Abstract
Objectives: To assess the constraints for Indian herbal drug industry with respect to manufacturing and commercialization of herbal medicines.
Methods: A questionnaire-based survey was conducted to obtain primary data on challenges faced during production, commercialization, and marketing approval for traditional or herbal drugs in India and abroad. Responses were collected from 150 companies by email, telephone, and in-person interviews from June 2009 to August 2010 and were analyzed to draw appropriate conclusions.
Results: The survey result showed that differing regulatory requirements and the limited market in foreign countries are the major hindrances for exporting. Standardization and quality control of raw materials and herbal formulations emerged as the major challenge for Indian herbal drug manufacturing firms. Insufficient regulatory guidelines, particularly guidelines for good manufacturing practices; nonimplementation of good agricultural and collection practices; and weak implementation of the Drugs and Cosmetics Act of 1940 are considered major drawbacks for the Indian herbal industry.
Conclusions: Proper implementation of the Drugs and Cosmetics Act of 1940, development of more elaborate guidelines on quality control aspects, and development of marker-based standards are needed to produce safe and effective herbal medicines in India. Because evidence-based studies are becoming increasingly essential for establishing the safety and efficacy of herbal products in the domestic and export market, more focus should be placed on scientific and technological advancement in the field of herbal medicine. Regulatory harmonization becomes essential to mitigate the delays in commercialization across countries.
Introduction
Worldwide there is a growing demand for Ayurveda and other traditional forms of medicine.1 In India, about 80% of the rural population uses medicinal herbs or indigenous systems of medicine.2 It is estimated that nearly 960 plant species are used by the Indian herbal industry, and the turnover of the industry is more than Rs 80 billion. Herbal exports include medicines of AYUSH (Ayurveda, Unani, Siddha, and homoeopathy) products, which occupy a share of 3% of total Indian pharmaceutical export. Seventy percent of export from the herbal sector consists largely of raw materials and is estimated to be Rs. 10 billion per annum. Thirty percent of the export consists of finished products, including herbal extracts.3 However, India's share in the global herbal export market is less than 1%.4 Although the AYUSH industry represents one of the oldest traditional forms of medicine in India, it has not been able to exploit the opportunities of the emerging market.5,6 To this end, the present study assessed the constraints that the Indian herbal drug industry is facing with respect to production, commercialization, and regulation for traditional or herbal drugs.
Methods
To understand the constraints in production and commercialization of herbal medicines, a 24-point questionnaire-based survey was conducted for Indian herbal drug companies involved in one or more activities of raw material collection/trading, extract preparation, drug manufacturing, or contract manufacturing only. The questionnaire had three modules. The first module elicited details on the representatives and information on general aspects of the company. The second module asked about problems encountered at each stage, from raw material collection to marketing of the products and quality control measures. The third module addressed exporting and importing and specific aspects of regulatory approvals, such as submission requirements, review of timelines, adequacy of guidelines, and support needed from government. Companies were also encouraged to offer their opinion about India's good manufacturing practice (GMP) regulation and regulatory guidelines for herbal drugs in the form of notes.
Two hundred companies were contacted for the study. Responses were collected from 150 companies that are involved in one or many aspects of herbal drug development by email, telephone, and personal interviews from June 2009 to August 2010. Responses were mainly collected from the directors/proprietors/ research and development heads of the companies as applicable.
Results and Discussion
Constraints for commercialization of herbal medicinal products
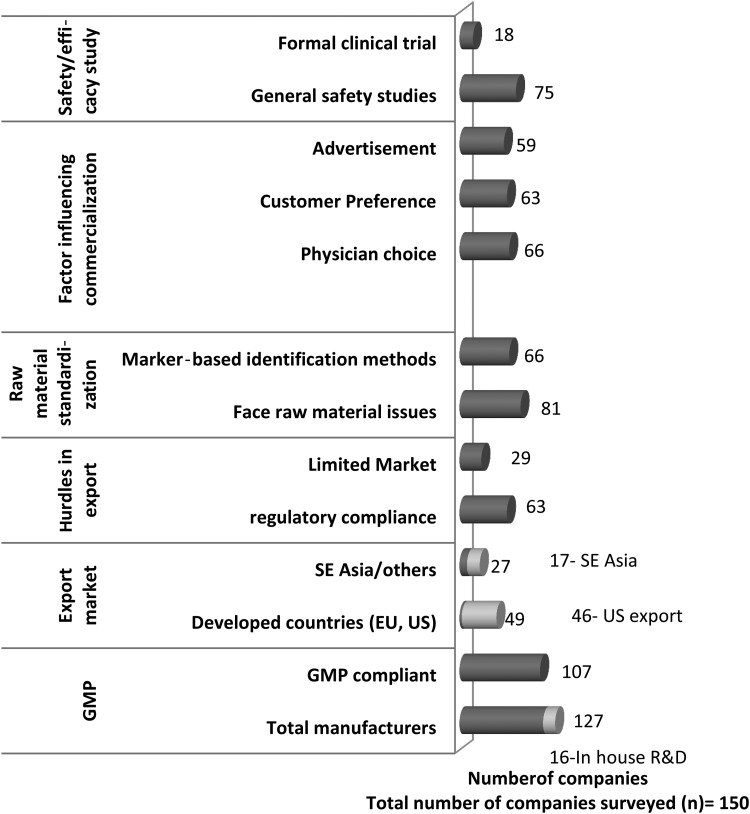
Among the survey respondents, 74 companies were found to export formulations to various countries. Of these, 46 companies export to the United States, 26 companies export to European countries, and 17 companies export to Southeast Asian countries (Fig. 1). Some companies also export to Australia and Middle Eastern and African countries. The survey revealed that achieving regulatory compliance is one of the major hurdles for exporters. Differences in the country-specific GMP standards and drug registration requirements are considered the major impediments for Indian manufacturers (Fig. 1).
FIG. 1.
Commercialization aspects of herbal drugs in India. (Field survey from June 2009 to August 2010. GMP, good manufacturing practice; R&D, research and development.)
Differing regulatory requirements
Differing regulatory requirements and subsequent delays in application submission and review process emerged as a major concern for the herbal medicine manufacturers from the survey results. Therefore, a comparative analysis of drug registration requirement was performed for countries such as India, the United States, and European nations to understand the differences in approval procedures and submission requirements. In India, the traditional herbal medicines, such as Ayurveda, Siddha, and Unani (ASU), are considered safe because of their long history of use. As such, no safety and efficacy studies are required for marketing approval, as per the Drugs and Cosmetics Act of 1940 (DCA).
The State Food and Drug Administration (SFDA) regulates manufacturing and marketing approvals. In the United States, most of the Indian herbal medicinal products are marketed as dietary supplements under the Dietary Supplement Health and Education Act of 1994. The Act does not require submission of any safety or efficacy data for marketing approval. The manufacturers have the responsibility of substantiating the safety of their products, and as such no health claim toward a particular disease or disorder is allowed. The manufacturers do not need to register their products with the US Food and Drug Administration (FDA) or get approval before producing or selling dietary supplements. The FDA is responsible for taking action against any unsafe dietary supplement product after it reaches the market. Maximum export to the United States (from the survey result) can be correlated to the relaxed requirements for the dietary supplements. Indian manufacturers prefer to sell their products as dietary supplements without any health claims because doing so does not require any scientific evidence.
In the European Union (EU), however, the application for marketing authorization for traditional medicinal products requires bibliographic evidence and preclinical safety data (such as the toxicologic and pharmacologic test data). As per the Traditional Herbal Medicinal Product Directive (2004/24/EC), to obtain traditional use registration, the applicant has to submit the quantitative and qualitative particulars of constituents of the medicinal product, a description of manufacturing methods, therapeutic indications, contraindications, adverse reaction, posology, and form and route of administration [Article 8(3)(a) to (h), (j) and (k)]. The application also requires the summary of product characteristics without the clinical particulars as specified in Article 11(4) of Directive 2001/83/EC.7,8 In case of combinations, information relating to the combination, pharmacologic effects or efficacy of the medicinal product, evidence for longstanding use, and experience is required. As per Directive 2004/24/EC, many products from non-EU countries that are yet to be used in the EU would be excluded because a minimum of 15 years of marketing history in EU is required. Under these circumstances, herbal medicines would be permitted only if they could successfully pass through a full regimen, which requires safety and efficacy data. This is likely to be very expensive for most Indian herbal medicine manufacturers. There is also a risk that herbal products, which should be considered as food supplements, will be considered as herbal medicinal products. Particular combinations of herbal products may be disallowed, and complex mixtures with significant levels of nutrients will be prohibited. Combinations with vitamins and minerals will be allowed only if the action of the nutrients is considered ancillary to that of the herbal ingredients; in addition, the applicant has to submit specifications for herbal substances/herbal preparations along with quality, specifications, and documentation for each vitamin and mineral. The cost of this is very high.
Therefore, the stringent regulatory requirements in the EU have made the United States a more favorable export destination than the EU, although demands for traditional Indian herbal medicines are increasing in both regions. However, Indian traditional herbal medicines are not getting their due recognition because they are sold as supplements rather than medicines. Furthermore, different countries have their own standards, which vary from those of India. A general comparison of the pharmacopeial standards reveals variation in plant-specific parameters and quality standards, such as permissible limits for heavy metals, pesticides, and microbial contamination in different countries. Country-specific standards, as well as regional guidelines, have evolved, only some of which have been adopted. Compliance with such multiple standards has become a major worry for Indian manufacturers and traders.
Limited market
The Limited market in foreign countries is considered the second major hindrance for exporting as revealed from the survey. Scarcity of herbal practitioners, particularly for traditional systems of Indian medicine, in overseas countries has resulted in limited recognition of Indian herbal medicines. The respondent companies believe that adequate support for promotion of Indian herbal medicines in foreign countries through exhibitions and trade fairs would definitely help increase the export potential. Further promotion of AYUSH education in foreign countries is needed to boost the herbal medicine sector. To enable greater recognition for traditional Indian systems of medicine, it is necessary to support, nourish, and strengthen the profession in other countries. the government of India has taken steps to propagate these systems by organizing major events and exhibitions and exchange of scholars, funding research, and providing technical support to universities. An Indo–US Centre for Research on Indian Systems of Medicine (CRISM) has been set up in the National Center for Natural Products Research, University of Mississippi.9 The primary mission of CRISM is to facilitate scientific validation and dissemination of information on ASU medicines through collaborative research. It is expected that the establishment of this center will help improve scientific acceptance of Indian systems of medicine.
Issues with standardization of raw materials
When the quality of an herbal product is questioned, standardization of raw material emerges as a major issue for the Indian herbal industry. As per the Department of AYUSH, nearly 600 medicinal plant products, 52 minerals, and 50 animal products are commonly used in traditional Ayurvedic preparations. Medicinal plants are easily contaminated during growth, collection, and processing. The survey revealed that more than 50% of companies face problems in collecting and authenticating raw material. Further, 54 companies (36%) consider adulteration of raw materials, which affects quality of the product, to be very common (Fig. 1).
Substitution, adulteration, and heavy metal contamination are the three major problems reported for Indian herbal medicines.10,11 The intentional or accidental presence of toxic heavy metals is reported at all steps, beginning from collection of raw materials to manufacturing.10–16 Microbial contaminants and mycotoxin (notably aflatoxin) contamination during preharvest and postharvest stages, including storage conditions, are also a major challenge for the manufacturers.17 Conventional quality control methods often become insufficient because of the complex nature of herbal medicines. To overcome this problem, one or more compounds are selected as markers for identification and quality assessment by the natural products analysts. Several markers, such as taxonomic, chemical, genomic, and proteomic markers, help identify herbal drug components.17–22 Although developed countries require chemical fingerprinting and marker-based assessment of raw materials and active ingredients for assuring its quality, in India this concept was only recently introduced. The Indian GMP regulation does not provide any guidelines for marker-based identification. Most companies recognize the need for marker-based identification, but only 66 companies (44%) perform chemical marker–based studies for their formulations, at government testing laboratories or private laboratories (Fig. 1).
In general, marker-based analysis is a costly process that requires sophisticated and expensive instruments. In India, most manufacturing firms are small and medium enterprises and don't have (or can't afford) such elaborate research facilities in their units. The survey revealed less than 10% of the firms have in-house research and development facilities (Fig. 1). Most manufacturers follow the traditional physical/chemical/ physiochemical methods for standardizing raw materials and formulations. Marker-based studies are further limited because reference standards are not available for all the herbs/plants used in medicinal preparations. Scarcity of third-party laboratories within and outside India for testing ingredients of Indian origin is also a major issue for the manufacturers.
Lack of regulatory guidelines
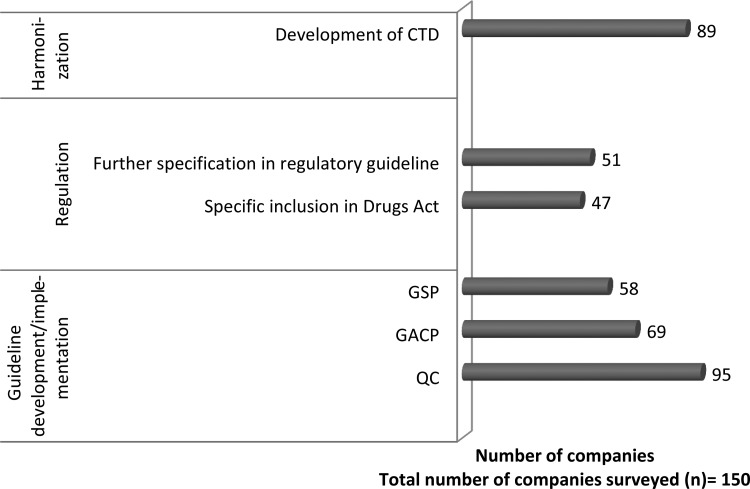
The survey revealed that insufficient regulatory guidelines for different aspects of production are an important reason for quality issues with herbal medicinal products. More than 60% of the respondents in the survey suggested that guidelines on quality control of herbal medicines be developed or elaborated (Fig. 2). Although guidelines have been issued for preclinical safety evaluation of ASU and other traditional medicines, further guidelines should also be developed for standardization of herbal preparation and marker-based identification of active components. Apart from quality control, good agricultural and collection practices (GACP) and good storage practices are also important in herbal drug manufacturing. In 2009, the National Medicinal Plants Board developed India-specific guidelines on good agriculture practices and good field collection practices in line with GACP developed by the World Health Organization.23–25 However, our interviews revealed that most of the manufacturers and raw material traders are not aware of these guidelines. Further, many associations or companies who are aware of these guidelines find them impractical because of lack of education and awareness among growers and the associated operational cost. Our interview analysis revealed that supply of standardized and certified raw materials and extracts that comply with standards of Ayurvedic pharmacopoeia of India is necessary for production of quality medicines. Establishment of government-certified raw-material supply centers in every state was suggested; this would help the manufacturers procure authentic raw materials. The respondents also suggested more elaborate regulatory guidelines in terms of raw material standardization and quality control during production.
FIG. 2.
Regulatory norms for development and implementation in herbal drug industry. (Field survey from June 2009 to August 2010. CTD, common technical dossier; GACP, good agricultural and collection practices; GSP, good storage practices; QC, quality control.)
The application of GMP is critical for the quality of the herbal medicines. The technical and nontechnical environment with regard to possible risks of adulteration and contamination, personnel, effectiveness of an independently run quality assurance system, and documentation are also the critical aspects of GMP. Most respondents (95 companies) suggest that apart from Schedule T, separate guidelines on quality control and quality assurance should be developed and that greater emphasis on the documentation practices is needed (Fig. 2).
In India, most traditional medicinal products are available as over the counter (OTC) drugs. Advertisement and customer preference are the major factors that influence the market for OTC herbal products, whereas prescription medicines are mainly controlled by physician choice. It is interesting to note that nearly half of the total interviewed companies are carrying out general safety studies for the medicines and 12% are conducting formal clinical trials at various medical colleges (Fig. 1). So far, no guideline has been issued for evaluation or clinical trials of herbal medicine in India. As a first attempt, the Drugs and Cosmetics 4th Amendment Rule 2008 provides guidelines for evaluation of ASU drugs (Rule 170). The new rule classifies the ASU medicines into four broad categories according to which clinical study requirement is prescribed. For the ASU drugs manufactured in accordance with formula, as per the definition given in Section 3(a) of DCA, and medicines based on aqueous extracts of medicinal plants for indications, as per text, no evidence of safety and efficacy (clinical evidence) is required. However, for proprietary ASU drugs, Indian ethno-medicine–based drugs, and hydroalcoholic extract–based drugs, safety and efficacy studies are mandatory.26 Hydroalcoholic extracts represent a different category than that recognized by Ayurveda through crude powders, decoctions, or aqueous extracts of medicinal plants. It is believed that the manufacturing process for hydroalcoholic extracts is a deviation from the fundamental principles of classic preparation. Therefore, hydroalcoholic extracts in any form should not be allowed for use in formulation that claims to be Ayurvedic. Clinical study is also necessary for medicines based on aqueous extracts for new indications.26
Another major drawback in the Indian herbal industry is the implementation of the DCA and its regulation. The field study revealed that only 107 of the surveyed companies were GMP compliant, even though GMP compliance as per Schedule T of the DCA has been compulsory since 2006. Further, survey responses revealed that the SFDA interprets the DCA differently; as a result, the same drug or formulation that is not permitted in one state is allowed to be manufactured in another state. The survey also identified nonuniformity in the drug registration timeline across states as a major issue. Development of unified protocols, defined timelines, and specific guidelines defining the meetings with regulators may help remove the anomalies with respect to state licensing authorities and establishing a unified system in the country. Most respondents suggested the need for scientific advice at the beginning of drug and formulation development, clinical trials, and dossier submission.
With more than 90% of the total herbal drug units in the country forming part of the small-scale sector, our interview analysis revealed that government support is required in many aspect of production. Supply of standardized raw material is the most common demand by the companies, as revealed in our study. Good-quality raw material can be produced if the growers and collectors are made aware and educated about the GACPs. More emphasis should be given to organic farming so that good-quality material can be produced. Initiative must be taken for cultivating some of the herbs predominantly used for herbal medicines. The government has already given many subsidies for the small and medium enterprises. But because of limited awareness, most small companies have not availed themselves of any help from the government. In such circumstances, it is essential to increase awareness about the facilities and support available from the government.
Compliance with different national regulatory standards was identified as a major hindrance for commercialization. More than half of the respondents recommended the need for development of a common technical dossier format for easier and faster approval (Fig. 2). Uniformity in herbal drug registration process and dossier submission requirements is also suggested. Pharmacopoeia harmonization and recognition of Indian monographs in other countries would be helpful for registration of drugs across countries.10 For example, in the United States, manufacturers face difficulties in marketing herbal products because unlike the U.S. Pharmacopoeia (USP) the Indian Pharmacopoeia (IP) does not have a separate section on dietary supplements. General notices of IP 2007 state that the mere presence of a monograph in the IP does not automatically mean that the formulation has been approved as a drug. However, as per the DCA, if a manufacturer wants to claim any substance to be IP grade, a drug license is required, even though the substance may not be meant to serve the drug industry. The drug rule also insists that an IP-grade substance cannot be manufactured at a site or with equipment with which a non-IP substance has been manufactured. It is not cost-effective for small and medium-sized manufacturers to establish manufacturing facilities dedicated to IP/USP-grade herbal extracts and preparations. Harmonization among the pharmacopoeia is necessary for ensuring uniformity of quality, safety, and efficacy of the same herbal medicines across countries. Harmonization of herbal drug registration requirements and dossier submission is essential to promoting global trade. Table 1 outlines suggested changes for the Indian herbal drug industry.
Table 1.
Major Suggested Changes for Indian Herbal Drug Industry (from Survey Responses)
| • Promotion of AYUSH education, with emphasis on technical education in AYUSH |
| • Popularization and promotion of Indian system of medicine in foreign countries |
| • Elaborate guidelines on quality control of herbal medicines |
| • Development of monographs and reference standards for marker-based analysis for all the plants used in medicinal preparations |
| • Supply of standardized and certified raw materials and extracts, sustainable cultivation of medicinal plants by identifying suitable zones |
| • Awareness regarding GAP, GACP, and GSP among growers and manufacturers |
| • Implementation and regulation of the DCA |
| • Development of unified protocols, defined timelines, and specific guidelines defining the meetings with regulators |
| • Capacity building and knowledge sharing within small to medium enterprises |
| • Financial assistance |
AYUSH, Ayurveda, Unani, Siddha, and homoeopathy; DCA, Drugs and Cosmetics Act of 1940; GACP, good agricultural and collection practices; GAP, good agriculture practices; GSP, good storage practices.
Conclusion
Although strengthening the regulatory mechanism with a view to ensuring quality of herbal medicines has become the prime concern for Indian drug regulators, drug manufacturers are grappling with increasing standards for herbal medicinal products. Fragmentation of the industry, lack of standardization of raw materials and finished products, inadequate research and development, slow pace of modernization, absence of focused marketing and branding, and inadequate emphasis on human resource development and education are the major reasons for slow growth of the Indian herbal industry. Proper implementation of DCA, development of more elaborate guidelines on quality control and quality assurance aspects, and development of marker-based standards are needed to produce safe and effective herbal medicines in India. Initiatives have been taken to address these issues by the Department of AYUSH. Schemes have been implemented to promote development of standardized herbal formulations. One such example is the New Millennium Indian Technology Leadership Initiative by the Council for Scientific and Industrial Research. Under this scheme, for the first time in India an Investigational New Drug application has been filed for an oral herbal formulation developed by extensive studies comprising finger printing, activity-guided fractionation, efficacy studies, toxicology, safety pharmacology, pharmacokinetics, and toxicokinetics for the treatment of psoriasis.
As evidence-based submissions are becoming increasingly essential for establishing the safety and efficacy of herbal products both in the domestic and the export market, more focus should be given on scientific and technological advancement in the field of herbal medicine. India must develop scientific cultivation, postharvest technology, processing, manufacturing, research and extension, patenting, and marketing strategy for medicinal plants and products. Regulatory harmonization becomes essential to mitigate the delays in commercialization across countries. Growing public demand for safe, high-quality, and efficacious integrative and complementary healthcare makes it imperative for AYUSH to urgently take steps in the fields of education, research, clinical medicine, pharmacopeial standards, health products, and services and improve regulatory mechanisms.
Disclosure Statement
The authors state that no competing financial interests exist.
References
- 1.World Health Organization Traditional medicine, fact sheet no. 134 [homepage on the Internet]. Online document at: http://www.who.int/mediacentre/factsheets/fs134/en/ Accessed March20, 2012
- 2.Mukherjee PK, Wahile A.Integrated approaches towards drug development from Ayurveda and other Indian system of medicines. J Ethnopharmacol 2006;103: 25–35 [DOI] [PubMed] [Google Scholar]
- 3.Working Group on “Access to Health Systems including AYUSH,” Government of India Planning Commission. Eleventh Five Year Plan (2007–2012) [homepage on the Internet] Online document at: http://planningcommission.nic.in/plans/planrel/11thf.htm Accessed March20, 2012
- 4.Singh H.Prospects and challenges for harnessing opportunities in medicinal plants sector in India. LEAD J 2006;2:198–211 [Google Scholar]
- 5.Wakdikar S.Global health care challenge: Indian experiences and new prescriptions. Electron J Biotechnol 2004;7:214–220 [Google Scholar]
- 6.Vaidya ADB, Devasagayam TPA.Current status of herbal drugs in India: an overview. J Clin Biochem Nutr 2007;4:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Directive 2004/24/EC of the European Parliament and of the Council [homepage on the Internet] Online document at: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2004:136:0085:0090:en:PDF Accessed March20, 2012
- 8.Directive 2001/83/EC of the European Parliament and of the Council of 6 November 2001 on the community code relating to medicinal products for human use [homepage on the Internet] Online document at: http://www.edctp.org/fileadmin/documents/ethics/DIRECTIVE_200183EC_OF_THE_EUROPEAN_PARLIAMENT.pdf Accessed March20, 2012
- 9.Centre for Research in Indian Systems of Medicine (CRISM) [homepage on the Internet] Online document at: www.crism.net Accessed June20, 2012
- 10.Sahoo N, Manchikanti P, Dey SH.Herbal drugs standards and regulation. Fitoterapia 2010;81:462–471 [DOI] [PubMed] [Google Scholar]
- 11.Mitra SK, Kannan R.A note on unintentional adulterations in ayurvedic herbs. Ethnobotanical Leaflets 2007;11:11–15 [Google Scholar]
- 12.Dargan PI, Gawarammana IB, Archer JRH, House IM, Shaw D, Wood D.Heavy metal poisoning from Ayurvedic traditional medicines: an emerging problem? Int J Env Health 2008;2:463–474 [Google Scholar]
- 13.Saper RB, Kales SN, Paquin J, et al. . Heavy metal content of ayurvedic herbal medicine products. JAMA 2004;292:2867–2873 [DOI] [PubMed] [Google Scholar]
- 14.Saper RB, Phillips RS, Sehgal A, et al. . Lead, mercury, and arsenic in US- and Indian-manufactured ayurvedic medicines sold via the internet. JAMA 2008;300:915–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ernst E.Toxic heavy metals and undeclared drugs in Asian herbal medicines. Trends Pharmacol Sci 2002;23:136–139 [DOI] [PubMed] [Google Scholar]
- 16.National Center for Complementary and Alternative Medicine (NCCAM) Ayurvedic medicine: an introduction [homepage on the Internet]. Online document at: http://nccam.nih.gov/health/ayurveda/introduction.htm Accessed January10, 2011
- 17.Bandaranayake WM. Quality control, screening, toxicity, and regulation of herbal drugs. In: Ahmad I, Aqil F, Owais M, eds. Modern Phytomedicine—Turning Medicinal Plants into Drugs. Weinheim, Germany: Wiley-VCH Verlag; 2003: 25–57 [Google Scholar]
- 18.Li S, Han Q, Qiao C, et al. . Chemical markers for the quality control of herbal medicines: an overview. Chin Med 2008;3:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saxena PK, Cole IB, Murch SJ.Approaches to quality plant based medicine: significance of chemical profiling. In: Applications of Plant Metabolic Engineering, Verpoorte R, Alfermann AW, Johnson TS, eds. Dordrecht, the Netherlands: Springer; 2007:311–330 [Google Scholar]
- 20.Fan X, Cheng Y, Ye Z, et al. . Multiple chromatographic fingerprinting and its application to the quality control of herbal medicines. Anal Chim Acta 2006;555:217–224 [Google Scholar]
- 21.Zeng Z, Liang Y, Chau F, et al. . Mass spectral profiling: an effective tool for quality control of herbal medicines. Anal Chim Acta 2007;604:89–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liang Y, Xie P, Chan K.Quality control of herbal medicines. J Chromatogr B 2004; 812:53–70 [DOI] [PubMed] [Google Scholar]
- 23.Guidelines on good agricultural practices, National Medicinal Plant Board, India [homepage on the Internet] Online document at: http://nmpb.nic.in/WriteReadData/links/8527013542Good%20Agricultural%20Practicies%20(GAPs)%20Booklet%20-%20Part%20-%20I.pdf Accesseed on June24, 2013
- 24.Guidelines on good field collection practices for Indian medicinal plants, National Medicinal plant Board, India [homepage on the Internet] Online document at: http://nmpb.nic.in/WriteReadData/links/7687590193Good%20Field%20Collection%20Practicies%20(GFCPs)%20Booklet%20-%20Part%20-%20I.pdf Accessed March20, 2012
- 25.World Health Organization Guidelines on good agricultural and collection practices for medicinal plants [homepage on the Internet]. Online document at: http://whqlibdoc.who.int/publications/2003/9241546271.pdf Accessed March20, 2012
- 26.Notification GSR No. 893(E) of 170 guidelines for evaluation of Ayurvedic. Siddha & Unani Drugs (2008) [homepage on the Internet]. Online document at: http://indianmedicine.nic.in/writereaddata/linkimages/4782799645-gaur.pdf Accessed January2, 2013