Abstract
The effects of lactoferrin (LF), an iron binding protein, on myelopoiesis have been studied extensively in vitro and in vivo in human and murine models over the past three decades. Due to the lack of high-quality homologous LFs, however, the conclusions are still unequivocal. Recently, both human and murine LFs have become available as recombinant products expressed in Chinese hamster ovary (CHO) cell lines showing mammalian type of glycosylation, thus apparently species compatible. In this study, we present the effects of homologous recombinant mouse LF (rmLF) on myelopoiesis in CBA mice. The myelocytic lineage has been assessed by their appearance in circulating blood and bone marrow, and induction of relevant mediators of inflammation. Intravenous injection of rmLF (100 μg/mouse) resulted in a significantly increased number of myelocytic cells in the circulating blood after 24 h. Mouse serum transferrin, used as a control protein, showed no stimulatory effect. The increase in output of neutrophil precursors, neutrophils, and eosinophils was correlated with a twofold increase of leukocyte concentrations. The analysis of the bone marrow sections confirmed increased myelopoiesis. The alterations in the bone marrow cell composition were statistically significant regarding mature neutrophils (10.8% vs. 27.7%), metamyelocytes (11.4% vs. 16.0%), and myelocytes (2.4% vs. 4.0%). The mobilization of the myelocytic cells in the bone marrow and the increased output of these cells into circulation were accompanied by elevated serum concentrations of interleukin-6 at 6 h and haptoglobin at 24 h following administration of rmLF. In conclusion, the homologous LF elicits significant and transient myelopoiesis in experimental mice.
Introduction
Myelopoiesis is a dynamic process dependent on a variety of mediators, which may stimulate or inhibit the proliferation and maturation of granulocyte and macrophage progenitors. The mutual interactions of many cell types and factors secreted by these cells maintain the number of granulocytes and monocytes/macrophages at constant levels inherent to the physiological state. These cells represent the first line of defense against pathogens; therefore, the regulation of their recruitment and release into the circulation is of major importance. Particularly meaningful is the regulation of granulopoiesis, since the complete differentiation of granulocytes lasts relatively long (10–14 days), although these cells live only 4–6 h after being released from bone marrow [1]. Thus, the cells must be continuously generated and released into the circulation. The demand for neutrophils significantly increases during infection [2], endotoxemia [3], and trauma [4]. The presence of endogenous glucocorticoids [5–7] plays an important role in triggering myelopoiesis. Cells of myeloid origin are recruited from pluripotential hematopoietic stem cells in the bone marrow [8]. Myelopoiesis is promoted by a number of cytokines, including interleukin (IL)-1 [9], IL-6 [10], granulocyte-macrophage colony-stimulating factor (GM-CSF), granulocyte CSF (G-CSF), and macrophage CSF (M-CSF) [11]. These cytokines are produced by various cell types (monocytes/macrophages, fibroblasts, endothelial cells, epithelial cells, osteoblasts, and lymphocytes) from many tissues and organs, including cells of the bone marrow microenvironment [12]. Interestingly, mice lacking the ability to produce CSFs were still able to generate macrophages and neutrophils in response to an inflammatory stimulus indicating that the requirement for CSFs in this process is not essential [13].
Lactoferrin (LF) is an iron-binding protein, contained in excretory secretions of mammals and secondary granules of neutrophils [14]. It constitutes an important element of the innate immunity system by regulating host immune responses to invading pathogens and also providing an environment for the development of adaptive immune responses. LF functions at the check points of many immune responses and provides homeostatic effects for a variety of stress-induced immune imbalances due to infection, trauma, and burns, showing prophylactic and therapeutic properties [15–17]. The involvement of LF in controlling the process of myelopoiesis has been a matter of controversy for the last three decades (reviewed in [18,19]). There are two opposing assessments of the LF role in myelopoiesis; the first view postulates a negative regulation [20–22], and the second one suggests the stimulatory function of LF in granulopoiesis in response to an infectious signal (a demand model) [23,24]. In addition, there are reports that LF has no effect on myelopoiesis [25,26]. Nevertheless, due to a variety of experimental models and lack of homologous LF, the controversy could not be satisfactorily resolved. Recently, a new protocol for the production of recombinant mouse LF (rmLF), bearing the mammalian glycosylation pattern, was developed in the Chinese hamster ovary (CHO) cell line and its biological potency was confirmed in protection of methicillin-resistant Staphylococcus aureus infected mice (M.L. Kruzel, pers. comm., May, 2013). This novel rmLF is fully homologous with the native mouse LF and became a valuable tool to verify the role of homologous LF in myelopoiesis. Although the effect of milk-derived mouse LF on myelopoiesis has been previously reported [23], here we also compare this effect with nonhomologous LFs, including bovine and human species.
The aim of this study was to analyze the effects of rmLF on cell compositions in the circulating blood and bone marrow, and the appearance of myelopoiesis relevant mediators in CBA mice. Based on this study, we postulate that the actual action of LF on myelopoiesis is regulatory leading to increased protection against potential microbial infection by alteration of distorted balance between the myeloid and lymphoid cell populations.
Materials and Methods
Mice
CBA female mice, 8 weeks old, from the Animal Facility in Ilkowice, Poland, were used in the study. The mice were fed granulated food and filtered tap water ad libitum. The study was approved by the local ethics committee.
Reagents
Low endotoxin rmLF and human recombinant lactoferrin (rhLF), (<1.0 EU/mg) were obtained from PharmaReview Corporation. Bovine milk-derived LF (bLF) was purchased from Sigma Chemical. Mouse transferrin (ChromPure Mouse Transferrin, code: 015-000-050) was from Jackson ImmunoResearch Laboratories. The ELISA kit for mouse IL-6 was obtained from eBioscience and the mouse haptoglobin ELISA kit was purchased from GenWay.
Treatment of mice with LF
LFs and transferrin (a control protein) were dissolved in 0.9% NaCl, filtered through 0.2-μm Millipore filters, and injected intravenously into mouse retro-orbital plexus (200 μL). Control mice were given 0.9% NaCL intravenously.
Analysis of peripheral blood and bone marrow compositions
The mice were anesthetized with isoflurane and blood was obtained from the retro-orbital plexus. Then, the mice were sacrificed by cervical dislocation, femurs were isolated, and bone marrow was obtained by eluting the bone content with a syringe equipped with 0.45-mm needle. The blood and bone marrow smears were prepared on microscopic slides. After drying, the smears were stained with Giemsa and May-Grünwald reagents. The smears were subsequently evaluated at 1,000× magnification (in immersion oil) in a Leica ATC 2000 microscope. Up to 100 cells were counted per slide. The results were presented as a percentage of cell types in the peripheral blood (mature neutrophils, neutrophil precursors—bands, eosinophils, lymphocytes, and monocytes). In the bone marrow, the following cell types were identified: myelocytes, metamyelocytes, bands, and mature neutrophils. The unidentified cells were classified as other. Mean values for each group are shown.
Determination of cytokines and haptoglobin in serum
The mice were anesthetized with isoflurane and blood was collected at 2, 6, and 24 h after intravenous injection of LF. The blood samples were centrifuged, the supernatants were collected, and subsequently frozen at −80°C. The levels of the examined factors were determined using commercial ELISA kits.
Preparation of histological sections
Mouse femurs were fixed in a 4.0% formalin solution for 24 h. Sections of femur were subjected to demineralization using a mixture of sodium citrate and formic acid at a 1:1 ratio, followed by dehydration and paraffin embedding. Fragments of the preparations were subsequently serially cut (6- to 7-μm sections) and stained with hematoxylin and eosin (H&E). The sections were evaluated using a light microscope Nikon Eclipse 80i at 1,000× magnification; the histologist (P.K.) viewing and interpreting the slides was blinded to the type of experiment and treatment.
Statistics
The results are presented as mean values±standard error. The Brown–Forsyth's test was used to determine the homogeneity of variance between groups. When the variance was homogenous, analysis of variance (one-way ANOVA) was applied, followed by post hoc comparisons with the Tukey's test to estimate the significance of the difference between groups. Nonparametric data were evaluated with the Kruskal–Wallis' analysis of variance, as indicated in the text. Significance was determined at P<0.05. Statistical analysis was performed using STATISTICA 6.1 for Windows.
Results
Effects of administration of selected LFs on blood and bone marrow cell composition
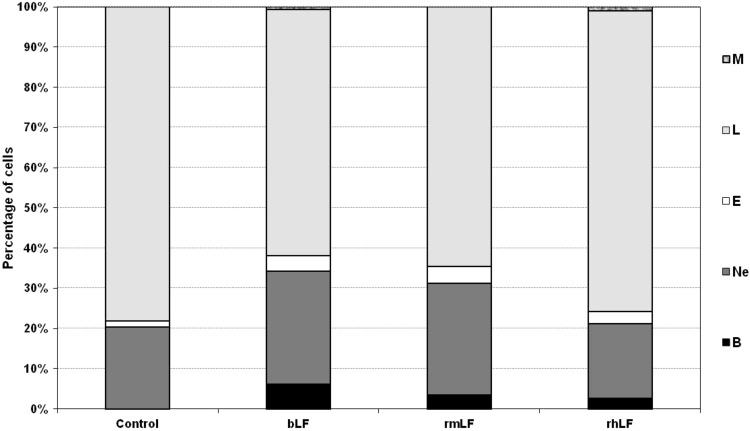
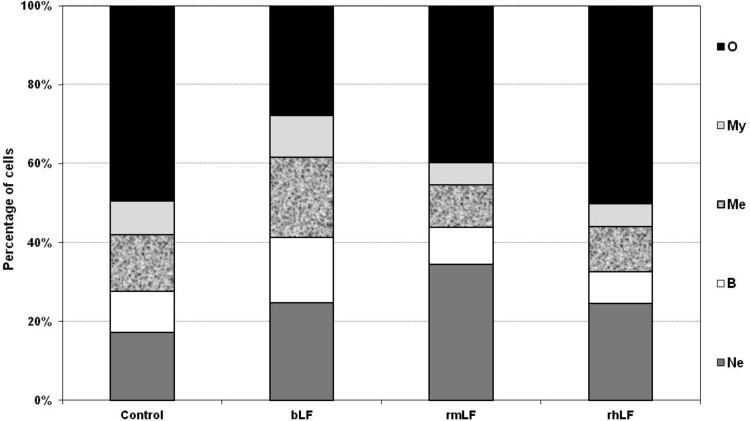
In a preliminary experiment, LFs from three different species (bovine milk-derived, recombinant mouse and recombinant human) were used. The homology of rmLF with the native mouse LF has been confirmed by the amino acid sequence (accession no. AAH06904), sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), and western blot analysis. LFs were given intravenously at 1 mg dose per mouse and after 24 h, the cell type compositions in the circulating blood and bone marrow were analyzed. bLF, which has already been shown to elicit myelopoiesis in mice [6] and humans [27], induced a significant rise in the content of myelocytic cells (bands, neutrophils, and eosinophils) in the circulating blood. A significant increase in the content of respective cell types of the myelocytic lineage also occurred upon administration of rmLF. However, in the case of human rhLF, the increase was less pronounced and did not regard the mature neutrophil pool (Fig. 1). Treatment of mice with LFs also affected the composition of bone marrow (Fig. 2). Interestingly, bLF proportionally augmented the content of each cell type of the myelocytic lineage (neutrophils, bands, metamyelocytes, and myelocytes) and overall, the increase in the content of these cells was the highest among the LFs studied. On the other hand, rmLF predominantly increased the pool of mature neutrophils with a concomitant decrease of less mature cell types, in particular, metamyelocytes and myelocytes. The total content of the myelocytic cells was not significantly changed by treatment with the rhLF. In this case, the percentage of neutrophils was elevated similarly as by bLF, but the content of less mature cells was diminished.
FIG. 1.
Analysis of circulating blood cell composition 24 h following administration of LFs. Mice were given LF (1 mg). The results are presented as the mean value of five mice per group. Statistics: Bands: control versus bLF, P=0.0001; control versus rmLF, P<0.0002; control versus rhLF, P<0.0005; neutrophils: control versus bLF, P<0.005; control versus rmLF, P<0.01; control versus rhLF, NS (P=0.75); eosinophils: control versus bLF, NS (P=0.06); control versus rmLF, P<0.025; control versus rhLF, NS (P=0.3); lymphocytes: control versus bLF, P<0.0002; control versus rmLF, P<0.0005; control versus rhLF, NS (P=0.4); monocytes: control versus bLF, P<0.02; control versus rmLF, NS (P=1.0); control versus rhLF, P<0.0005. B, bands; Ne, neutrophils; E, eosinophils; L, lymphocytes; M, monocytes; NS, not significant; LF, lactoferrin; rmLF, recombinant mouse LF; rhLF, human recombinant lactoferrin; bLF, bovine milk-derived LF.
FIG. 2.
Analysis of bone marrow cell types 24 h following administration of LFs. Mice were given LF (1 mg). The results are presented as the mean value of five mice per group. Statistics: Neutrophils: control versus bLF, NS (P=0.1699); control versus rmLF, P<0.0005; control versus rhLF, NS (P=0.12); bands: control versus bLF, P<0.05; control versus rmLF, NS (P=0.94); control versus rhLF, NS (P=0.5); metamyelocytes: control versus bLF, P<0.01; control versus rmLF, NS (P=0.08); control versus rhLF, NS (P=0.22); myelocytes: control versus bLF, NS (P=0.5); control versus rmLF, NS (P=0.16); control versus rhLF, NS (P=0.2); other: control versus bLF, P<0.0002; Control versus rmLF, P<0.02; control versus rhLF, NS (P=0.98). Ne, neutrophils; B, bands; Me, metamyelocytes; My, myelocytes; O, other.
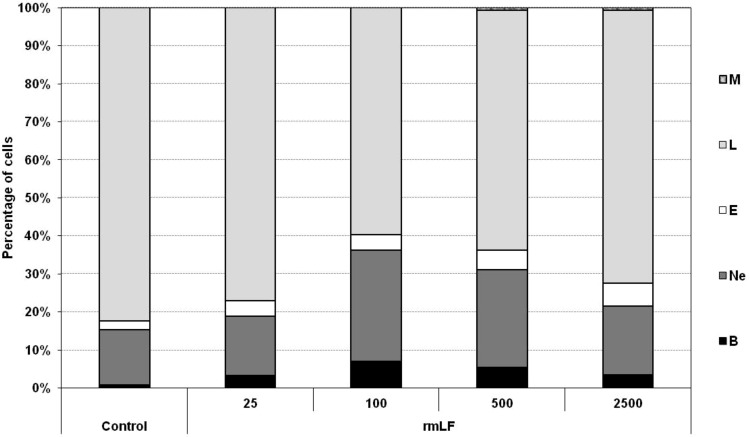
Determination of optimal dose of rmLF
For subsequent experiments, it was important to establish the optimal dose of rmLF, which would induce the highest output of myeloid-derived cells to the circulation. The following doses of rmLF (per mouse) were tested: 25, 100, 500, and 2,500 μg. The best effect was achieved at 100 μg dose (40% of myelocytic cells vs. 17.6% in NaCl control). Significant increases of the respective cell types in the peripheral blood were registered: bands (0.8% vs. 6.2%), neutrophils (14.6% vs. 29.4%), and eosinophils (2.2% vs. 4.0%). Higher doses were less effective with exception of the eosinophil levels (Fig. 3).
FIG. 3.
Release of myeloid lineage cells into circulation–determination of optimal dose of rmLF. Mice were given rmLF in the following doses: 25, 100, 500, and 2,500 μg. Samples of blood were taken 24 h after administration of LF. The results are presented as the mean value of five mice per group. Statistics: Bands: control versus rmLF 25, P<0.02; control versus rmLF 100, P<0.0002; control versus rmLF 500, P<0.0002; control versus rmLF 2,500, P<0.01; neutrophils: control versus rmLF 25, NS (P=0.99); control versus rmLF 100, P<0.002; control versus rmLF 500, P<0.05; control versus rmLF 2,500, NS (P=0.9); eosinophils: control versus rmLF 25, P<0.02; control versus rmLF 100, P<0.02; control versus rmLF 500, P<0.0005; control versus rmLF 2,500, P<0.0002; lymphocytes: control versus rmLF 25, NS (P=0.24); control versus rmLF 100, P<0.0002; control versus rmLF 500, P<0.0002; control versus rmLF 2,500, P<0.0005; monocytes: control versus rmLF 25, NS (P=1.0); control versus rmLF 100, NS (P=1.0); control versus rmLF 500, NS (P=0.083); control versus rmLF 2,500, NS (P=0.083). B, bands; Ne, neutrophils; E, eosinophils; L, lymphocytes; M, monocytes.
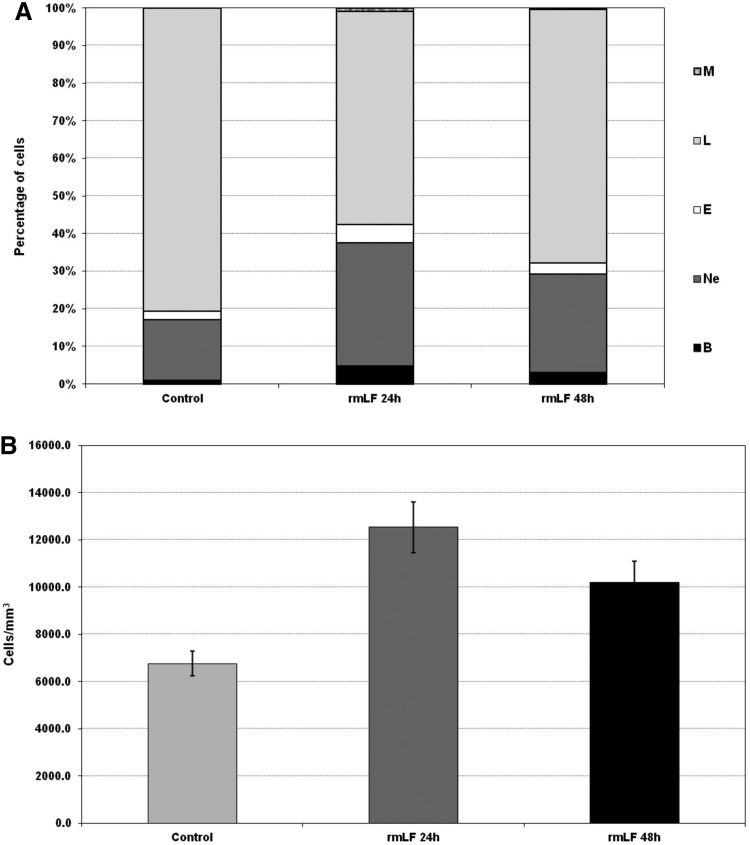
LF-induced myelopoiesis is correlated with increase of blood leukocytes
In the next experiment, we demonstrated that the increases in the myelocytic cell-type content in blood after 24 and 48 h following administration of the optimal rmLF dose (100 μg/mouse) (Fig. 4A) were closely correlated with a significant rise in the leukocyte numbers (Fig. 4B).
FIG. 4.
Blood cell composition (A) and numbers of circulating leukocytes (B) at 24 and 48 h after administration of rmLF. Mice were given rmLF (100 μg). The results are presented as the mean value of five mice per group. Statistics: (A) Bands: control 24 h versus rmLF 24 h, P<0.001; control 48 h versus rmLF 48 h, P<0.05; rmLF 24 h versus rmLF 48 h, NS (P=0.17); neutrophils: control 24 h versus rmLF 24 h, P<0.0005; control 48 h versus rmLF 48 h, P<0.01; rmLF 24 h versus rmLF 48 h, NS (P=0.069); eosinophils: control 24 h versus rmLF 24 h, P<0.0005; control 48 h versus rmLF 48 h, NS (P=0.20); rmLF 24 h versus rmLF 48 h, P<0.005; lymphocytes: control 24 h versus rmLF 24 h, P<0.0002; control 48 h versus rmLF 48 h, P<0.005; rmLF 24 h versus rmLF 48 h, P<0.02; monocytes: control 24 h versus rmLF 24 h, P<0.0005; control 48 h versus rmLF 48 h, NS (P=0.16); rmLF 24 h versus rmLF 48 h, P<0.005. (B) Control 24 h versus rmLF 24 h, P<0.001; control 48 h versus rmLF 48 h, P<0.05; rmLF 24 h versus rmLF 48 h, NS (P=0.17). There were no statistical differences between control 24 h and 48 h—in figure only 24 h control was shown. B, bands; Ne, neutrophils; E, eosinophils; L, lymphocytes; M, monocytes.
Changes in blood and bone marrow cell composition 24 and 48 h following administration of the optimal dose of rmLF
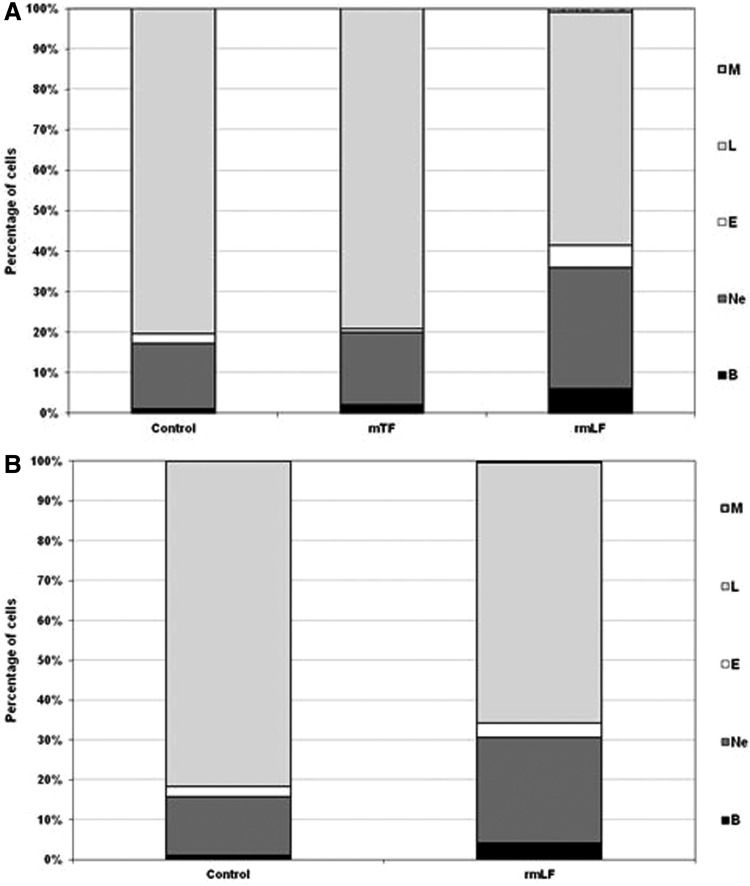
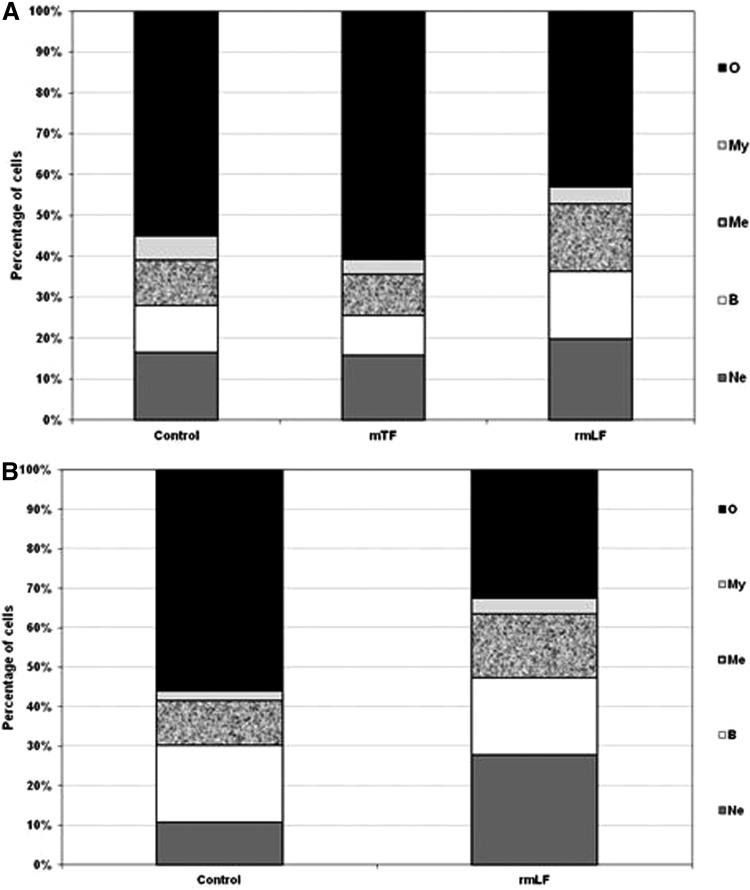
After establishing the optimal dose of rmLF for the induction of myelopoiesis, we compared the effects of rmLF on blood and bone marrow cell composition at 24 and 48 h following administration of the protein. In addition, we applied mouse serum transferrin (mTF), due to its close functional relation, as a control. The results are shown in Figures 5A, B and 6A, B. It appeared that the total content of the myelocytic cell lineage in the circulating blood at 24 h rose from 18.5% in the control to 43.33% in rmLF-treated mice (Fig. 5A) However, 48 h following the rmLF injection, the increase was somewhat lower (from 19.25% to 34.57%) (Fig. 5B). mTF exerted nonsignificant changes in the blood and bone marrow cell compositions (Figs. 5A and 6A) after 24 h. Due to the lack of myelopoietic activity of mTF after 24 h, the 48-h measurement was not performed in this group. Contrary to the blood cell composition, the total content of the myelocytic cell lineage in the bone marrow after 48 h was higher (Fig. 6B) than after 24 h (Fig. 6A) (increase from 42.5% to 67.67% vs. 45.2% to 57.0%). More specifically, the highest, relative increase was observed in cases of mature neutrophils (15.80% vs. 27.71%) followed by metamyelocytes (10.00% vs. 16.00%) and bands (16.43% vs. 19.29%).
FIG. 5.
Composition of the peripheral blood cell picture at 24 h (A) and 48 h (B) following administration of rmLF and mTF—a control protein. Mice were given rmLF and mTF (100 μg). The results are presented as the mean value of five mice per group. Statistics: (A) Bands: control versus mTF, NS (P=0.62); control versus rmLF, P<0.0002; mTF versus rmLF, P<0.0005; neutrophils: control versus mTF, NS (P=0.98); control versus rmLF, P<0.0005; mTF versus rmLF, P<0.002; eosinophils: control versus mTF, NS (P=0.07); control versus rmLF, P<0.0002; mTF versus rmLF, P<0.0002; lymphocytes: control versus mTF, NS (P=0.73); control versus rmLF, P<0.0002; mTF versus rmLF, P<0.0002; monocytes: control versus mTF, NS (P=1.0); control versus rmLF, NS (P=0.12); mTF versus rmLF, NS (P=0.12). (B) Bands: control versus rmLF, P<0.002; neutrophils: control versus rmLF, P<0.002; eosinophils: control versus rmLF, NS (P=0.31); lymphocytes: control versus rmLF, P<0.0002; monocytes: control versus rmLF, NS (P=0.85). B, bands; Ne, neutrophils; E, eosinophils; L, lymphocytes; M, monocytes; mTF, mouse serum transferrin.
FIG. 6.
Cellular composition of the bone marrow 24 h (A) and 48 h (B) following rmLF treatment. Mice were given rmLF and mTF (100 μg). The results are presented as the mean value of five mice per group. Statistics: (A) Neutrophils: control versus mTF, NS (P=0.89); control versus rmLF, NS (P=0.06); mTF versus rmLF, P<0.01; bands: control versus mTF, NS (P=0.99); control versus rmLF, NS (P=0.61); mTF versus rmLF, NS (P=0.45); metamyelocytes: control versus mTF, NS (P=0.97); control versus rmLF, NS (P=0.11); mTF versus rmLF, P<0.05; myelocytes: control versus mTF, NS (P=0.19); control versus rmLF, NS (P=0.44); mTF versus rmLF, NS (P=0.98); other: control versus mTF, NS (P=0.38); control versus rmLF, P<0.02; mTF versus rmLF, P<0.0005. (B) Neutrophils: control versus rmLF, P<0.001; bands: control versus rmLF, P<0.02; metamyelocytes: control versus rmLF, NS (P=0.21); myelocytes: control versus rmLF, NS (P=0.48); others: control versus rmLF, P<0.0002. Ne, neutrophils; B, bands; Me, metamyelocytes; My, myelocytes; O, other.
Induction of IL-6 and haptoglobin by rmLF
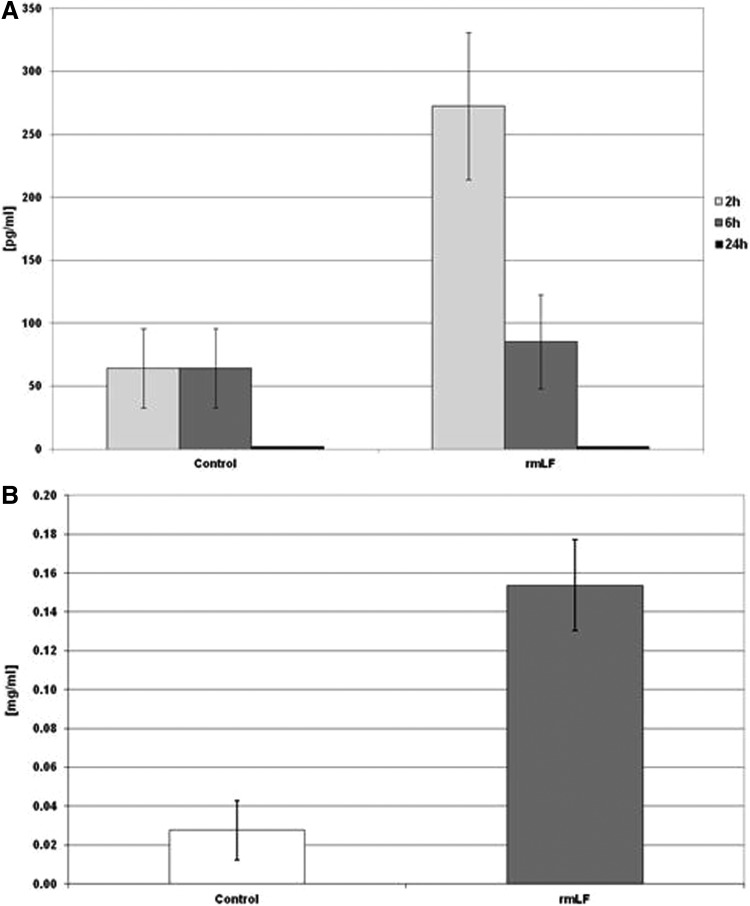
Determination of IL-6 serum levels (Fig. 7A) revealed about fourfold increase of concentration of this cytokine at 2 h postinjection, compared to control mice. IL-6 concentrations fell sharply at 6 h (no difference with the control) and were not detectable after 24 h. There was no significant IL-1 and GM-CSF serum concentration increase at indicated time intervals (not shown). In addition, 24 h after rmLF administration, increased concentrations of plasma haptoglobin (about fivefold above control) were determined (Fig. 7B).
FIG. 7.
IL-6 (A) and haptoglobin (B) serum levels. IL-6 concentration was determined at: 2, 6, and 24 h and haptoglobin level was measured at 24 h following rmLF injection. The results are presented as the mean value of seven mice per group. Statistics: (A) Control 2 h versus rmLF 2 h, P<0.05; control 6 h versus rmLF 6 h, NS (P=0.69); control 24 h versus rmLF 24 h, UD. (B) Control versus rmLF, P<0.005. UD, undetectable; IL, interleukin.
Histological evaluation of the bone marrow
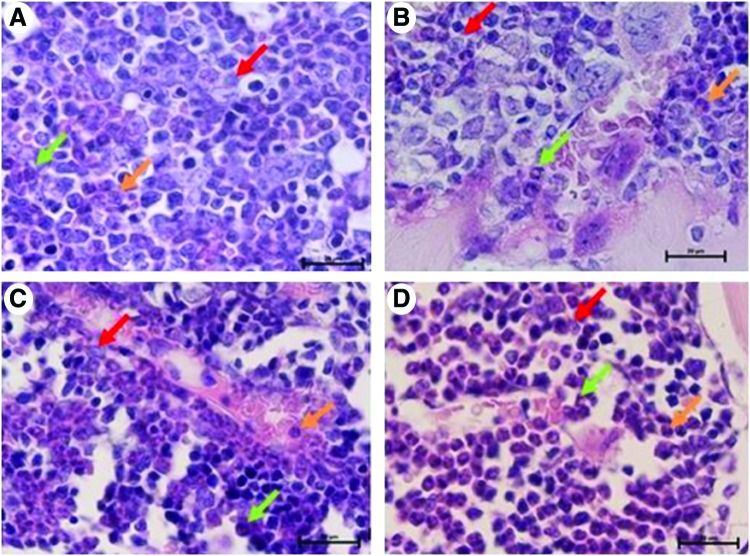
The analysis of bone marrow sections after 24 and 48 h following administration of rmLF indicates induction of myelopoiesis and to a lesser degree in relation to erythroid lineages. Neither growth of adipocytes nor fibroblast lineage cells was observed. After 24 h following rmLF administration (Fig. 8C), an increase in the number of myelocytes and band forms is observed in comparison to control bone marrow. After 48 h (Fig. 8D), even higher mobilization of the myelocytic cell lineage is registered, with a predominance of metamyelocytes, bands, and neutrophils. In mice given mTF (Fig. 8B), the composition of cell types resembled that of control, saline-treated mice (Fig. 8A).
FIG. 8.
Visualization of myelopoiesis in sections of femurs derived from mice treated under various protocols (1,000× magnification, H&E staining). (A) Control mouse treated with 0.9% NaCl. In the bone marrow, colonies of all cell lineages are seen with a not significant prevalence of the erythropoietic lineage. Within leukopoiesis, the most dominant is myelopoiesis. (B) Control mouse treated with transferrin. The picture does not differ from the previous one. (C) Mouse treated with rmLF after 24 h shows activation of the bone marrow. An increase of the myelocyte number and band forms in bone marrow sinusoids as compared to control mice. (D) Mouse treated with rmLF after 48 h. Even more intense myelopoiesis; the strongest for metamyelocytes, bands, and neutrophils. myelocyte, red arrow; band, orange arrow; neutrophil, green arrow; H&E, hematoxylin and eosin. Color images available online at www.liebertpub.com/scd
Discussion
Investigations on the role of LF in regulation of myelopoiesis have a long history, yet there is no consensus and no definite conclusions have been drawn [19]. The primary obstacle was the difficulty in obtaining homologous LF, used only in one study [23], to perform in vivo experiments with confidence of species-specific responses.
In this investigation, we demonstrated that novel, fully homologous rmLF given intravenously induced myelopoiesis in mice. The homology of rmLF with the native mouse LF has been confirmed earlier by the amino acid sequence, SDS-PAGE, and western blot analyses and further supported by use of the CHO expression system that provides mammalian type of post-translational modifications, including glycosylation. Importantly, serum mouse transferrin used in this study as a control protein showed no such effect. Although this result only confirmed the original finding [23] with a milk-derived native mouse LF, our study added more information on the kinetics of LF-induced myelopoiesis as well as presented histological examinations of the circulating blood and bone marrow. In the original report [23], the authors showed that intravenous injection of mouse LF (2 mg/mouse) led to an increase of plasma colony stimulating factors after 12 h, followed by the increase in bone marrow granulocyte-macrophage progenitor cells at 48 h. Of particular importance in our study is the fact that rmLF is not immunogenic in mice as both amino acid sequence and the glycan portion of rmLF are fully compatible with the host. Therefore, the signals transmitted to cells of the mouse immune system by exogenous rmLF could not be associated with antigen recognition, which may occur upon injection of LF from other species.
The analysis of blood and bone marrow cell composition confirmed that mouse bone marrow is a significant reservoir of mature granulocytes [28] such that, at 24 h after rmLF injection, a strong increase in the content of neutrophils and their immediate precursors as well as eosinophils in the peripheral blood could be attained. Following 48 h, the histological analysis of femur bone marrow sections confirmed a restoration of the neutrophil content, accompanied by increases in the fractions of metamyelocytes and myelocytes. In addition, a penetration of immature and mature neutrophils into bone marrow blood vessels could be demonstrated indicating the process of cell release into the circulation.
In addition, our study showed a difference in the ability of mouse, bovine, and human LFs to induce myelopoiesis, which could be due to the fine specificity in the reaction of rmLF with target cells. The potent action of bovine milk LF in this process was probably associated with a high content of mannose in its glycan component [29], thus resembling the sugar composition of yeast and bacteria [30], and with immunogenicity associated with only partial amino acid sequence homology with mouse LF. In bovine LF, the glycan moiety constitutes 10% of the molecular weight and is exceptionally rich in mannose [29]. Human LF contains a smaller glycan component and a much lower amount of mannose residues which are, in addition, not exposed for interactions with potential cell surface receptors [31].
The sequence homology between human LF and bovine is 69% and between bovine and mouse is 64%. Therefore, providing similar amino acid sequence of these proteins seems that the glycan characteristics could account for its potent adjuvant properties in mice [32] as well as for the induction of myelopoiesis in volunteers [27] and in mice [6,33].
The induction of myelopoiesis by LF resembles in some respects the phenomenon of sterile inflammation, as in the case of casein-induced neutrophilia [33]. In that model, casein induced serum IL-6 and G-CSF, however, TNF-α, IL-1, IFN-γ, and GM-CSF were not detectable. In our investigation, the appearance of IL-6 was evident, but we also could not demonstrate the presence of IL-1 and GM-CSF. In addition, similarly, as in the case of sterile inflammation [34], LF-induced neutrophilia was protective against subsequent infection of mice with Escherichia coli [33]. The elevation of haptoglobin serum concentrations, one of the acute-phase proteins, could be expected as IL-6 was shown to induce acute-phase protein production in the liver [35]. In addition, haptoglobin may be released from neutrophils upon activation [36].
It is obvious that endogenous LF, as an acute-phase protein, represents only one element in a chain of reactions in response to septic [37] or sterile stimuli [38]. So, it is not surprising that the administration of LF to normal healthy animals may result in inflammatory-like phenomena [39,40]. Therefore, the actual immunoregulatory roles of LF may be properly evaluated only in in vivo models, where animals are subjected to pathologic conditions. In our in vivo experimental models, both up- and downregulatory actions of LF, given orally or intraperitoneally, were documented. For example, a stimulation of myelopoiesis by pretreatment of mice with LF was demonstrated in bacteremia, which was associated with protection [33]. Similarly, in mice subjected to chemotherapy and bone marrow transplant, the bone marrow was more rapidly repopulated in LF treated than in control mice [41]. On the other hand, in mice given a sublethal dose of cyclophosphamide (CP), LF inhibited a rebound in CP-induced neutrophilia and eosinophilia and led to normalization of the blood picture (increased percentage of lymphocytes) [42]. That action was also correlated with decreased production of IL-6 in cultures of alveolar macrophages [43].
In summary, we demonstrated that mouse recombinant LF can induce myelopoiesis, as evidenced by significant changes in blood and bone marrow compositions, which were accompanied by the release of IL-6 and haptoglobin into the circulation. We propose that the physiologic role of LF in myelopoiesis should be discussed in a broad context taking into consideration the metabolic disturbances due to trauma, infection, or therapeutic interventions. The understanding of the nature of LF has substantially evolved and at present, the protein is commonly perceived as a sensor of immune status. Thus, regarding the process of myelopoiesis, LF may, in our opinion, inhibit or enhance that process, and in both cases these actions serve as preventive or protective measures for the immune system against a wide range of metabolic disturbances, including infection or therapy-induced insults.
Acknowledgments
This work was supported, in part, by NIH grant 2R42A1051 050-04. We also acknowledge PharmaReview, Corp. (Houston, TX) for the kind gifts of rLFs.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Quinton LJ, Dale DC. and Nelson. S. (2005). Use of colony-stimulating factors for treatment of neutropenia and infectious diseases. In: The Neutrophils: New Outlook for Old Cells. Galbrilovich D, ed., 2nd edn. Imperial Collage Press, London, pp. 301–306 [Google Scholar]
- 2.Delano MJ, Kelly-Scumpia KM, Thayer TC, Winfield RD, Scumpia PO, Cuenca AG, Harrington PB, O'Malley KA, Warner E, et al. (2011). Neutrophil mobilization from the bone marrow during polymicrobial sepsis is dependent on CXCL12 signaling. J Immunol 187:911–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chandra R, Villanueva E, Feketova E, Machiedo GW, Hasko G, Deitch EA. and Spolarics Z. (2008). Endotoxemia down-regulates bone marrow lymphopoiesis but stimulates myelopoiesis: the effect of G6PD deficiency. J Leukoc Biol 83:1541–1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Noel JG, Valente JF, Ogle JD, Cornelius J, Custer DA, Li BG, Alexander JW. and Ogle CK. (2002). Changes in bone marrow-derived myeloid cells from thermally injured rats reflect changes in the progenitor cell population. J Burn Care Rehabil 23:75–86 [DOI] [PubMed] [Google Scholar]
- 5.Laakko T. and Fraker P. (2002). Rapid changes in the lymphopoietic and granulopoietic compartments of the marrow caused by stress levels of corticosterone. Immunology 105:111–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zimecki M, Artym J. and Kocieba M. (2009). Endogenous steroids are responsible for lactoferrin-induced myelopoiesis in mice. Pharmacol Rep 61:705–710 [DOI] [PubMed] [Google Scholar]
- 7.Mountford JC, Bunce CM, Hughes SV, Drayson MT, Webb D, Brown G. and Hewison M. (1999). Estrone potentiates myeloid cell differentiation: a role for 17 beta-hydroxysteroid dehydrogenase in modulating hemopoiesis. Exp Hematol 27:451–460 [DOI] [PubMed] [Google Scholar]
- 8.Katsura Y. and Kawamoto H. (2001). Stepwise lineage restriction of progenitors in lympho-myelopoiesis. Int Rev Immunol 20:1–20 [DOI] [PubMed] [Google Scholar]
- 9.Crown J, Jakubowski A. and Gabrilove J. (1993). Interleukin-1: biological effects in human hematopoiesis. Leuk Lymphoma 9:433–440 [DOI] [PubMed] [Google Scholar]
- 10.Hauser SP, Kajkenova O. and Lipschitz DA. (1997). The pivotal role of interleukin 6 in formation and function of hematopoietically active murine long-term bone marrow cultures. Stem Cells 15:125–132 [DOI] [PubMed] [Google Scholar]
- 11.Broxmeyer HE, Williams DE, Cooper S, Ralph P, Gillis S, Bicknell DC, Hangoc G, Drummond R. and Lu L. (1988). Synergistic interaction of hematopoietic colony stimulating and growth factors in the regulation of myelopoiesis. Behring Inst Mitt 83:80–84 [PubMed] [Google Scholar]
- 12.Johnson A. and Dorshkind K. (1986). Stromal cells in myeloid and lymphoid long-term bone marrow cultures can support multiple hemopoietic lineages and modulate their production of hemopoietic growth factors. Blood 68:1348–1354 [PubMed] [Google Scholar]
- 13.Hibbs ML, Quilici C, Kountouri N, Seymour JF, Armes JE, Burgess AW. and Dunn AR. (2007). Mice lacking three myeloid colony-stimulating factors (G-CSF, GM-CSF, and M-CSF) still produce macrophages and granulocytes and mount an inflammatory response in a sterile model of peritonitis. J Immunol 178:6435–6443 [DOI] [PubMed] [Google Scholar]
- 14.Masson PL, Heremans JF. and Schonne E. (1969). Lactoferrin, an iron-binding protein in neutrophilic leukocytes. J Exp Med 130:643–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Legrand D, Elass E, Carpentier M. and Mazurier J. (2006). Interactions of lactoferrin with cells involved in immune function. Biochem Cell Biol 84:282–290 [DOI] [PubMed] [Google Scholar]
- 16.Kruzel ML, Actor JK, Boldogh I. and Zimecki M. (2007). Lactoferrin in health and disease. Postepy Hig Med Dosw (Online) 61:261–267 [PubMed] [Google Scholar]
- 17.Zimecki M. and Kruzel ML. (2007). Milk-derived proteins and peptides of potential therapeutic and nutritive value. J Exp Ther Oncol 6:89–106 [PubMed] [Google Scholar]
- 18.Bagby GC., Jr. (1989). Regulation of granulopoiesis: the lactoferrin controversy. Blood Cells 15:386–399 [PubMed] [Google Scholar]
- 19.Artym J. and Zimecki M. (2007). The effects of lactoferrin on myelopoiesis: can we resolve the controversy? Postepy Hig Med Dosw (Online) 61:129–150 [PubMed] [Google Scholar]
- 20.Broxmeyer HE. (1979). Lactoferrin acts on Ia-like antigen-positive subpopulations of human monocytes to inhibit production of colony stimulatory activity in vitro. J Clin Invest 64:1717–1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gentile P. and Broxmeyer HE. (1983). Suppression of mouse myelopoiesis by administration of human lactoferrin in vivo and the comparative action of human transferrin. Blood 61:982–993 [PubMed] [Google Scholar]
- 22.Zucali JR, Broxmeyer HE, Levy D. and Morse C. (1989). Lactoferrin decreases monocyte-induced fibroblast production of myeloid colony-stimulating activity by suppressing monocyte release of interleukin-1. Blood 74:1531–1536 [PubMed] [Google Scholar]
- 23.Sawatzki G. and Rich IN. (1989). Lactoferrin stimulates colony stimulating factor production in vitro and in vivo. Blood Cells 15:371–385 [PubMed] [Google Scholar]
- 24.Rich IN. and Sawatzki G. (1989). Lactoferrin, the signal for colony stimulating factor production? Negative-feedback regulation versus supply-and-demand regulation of myelopoiesis. Blood Cells 15:400–406 [PubMed] [Google Scholar]
- 25.Stryckmans P, Delforge A, Amson RB, Prieels JP, Telerman A, Bieva C, Deschuyteneer M. and Ronge-Collard E. (1984). Lactoferrin: no evidence for its role in regulation of CSA production by human lymphocytes and monocytes. Blood Cells 10:369–395 [PubMed] [Google Scholar]
- 26.Poppas A, Faith MR. and Bierman HR. (1986). In vivo study of lactoferrin and murine rebound myelopoiesis. Am J Hematol 22:1–8 [DOI] [PubMed] [Google Scholar]
- 27.Zimecki M, Spiegel K, Wlaszczyk A, Kubler A. and Kruzel ML. (1999). Lactoferrin increases the output of neutrophil precursors and attenuates the spontaneous production of TNF-alpha and IL-6 by peripheral blood cells. Arch Immunol Ther Exp (Warsz) 47:113–118 [PubMed] [Google Scholar]
- 28.Chervenick PA, Boggs DR, Marsh JC, Cartwright GE. and Wintrobe MM. (1968). Quantitative studies of blood and bone marrow neutrophils in normal mice. Am J Physiol 215:353–360 [DOI] [PubMed] [Google Scholar]
- 29.Coddeville B, Strecker G, Wieruszeski JM, Vliegenthart JF, van Halbeek H, Peter-Katalinic J, Egge H. and Spik G. (1992). Heterogeneity of bovine lactotransferrin glycans. Characterization of alpha-D-Galp-(1→3)-beta-D-Gal- and alpha-NeuAc-(2→6)-beta-D-GalpNAc-(1→4)- beta-D-GlcNAc-substituted N-linked glycans. Carbohydr Res 236:145–164 [DOI] [PubMed] [Google Scholar]
- 30.Lengeler KB, Tielker D. and Ernst JF. (2008). Protein-O-mannosyltransferases in virulence and development. Cell Mol Life Sci 65:528–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Graham SA, Antonopoulos A, Hitchen PG, Haslam SM, Dell A, Drickamer K. and Taylor ME. (2011). Identification of neutrophil granule glycoproteins as Lewis(x)-containing ligands cleared by the scavenger receptor C-type lectin. J Biol Chem 286:24336–24349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zimecki M, Kocieba M. and Kruzel M. (2002). Immunoregulatory activities of lactoferrin in the delayed type hypersensitivity in mice are mediated by a receptor with affinity to mannose. Immunobiology 205:120–131 [DOI] [PubMed] [Google Scholar]
- 33.Zimecki M, Artym J, Chodaczek G, Kocieba M. and Kruzel ML. (2004). Protective effects of lactoferrin in Escherichia coli-induced bacteremia in mice: relationship to reduced serum TNF alpha level and increased turnover of neutrophils. Inflamm Res 53:292–296 [DOI] [PubMed] [Google Scholar]
- 34.Noursadeghi M, Bickerstaff MC, Herbert J, Moyes D, Cohen J. and Pepys MB. (2002). Production of granulocyte colony-stimulating factor in the nonspecific acute phase response enhances host resistance to bacterial infection. J Immunol 169:913–919 [DOI] [PubMed] [Google Scholar]
- 35.Lin BF, Ku NO, Zahedi K, Whitehead AS. and Mortensen RF. (1990). IL-1 and IL-6 mediate increased production and synthesis by hepatocytes of acute-phase reactant mouse serum amyloid P-component (SAP). Inflammation 14:297–313 [DOI] [PubMed] [Google Scholar]
- 36.Theilgaard-Monch K, Jacobsen LC, Nielsen MJ, Rasmussen T, Udby L, Gharib M, Arkwright PD, Gombart AF, Calafat J, et al. (2006). Haptoglobin is synthesized during granulocyte differentiation, stored in specific granules, and released by neutrophils in response to activation. Blood 108:353–361 [DOI] [PubMed] [Google Scholar]
- 37.Gutteberg TJ, Rokke O, Andersen O. and Jorgensen T. (1989). Early fall of circulating iron and rapid rise of lactoferrin in septicemia and endotoxemia: an early defence mechanism. Scand J Infect Dis 21:709–715 [DOI] [PubMed] [Google Scholar]
- 38.Shastri KA, Logue GL, Stern MP, Raza S, O'Connor BM, Bovill JJ. and Hoover EL. (1994). Neutrophil lactoferrin release during open heart surgery is unrelated to complement activation. Asaio J 40:56–61 [PubMed] [Google Scholar]
- 39.Asako H, Kurose I, Wolf RE. and Granger DN. (1994). Mechanisms of lactoferrin-induced leukocyte-endothelial cell adhesion in postcapillary venules. Microcirculation 1:27–34 [DOI] [PubMed] [Google Scholar]
- 40.Kurose I, Yamada T, Wolf R. and Granger DN. (1994). P-selectin-dependent leukocyte recruitment and intestinal mucosal injury induced by lactoferrin. J Leukoc Biol 55:771–777 [DOI] [PubMed] [Google Scholar]
- 41.Artym J, Zimecki M, Kuryszko J. and Kruzel ML. (2005). Lactoferrin accelerates reconstitution of the humoral and cellular immune response during chemotherapy-induced immunosuppression and bone marrow transplant in mice. Stem Cells Dev 14:548–555 [DOI] [PubMed] [Google Scholar]
- 42.Artym J, Zimecki M. and Kruzel M. (2004). Normalization of peripheral blood cell composition by lactoferrin in cyclophosphamide-treated mice. Med Sci Monit 10:BR84–BR89 [PubMed] [Google Scholar]
- 43.Artym J, Zimecki M. and Kruzel ML. (2004). Effects of lactoferrin on IL-6 production by peritoneal and alveolar cells in cyclophosphamide-treated mice. J Chemother 16:187–192 [DOI] [PubMed] [Google Scholar]