Abstract
Lactate has been regarded as a waste product of anaerobic metabolism of glucose. Evidence also suggests, however, that the brain may use lactate as an alternative fuel. Our aim was to determine the extent of lactate uptake from the circulation into the brain after traumatic brain injury (TBI) and to compare it with levels of lactate in the brain extracellular fluid. We recruited 19 patients with diffuse TBI, monitored with cerebral microdialysis and jugular bulb catheters. Serial arteriovenous (AV) concentration differences of glucose and lactate were calculated from arterial and jugular blood samples, providing a measure of net uptake or export by the brain. Microdialysis was used to measure brain extracellular glucose and lactate. In 17/19 patients studied for 5 days post-injury, there were periods of net lactate uptake into the brain, most frequently on day 3 after injury. Brain microdialysate lactate had a median (interquartile range [IQR]) concentration of 2.5 (1.5–3.2) mmol/L during lactate uptake and 2.2 (1.7–3.0) mmol/L during lactate export. Lactate uptake into the brain occurred at a median (IQR) arterial lactate concentration of 1.6 (1.0–2.2) mmol/L. Lactate uptake was associated with significantly higher AV difference in glucose values with a median (IQR) of 0.4 (0.03–0.7) mmol/L during uptake and 0.1 (−0.2–0.3) mmol/L during lactate export (Mann-Whitney U p=0.003). Despite relatively high brain lactate compared with arterial lactate concentrations, the brain appears to up-regulate lactate transport into the brain after TBI. This may serve to satisfy greater demands for energy substrate from the brain after TBI.
Key words: : metabolism, microdialysis, traumatic brain injury (human)
Introduction
The pathophysiology of traumatic brain injury (TBI) involves complex and variable changes to cerebral energy metabolism. Some of the earliest evidence in support of changes to energy metabolism originated from studies that found elevated lactate levels in the cerebrospinal fluid of patients with TBI.1 More recently, microdialysis, which permits the sampling of molecules from the brain extracellular fluid, reveals elevated lactate levels after TBI that relate to unfavorable outcomes, particularly during the first 72 hours after injury.2
Lactate has been perceived as a waste product of anaerobic metabolism, produced when oxygen provision is insufficient to meet demand. This cannot be the complete picture, however, because elevated lactate levels are observed after TBI despite seemingly adequate oxygen delivery.3 Evidence also points toward the importance of mitochondrial dysfunction after TBI.4–6 A failure of mitochondrial enzymes, including pyruvate dehydrogenase, which funnels pyruvate into the tricarboxylic acid (TCA) cycle, increases the amount of lactate generated from pyruvate.7 As well as reflecting hypoxia and/or mitochondrial dysfunction, elevated lactate levels may also reflect enhanced uptake of lactate from the circulation.
Carbon-14 (14C) lactate autoradiography experiments demonstrate accumulation of radiolabel at the injury site after fluid percussion injury in rats.8 In addition, evidence exists for lactate uptake from the circulation into the human brain in normal volunteers (with lactate infused intravenously and/or produced endogenously by exercise),9,10 as well as in sedated, ventilated patients with TBI.11,12 Further, lactate infused via a microdialysis catheter into the human brain after TBI has been shown using carbon-13 (13C) labeling and nuclear magnetic resonance (NMR) spectroscopy to be used via the TCA cycle.13
The concept of lactate functioning as a cerebral energy source has been suggested by Pellerin and Magistretti,14,15 who in 1994 introduced the principles of a mechanism that later became known as the astrocyte-neuron lactate shuttle (ANLS) hypothesis. The ANLS model describes a metabolic relationship between neurons and astrocytes whereby neurons preferentially metabolize lactate (which is synthesized from blood-borne glucose by astrocytes) as an oxidative fuel.14,15 As a logical extension of the hypothesis, the elevated lactate levels found in the brain after TBI may not only reflect increased generation from hypoxia and mitochondrial dysfunction, but also increased uptake of lactate from the circulation for use as an energy substrate.15
The aim of the present study was to explore the uptake and release of endogenous lactate by the injured brain using a combination of arteriovenous (AV) concentration gradients and cerebral microdialysis in patients with TBI. In particular, this study investigated the inter-relationships between arterial lactate concentration, brain extracellular lactate concentration, and lactate import versus export by the brain, as well as the time-variant nature of the process, during the first days after injury.
Methods
Patients
This study was approved by the Cambridgeshire (2) Local Research Ethics Committee. Patients (more than 16 years old) with severe TBI, defined as those suffering cranial trauma with consistent CT scan findings and with a post-resuscitation Glasgow Coma Scale≤8 were recruited. Patients were treated according to local TBI management protocols, which include endotracheal intubation, ventilation, sedation, muscular paralysis, and jugular bulb oximetry. For the purposes of this study, monitoring data were analyzed for 5 days from recruitment.
Invasive brain monitoring
A triple lumen cranial access device (Technicam, Newton Abbot, UK) was used for each patient. This permitted placement of an intracranial pressure (ICP) monitor (Codman, Raynham, MA), a microdialysis catheter (CMA 71, M Dialysis AB, Stockholm, Sweden), and a Licox® brain tissue oxygen sensor (GMS, Kiel-Mielkendorf, Germany) into the right frontal white matter. The microdialysis catheter was perfused with CNS perfusion fluid (M Dialysis AB), which consists of NaCl (147 mM), KCl (2.7 mM), CaCl2 (1.2 mM), and MgCl2 (0.85 mM) in water and does not contain any lactate or any other additives, at a rate of 0.3 μL/min using CMA 106 microdialysis pumps (M Dialysis AB). Microdialysis collection vials were changed hourly and analyzed at the bedside using an automated enzymatic colorimetric analyzer (ISCUS, M Dialysis AB) as per standard clinical protocols. ICP, cerebral perfusion pressure, jugular venous oxygen saturation (SjO2), and brain tissue oxygen (PbO2) data were recorded at the bedside using ICM+software (ICM+, University of Cambridge, UK).
Plasma sampling
Plasma sampling was undertaken twice daily from an arterial line and from a right-sided jugular venous catheter. We chose to use the right jugular vein because this usually constitutes the dominant venous drainage of the brain.16 The first 1 mL was discarded and then 5 mL were collected into an ethylenediaminetetraacetic acid tube and immediately centrifuged for 15 min at 1500 g at 4°C. The supernatant (plasma) was decanted and stored at −80°C until further processing was performed. Thawed plasma was filtered through 10 kDa molecular weight cut-off Amicon Ultra centrifugal filters (Millipore, Cork, Ireland) for 60 min at 8700 x g at 4°C, to remove large protein molecules. The resulting solution was analyzed for glucose and lactate concentrations using an ISCUS analyzer.
Statistical analysis
All statistical analyses were performed using SPSS19 for Mac (IBM SPSS Statistics, NY). Microdialysate sample results were compared with plasma sample results collected concurrently to provide a direct comparison in each patient at a specific time point. Statistical methods included non-parametric tests (Mann-Whitney U test and Kruskal-Wallis one-way analysis of variance) with a preselected p value of 0.05 used to indicate statistical significance. Average values are presented as median values with the interquartile range given in parentheses. Plasma sampling and invasive brain monitoring data were pooled from multiple patients to calculate pre- and post-72 h median values.
AV difference values for lactate (AVlac) were calculated as the arterial concentration minus the venous concentration and dichotomized into groups of uptake (positive values) and export (negative values). To ensure that positive and negative AVlac values robustly reflected uptake and export, respectively, values close to zero were excluded when dichotomizing the data. We conservatively selected an error margin of±4% based on the precision of analysis for the ISCUS analyzer of 1.6 to 2.7%.17 This resulted in the exclusion of AVlac measurements from −0.05 to +0.05 mmol/L (because 0.05 mmol/L is 4% of the mean arterial lactate concentration of 1.36 mmol/L found in our patients).
Results
Patients
Nineteen patients with TBI were included in this study (Table 1). Patients' microdialysis monitoring commenced at a median of 18 h (range 9–46 h) from their injury. The first plasma samples were withdrawn a median of 30 h (range 15–101 h) from injury with 29% of measurements obtained during the first 72 h after injury.
Table 1.
Patient Demography
| Patient | Age | Sex | Mechanism of injury | Post-resuscitation GCS | Pattern of injury |
|---|---|---|---|---|---|
| 1 |
44 |
F |
RTA |
E1 V1 M1 |
Diffuse |
| 2 |
25 |
M |
RTA |
E1 V1 M1 |
Diffuse |
| 3 |
27 |
F |
RTA |
E1 V1 M1 |
Diffuse |
| 4 |
58 |
F |
RTA |
E1 V1 M1 |
Diffuse |
| 5 |
61 |
F |
RTA |
E1 V1 M1 |
Diffuse |
| 6 |
49 |
M |
RTA |
E1 V2 M5 |
Contusions |
| 7 |
60 |
M |
RTA |
E1 V1 M5 |
ICH/IVH/diffuse |
| 8 |
30 |
M |
Fall |
E1 V2 M2 |
Evacuated EDH/diffuse |
| 9 |
39 |
F |
RTA |
E1 V1 M1 |
Diffuse |
| 10 |
41 |
F |
Fall |
E1 V1 M1 |
Diffuse |
| 11 |
37 |
M |
RTA |
E1 V1 M1 |
Diffuse |
| 12 |
43 |
M |
RTA |
E1 V1 M1 |
Diffuse |
| 13 |
22 |
M |
RTA |
E1 V1 M1 |
Diffuse |
| 14 |
18 |
F |
RTA |
E1 V1 M5 |
Evacuated EDH/diffuse |
| 15 |
46 |
F |
RTA |
E1 V1 M2 |
Small SDH/diffuse |
| 16 |
27 |
M |
RTA |
E1 V2 M5 |
Diffuse |
| 17 |
47 |
M |
RTA |
E1 V1 M3 |
Diffuse |
| 18 |
28 |
F |
Fall |
E1 V1 M1 |
Diffuse |
| 19 | 51 | M | Assault | E1 V2 M5 | Diffuse/contusions |
A total of 19 patients were recruited including 9 females. All patients presented with a diffuse injury and GCS from 3–8. GCS, Glasglow Coma Scale; RTA, road traffic accident; ICH, intracerebral hematoma; IVH, intraventricular hemorrhage; EDH, extradural hematoma; SDH, subdural hematoma.
Arterial glucose and lactate values
Arterial glucose values ranged from 4.1 to 13.4 mmol/L with 65.7% of values between 4 and 8 mmol/L reflecting the goal-directed management of blood glucose. Arterial glucose values were lower in the first 72 h after injury with a median concentration of 6.97 (5.92–7.85) and 7.46 (6.48–8.16) mmol/L after this period (p=0.049). Arterial lactate concentrations were stable, with median concentrations of 1.12 (0.87–1.68) in the first 72 h, and 1.02 (0.69–1.59) mmol/L after this period (p=0.074) (Table 2).
Table 2.
Comparison between Measurements Taken Early (<72 h) and Later (>72 h) from Time of Injury
| First 72 h – median (IQR) | Beyond 72 h – median (IQR) | p value | |
|---|---|---|---|
| Arterial glucose (mmol/L) |
6.97 (5.92 – 7.85) |
7.46 (6.48 – 8.16) |
0.049 |
| Arterial lactate (mmol/L) |
1.12 (0.87 – 1.68) |
1.02 (0.69 – 1.59) |
0.074 |
| AV difference glucose (mmol/L) |
0.16 (−0.24 – 0.41) |
0.18 (−0.10 – 0.54) |
0.299 |
| AV difference lactate (mmol/L) |
−0.07 (−0.13 – 0.02) |
−0.05 (−0.11 – 0.04) |
0.621 |
| Brain glucose (mmol/L) |
0.77 (0.49 – 1.27) |
0.54 (0.38 – 0.86) |
0.018 |
| Brain lactate (mmol/L) |
2.37 (1.67 – 2.87) |
2.65 (1.79 – 3.25) |
0.133 |
| Brain/arterial glucose ratio |
0.11 (0.07 – 0.18) |
0.08 (0.05 – 0.11) |
0.003 |
| Lactate/pyruvate ratio |
26.5 (23.5 – 28.8) |
26.1 (22.6 – 28.7) |
0.883 |
| PbO2 (mm Hg) |
20.4 (15.7 – 28.8) |
26.3 (18.7 – 32.0) |
0.019 |
| SjO2 (%) |
77.5 (70.0 – 83.0) |
78.0 (69.5 – 83.3) |
0.982 |
| CPP (mm Hg) | 76 (72 – 81) | 74 (69 – 83) | 0.485 |
Arterial glucose was significantly lower during the early period while brain glucose and the brain/arterial glucose ratio were significantly higher. p values refer to Mann-Whitney U tests of significance. IQR, interquartile range; AV, arteriovenous; PbO2, brain tissue oxygen; SjO2, jugular venous oxygen saturation; CPP; cerebral perfusion pressure.
Glucose and lactate uptake into the brain
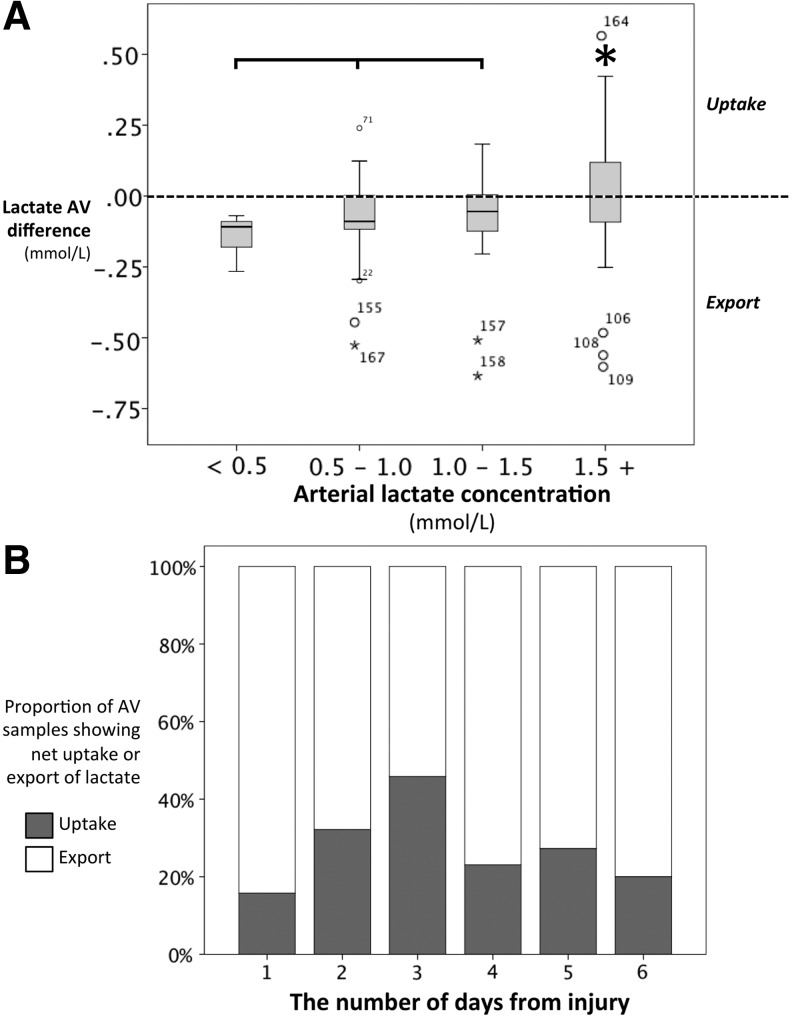
The median AV difference in glucose (AVglc) was 0.17 (−0.15–0.50) mmol/L. Positive values for AVglc indicate net uptake of glucose into the brain. The median AVlac was −0.06 (−0.11–0.02) mmol/L. Lactate uptake by the brain was demonstrated by positive AVlac values>0.05 mmol/L. This was observed in 17/19 patients and in 29% of samples. Greater AVlac values were associated with higher arterial lactate concentrations (Fig. 1A), significantly so at arterial lactate concentrations >1.5 mmol/L (p=0.001). A median arterial lactate concentration of 1.63 (0.99–2.24) mmol/L was associated with brain lactate uptake and 0.89 (0.63–1.23) mmol/L with brain lactate export (p<0.001). Lactate uptake was associated with higher AVglc values with a median of 0.42 (0.03–0.67) during uptake and 0.07 (−0.19–0.31) mmol/L during lactate export (p=0.003). The highest proportion of lactate uptake into the brain was observed on day 3 after injury (Fig. 1B).
FIG. 1.
Lactate uptake at different arterial lactate concentrations and on different days after TBI. (A) The distribution of values for arteriovenous lactate (AVlac) were significantly different across binned arterial lactate concentrations (Kruskal-Wallis p=0.004). Arterial lactate concentrations greater than 1.5 mmol/L were associated with significantly higher AVlac values, when compared with arterial lactate concentrations less than 1.5 mmol/L, as denoted by the asterisk (Mann-Whitney U p=0.001). (B) A greater proportion of lactate uptake (grey), as opposed to lactate export (white), was observed 3 days after the injury. For a definition of lactate “uptake” and “export” see Methods.
Brain glucose and lactate values
Median brain glucose and lactate concentrations, measured in microdialysis samples, were 0.66 (0.42–0.96) mmol/L and 2.46 (1.72–3.21) mmol/L, respectively. Brain glucose concentrations were statistically not significantly different during periods of lactate uptake or export with median concentrations of 0.72 (0.47–1.17) versus 0.57 (0.35–0.85) mmol/L, respectively (p=0.147). Brain lactate levels were also not significantly different during periods of lactate uptake or export with median concentrations of 2.46 (1.50–3.18) and 2.19 (1.72–3.02) mmol/L, respectively (p=0.546). The lactate/pyruvate (LP) ratio was also not significantly different with a median of 24.7 (22.9–28.6) during lactate uptake and 26.4 (23.4–28.7) during export (p=0.452).
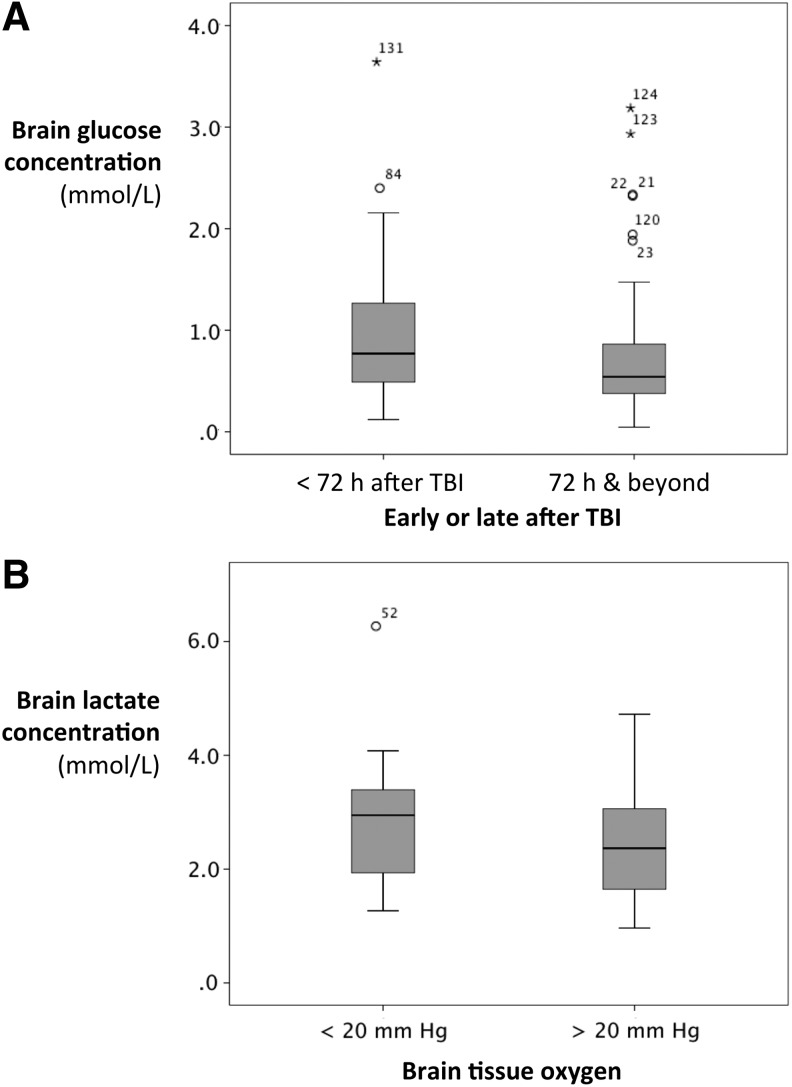
Brain glucose concentrations were higher in the first 72 h after injury when compared with values measured after this period with median concentrations of 0.77 (0.49–1.27) and 0.54 (0.38–0.86) mmol/L, respectively (p=0.018) (Table 2 and Fig. 2A). This was despite lower arterial glucose values during the first 72 h. Reflecting this, the ratio of brain/arterial glucose was higher, although AVglc values did not change significantly between these periods.
FIG. 2.
Brain glucose and lactate concentrations. (A) Brain glucose concentrations were significantly higher in the first 72 h after injury (0.77 vs. 0.54 mmol/L; Mann-Whitney U p=0.018). (B) A brain tissue oxygen partial pressure of <20 mm Hg was associated with significantly higher brain lactate concentrations as measured by microdialysis. Median brain lactate was 2.94 mmol/L when PbO2 was <20 mm Hg and 2.37 when PbO2 was >20 mm Hg (Mann Whitney U p=0.026). TBI, traumatic brain injury.
Cerebral perfusion/oxygenation
The majority of plasma and microdialysis samples were taken when either PbO2 or SjO2 indicated adequate cerebral perfusion. Insufficient perfusion, defined as the combination of SjO2<60% and PbO2<20 mm Hg, was observed at 4/126 sampling periods in three patients. A PbO2<20 mm Hg, suggestive of focal ischemia in the region of the Licox probe, was associated with a median microdialysate lactate of 2.94 (1.94–3.39) mmol/L. The median microdialysate lactate was 2.37 (1.65–3.06) mmol/L when PbO2 was >20 mm Hg (p=0.026) (Fig. 2B). Brain glucose, brain LP ratio, AVglc, and AVlac were not significantly different between instances of apparent ischemia and non-ischemia.
Discussion
The key findings of this study are that the injured brain imports lactate from the circulation despite relatively high brain lactate concentrations and that glucose uptake is maintained during periods of lactate uptake.
There are periods of net lactate uptake into the brain after TBI
The human brain, in resting control subjects, normally exports a small net amount of lactate into the circulation reflected by negative AV difference in lactate (AVlac) values, typically between −0.03 and −0.1 mmol/L.11,18,19 In the present study, periods of positive AVlac values, indicating net lactate uptake, were found in 17/19 patients after TBI, representing 29% of samples, peaking on day 3 after injury.
A switch to lactate uptake may be important after TBI because of a change in the metabolic priorities of glucose. After TBI, a greater proportion of glucose is used for potentially neuroprotective pathways, such as the pentose phosphate pathway (PPP), which, in contrast to glycolysis or the TCA cycle/oxidative phosphorylation, does not generate any energy. The PPP protects against oxidative stress and produces molecules that may be used in biosynthesis and cell repair. Increased glucose metabolism via the PPP has been demonstrated in patients after TBI and has also been demonstrated after experimental injury in rats.20,21 Hence, alternative fuel substrates may become recruited in an effort to maintain adequate cellular energy reserves. Lactate delivered to the human brain intravenously or via cerebral microdialysis has been shown by 13C-lactate labeling studies to be metabolized by the TCA cycle.9,13 In hippocampal slices, lactate can support normal synaptic function even in the absence of glucose,22 and in humans, lactate uptake may be associated with a concomitant reduction in glucose uptake.9,23 Hence, it appears that, not only can the brain take up lactate, but it can in at least some cases also be used preferentially over glucose for the purposes of energy generation.
Lactate uptake into the injured brain may also reflect differing susceptibilities of neurons and astrocytes to injury and different metabolic profiles given their structural and functional differences. It is also not surprising that they appear to have a degree of metabolic interdependency given the kinship that is needed for effective neurotransmission. The most well described metabolic relationship is the ANLS hypothesis proposed by Magistretti and Pellerin.14,15 In this hypothesis, astrocytes preferentially metabolize glucose to lactate, which is then taken up by neurons and converted to pyruvate for metabolism through the TCA cycle. Our results are compatible with the ANLS hypothesis. Increased lactate demand from the injured brain may reflect a neuronal energy deficit that astrocytes are not able to support, especially if the astrocytes themselves are injured or compromised by the TBI.
The significance of a peak in lactate uptake on day 3 in this group of patients is not entirely clear. Many of the pathophysiological mechanisms identified in TBI, such as cell swelling and membrane disruption, inflammatory response, mitochondrial dysfunction, and electrophysiological disturbances, place demands on cellular energy metabolism. Changes with time in brain extracellular concentrations of glucose, lactate, and other small molecules occur in the course of days post-injury, demonstrated in a microdialysis study of 223 patients with TBI.2 The overall pattern was a decline in glucose and a rise in lactate during the course of a week of monitoring. A similar pattern was seen in the present study of 12 patients, for the median brain microdialysate concentrations of glucose (first 72 h>beyond 72 h) and lactate (first 72 h<beyond 72 h) (Table 2).
Comparing these TBI data with “normal” brain extracellular physiological ranges,24 by day 3 post-injury brain extracellular glucose appears to have declined to a critical point, to which the injured brain responds by importing more lactate from the bloodstream, presumably to act as a complementary energy source. There were also time-dependent post-TBI changes in the brain microdialysate cytokine and chemokine profile, in a detailed 42-plex study of 12 patients.25,26 Day 3 post-injury appears to be in a transition period, in terms of identified cytokines and chemokines that are produced in defined time frames (e.g., interleukin [IL]-6 at 24–48 h, IL-10 at 96–144 h) and discriminate between different temporal phases of the inflammatory response. We therefore speculate that Day 3 post-injury represents the period when different pathophysiological mechanisms combine to exert the greatest metabolic demand.
The brain imports lactate against a concentration gradient
Lactate uptake into the brain is observed in several situations other than TBI. If arterial lactate concentrations are elevated sufficiently, a concentration gradient is established so that lactate is transported from the blood to the brain. Arterial lactate concentrations can be elevated experimentally by administering a lactate infusion or can dramatically rise during intense exercise.9,19,27,28 Exercise-induced arterial lactate elevation to 2.5–3.5 mmol/L results in lactate uptake by the brain as measured using AV concentration differences.19,28 In our patients, however, a median arterial lactate concentration of only 1.63 mmol/L was associated with lactate uptake by the brain against a concentration gradient (median brain extracellular lactate concentration in our patients was 2.45 mmol/L, in agreement with other TBI microdialysis studies).2,12 This suggests that lactate transporters must considerably up-regulate their capacity to transport lactate across the blood brain barrier (BBB) against a concentration gradient. 14C-labeled lactate autoradiography and 13C-labeled lactate NMR experiments support the evidence here that the injured brain takes up more lactate even at normal circulating systemic concentrations.13,29
Lactate transport in the brain is facilitated by a series of monocarboxylate transporters (MCTs) that co-transport protons. MCT1 transporters are mainly expressed on endothelial cells and astrocytes, MCT2 transporters are predominantly neuronal, and MCT4 predominantly glial.28,30,31 The behavior of MCTs is complex and multifactorial. Movement of lactate “uphill” by MCT1 against a lactate concentration gradient may be facilitated by a “downhill” proton concentration gradient.32 Cerebral extracellular pH measurement was not possible in the present study, because of discontinuation of clinical sensor (Neurotrend) by its manufacturers, and no current alternative.
Various physiological and pathological conditions have been shown to induce the expression of MCT transporters. After cortical contusion injury in rats, immunohistochemistry and Western blot analysis demonstrated a 26 to 28% increase in expression of MCT2 transporters.33 This induction of MCT2 expression occurred acutely, apparent at 3 h after TBI. The induction of MCT2 expression was found primarily within the vasculature. This potentially correlates with the increase in lactate transport across the BBB that we observed. It is unclear how TBI might trigger up-regulation of MCT transporters. Conceivably, it may be driven by metabolism such that cellular demand for energy, at a time when glucose uptake is insufficient to meet this demand, promotes increased MCT activity. In addition, mediators that increase after TBI may induce MCT transporter expression. Vascular endothelial growth factor, hypoxia-inducible factor 1, and noradrenaline are found to be elevated after TBI and have all been implicated in affecting MCT transporter expression.30,34
Changes to glucose uptake and brain extracellular concentrations during lactate import
Lactate uptake was associated with higher AVglc values. A median AVglc value of 0.42 mmol/L was observed when AVlac values indicated lactate uptake and 0.07 mmol/L when AVlac values indicated lactate export. This value for AVglc observed during lactate uptake is similar to values found in control subjects.10,11,18,35,36 The association of lactate uptake with normal or higher glucose uptake may reflect a more favorable response to the energy crisis caused by TBI, with the corollary being that depressed glucose uptake and lactate export are unfavorable responses. Depressed glucose uptake and lactate export may also simply reflect a reduction in the need for energy substrate—for example, from cell loss. In contrast, Meierhans and coworkers12 found that increased lactate uptake was associated with reduced glucose uptake. Also, healthy human subjects demonstrate a reduction in glucose uptake when given a lactate infusion.9 It seems likely then that injured brains differ in their capacities to favorably regulate glucose and lactate uptake.
A possible association of increased substrate availability from increased uptake of glucose or lactate might be a change in the LP ratio, frequently used as a marker of energy dysfunction and metabolic crisis. The LP ratio reflects the redox status of the cell, which in turn reflects the balance of oxidative glucose and non-oxidative metabolism.37 Hence, it increases during hypoxia or mitochondrial dysfunction. Meierhans and associates38 found that brain glucose concentrations greater than 3 mmol/L were associated with a lower LP ratio. We found no statistically significant relationship between LP ratio and brain glucose, brain lactate, AVglc, and AVlac values. This most likely reflects the more significant role that other factors have in influencing the LP ratio, such as tissue oxygen levels and mitochondrial dysfunction.
Elevated brain lactate levels are associated with tissue hypoxia
Our results suggest that the increased brain lactate observed after TBI partly reflects periods of uptake from the circulation. Our results also demonstrate that brain tissue hypoxia contributes to brain extracellular lactate elevation. Brain tissue oxygen levels that are considered consistent with ischemia (albeit rare events) were found in this study to be associated with higher concentrations of brain lactate. There was no relationship between brain tissue oxygen and AVlac values.
Limitations
Arterial and venous measurements provided us with only a snapshot view of cerebral metabolism with sampling limited to twice daily. For comparisons with brain extracellular concentrations, only those microdialysis measurements taken at the same time points of blood sampling were analyzed. A limitation is that we did not measure cerebral blood flow (CBF). Uptake of glucose or lactate into the brain is influenced by CBF. Decrease in CBF would lead to a higher percentage extraction of blood-borne glucose and lactate by the brain and potentially result in a higher AV difference, although the picture is complicated by the brain's ability to produce and export lactate. We consider that our approach of simply dichotomizing the AV lactate data into net “uptake” or “export” diminishes the influence of CBF on our interpretation, and we regard our approach as a conservative assessment of net lactate uptake. If CBF were to fall to an ischemic state, brain lactate production from anaerobic glycolysis would increase and export from the brain might be expected to increase venous levels of lactate, thereby reducing the chance of observing a positive lactate AV gradient. Our patients did not have overt ischemia. In a clinical study of carotid endarterectomy, increases were seen during clamping both for AV difference in glucose concentration and for % incidence of positive lactate AV gradient.39 We therefore think that our conclusions are robust and that CBF differences are unlikely to have changed our principal finding of lactate uptake into the injured brain.
Jugular venous sampling is not entirely representative of the entire brain, and there may be differences between the left and right sides. We attempted to minimize the effect of this asymmetry by selecting patients with diffuse TBI so that metabolic changes in the left and right hemisphere were likely to be similar. Microdialysis underestimates the true extracellular concentration of glucose and lactate in brain extracellular fluid. The “recovery” of glucose and lactate depend on the microdialysis membrane and the perfusion flow rate.40 Slower rates of perfusion result in greater recovery at the expense of smaller volumes of microdialysate returned. We chose a catheter and standard flow rate (0.3 μL/min) that recovers approximately 70% of glucose and lactate and produces sufficient microdialysate to make hourly measurements practicable.40 The underestimation of brain glucose and lactate concentration does not impact on our AV gradient measurements; however, it reinforces the observation that lactate can be imported into the brain against a concentration gradient.
Conclusion
Our findings suggest that the injured brain takes up lactate, which can be oxidatively metabolized.13 Lactate uptake occurs despite relatively high brain lactate levels after TBI suggesting up-regulation of MCT transporters. Glucose delivery to brain cells is maintained during periods of lactate uptake. Hence, lactate uptake may reflect an adaptive response to the increased energy demands and change in metabolic priorities of the injured brain.
The injured brain's capacity to use endogenous lactate as an alternative fuel implies that exogenous lactate may be therapeutic in TBI patients. Accordingly, rats given intravenous lactate after fluid percussion injury performed better than those given saline.41,42 Glycemic control of neurocritical care patients is necessary to avoid both hypo- and hyperglycemia, although tight glycemic control may be too restrictive for optimal cerebral metabolism and less rigid control may be preferable.43 Lactate administration may have a role in supporting energy metabolism in this context. Ongoing and future clinical studies will elucidate whether lactate administration improves outcomes.
Acknowledgments
We thank Mr. M. Guilfoyle for statistical advice. We gratefully acknowledge financial support as follows. Study support: Medical Research Council (Grant Nos. G0600986 ID79068 and G1002277 ID98489) and National Institute for Health Research Biomedical Research Centre Cambridge. Authors' support: I.J. – Medical Research Council (Grant no. G1002277 ID 98489) and National Institute for Health Research Biomedical Research Centre Cambridge; A.H. – Medical Research Council/ Royal College of Surgeons of England Clinical Research Training Fellowship (Grant no. G0802251) and Raymond and Beverly Sackler Fellowship; K.L.H.C. – National Institute for Health Research Biomedical Research Centre Cambridge; RJS – National Institute for Health Research Flexibility and Sustainability Fund; P.J.H. – Academy of Medical Sciences/Health Foundation Senior Surgical Scientist Fellowship and National Institute for Health Research Biomedical Research Centre Cambridge.
Author Disclosure Statement
P.J.H. is a Director of Technicam. For the remaining authors, no competing financial interests exist.
References
- 1.Enevoldsen E.M., and Jensen F.T. (1977). Cerebrospinal fluid lactate and pH in patients with acute severe head injury. Clin. Neurol. Neurosurg. 80, 213–225 [DOI] [PubMed] [Google Scholar]
- 2.Timofeev I., Carpenter K.L., Nortje J., Al-Rawi P.G., O'Connell M.T., Czosnyka M., Smielewski P., Pickard J.D., Menon D.K., Kirkpatrick P.J., Gupta A.K., and Hutchinson P.J. (2011). Cerebral extracellular chemistry and outcome following traumatic brain injury: a microdialysis study of 223 patients. Brain 134, 484–494 [DOI] [PubMed] [Google Scholar]
- 3.Vespa P., Bergsneider M., Hattori N., Wu H.M., Huang S.C., Martin N.A., Glenn T.C., McArthur D.L., and Hovda D.A. (2005). Metabolic crisis without brain ischemia is common after traumatic brain injury: a combined microdialysis and positron emission tomography study. J. Cereb. Blood Flow Metab. 25, 763–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verweij B.H., Muizelaar J.P., Vinas F.C., Peterson P.L., Xiong Y., and Lee C.P. (2000). Impaired cerebral mitochondrial function after traumatic brain injury in humans. J. Neurosurg. 93, 815–820 [DOI] [PubMed] [Google Scholar]
- 5.Gilmer L.K., Roberts K.N., Joy K., Sullivan P.G., and Scheff S.W. (2009). Early mitochondrial dysfunction after cortical contusion injury. J. Neurotrauma 26, 1271–1280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mazzeo A.T., Beat A., Singh A., and Bullock M.R. (2009). The role of mitochondrial transition pore, and its modulation, in traumatic brain injury and delayed neurodegeneration after TBI. Exp. Neurol. 218, 363–370 [DOI] [PubMed] [Google Scholar]
- 7.Robertson C.L., Scafidi S., McKenna M.C., and Fiskum G. (2009). Mitochondrial mechanisms of cell death and neuroprotection in pediatric ischemic and traumatic brain injury. Exp. Neurol. 218, 371–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen T., Qian Y.Z., Rice A., Zhu J.P., Di X., and Bullock R. (2000). Brain lactate uptake increases at the site of impact after traumatic brain injury. Brain Res. 861, 281–287 [DOI] [PubMed] [Google Scholar]
- 9.van Hall G., Strømstad M., Rasmussen P., Jans Ø., Zaar M., Gam C., Quistorff B., Secher N.H., and Nielsen H.B. (2009). Blood lactate is an important energy source for the human brain. J. Cereb. Blood Flow Metab. 29, 1121–1129 [DOI] [PubMed] [Google Scholar]
- 10.Ide K., Schmalbruch I., and Quistorff B. (2000). Lactate, glucose and O2 uptake in human brain during recovery from maximal exercise. J. Physiol. 522,159–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glenn T.C., Kelly D.F., Boscardin W.J., McArthur D.L., Vespa P., Oertel M., Hovda D.A., Bergsneider M., Hillered L., and Martin N.A. (2003). Energy dysfunction as a predictor of outcome after moderate or severe head injury: indices of oxygen, glucose, and lactate metabolism. J. Cereb. Blood Flow Metab. 23, 1239–1250 [DOI] [PubMed] [Google Scholar]
- 12.Meierhans R., Brandi G., Fasshauer M., Sommerfeld J., Schüpbach R., Béchir M., and Stover J. (2012). Arterial lactate above 2 mM is associated with increased brain lactate and decreased brain glucose in patients with severe traumatic brain injury. Minerva Anestesiol. 78, 185–193 [PubMed] [Google Scholar]
- 13.Gallagher C.N., Carpenter K.L., Grice P., Howe D.J., Mason A., Timofeev I., Menon D.K., Kirkpatrick P.J., Pickard J.D., Sutherland G.R., and Hutchinson P.J. (2009). The human brain utilizes lactate via the tricarboxylic acid cycle: a 13C-labelled microdialysis and high-resolution nuclear magnetic resonance study. Brain 132, 2839–2849 [DOI] [PubMed] [Google Scholar]
- 14.Pellerin L., and Magistretti P.J. (1994). Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc. Natl. Acad. Sci. U S A 91, 10625–10629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pellerin L., and Magistretti P.J. (2012). Sweet sixteen for ANLS. J. Cereb. Blood Flow Metab. 32, 1152–1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beards S.C., Yule S., Kassner A., and Jackson A. (1998). Anatomical variation of cerebral venous drainage: the theoretical effect on jugular bulb blood samples. Anaesthesia 53, 627–633 [DOI] [PubMed] [Google Scholar]
- 17.CMA Microdialysis AB. (2008). Technical Manual for ISCUS Flex Microdialysis Analyzer. CMA AB, Solna, Sweden, pps. 16–19 [Google Scholar]
- 18.Larsen T.S., Rasmussen P., Overgaard M., Secher N.H., and Nielsen H.B. (2008). Non-selective β-adrenergic blockade prevents reduction of the cerebral metabolic ratio during exhaustive exercise in humans. J. Physiol. 586, 2807–2815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quistorff B., Secher N.H., and Van Lieshout J.J. (2008). Lactate fuels the human brain during exercise. FASEB J. 22, 3443–3449 [DOI] [PubMed] [Google Scholar]
- 20.Dusick J.R., Glenn T.C., Lee W.N., Vespa P.M., Kelly D.F., Lee S.M., Hovda D.A., and Martin N.A. (2007). Increased pentose phosphate pathway flux after clinical traumatic brain injury: a [1,2-13C2]glucose labeling study in humans. J. Cereb. Blood Flow Metab. 27, 1593–1602 [DOI] [PubMed] [Google Scholar]
- 21.Bartnik B.L., Sutton R.L., Fukushima M., Harris N.G., Hovda D.A., and Lee S.M. (2005). Upregulation of pentose phosphate pathway and preservation of tricarboxylic acid cycle flux after experimental brain injury. J. Neurotrauma 22, 1052–1065 [DOI] [PubMed] [Google Scholar]
- 22.Schurr A., West C.A., and Rigor B.M. (1988). Lactate-supported synaptic function in the rat hippocampal slice preparation. Science 240, 1326–1328 [DOI] [PubMed] [Google Scholar]
- 23.Smith D., Pernet A., Hallett W.A., Bingham E., Marsden P.K., and Amiel S.A. (2003). Lactate: a preferred fuel for human brain metabolism in vivo. J. Cereb. Blood Flow Metab. 23, 658–664 [DOI] [PubMed] [Google Scholar]
- 24.Reinstrup P., Ståhl N., Mellergård P., Uski T., Ungerstedt U., and Nordström C.H. (2000). Intracerebral microdialysis in clinical practice: baseline values for chemical markers during wakefulness, anesthesia, and neurosurgery. Neurosurgery 47, 701–710 [DOI] [PubMed] [Google Scholar]
- 25.Helmy A., Carpenter K.L., Menon D.K., Pickard J.D., and Hutchinson P.J. (2010). The cytokine response to human traumatic brain injury: temporal profiles and evidence for cerebral parenchymal production. J. Cereb. Blood Flow Metab. 31, 658–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Helmy A., Antoniades C.A., Guilfoyle M.R., Carpenter K.L., and Hutchinson P.J. (2012). Principal component analysis of the cytokine and chemokine response to human traumatic brain injury. PLoS One 7, e39677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dager S.R., Marro K.I., Richards T.L., and Metzger G.D. (1992). Localized magnetic resonance spectroscopy measurement of brain lactate during intravenous lactate infusion in healthy volunteers. Life Sci. 51, 973–985 [DOI] [PubMed] [Google Scholar]
- 28.Dalsgaard M.K. (2006). Fuelling cerebral activity in exercising man. J. Cereb. Blood Flow Metab. 26, 731–750 [DOI] [PubMed] [Google Scholar]
- 29.Chen T., Qian Y.Z., Di X., Zhu J.P., and Bullock R. (2000). Evidence for lactate uptake after rat fluid percussion brain injury. Acta. Neurochir. Suppl 76, 359–364 [DOI] [PubMed] [Google Scholar]
- 30.Pierre K., and Pellerin L. (2005). Monocarboxylate transporters in the central nervous system: distribution, regulation and function. J. Neurochem. 94, 1–14 [DOI] [PubMed] [Google Scholar]
- 31.Hutchinson P.J., O'Connell M.T., Seal A., Nortje J., Timofeev I., Al-Rawi P.G., Coles J.P., Fryer T.D., Menon D.K., Pickard J.D., and Carpenter K.L. (2009). A combined microdialysis and FDG-PET study of glucose metabolism in head injury. Acta Neurochir. (Wien) 151, 51–61 [DOI] [PubMed] [Google Scholar]
- 32.Halestrap A.P., and Price N.T. (1999). The proton-linked monocarboxylate transporter (MCT) family: structure, function and regulation. Biochem. J. 343, 281–299 [PMC free article] [PubMed] [Google Scholar]
- 33.Prins M.L., and Giza C.C. (2006). Induction of monocarboxylate transporter 2 expression and ketone transport following traumatic brain injury in juvenile and adult rats. Dev. Neurosc.i 28, 447–456 [DOI] [PubMed] [Google Scholar]
- 34.Pierre K., Debernardi R., Magistretti P.J., and Pellerin L. (2003). Noradrenaline enhances monocarboxylate transporter 2 expression in cultured mouse cortical neurons via a translational regulation. J. Neurochem. 86, 1468–1476 [DOI] [PubMed] [Google Scholar]
- 35.Gibbs E., Lennox W., and Nims L. (1942). Arterial and cerebral venous blood. J Biol Chem. 144, 325–332 [Google Scholar]
- 36.Leegsma-Vogt G., Venema K., Postema F., and Korf J. (2001). Monitoring arterio-venous differences of glucose and lactate in the anesthetized rat with or without brain damage with ultrafiltration and biosensor technology. J. Neurosci. Res. 66, 795–802 [DOI] [PubMed] [Google Scholar]
- 37.Sun F., Dai C., Xie J., and Hu X. (2012). Biochemical issues in estimation of cytosolic free NAD/NADH ratio. PLoS One 7, e34525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meierhans R., Béchir M., Ludwig S., Sommerfeld J., Brandi G., Haberthür C., Stocker R., and Stover J.F. (2010). Brain metabolism is significantly impaired at blood glucose below 6 mM and brain glucose below 1 mM in patients with severe traumatic brain injury. Crit. Care 14, R13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Espenell A.E., McIntyre I.W., Gulati H., Girling L.G., Wilkinson M.F., Silvaggio J.A., Koulack J., West M., Harding G.E., Kaufmann A.M., and Mutch W.A. (2010). Lactate flux during carotid endarterectomy under general anesthesia: correlation with various point-of-care monitors. Can. J. Anesth. 57, 903–912 [DOI] [PubMed] [Google Scholar]
- 40.Hutchinson P.J., O'Connell M.T., Al-Rawi P.G., Maskell L.B., Kett-White R., Gupta A.K., Richards H.K., Hutchinson D.B., Kirkpatrick P.J., and Pickard J.D. (2000). Clinical cerebral microdialysis: a methodological study. J. Neurosurg. 93, 37–43 [DOI] [PubMed] [Google Scholar]
- 41.Rice A.C., Zsoldos R., Chen T., Wilson M.S., Alessandri B., Hamm R.J., and Bullock M.R. (2002). Lactate administration attenuates cognitive deficits following traumatic brain injury. Brain Res. 928, 156–159 [DOI] [PubMed] [Google Scholar]
- 42.Holloway R., Zhou Z., Harvey H.B., Levasseur J.E., Rice A.C., Sun D., Hamm R.J., and Bullock M.R. (2007). Effect of lactate therapy upon cognitive deficits after traumatic brain injury in the rat. Acta Neurochir. (Wien) 149, 919–927 [DOI] [PubMed] [Google Scholar]
- 43.Kramer A.H., Roberts D.J., and Zygun D.A. (2012). Optimal glycemic control in neurocritical care patients: a systematic review and meta-analysis. Crit. Care 16, R203. [DOI] [PMC free article] [PubMed] [Google Scholar]