Abstract
Background: In Graves' orbitopathy (GO), increased proliferation, excess adipogenesis, and hyaluronan overproduction produce GO exophthalmos. Enophthalmos occurs in some glaucoma patients treated with Bimatoprost (prostaglandin F2α, PGF2α) eye drops. We hypothesized that enophthalmos is secondary to reductions in orbital tissue proliferation, adipogenesis, and/or increased lipolysis. We aimed to determine which of these is affected by PGF2α by using the 3T3-L1 murine preadipocyte cell line and primary human orbital fibroblasts (OFs) from GO patients (n=5) and non-GO (n=5).
Methods: 3T3-L1 cells and orbital OFs were cultured alone or with PGF2α (all experiments used 10−8 to 10−6 M) and counted on days 1/2/3 or 5, respectively; cell cycle analysis (flow cytometry) was applied. Adipogenesis (in the presence/absence of PGF2α) was evaluated (day 7 or 15 for 3T3-L1 and primary cells, respectively) morphologically by Oil Red O staining and quantitative polymerase chain reaction measurement of adipogenesis markers (glycerol-3-phosphate dehydrogenase and lipoprotein lipase, respectively). For lipolysis, in vitro–differentiated 3T3-L1 or mature orbital adipocytes were incubated with norepinephrine and PGF2α and free glycerol was assayed. Appropriate statistical tests were applied.
Results: The population doubling time of 3T3-L1 was 27.3±1.4 hours—significantly increased by dimethyl sulfoxide 0.02% to 44.6±4.8 hours (p=0.007) and further significantly increased (p=0.049 compared with dimethyl sulfoxide) by 10−8 M PGF2α to 93.6±19.0 hours, indicating reduced proliferation, which was caused by prolongation of G2/M. GO OFs proliferated significantly more rapidly than non-GO (population doubling time 5.36±0.34 or 6.63±0.35 days, respectively, p=0.035), but the proliferation of both was significantly reduced (dose dependent from 10−8 M) by PGF2α, again with prolongation of G2/M. Adipogenesis in 3T3-L1 cells was minimally affected by PGF2α when assessed morphologically, but the drug significantly reduced transcripts of the glycerol-3-phosphate dehydrogenase differentiation marker. GO OFs displayed significantly higher adipogenic potential than non-GO, but in both populations, adipogenesis, evaluated by all 3 methods, was significantly reduced (dose dependent from 10−8 M) by PGF2α. There was no effect of PGF2α on basal or norepinephrine-induced lipolysis, in 3T3-L1 or human OFs, either GO or non-GO.
Conclusions: The results demonstrate that PGF2α significantly reduces proliferation and adipogenesis and that human OFs are more sensitive to its effects than 3T3-L1. Consequently, PGF2α could be effective in the treatment of GO.
Introduction
Prostaglandin F2α (PGF2α) analogs, including Bimatoprost, have proven efficacy when used topically during glaucoma therapy to lower intraocular pressure. It is believed that PGF2α lowers intraocular pressure by increasing aqueous humor outflow through both the uveoscleral and the trabecular meshwork routes by mimicking the action of naturally occurring prostamides (1). These positive effects of PGF2α are not without the balance of some adverse effects. There are emerging case reports of deepening of lid sulcus and/or enophthalmos developing in patients treated with bimatoprost worldwide although in small numbers (2–7). This side effect is more noticeable if only one eye is exposed to treatment as the treated eye is easily comparable with the unexposed eye. However, since most patients receive treatment to both eyes, it is possible that the incidence of enophthalmos in bimatoprost-treated patients has been underestimated. This effect can be reversed by discontinuation of the prostaglandin analog therapy. A possible mechanism by which PGF2α agonists might produce enophthalmos is through reduction of orbital fat volume (8). A PGF2α receptor agonist has been shown to be a potent inhibitor of adipose differentiation in newborn rat precursor cells (9). This raises the possibility that PGF2α exerts direct effects on adipose tissue precursors. The inhibition of this adipose tissue differentiation may be mediated via PGF2α binding to a member of the G-protein–coupled receptor family known as prostaglandin F receptor or FP (10). This receptor is encoded by the PTGFR gene located on chromosome 1 in human (11) and chromosome 3 in mouse (12). The FP receptor is a Gq-coupled receptor, which once activated leads to release of inositol-1,4,5-trisphosphate and diacylglycerol, which in turn increases the Ca2+ level (11,13,14). Previous work from others in 3T3-L1 reports that PGF2α inhibits adipogenesis via a calcineurin-dependent mechanism by blocking expression of critical adipogenic transcription factors PPARγ and C/EBPα (15). This mechanism can be negated by calcineurin inhibitors such as cyclosporine and tacrolimus (FK506) (16), which might explain inconsistent results in the management of Graves' orbitopathy (GO) with these agents (17,18).
The opposite of enophthalmos, that is, exophthalmos, is a feature of GO. This represents a poorly understood component of Graves' disease. Graves' disease is caused by thyroid-stimulating antibody, and there are indirect demonstrations that they might also be important in GO (19). The main features of GO include orbital connective tissue fat pad expansion, tissue and extraocular muscle infiltration with mononuclear cells, and tissue remodeling that leads to fibrosis and reduced eye motility. GO has an annual adjusted incidence rate of 3 men and 16 women per 100,000 populations (20). The so-called subclinical involvement is quite common, approaching up to 70% of adults with Graves' disease detected via magnetic resonance imaging or computed tomography scanning (21).
We hypothesized that the observed enophthalmos in patients treated with PGF2α is secondary to reductions in orbital tissue proliferation, adipogenesis, and/or increased lipolysis. Our aim was to investigate which of these is affected, by using cell lines and human orbital fibroblasts, to determine whether the drug might be useful as a treatment for GO.
Materials and Methods
All tissue culture components were obtained from Lonza (Verviers, Belgium) and reagents from Sigma-Aldrich (St. Louis, MO) unless otherwise stated. 17-Phenyl trinor PGF2α was obtained from Cayman Chemical (Ann Arbor, MI) and diluted in dimethyl sulfoxide (DMSO) to produce a stock solution of 1 millimolar (10−3 M). Working concentrations were from 10−8 to 10−6 molar concentration.
Tissue specimen and preparation
The 3T3-L1 preadipocyte cell line was obtained from the American Type Culture Collection (Rockville, MD). Orbital tissue samples were collected, with informed consent and local research ethics committee's approval. GO patients (n=5; 3 women and 2 men with median age of 50 years, range 39–54 years) were diagnosed on clinical grounds based on the presence of typical clinical features in the context of autoimmune thyroid disease. The GO samples were obtained from patients undergoing decompression surgery and having inactive disease with a clinical activity score below 2. None of these patients had previous orbital radiotherapy. Only one patient had steroid treatment and was on the treatment during orbital decompression. The non-GO samples (n=5; 3 men and 2 women with median age of 53 years, range 52–60 years) were from individuals free of thyroid or other inflammatory eye disease who underwent augmented blepharoplasty. Orbital preadipocyte/fibroblasts (OFs) were obtained from explant cultures as previously described (22). Briefly, orbital fat biopsies were diced and placed in six-well plates in a complete medium (CM; Dulbecco's modified Eagle's medium, Hams F12, 10% fetal calf serum [FCS], penicillin/streptomycin, pyruvate, and bicarbonate) and allowed to attach so that OFs migrated out from the tissue. Once OFs were adherent, the plates were washed with the culture medium and OFs were grown to confluence replacing the medium every 7 days. The cells were trypsinized and frozen in liquid nitrogen until further use. Cells were used at a low passage number (≤3); thus, not every sample was used for each experiment.
Preadipocyte/fibroblast culture and cell counting
Five thousand cells (3T3-L1 or OF) were plated in the CM and allowed to attach for 1 day; PGF2α at a 10−8 M concentration was then added (either on day 0 alone or daily to test for reversibility and mimic current topical application in clinical practice), whereas control cells were cultured in the CM containing 0.02% v/v DMSO. Because of the very short half-life of the product and rapid proliferation rate of the 3T3-L1 cell line, direct cell counting (Cellometer®) was performed on days 1, 2, and 3. In contrast, the low population doubling time (PDT) of primary OFs required that cell counts were performed on day 5. Individual experiments were done in triplicate and repeated at least twice. Trypan blue analysis (0.2%) was carried out after 24-hour exposure to 10−8 to 10−6 molar PGF2α.
In vitro adipogenesis
The various cell populations were plated in six-well plates in the CM. Adipogenesis was induced in confluent cells by replacing with a differentiation medium (DM) containing 10% FCS (5% FCS for 3T3-L1 cell line), biotin (33 μM), pantothenate (17 μM), T3 (1 nM), dexamethasone (100 nM), thiazolidinedione (1 μM), and insulin (500 nM) for 7 (3T3-L1) or 15 (OF) days. PGF2α was introduced together with the DM on day 0 at 10−8, 10−7, and 10−6 M concentrations; DM±PGF2α was changed every 3 days. Reversibility was tested by applying PGF2α (10−6 M) for varying numbers of days (3, 6, 9, 12, or 15 days) to allow cell recovery. Adipogenesis was assessed microscopically to detect the accumulation of lipid droplets. Oil Red O staining was performed, and this was followed by extraction of the absorbed dye with 100% isopropanol and measurement of the OD490. Transcript measurements of adipogenic markers glycerol-3-phosphate dehydrogenase (GPDH) for 3T3-L1 and lipoprotein lipase (LPL) for primary OFs were performed by quantitative polymerase chain reaction (Q-PCR). RNA was extracted and reverse transcribed using standard protocol, (23) and transcript copy numbers for the genes were measured using SYBR green and a Stratagene (La Jolla, CA) MX3000 light cycler. Primer details are provided in Table 1. Comparison with plasmid standard curves (included in each experiment) permitted calculation of absolute values for each sample (transcripts per microgram input RNA). In addition, transcripts for a housekeeping gene, APRT (ARP for 3T3-L1), were measured so that values could be expressed relative to this (transcripts per 1000 APRT/ARP). In a single Q-PCR experiment, all measurements were made in duplicate.
Table 1.
Polymerase Chain Reaction Primers Used Indicating Exon Location and Size of Amplicon
| Forward primer | Reverse primer | |
|---|---|---|
| mARP (72 bp) |
GAG GAA TCA GAT GAG GAT ATG GGA (exon 7) |
AAG CAG GCT GAC TTG GTT GC (exon 7) |
| mGPDH (124 bp) |
ATG CTC GCC ACA GAA TCC ACA C (exon 8) |
AAC CGG CAG CCC TTG ACT TG (exon 8) |
| hAPRT (247 bp) |
GCT GCG TGC TCA TCC GAA AG (exon 3) |
CCT TAA GCG AGG TCA GCT CC (exon 5) |
| hLPL (275 bp) | GAG ATT TCT CTG TAT GGA CC (exon 7) | CTG CAA ATG AGA CAC TTT CTC (exon 9) |
Lipolysis
Adipogenesis was induced in vitro using the DM (as above) in 3T3-L1; on day 7, the DM was replaced with a serum-free medium. Mature human orbital adipocytes were obtained by collagenase digest and centrifugation on a phthalic acid dinonyl ester gradient as previously described (24) and resuspended in the serum-free medium. In both cases, varying concentrations of PGF2α were introduced alone (unstimulated lipolysis) or combined with 10−8 to 10−6 M L-norepinephrine (stimulated lipolysis) for 4 hours. Cell suspensions/supernatants were extracted, and free glycerol assays (Cayman Chemical) were performed according to the manufacturer's instructions; after 15 minutes of incubation with the assay reagents, the optical density was read at 490 nm.
Cell cycle analysis
3T3-L1 cells and OFs were plated in a 75 mL flask, allowed to attach for 24 hours and then treated with 10−6 M PGF2α for 48 hours and 5 days, respectively. The cells were trypsinized, fixed in ice-cold 70% ethanol overnight at 4°C, and stored at −20°C. Before analysis, ethanol was removed and samples were resuspended in 500 μL phosphate-buffered saline–containing propidium iodide (50 μg/mL) and RNAse A (50 μg/mL) at 37°C for 20 minutes. Flow cytometry was performed on BD FACS Canto II using FACSDiva 6.0 software from Becton Dickinson and Co. (Mountain View, CA). Propidium iodide was detected using the 575/26 nm channel. Forward light scatter, side light scatter, and fluorescence emissions were collected for 10,000 cells. Results were analyzed using FlowJo software version 10.0.5 (Tree Star, Inc., Ashland, OR).
Statistical analysis
For statistical analysis, we used SPSS 18.0 software. Where appropriate, data were analyzed using the Student's t-test for parametric and Mann–Whitney for nonparametric. Multiple comparisons of group means were analyzed using one-way ANOVA with post hoc Tukey HSD. In all cases, p<0.05 was considered significant. The statistical analysis applied is indicated in the tables and figure legends. All parametric data are presented as mean±SEM, and median±interquartile range for nonparametric.
Results
PGF2α reduces 3T3-L1 cell proliferation by prolonging the G2/M phase
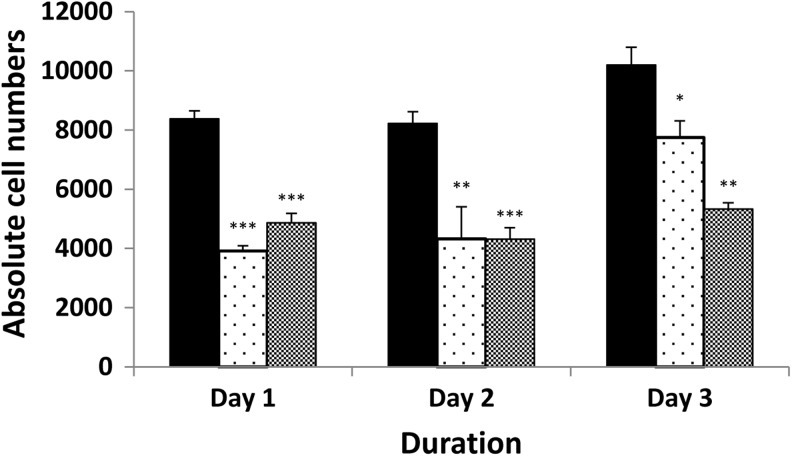
In the 3T3-L1 cell line, the PDT of untreated cells was 27.3±1.4 hours. The DMSO control at 0.02% significantly increased the PDT to a mean of 44.6±4.8 hours (p=0.007 compared with the untreated cells). PGF2α at a 10−8 M concentration significantly reduced 3T3-L1 cell proliferation with PDT of 93.6±19.0 hours (p=0.049 compared with DMSO control). However, the limited half-life of the compound resulted in recovery of cell proliferation, with cell numbers returning toward untreated levels by day 3. In contrast, daily administration of PGF2α produced a sustained significant reduction in proliferation (Fig. 1). To exclude simple toxicity as the cause of reduced growth, trypan blue exclusion was performed and indicated >90% survival across three concentrations of PGF2α (10−8 to 10−6 M), suggesting that this is not the case. In the same experiment, we also obtained a dose-dependent decrease in proliferation (data not shown).
FIG. 1.
Direct cell counting to assess the effects of PGF2α on proliferation of 3T3-L1 cells cultured alone (black bars, DMSO control), with addition of 10−8 M PGF2α on day 0 (stippled bars) or with daily addition of PGF2α (gray bars). Results are expressed as mean±SEM of two individual experiments all performed in triplicate. *p<0.05; **p<0.01; ***p<0.001 compared with the control on respective days. DMSO, dimethyl sulfoxide; PGF2α, prostaglandin F2α.
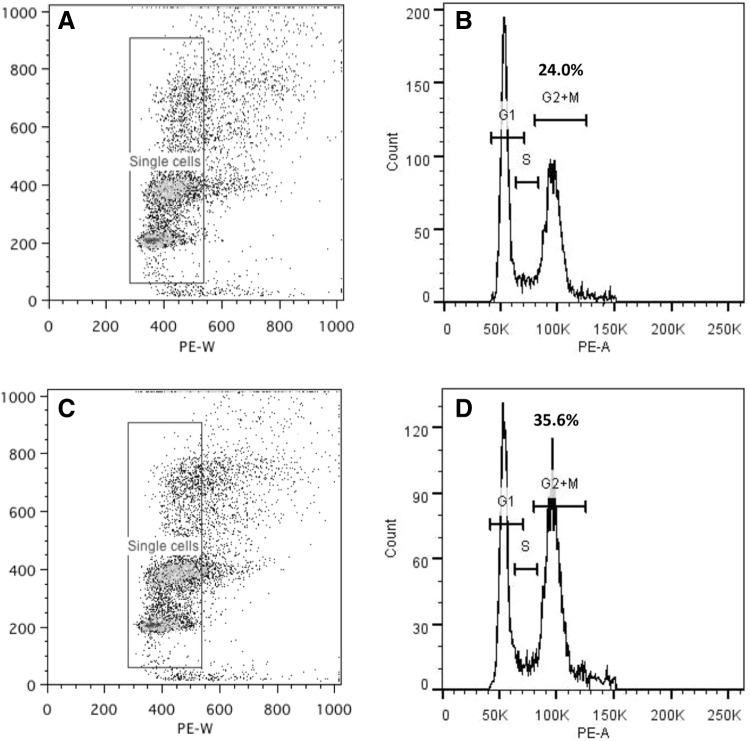
To determine whether an increase in apoptosis or cell cycle disruption was responsible for the increased PDT, cell cycle analysis was undertaken. Our results illustrate that PGF2α significantly increased the percentage of cells in the G2/M phase from 23.97±2.87 in untreated to 35.65±1.29 in treated cells, p=0.04 (Fig. 2), indicating prolongation of this stage, but did not increase the proportion of cells undergoing apoptosis (as defined by the pre-G1 peak).
FIG. 2.
Cell cycle analysis of 3T3-L1, to assess PGF2α effects, presented as scatter plots (A, C) and histograms (B, D) in control (A, B) and 10−6 M PGF2α-treated (C, D) cells. The x-axis on the scatter plot represents cell size where the arbitrary box was drawn to gate single cells, and the y-axis represents fluorescence intensity of DNA dye (propidium iodide). The histograms show the G1, S, and G2/M phases (figures above=percentage of cells) in (B) control (+DMSO) medium or (D) treated with PGF2α. The histogram reports DNA content (x-axis) and cell number (y-axis). The figure is a representative experiment of two performed, both in duplicate.
PGF2α reduced in vitro–induced adipogenesis in 3T3-L1
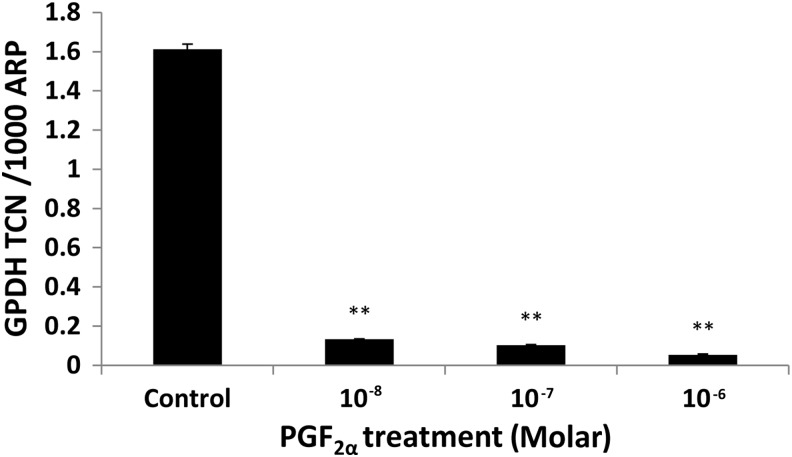
In the DM, 3T3-L1 cells were induced to undergo adipogenesis, and more than 70% of the cells acquired a rounded appearance and intracellular lipid droplet accumulation. In our system, we managed to induce an approximately 14-fold increase in GPDH transcripts by day 7 as compared with day 0 (p=0.002). In view of the proliferation results, which indicated that PGF2α has a short half-life, the drug was added at every medium change throughout the period of differentiation. When assessing adipogenesis morphologically, in both treated and control groups, by day 3, adipogenic changes were seen, but with no difference in the number of adipogenic foci. At day 7, the increase in numbers of lipid droplets was similar in PGF2α-treated cells and controls. When using Q-PCR measurement of markers of differentiation, the presence of 10−8 M PGF2α reduced transcripts for GPDH by about 12-fold (p=0.01) with a dose-dependent response (Fig. 3).
FIG. 3.
In vitro–induced adipogenesis of 3T3-L1 cells assessed by Q-PCR measurement of GPDH transcripts expressed as TCN per 1000 copies of the acidic ribophosphoprotein (ARP) housekeeper gene after 7 days of exposure to control (DM+DMSO) and treatment (DM+PGF2a). Data shown (mean±SEM) are from a representative experiment of two performed in duplicate. Error bar represents±SEM; **p<0.01. DM, differentiation medium; GPDH, glycerol-3-phosphate dehydrogenase; Q-PCR, quantitative polymerase chain reaction; TCN, transcript copy number.
PGF2α had no effect on lipolysis on differentiating 3T3-L1
To study the effects of PGF2α on 3T3-L1 lipolysis, we induced adipogenesis by culturing confluent cells in the DM for 7 days. As noted above, at this point,>70% of the cells had undergone differentiation and contained large lipid droplets although none were fully mature adipocytes with a single lipid vacuole, since these cells are not adherent and are removed during manipulations.
The effects of 10−8 to 10−6 M PGF2α on lipolysis were assessed in unstimulated in vitro–differentiated cells and also in cells in which lipolysis was induced using 10−8 to 10−6 M norepinephrine (produced a dose-dependent increase in free glycerol peaking at 300%). PGF2α alone did not induce lipolysis and had no effect on norepinephrine-mediated lipolysis (data not shown).
PGF2α reduced cell proliferation in GO and non-GO orbital preadipocytes
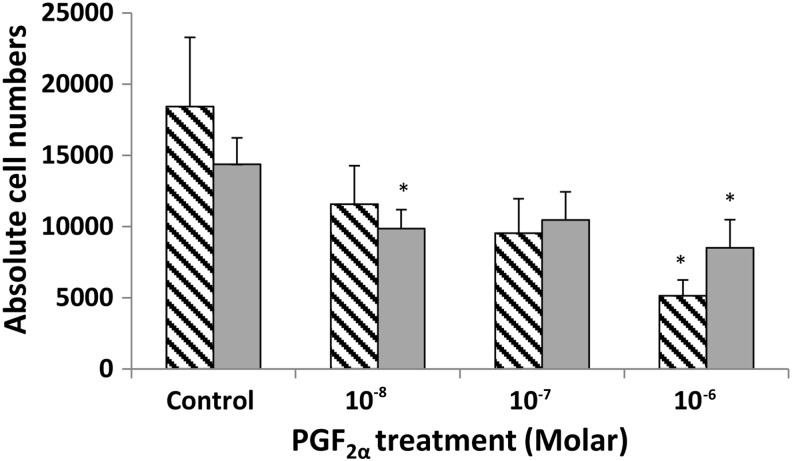
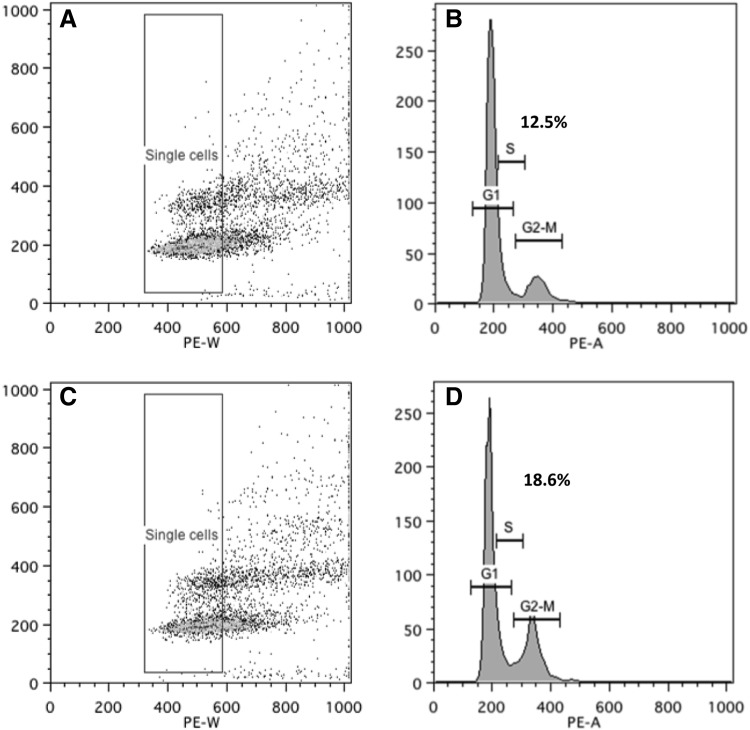
We subsequently performed the experiments in primary OFs obtained from patients with GO and individuals free of thyroid or other inflammatory eye disease. The average PDT for orbital cells from GO patients (n=3) was 5.36±0.34 days, but for non-GO orbits (n=3), it was 6.63±0.35 days; the difference was significant (p=0.035) with GO orbital cells proliferating more rapidly. In view of the longer PDT of primary orbital cells, the effects of PGF2α on proliferation were assessed by cell counting 5 days after plating in CM. Similar results to those for 3T3-L1 were obtained, in that PGF2α reduced orbital cell proliferation in both GO and non-GO patients compared with controls (Fig. 4). Cell cycle analysis of the primary OFs also demonstrated G2/M phase arrest in both GO and non-GO populations. In non-GO OFs, the percentage of cells in G2/M in the controls was 17.14±0.72 and increased to 22.43±0.89 (p=0.003), whereas in GO OFs, PGF2α increased the percentage to 18.57±0.17 from 12.49±0.30 in the DMSO control (p<0.001; Fig. 5).
FIG. 4.
Direct cell counting to assess the effects of PGF2α on proliferation of human OFs cultured for 5 days alone (DMSO control) or with 10−8 to 10−6 M PGF2α. Stippled bars are Graves' OFs (n=3) and gray bars non-GO (n=3). Results are presented as mean±SEM; *p<0.05 compared with the control on each respective day. OFs, orbital fibroblasts.
FIG. 5.
Cell cycle analysis was performed in OFs, to assess PGF2α effects, presented as scatter plots (A, C) and histograms (B, D) in the control (A, B), and 10−6 M PGF2α-treated (C, D) cells. The x-axis on the scatter plot represents cell size where the arbitrary box was drawn to gate single cells, and the y-axis represents fluorescence intensity of DNA dye (propidium iodide). The histograms show the G1, S, and G2/M (figures above=percent of cells) phases in (B) control (+DMSO) medium or (D) treated with PGF2α. The histogram reports DNA content (x-axis) and cell number (y-axis). The figure presents data from Graves' OFs and is a representative experiment of two performed (using OFs from different donors), both in duplicate.
PGF2α reduced in vitro–induced adipogenesis in GO and non-GO preadipocytes
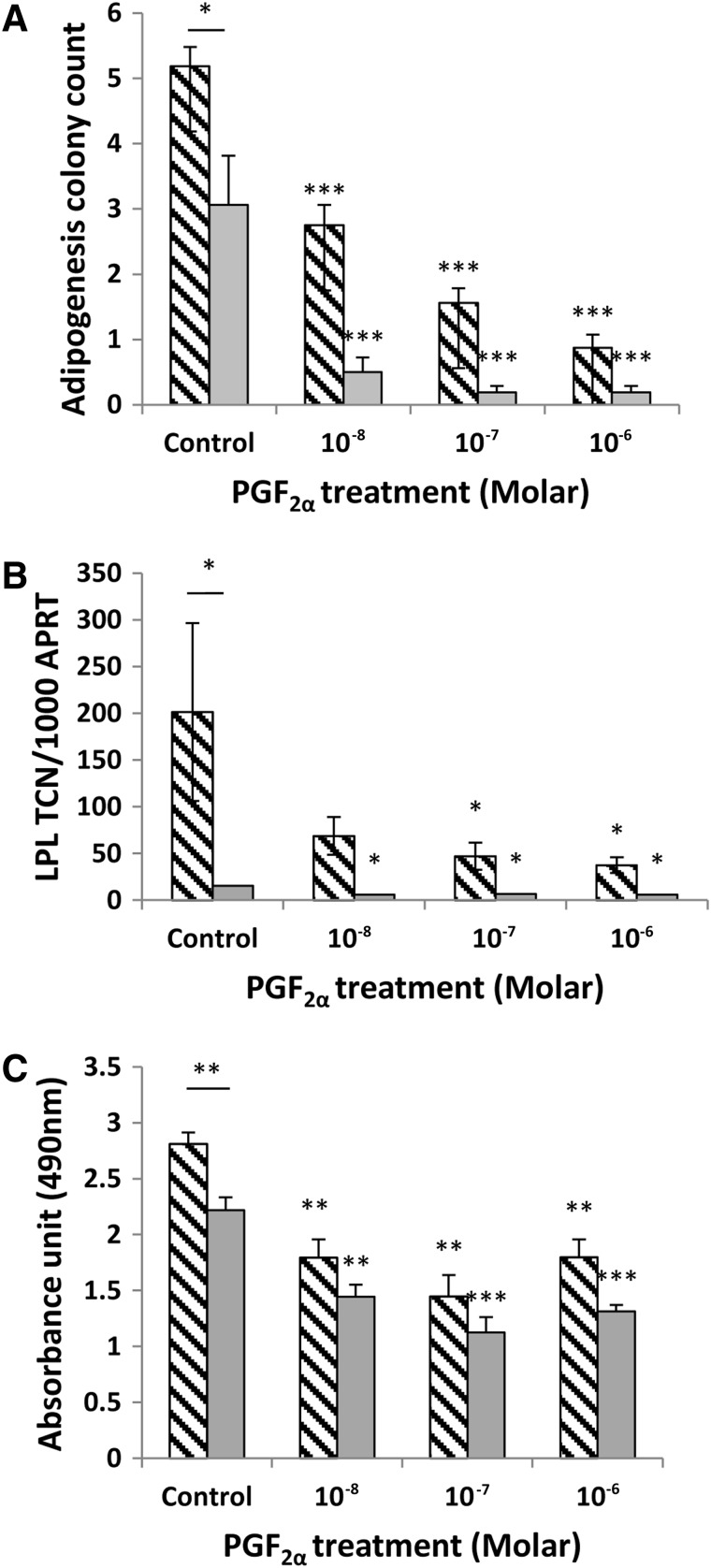
After 15 days of incubation in DM, preadipocytes from GO and non-GO orbits were seen to be undergoing adipogenesis. Compared with the 3T3-L1 cell line, only a small proportion (up to 10%) of the primary cells differentiate by assuming a rounded appearance and forming lipid vacuoles. In our in vitro model, the addition of DM induced at least a 500-fold increase in LPL transcripts in GO orbital cells and an approximately 150-fold increase in those from non-GO orbits compared with day 0 (p<0.001). The increased adipogenic potential of the GO cells was further supported by their significantly higher colony count (p=0.013) and Oil Red O absorbance data (p=0.008), shown in Figure 6.
FIG. 6.
In vitro–induced adipogenesis (15 days in DM) in orbital preadipocytes in the control (DMSO), and PGF2α-treated cells was assessed by (A) counting foci of differentiation, (B) Q-PCR measurement of LPL transcripts, and (C) quantification of Oil Red O staining. (A) Colony counts (n=3) are expressed as the mean±SEM from 4 representative quadrants of the well. (B) Q-PCR results (n=5) expressed as mean±SEM of TCN per 1000 copies of housekeeper gene (adenosine phosphoribosyl transferase, APRT). (C) Oil Red O staining (n=2) expressed as the mean±SEM of the OD490 absorbance. Stippled bars are Graves' OFs and gray bars non-GO. In all cases, the bar above the control represents statistical comparison between TCN in GO and non-GO orbital cells; other comparisons are between treated and control. *p<0.05; **p<0.01; ***p<0.001. LPL, lipoprotein lipase.
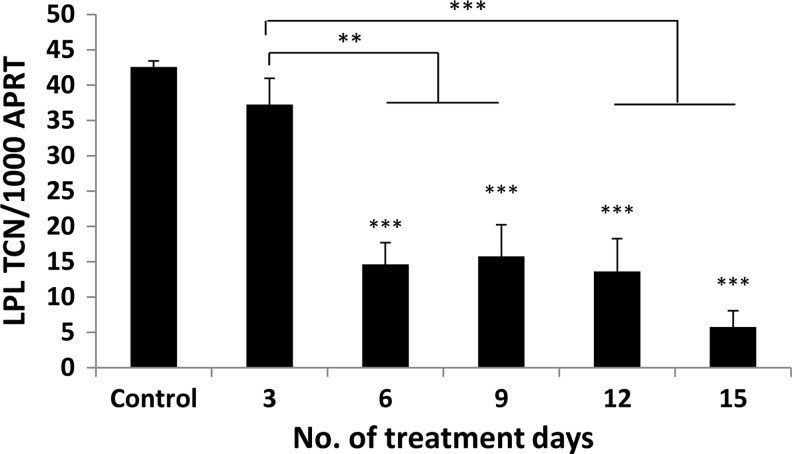
The addition of PGF2α significantly inhibited adipogenesis in GO and non-GO OFs when assessed by all three measures of differentiation (Fig. 6A–C). The drug produced visible signs of reduced adipocyte colony formation and Oil Red O staining, but its effects on adipogenesis were most apparent when comparing transcript levels for LPL. A significant reduction in LPL transcripts was observed in cells from GO and non-GO orbits at all concentrations of PGF2α from 10−8 to 10−6 M (p<0.01). The effects were partly reversible as illustrated in Figure 7, in which OFs exposed to the drug for 3 days had no significant reduction in adipogenesis, and cells exposed for 6, 9, 12, or 15 days all demonstrated a significant reduction in differentiation but with no significant difference when comparing cells exposed for 15 days with those exposed for 6, 9, or 12 days.
FIG. 7.
The effects of PGF2α on adipogenesis are reversible; confluent Graves' OFs (n=2) were treated with the differentiation medium alone or supplemented with PGF2α 10−6 M for varying periods during differentiation as indicated in the graph. LPL transcripts were measured on day 15 and expressed as mean±SEM of transcript copy number (TCN) per 1000 copies of housekeeper gene (APRT). In all cases, comparisons are between treated and control. **p<0.01; ***p<0.001.
PGF2α had no effect on lipolysis on mature orbital adipocytes
We obtained mature adipocytes from two GO patients and treated them with PGF2α alone or in the presence of norepinephrine, as described above for the 3T3-L1 cell line. No concentration of PGF2α induced lipolysis in basal conditions. Norepinephrine produced a dose-dependent increase in glycerol with a maximum of 260% compared with unstimulated cells, but as with the cell line, this was not altered by PGF2α (data not shown).
Discussion
The enophthalmos in people treated with eye drops containing PGF2α for glaucoma (2–7) has been suggested to be caused by fat atrophy (8). With this in mind, we have investigated whether the reduced orbital fat volume is the result of diminished proliferation, inhibition of adipogenesis, and/or increased lipolysis. We have used a well-established preadipocyte cell line and also human primary OFs. In addition, recognizing a potential application for PGF2α in treating GO, we have compared the effects in OFs originating in people with GO and free of the disease.
In the 3T3-L1 cell line, proliferation and adipogenesis were inhibited by PGF2α in a dose-dependent manner, from 10−8 M. The inhibitory effects of PGF2α on adipogenesis are in agreement with the findings of several other authors, including the Clipstone group (15,16), who demonstrated that the mechanism involved calcium/calcineurin signaling. They also concur with Casimir et al. (25), who reported that endogenous PGF2α production is lower in differentiating cells and postulated that its release from preadipocytes provides a control mechanism to limit adipogenesis. We are unaware of any studies investigating whether proliferation of 3T3-L1 is modified by PGF2α, and we also demonstrated that the reduced proliferation is not caused by cytotoxicity, but by a prolongation of the G2/M phase of the cell cycle. We were surprised to note that the vehicle, DMSO, exerted significant inhibition of proliferation, even at very low concentrations. DMSO is widely used to differentiate cells and its mode of action includes cell cycle arrest (26), but it should be noted that PGF2α exerted an additional significant reduction in proliferation.
In contrast, we were unable to demonstrate any modification of lipolysis, either basal or norepinephrine-induced, by PGF2α. We used in vitro–differentiated 3T3-L1, which displayed abundant lipid accumulation, but this had not coalesced into the single vacuole, which typifies a mature adipocyte. Since mature adipocyte cells are nonadherent, they are lost from the culture well during medium changes, but the immaturity of the cells used might provide some explanation for the absence of any effect. However, that we did not observe any effect of PGF2α on lipolysis when using freshly isolated mature adipocytes from human orbital adipose tissues suggests that this limitation had minimal impact on the interpretation of the results.
Experiments using OFs from human orbits revealed disease-associated differences, for example, significantly higher proliferation and adipogenic potential in cells from GO patients compared with those from donors free of any inflammatory eye problem or thyroid disease, even though the GO tissues were obtained from patients with apparently inactive disease. The increased proliferation in GO has been reported previously (27) and the enhanced adipogenesis agrees with previous ex vivo data from ourselves (23) and others (28).
PGF2α significantly inhibited the proliferation of OFs, originating in GO and non-GO orbits, and agree with the results of Seibold et al. (29) obtained with human subcutaneous preadipocytes. Our subsequent cell cycle analyses revealed that, in common with the 3T3-L1 cell line, there was an accumulation of OFs in the G2/M phase, with GO and non-GO cells being similarly affected.
PGF2α also inhibited adipogenesis in OFs, as reported by Choi and colleagues (30), although these authors did not investigate cells from GO orbits, whereas our study illustrates that they remain highly responsive to the drug and have not become resistant, for example, by losing PGF2α receptors.
We recognize that our study has limitations, including the use of in vitro models devoid of the inflammatory cytokines and cell–cell interactions operating in GO orbits. However, the models have been used by ourselves (31) and others (28,32) to provide valuable insights into the tissue remodeling leading to GO, and in the absence of a robust animal model is the best currently available. The number of GO patients who could benefit from the drug may be small, if the enophthalmos side effect is uncommon. However, since most patients receive treatment to both eyes, it is possible that the incidence of enophthalmos in bimatoprost-treated patients has been underestimated.
The results obtained are encouraging, since they indicate that several of the mechanisms which contribute to the expansion of the orbital volume, and responsible for the exophthalmos in GO, are inhibited by PGF2α. The inhibitory actions on proliferation and differentiation were obtained in the range of 10−8 to 10−6 M PGF2α, and even the most concentrated is 3 orders of magnitude less than the 0.03% used in eye drops, although this is a prodrug. The prodrug, 17-phenyl trinor PGF2α ethyl amide, needs to be converted by an amidase enzyme present in the human cornea, to the corresponding free acid, 17-phenyl trinor PGF2α (33,34). The latter free acid compound, which we used in these experiments to negate the requirement of the amidase enzyme, is a potent FP receptor agonist but has a short half-life, as illustrated by the rapid recovery of 3T3-L1 proliferation and OF adipogenesis when the agent was withdrawn, and indicates that daily administration would be required.
We would predict that the drug will reach the retro-orbital space to exert its intended effect, since topical ocular administration of PGF2α leads to its detection in the aqueous humor and systemic circulation (35,36); the existence of reduced periorbital fat in bimatoprost-treated patients also supports the prediction (8).
Fortunately, drug formulations containing PGF2α have been in regular use for glaucoma (on a daily basis) for some time, and indeed PGF2α preparations are available over the counter for cosmetic application; thus, their safety is well established. We conclude that clinical trials of PGF2α and/or associated products are warranted in GO.
Author Disclosure Statement
No conflict of interest declared.
References
- 1.Eisenberg DL, Toris CB, Camras CB.2002Bimatoprost and travoprost: a review of recent studies of two new glaucoma drugs. Surv Ophthalmol 47Suppl 1:S105–S115 [DOI] [PubMed] [Google Scholar]
- 2.Peplinski LS, Albiani Smith K.2004Deepening of lid sulcus from topical bimatoprost therapy. Optom Vis Sci 81:574–577 [DOI] [PubMed] [Google Scholar]
- 3.Filippopoulos T, Paula JS, Torun N, Hatton MP, Pasquale LR, Grosskreutz CL.2008Periorbital changes associated with topical bimatoprost. Ophthal Plast Reconstr Surg 24:302–307 [DOI] [PubMed] [Google Scholar]
- 4.Tappeiner C, Perren B, Iliev ME, Frueh BE, Goldblum D.2008[Orbital fat atrophy in glaucoma patients treated with topical bimatoprost—can bimatoprost cause enophthalmos?]. Klin Monbl Augenheilkd 225:443–445 [DOI] [PubMed] [Google Scholar]
- 5.Yam JC, Yuen NS, Chan CW.2009Bilateral deepening of upper lid sulcus from topical bimatoprost therapy. J Ocul Pharmacol Ther 25:471–472 [DOI] [PubMed] [Google Scholar]
- 6.Aydin S, Isikligil I, Teksen YA, Kir E.2010Recovery of orbital fat pad prolapsus and deepening of the lid sulcus from topical bimatoprost therapy: 2 case reports and review of the literature. Cutan Ocul Toxicol 29:212–216 [DOI] [PubMed] [Google Scholar]
- 7.Park J, Cho HK, Moon JI.2011Changes to upper eyelid orbital fat from use of topical bimatoprost, travoprost, and latanoprost. Jpn J Ophthalmol 55:22–27 [DOI] [PubMed] [Google Scholar]
- 8.Jayaprakasam A, Ghazi-Nouri S.2010Periorbital fat atrophy—an unfamiliar side effect of prostaglandin analogues. Orbit 29:357–359 [DOI] [PubMed] [Google Scholar]
- 9.Serrero G, Lepak NM.1997Prostaglandin F2alpha receptor (FP receptor) agonists are potent adipose differentiation inhibitors for primary culture of adipocyte precursors in defined medium. Biochem Biophys Res Commun 233:200–202 [DOI] [PubMed] [Google Scholar]
- 10.Balapure AK, Rexroad CE, Jr, Kawada K, Watt DS, Fitz TA.1989Structural requirements for prostaglandin analog interaction with the ovine corpus luteum prostaglandin F2 alpha receptor. Implications for development of a photoaffinity probe. Biochem Pharmacol 38:2375–2381 [DOI] [PubMed] [Google Scholar]
- 11.Abramovitz M, Boie Y, Nguyen T, Rushmore TH, Bayne MA, Metters KM, Slipetz DM, Grygorczyk R.1994Cloning and expression of a cDNA for the human prostanoid FP receptor. J Biol Chem 269:2632–2636 [PubMed] [Google Scholar]
- 12.Sugimoto Y, Hasumoto K, Namba T, Irie A, Katsuyama M, Negishi M, Kakizuka A, Narumiya S, Ichikawa A.1994Cloning and expression of a cDNA for mouse prostaglandin F receptor. J Biol Chem 269:1356–1360 [PubMed] [Google Scholar]
- 13.Black FM, Wakelam MJ.1990Activation of inositol phospholipid breakdown by prostaglandin F2 alpha without any stimulation of proliferation in quiescent NIH-3T3 fibroblasts. Biochem J 266:661–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakao A, Watanabe T, Taniguchi S, Nakamura M, Honda Z, Shimizu T, Kurokawa K.1993Characterization of prostaglandin F2 alpha receptor of mouse 3T3 fibroblasts and its functional expression in Xenopus laevis oocytes. J Cell Physiol 155:257–264 [DOI] [PubMed] [Google Scholar]
- 15.Liu L, Clipstone NA.2007Prostaglandin F2alpha inhibits adipocyte differentiation via a G alpha q-calcium-calcineurin-dependent signaling pathway. J Cell Biochem 100:161–173 [DOI] [PubMed] [Google Scholar]
- 16.Neal JW, Clipstone NA.2002Calcineurin mediates the calcium-dependent inhibition of adipocyte differentiation in 3T3-L1 cells. J Biol Chem 277:49776–49781 [DOI] [PubMed] [Google Scholar]
- 17.Krassas GE, Heufelder AE.2001Immunosuppressive therapy in patients with thyroid eye disease: an overview of current concepts. Eur J Endocrinol 144:311–318 [DOI] [PubMed] [Google Scholar]
- 18.Bartalena L, Pinchera A, Marcocci C.2000Management of Graves' ophthalmopathy: reality and perspectives. Endocr Rev 21:168–199 [DOI] [PubMed] [Google Scholar]
- 19.Zhang L, Bowen T, Grennan-Jones F, Paddon C, Giles P, Webber J, Steadman R, Ludgate M.2009Thyrotropin receptor activation increases hyaluronan production in preadipocyte fibroblasts: contributory role in hyaluronan accumulation in thyroid dysfunction. J Biol Chem 284:26447–26455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bartley GB.1994The epidemiologic characteristics and clinical course of ophthalmopathy associated with autoimmune thyroid disease in Olmsted County, Minnesota. Trans Am Ophthalmol Soc 92:477–588 [PMC free article] [PubMed] [Google Scholar]
- 21.Forbes G, Gorman CA, Brennan MD, Gehring DG, Ilstrup DM, Earnest Ft.1986Ophthalmopathy of Graves' disease: computerized volume measurements of the orbital fat and muscle. Am J Neuroradiol 7:651–656 [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang L, Baker G, Janus D, Paddon CA, Fuhrer D, Ludgate M.2006Biological effects of thyrotropin receptor activation on human orbital preadipocytes. Invest Ophthalmol Vis Sci 47:5197–5203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Starkey KJ, Janezic A, Jones G, Jordan N, Baker G, Ludgate M.2003Adipose thyrotrophin receptor expression is elevated in Graves' and thyroid eye diseases ex vivo and indicates adipogenesis in progress in vivo. J Mol Endocrinol 30:369–380 [DOI] [PubMed] [Google Scholar]
- 24.Crisp M, Starkey KJ, Lane C, Ham J, Ludgate M.2000Adipogenesis in thyroid eye disease. Invest Ophthalmol Vis Sci 41:3249–3255 [PubMed] [Google Scholar]
- 25.Casimir DA, Miller CW, Ntambi JM.1996Preadipocyte differentiation blocked by prostaglandin stimulation of prostanoid FP2 receptor in murine 3T3-L1 cells. Differentiation 60:203–210 [DOI] [PubMed] [Google Scholar]
- 26.Lalic H, Lukinovic-Skudar V, Banfic H, Visnjic D.2012Rapamycin enhances dimethyl sulfoxide-mediated growth arrest in human myelogenous leukemia cells. Leuk Lymphoma 53:2253–2261 [DOI] [PubMed] [Google Scholar]
- 27.Meyer zu Horste M, Stroher E, Berchner-Pfannschmidt U, Schmitz-Spanke S, Pink M, Gothert JR, Fischer JW, Gulbins E, Eckstein AK.2011 A novel mechanism involved in the pathogenesis of Graves ophthalmopathy (GO): clathrin is a possible targeting molecule for inhibiting local immune response in the orbit. J Clin Endocrinol Metab 96:E1727–E1736 [DOI] [PubMed] [Google Scholar]
- 28.Kumar S, Coenen MJ, Scherer PE, Bahn RS.2004Evidence for enhanced adipogenesis in the orbits of patients with Graves' ophthalmopathy. J Clin Endocrinol Metab 89:930–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seibold LK, Ammar DA, Kahook MY.2013Acute effects of glaucoma medications and benzalkonium chloride on pre-adipocyte proliferation and adipocyte cytotoxicity in vitro. Curr Eye Res 38:70–74 [DOI] [PubMed] [Google Scholar]
- 30.Choi HY, Lee JE, Lee JW, Park HJ, Jung JH.2012In vitro study of antiadipogenic profile of latanoprost, travoprost, bimatoprost, and tafluprost in human orbital preadiopocytes. J Ocul Pharmacol Ther 28:146–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Starkey K, Heufelder A, Baker G, Joba W, Evans M, Davies S, Ludgate M.2003Peroxisome proliferator-activated receptor-gamma in thyroid eye disease: contraindication for thiazolidinedione use? J Clin Endocrinol Metab 88:55–59 [DOI] [PubMed] [Google Scholar]
- 32.Smith TJ, Koumas L, Gagnon A, Bell A, Sempowski GD, Phipps RP, Sorisky A.2002Orbital fibroblast heterogeneity may determine the clinical presentation of thyroid-associated ophthalmopathy. J Clin Endocrinol Metab 87:385–392 [DOI] [PubMed] [Google Scholar]
- 33.Maxey KM, Johnson JL, LaBrecque J.2002The hydrolysis of bimatoprost in corneal tissue generates a potent prostanoid FP receptor agonist. Surv Ophthalmol 47Suppl 1:S34–S40 [DOI] [PubMed] [Google Scholar]
- 34.Hellberg MR, Ke T-L, Haggard K, Klimko PG, Dean TR, Graff G.2003The hydrolysis of the prostaglandin analog prodrug bimatoprost to 17-phenyl-trinor PGF2alpha by human and rabbit ocular tissue. J Ocul Pharmacol Ther 19:97–103 [DOI] [PubMed] [Google Scholar]
- 35.Woodward DF, Krauss AH, Chen J, Lai RK, Spada CS, Burk RM, Andrews SW, Shi L, Liang Y, Kedzie KM, Chen R, Gil DW, Kharlamb A, Archeampong A, Ling J, Madhu C, Ni J, Rix P, Usansky J, Usansky H, Weber A, Welty D, Yang W, Tang-Liu DD, Garst ME, Brar B, Wheeler LA, Kaplan LJ.2001The pharmacology of bimatoprost (Lumigan). Surv Ophthalmol 45Suppl 4:S337–S345 [DOI] [PubMed] [Google Scholar]
- 36.Ichhpujani P, Katz LJ, Hollo G, Shields CL, Shields JA, Marr B, Eagle R, Alvim H, Wizov SS, Acheampong A, Chen J, Wheeler LA.2012Comparison of human ocular distribution of bimatoprost and latanoprost. J Ocul Pharmacol Ther 28:134–145 [DOI] [PubMed] [Google Scholar]