondrial membrane and uncoupling of the electron transport chain.25 In our study, DGT-dependent ROS accumulation was sensitive to inhibition of mitoKATP (Figure 2), thus supporting the role of this channel in NOX2-mediated mitochondrial ROS production. The mitochondrial ROS response is likely to be further amplified by ROS-induced ROS release from a larger mitochondrial pool via MPTP.29,30 This could account for the relatively large magnitude of the ‘global’ ROS response when compared with the apparently ‘local’ ROS attributable to NOX2 alone that was, virtually undetectable with our cytosolic ROS indicator in myocytes overexpressing SOD2 or exposed to the mitoKATP inhibitor, 5-HD (Figures 1, 2, and 3). Previously, NOX-generated ROS has been shown to stimulate mitochondrial ROS production in endothelial and vascular smooth muscle cells.31,32 Our study shows that NOX2-derived ROS are able to induce the release of ROS from cardiac myocyte mitochondria to mediate the arrhythmogenic effects of CGs.
As for the upstream signalling steps leading to NOX2 activation, our study demonstrated that ROS generation is prevented by inhibition of PI3K and PKC (but not specific inhibition of PKCε) (Figure 2). Binding of CGs to NKA has been shown to activate Src kinase12,33 with a subsequent stimulation of the PI3K and PKC pathways.19 PI3K along with PKC, on the other hand, have been linked to NOX2 activation in various systems.20–22 Based on the evidence presented here, PI3K and PKC mediate the NOX2-dependent oxidant production required for the arrhythmogenic action of DGT.
4.2. Oxidized CaMKII phosphorylation of RyR2 as the downstream mechanism for CG-induced ROS
CaMKII has emerged as an important integrating molecule in cardiac myocyte Ca2+ and ROS signalling involved in various pathologies including vascular disease, heart failure, and arrhythmias.26 Here we show that ox-CaMKII is a key mediator of the CGs ROS-induced arrhythmogenic effects on myocyte Ca2+ handling. In particular, DGT treatment increases ox-CaMKII levels in WT (but not in NOX2KO) myocytes (Figure 5). These increases in CaMKII oxidation were paralleled by increased phosphorylation of RyR2 at the CaMKII site Ser 2814. Importantly, the arrhythmogenic effects of DGT were markedly decreased in myocytes from mice lacking this site (RyR2-S2814A mice), thus providing direct evidence for the role of RyR2 CaMKII phosphorylation in the arrhythmogenic effects of DGT (Figure 6). Interestingly, RyR2-S2814A myocytes along with NOX2KO myocytes displayed a higher tolerance to the proarrhythmic action of DGT than myocytes overexpressing SOD2 (Figures 1, 3, and 6). This difference could be ascribed to the finding that in our experimental settings, both NOX2 and RyR2 phosphorylation are required for the effects of DGT, as an essential upstream source of ROS and a critical downstream (via CaMKII) target, respectively (Figure 7). SOD2 overexpression, on the other hand, only attenuates ROS produced by the mitochondria apparently without completely suppressing ROS signalling that leads to RyR2 phosphorylation at Ser 2814.
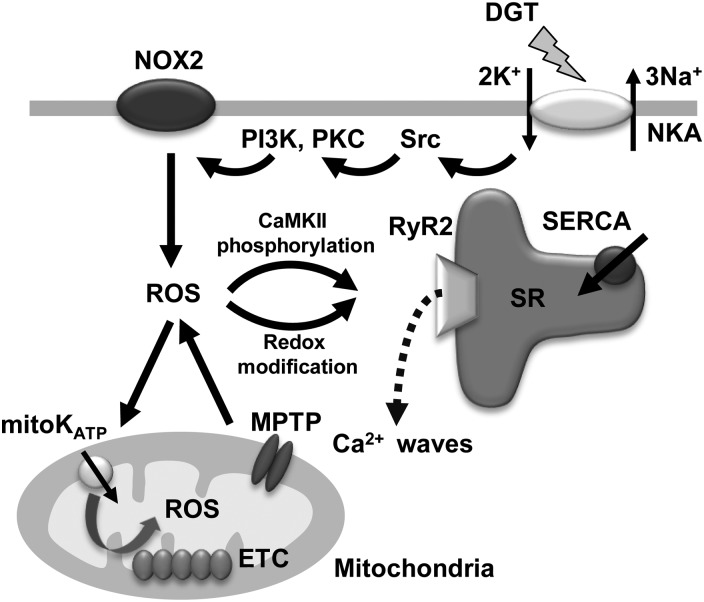
Figure 7.
The mechanism of ROS-dependent arrhythmogenic effect of DGT. Binding of digitoxin (DGT) to Na+/K+ ATPase (NKA) initiates a signalling cascade that involves Src, PI3K, and PKC and NOX2 to produce ROS; ROS supplied by NOX2 induce the release of more ROS from mitochondria via activation of the mitochondrial KATP channel (mitoKATP) and opening of the permeability transition pore (MPTP). DGT-induced ROS activate CaMKII in turn increasing phosphorylation of RyR2 at Ser 2814 and cause redox modifications of RyR2 thiols. NOX2, NADPH oxidase type 2; PI3K, phosphatidylinositol 3 kinase; PKC, protein kinase C; ROS, reactive oxygen species; ETC, electron transport chain; SERCA, sarcoplasmic reticulum calcium transport ATPase; SR, sarcoplasmic reticulum; RyR2, ryanodine receptor 2.
The role of RyR2 CaMKII phosphorylation in DGT-induced arrhythmogenesis is consistent with previous reports showing the importance of this modification in different experimental and pathological settings including cardiac hypertrophy, non-ischaemic HF, and post-infarction sudden cardiac death.34 As recently shown, CaMKII phosphorylation accelerates the recovery of Ca2+ release from refractoriness, thus increasing the possibility of proarrhythmic spontaneous release.35 It is interesting to note that DGT can also lead to oxidation of RyR212 (Supplementary material online, Figure S6). Redox modification increases RyR2 activity that in itself can also be proarrhythmic.36,37 Although it does not refute the potential role of RyR2 oxidation, our study demonstrates that CaMKII phosphorylation of RyR2 at Ser 2814 is essential for dysregulated Ca2+ handling caused by DGT.
Previously, Gonano et al.38 suggested that ouabain causes arrhythmias via Ca2+-dependent activation of CaMKII with subsequent phosphorylation of RyR2 leading to increased propensity for spontaneous Ca2+ release. While reaffirming the role of CaMKII in CG toxicity, our study suggests that CaMKII activation was due, at least in part, to increased ROS and that RyR2 is a critical principal target for the pathological actions of CaMKII in the presence of DGT. Of note, consistent with the findings of Gonano et al.,38 we did observe increased phosphorylation of PLB at Thr 17 in response to DGT; however, the rate of decay of the Ca2+ transient was unchanged suggesting that stimulation of SERCA plays a limited role in the arrhythmogenic effects of DGT under our experimental conditions.
While demonstrating that the CGs can lead to ROS/CaMKII-dependent phosphorylation of RyR2 to increase propensity for Ca2+ waves, our results do not necessarily rule out a role of the classical mechanisms in arrhythmogenesis involving Na+/Ca2+ exchanger and cellular accumulation of Na+ and Ca2+.1 Nevertheless, our findings emphasize the importance of reevaluating the mechanisms of CG-induced arrhythmias commonly attributed to ionic mechanisms.
4.3. Clinical significance
Although the clinical use of CGs has declined in recent years, digoxin is still in the top 100 most prescribed medications and is widely employed accounting for 20 million drug prescriptions annually in the USA.39,40 Digoxin is a treatment of choice for patients with HF who are resistant to standard therapy and for controlling ventricular rate in atrial fibrillation.41 Thus, CG toxicity, including life-threatening arrhythmias, remains a serious health care problem. Despite their adverse effects and increasing availability of alternative therapies, CGs continue to hold promise and substantial efforts are on the way to optimize the clinical use of these time-tested drugs.1,42 For example, combination of digoxin and beta-blockers proved to be highly efficient in the treatment of patients suffering from diastolic HF and atrial fibrillation.43 An important finding of the present study is that inhibition of ROS production by NOX2 or mitochondria prevents the arrhythmogenic effects while preserving potentiation of the amplitude of the Ca2+ transient by DGT. These results suggest that combining DGT therapy with administration of antioxidants or inhibitors of NOX, PI3K, PKC, or CaMKII pathway could offer a rational strategy for the optimization of HF treatment with CGs.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Funding
This work was supported by National Institutes of Health grants HL074045 and HL063043 (to S.G.) and HL114940 (to B.J.B.). H.T.H was supported by a Predoctoral Fellowship from Great Rivers Affiliate, American Heart Association.
Supplementary Material
Acknowledgments
Conflict of interest: none declared.
References
- 1.Wasserstrom JA, Aistrup GL. Digitalis: new actions for an old drug. Am J Physiol Heart Circ Physiol. 2005;289:H1781–H1793. doi: 10.1152/ajpheart.00707.2004. [DOI] [PubMed] [Google Scholar]
- 2.Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med. 1997;336:525–533. doi: 10.1056/NEJM199702203360801. [DOI] [PubMed] [Google Scholar]
- 3.Ferrier GR. Digitalis arrhythmias: role of oscillatory afterpotentials. Prog Cardiovasc Dis. 1977;19:459–474. doi: 10.1016/0033-0620(77)90010-x. [DOI] [PubMed] [Google Scholar]
- 4.Currie GM, Wheat JM, Kiat H. Pharmacokinetic considerations for digoxin in older people. Open Cardiovasc Med J. 2011;5:130–135. doi: 10.2174/1874192401105010130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roever C, Ferrante J, Gonzalez EC, Pal N, Roetzheim RG. Comparing the toxicity of digoxin and digitoxin in a geriatric population: should an old drug be rediscovered? South Med J. 2000;93:199–202. [PubMed] [Google Scholar]
- 6.Reuter H, Henderson SA, Han T, Ross RS, Goldhaber JI, Philipson KD. The Na+-Ca2+ exchanger is essential for the action of cardiac glycosides. Circ Res. 2002;90:305–308. doi: 10.1161/hh0302.104562. [DOI] [PubMed] [Google Scholar]
- 7.Altamirano J, Li Y, DeSantiago J, Piacentino V, Houser SR, Bers DM. The inotropic effect of cardioactive glycosides in ventricular myocytes requires Na+–Ca2+ exchanger function. J Physiol. 2006;575:845–854. doi: 10.1113/jphysiol.2006.111252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wier WG, Hess P. Excitation-contraction coupling in cardiac Purkinje fibers. Effects of cardiotonic steroids on the intracellular [Ca2+] transient, membrane potential, and contraction. J Gen Physiol. 1984;83:395–415. doi: 10.1085/jgp.83.3.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eisner DA, Kashimura T, Venetucci LA, Trafford AW. From the ryanodine receptor to cardiac arrhythmias. Circ J. 2009;73:1561–1567. doi: 10.1253/circj.cj-09-0478. [DOI] [PubMed] [Google Scholar]
- 10.Pasdois P, Quinlan CL, Rissa A, Tariosse L, Vinassa B, Costa ADT, et al. Ouabain protects rat hearts against ischemia-reperfusion injury via pathway involving src kinase, mitoKATP, and ROS. Am J Physiol Heart Circ Physiol. 2007;292:H1470–H1478. doi: 10.1152/ajpheart.00877.2006. [DOI] [PubMed] [Google Scholar]
- 11.Liu T, Brown DA, O'Rourke B. Role of mitochondrial dysfunction in cardiac glycoside toxicity. J Mol Cell Cardiol. 2010;49:728–736. doi: 10.1016/j.yjmcc.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ho H-T, Stevens SCW, Terentyeva R, Carnes CA, Terentyev D, Györke S. Arrhythmogenic adverse effects of cardiac glycosides are mediated by redox modification of ryanodine receptors. J Physiol. 2011;589:4697–4708. doi: 10.1113/jphysiol.2011.210005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pollock JD, Williams DA, Gifford MAC, Li LL, Du X, Fisherman J, et al. Mouse model of X–linked chronic granulomatous disease, an inherited defect in phagocyte superoxide production. Nat Genet. 1995;9:202–209. doi: 10.1038/ng0295-202. [DOI] [PubMed] [Google Scholar]
- 14.Usui S, Komeima K, Lee SY, Jo Y-J, Ueno S, Rogers BS, et al. Increased expression of catalase and superoxide dismutase 2 reduces cone cell death in retinitis pigmentosa. Mol Ther. 2009;17:778–786. doi: 10.1038/mt.2009.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chelu MG, Sarma S, Sood S, Wang S, van Oort RJ, Skapura DG, et al. Calmodulin kinase II-mediated sarcoplasmic reticulum Ca2+ leak promotes atrial fibrillation in mice. J Clin Invest. 2009;119:1940–1951. doi: 10.1172/JCI37059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Y, Trush MA. Diphenyleneiodonium, an NAD(P)H oxidase inhibitor, also potently inhibits mitochondrial reactive oxygen species production. Biochem Biophys Res Commun. 1998;253:295–299. doi: 10.1006/bbrc.1998.9729. [DOI] [PubMed] [Google Scholar]
- 17.Mondin L, Balghi H, Constantin B, Cognard C, Sebille S. Negative modulation of inositol 1,4,5-trisphosphate type 1 receptor expression prevents dystrophin-deficient muscle cells death. Am J Physiol Cell Physiol. 2009;297:C1133–C1145. doi: 10.1152/ajpcell.00048.2009. [DOI] [PubMed] [Google Scholar]
- 18.Zhang G-X, Lu X-M, Kimura S, Nishiyama A. Role of mitochondria in angiotensin II-induced reactive oxygen species and mitogen-activated protein kinase activation. Cardiovasc Res. 2007;76:204–212. doi: 10.1016/j.cardiores.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 19.Liu L, Zhao X, Pierre SV, Askari A. Association of PI3K-Akt signaling pathway with digitalis-induced hypertrophy of cardiac myocytes. Am J Physiol Cell Physiol. 2007;293:C1489–C1497. doi: 10.1152/ajpcell.00158.2007. [DOI] [PubMed] [Google Scholar]
- 20.Yamamori T, Inanami O, Nagahata H, Kuwabara M. Phosphoinositide 3-kinase regulates the phosphorylation of NADPH oxidase component p47(phox) by controlling cPKC/PKCdelta but not Akt. Biochem Biophys Res Commun. 2004;316:720–730. doi: 10.1016/j.bbrc.2004.02.108. [DOI] [PubMed] [Google Scholar]
- 21.Frey RS, Gao X, Javaid K, Siddiqui SS, Rahman A, Malik AB. Phosphatidylinositol 3-kinase gamma signaling through protein kinase czeta induces NADPH oxidase-mediated oxidant generation and NF-kappaB activation in endothelial cells. J Biol Chem. 2006;281:16128–16138. doi: 10.1074/jbc.M508810200. [DOI] [PubMed] [Google Scholar]
- 22.Chatterjee S, Browning EA, Hong N, DeBolt K, Sorokina EM, Liu W, et al. Membrane depolarization is the trigger for PI3 K/Akt activation and leads to the generation of ROS. Am J Physiol Heart Circ Physiol. 2012;302:H105–H114. doi: 10.1152/ajpheart.00298.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohnuma Y, Miura T, Miki T, Tanno M, Kuno A, Tsuchida A, et al. Opening of mitochondrial KATP channel occurs downstream of PKC-ɛ activation in the mechanism of preconditioning. Am J Physiol Heart Circ Physiol. 2002;283:H440–H447. doi: 10.1152/ajpheart.00434.2001. [DOI] [PubMed] [Google Scholar]
- 24.Zhang DX, Chen Y-F, Campbell WB, Zou A-P, Gross GJ, Li P-L. Characteristics and superoxide-induced activation of reconstituted myocardial mitochondrial ATP-sensitive potassium channels. Circ Res. 2001;89:1177–1183. doi: 10.1161/hh2401.101752. [DOI] [PubMed] [Google Scholar]
- 25.Daiber A. Redox signaling (cross-talk) from and to mitochondria involves mitochondrial pores and reactive oxygen species. Biochim Biophys Acta BBA Bioenerg. 2010;1797:897–906. doi: 10.1016/j.bbabio.2010.01.032. [DOI] [PubMed] [Google Scholar]
- 26.Erickson JR, He BJ, Grumbach IM, Anderson ME. CaMKII in the cardiovascular system: sensing redox states. Physiol Rev. 2011;91:889–915. doi: 10.1152/physrev.00018.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bers DM. Excitation-Contraction Coupling and Cardiac Contractile Force. Springer; 2001. [Google Scholar]
- 28.Matejíková J, Kucharská J, Pintérová M, Pancza D, Ravingerová T. Protection against ischemia-induced ventricular arrhythmias and myocardial dysfunction conferred by preconditioning in the rat heart: involvement of mitochondrial K(ATP) channels and reactive oxygen species. Physiol Res Acad Sci Bohemoslov. 2009;58:9–19. doi: 10.33549/physiolres.931317. [DOI] [PubMed] [Google Scholar]
- 29.Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial ROS-induced ROS release: An update and review. Biochim Biophys Acta BBA Bioenerg. 2006;1757:509–517. doi: 10.1016/j.bbabio.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 30.Costa ADT, Garlid KD. Intramitochondrial signaling: interactions among mitoKATP, PKCɛ, ROS, and MPT. Am J Physiol Heart Circ Physiol. 2008;295:H874–H882. doi: 10.1152/ajpheart.01189.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kimura S, Zhang G-X, Nishiyama A, Shokoji T, Yao L, Fan Y-Y, et al. Mitochondria-derived reactive oxygen species and vascular MAP kinases: comparison of angiotensin II and diazoxide. Hypertension. 2005;45:438–444. doi: 10.1161/01.HYP.0000157169.27818.ae. [DOI] [PubMed] [Google Scholar]
- 32.Doughan AK, Harrison DG, Dikalov SI. Molecular mechanisms of angiotensin II-mediated mitochondrial dysfunction: linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ Res. 2008;102:488–496. doi: 10.1161/CIRCRESAHA.107.162800. [DOI] [PubMed] [Google Scholar]
- 33.Haas M, Askari A, Xie Z. Involvement of Src and epidermal growth factor receptor in the signal-transducing function of Na+/K+-ATPase. J Biol Chem. 2000;275:27832–27837. doi: 10.1074/jbc.M002951200. [DOI] [PubMed] [Google Scholar]
- 34.Zhang T, Maier LS, Dalton ND, Miyamoto S, Ross J, Bers DM, et al. The δC isoform of CaMKII is activated in cardiac hypertrophy and induces dilated cardiomyopathy and heart failure. Circ Res. 2003;92:912–919. doi: 10.1161/01.RES.0000069686.31472.C5. [DOI] [PubMed] [Google Scholar]
- 35.Belevych AE, Terentyev D, Terentyeva R, Ho H-T, Gyorke I, Bonilla IM, et al. Shortened Ca2+ signaling refractoriness underlies cellular arrhythmogenesis in a postinfarction model of sudden cardiac death. Circ Res. 2012;110:569–577. doi: 10.1161/CIRCRESAHA.111.260455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zima AV, Blatter LA. Redox regulation of cardiac calcium channels and transporters. Cardiovasc Res. 2006;71:310–321. doi: 10.1016/j.cardiores.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 37.Belevych AE, Terentyev D, Viatchenko-Karpinski S, Terentyeva R, Sridhar A, Nishijima Y, et al. Redox modification of ryanodine receptors underlies calcium alternans in a canine model of sudden cardiac death. Cardiovasc Res. 2009;84:387–395. doi: 10.1093/cvr/cvp246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gonano LA, Sepúlveda M, Rico Y, Kaetzel M, Valverde CA, Dedman J, et al. Calcium-Calmodulin kinase II mediates digitalis-induced arrhythmias. Circ Arrhythm Electrophysiol. 2011;4:947–957. doi: 10.1161/CIRCEP.111.964908. [DOI] [PubMed] [Google Scholar]
- 39.Bartholow M. Top 200 prescription drugs of 2009. Pharm Times. 2010;76:34–36. [Google Scholar]
- 40.Mittal MK, Chockalingam P, Chockalingam A. Contemporary indications and therapeutic implications for digoxin use. Am J Ther. 2011;18:280–287. doi: 10.1097/MJT.0b013e3181c6c0d2. [DOI] [PubMed] [Google Scholar]
- 41.Gheorghiade M, Adams KF, Colucci WS. Digoxin in the management of cardiovascular disorders. Circulation. 2004;109:2959–2964. doi: 10.1161/01.CIR.0000132482.95686.87. [DOI] [PubMed] [Google Scholar]
- 42.Aditya S, Rattan A. Istaroxime: a rising star in acute heart failure. J Pharmacol Pharmacother. 2012;3:353–355. doi: 10.4103/0976-500X.103705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khand AU, Rankin AC, Martin W, Taylor J, Gemmell I, Cleland JGF. Carvedilol alone or in combination with digoxin for the management of atrial fibrillation in patients with heart failure? J Am Coll Cardiol. 2003;42:1944–1951. doi: 10.1016/j.jacc.2003.07.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.