Abstract
Despite the immunosuppressive, homing, and regenerative capabilities of mesenchymal stem cells (MSCs), their ability to migrate to arthritic joints and influence the course of arthritis in vivo remains poorly understood. The objective of this study was to determine if allogeneic MSCs migrate to inflamed joints in vivo and to determine if MSCs expressing the costimulation blocker cytotoxic T lymphocyte associated antigen-4 coupled to immunoglobulin-G (CTLA4Ig) could be used to ameliorate collagen induced arthritis (CIA). The migration of systemically delivered inbred mouse strain (FVB) MSCs to migrate to inflamed joints in CIA was studied using real-time quantitative polymerase chain reaction. Furthermore, the effect of BALB/c MSCs modified with an adenoviral vector to express CTLA4Ig, on T cell function in vitro and on CIA in vivo was assessed. After systemic delivery of FVB MSCs, eGFP DNA was detectable in the joints of mice with CIA confirming that some MSCs had reached to inflamed joints. BALB/c MSCs suppressed the secretion of both TNFα and IFNγ, and reduced the ratio of Th1:Th2 cytokine expression, by DBA/1 T cells in vitro irrespective of viral modification. The expression of CTLA4Ig did not augment this effect. Despite a worsening of disease scores after infusion of BALB/c MSCs in vivo, BALB/c MSCs expressing CTLA4Ig significantly delayed the onset of inflammatory arthritis in CIA. These data demonstrate that allogeneic MSCs can migrate to the inflamed joints of CIA in vivo and that genetically modified allogeneic MSCs may be considered for development of gene therapy strategies for inflammatory arthritis
Introduction
Rheumatoid arthritis (RA) is an inflammatory joint disease with the synovium as the principle site of pathology. Inflammation is facilitated by a complex network of cytokines, predominantly TNFα, IFNγ, and IL-17, mediated by macrophage, B lymphocyte, and T lymphocyte activation [1,2]. Strategies used to treat RA now include biologic therapies, which target TNFα, B cells, T cells, and IL-6 [3–6]. Despite recent advances, a proportion of patients remain refractory to treatment and the need for new targeted therapies remains.
Mesenchymal stem cells (MSCs) have the potential to modulate T cell mediated immune responses, as well as facilitate repair and regeneration of injured tissues [7,8]. As MHC II and costimulatory molecules are not expressed on MSCs it is hypothesized that allogeneic MSCs may be of use in the treatment of inflammatory conditions, such as RA [9–11]. Furthermore, evidence suggests that MSCs may preferentially migrate to sites of tissue injury, such as bone fractures and metastatic disease in vivo [12,13]. Despite this, it has not been consistently demonstrated that MSCs have a therapeutic role in murine models of inflammatory arthritis. MSCs have been reported to ameliorate disease in some studies [14–17], while in other reports there is either no effect, or indeed a deleterious effect, on disease progression [18–22]. There is evidence to suggest that the inflammatory milieu of arthritis may impact on the immunosuppressive functions of MSCs in mouse collagen induced arthritis (CIA) model, rat adjuvant induced arthritis (AIA), and spontaneous severe erosive arthritis (SSEA) in K/BxN mice [18,23]. In SSEA and AIA, the genetic background of the MSC did not appear to affect in vivo function, while in the more commonly used CIA model allogeneic MSCs significantly exacerbated disease progression [18,23]. Despite these conflicting results, MSCs have been used successfully as vehicles for delivery of gene therapy in experimental CIA, with successful disease suppression using transforming growth factor β and Il-10 transduced syngeneic MSCs [22,24]. Taking these gene therapy results, together with the finding that abrogation of the inflammatory milieu with a protease inhibitor facilitated suppression of AIA and SSEA with allogeneic MSCs [23], we sought to determine if these cells expressing the T cell costimulation blocker cytotoxic T lymphocyte associated antigen-4 coupled to immunoglobulin-G (CTLA4Ig) would suppress disease activity in CIA when delivered in early disease. CTLA4 is a homologue of CD28, which binds competitively to the B7 complex on antigen presenting cells thereby preventing the costimulation of activated T cells and resulting in T cell anergy [25]. MSCs do not express the B7 complex and indeed can prevent the proliferation of T cells stimulated via the CD28 pathway [26]. In addition to this, MSCs inhibit the proliferation of CD4+ and CD8+ T cells with reduced expression of activation markers [9,27] and can mediate decreased Th2 cell production of IFNγ [28,29]. Taken together, MSCs and CTLA4Ig may work synergistically to abrogate the inflammatory cascade mediated by the T cells which populate the inflamed synovium.
The use of modified MSCs to ameliorate disease has been studied in both models of arthritis and malignancy [30–32]. To date, syngeneic MSCs have been successfully used to deliver either IL-10 or TGFβ to DBA/1 mice with CIA [22,24]. The use of allogeneic cells would increase the potential use of this therapy and therefore, the use of MHC mismatched MSCs [18] was used to determine migration to the joint in vivo and the impact of allogeneic MSCs secreting CTLA4Ig on disease progression in CIA in DBA/1 mice. CTLA4Ig is currently licensed for clinical use in RA and MSCs expressing CTLA4Ig have been shown to be of therapeutic benefit in animal models of graft versus host disease [33] and autoimmune thyroiditis [34]. The cotransfection of MSCs may also be used to facilitate other gene therapy strategies. MSCs transduced to express BMP2 with an adenoviral construct had been shown to stimulate osteogenesis; however, effects were limited by immune reactions against the viral construct. Cotransfection of MSCs with adenoviral vectors expressing CTLA4Ig and BMP2 resulted in stimulation of osteogenesis in vitro with a blunting of the immune response against the adenoviral vector demonstrated in mixed lymphocyte reactions [35].
Materials and Methods
MSC isolation and characterization
Bone marrow MSCs were isolated from 8–10 week old BALB/c (Harlan Labs.) and FVB.Cg-Tg(GFPU)5Nagy/J (FVB-GFP); (Jackson Labs.) mice [18]. Briefly, cells from two femurs were removed by centrifugation and cultured in a T-175 flask under standard culture conditions. After formation of colonies, cells were replated in complete expansion medium (CEM; Iscove's modified eagle's medium, 9% horse and 9% fetal bovine serum, 1% penicillin and streptomycin, 1% L-glutamine) at a density of 50 cells/cm2 and subsequently at 4,500 cells/cm2. Cell surface antigens CD73, CD34, CD31, Sca1, CD105, CD44, and CD31, were assessed by flow cytometry as previously described [18].
Differentiation of MSCs
Adipogenesis, osteogenesis, and chondrogenesis were induced in murine MSCs [18]. Adipogenesis was demonstrated by staining of lipid vacuoles with Oil Red O. In osteogenic cultures, calcium content was quantified using the Calcium Liquicolour Kit (StanBio, Texas USA) and von Kossa staining was used to demonstrate Calcium deposition. MSCs were incubated in 3% silver nitrate solution in the dark for 10 min before rinsing with deionised water three times. The last rinse was left on the cells and they were exposed to bright warm light for 15 min. Cells were rinsed again and photographed on an Olympus IX71 inverted microscope camera utilising CellIP imaging software (Olympus). A silver black color confirmed the presence of calcium deposits. Chondrogenesis assays were carried out using a pellet culture system. After digestion with papain, the dimethymethylene blue assay was used to demonstrate glycosaminoglycan (GAG) content, while formalin fixed pellets were sectioned and stained for GAG with toluidine blue.
MSC viral transduction
Optimization of viral transduction was carried out using first generation E1/E3-deleted type V adenovirus encoding green fluorescent protein under a cytomegalovirus promoter (AdGFP) [36]. Cells were plated at 5×104 cells per well in a 12-well plate in CEM and allowed to adhere for 24 h after which medium was removed and 300 μL of CEM containing AdGFP was added to appropriate wells. In initial experiments, virus was added to the cells at a multiplicity of infection (MOI) of 1,000, 500, or 100. Control wells had 300 μL of CEM alone added. Cells were either incubated at 37°C and 5% CO2 for 90 min or centrifuged at 2,000 g at 37°C for 90 min. After this, medium was replaced with 2 mL CEM. Cells were incubated for 48 h at 37°C, 5% CO2 before washing with phosphate buffered saline (PBS) and trypsinisation. Cells were stained with 7-aminoactinomycin D (7-AAD) to identify dead cells and fixed in paraformaldehyde. Both 7-AAD and GFP expression were analyzed on a Guava Flow Cytometer using eXpress Plus™ software and GFP transduction was recorded as the percentage GFP expression in the cell population.
Using the centrifugation method described above, MSCs were transduced with a type V adenovirus expressing CTLA4Ig (AdCTLA4Ig) [37] at MOI 500. Medium (500 μL) was collected every 24 h for 15 days from transduced and control wells. After each collection, the medium was replaced with fresh CEM. An ELISA for human IgG (Serazym®Human IgG; Seramun Diagnostica GmbH) was performed on the stored supernatants according to the manufacturer's instructions. Absorbance was read at 450 nm using Wallac™ workstation software.
In vitro T cell stimulation studies
Submandibular lymph nodes and spleen were recovered from DBA/1 mice and shredded before passing through a 70 μm mesh to form a single cell suspension. CD4+ T cells were isolated and stimulated with anti-CD3/anti-CD28 beads as previously described [18]. Briefly, CD4 Dynabeads (Miltenyi Biotech) were used to positively select CD4+ cells on an octoMACS separator (Miltenyi Biotech). T cells were activated with beads at a 1:1 ratio for 24 h and subsequently plated with adherent BALB/c MSCs at either a 1:10 or 1:1 ratio (ratio of MSC:T cells). MSCs were either untransduced or transduced with an Adnull vector or AdCTLA4Ig as described. Control wells included MSC cultures alone and unstimulated CD4+ T cells. Culture supernatants were collected at 24 and 72 h and stored at −20°C. The concentration of cytokines in the supernatants was determined using eBioscience™ Mouse Th1/Th2 and Mouse TNFα Ready-SET-Go! ELISA as per the manufacturer's instructions.
Collagen induced arthritis
Approval from the local Institutional Animal Care and Use Committee was provided for all animal work and mice were housed in groups of two to three at room temperature and with a ready supply of food and water. Inflammatory arthritis was induced in male DBA/1 mice aged 7–9 weeks as described [18]. Briefly, mice were immunized with an intradermal injection of bovine type II collagen (50 μL; MD BioSciences) emulsified in complete (Freund's adjuvant (50 μL; MD Biosciences). This was followed by a booster injection of type II collagen in incomplete Freund's adjuvant (MD Biosciences) after 21 days. For homing experiments, 1×106 passage 5 FVB-GFP MSCs in 100 μL PBS were delivered intravenously (iv) on day 21 or 32 allowing quantification of the MSCs by quantitative real-time–polymerase chain reaction (qRT-PCR). In subsequent experiments, appropriate groups had 100 μL of PBS containing 1×105 MSCs, MSCs Adnull or MSCs AdCTLA4Ig injected into the lateral tail vein at the time of booster immunization. Control groups received PBS only and all cells were used within 30 min of harvesting. The clinically approved human drug CTLA4Ig (Abatacept; Bristol Myers Squibb) was delivered intraperitoneally (ip) as a positive treatment control; five doses at 72 h intervals, starting on day 21, were administered to the animals. A clinical score was based on swelling across the metatarsophalangeal joints (measured using a digital caliper) and the presence of dactylitis. The scoring system was designed to ensure that dactylitis was included in the assessment and to avoid reliance on subjective measures, such as erythema and increments in paw swelling of <1 mm which may be unreliable. The score was recorded as follows paw<2 mm=0 points, paw 2–3 mm=2 points, paw 3–4 mm=3 points, paw 4–5 mm=4 points, paw 5–6 mm=5 points, swollen wrist or ankle=3 points, swollen digit=1 point.
DNA isolation and real-time PCR
Mice were sacrificed either 5 or 14 days after iv injection of FVB-GFP MSCs and lung, liver, knees and paws were removed. Harvested tissues were digested with 5% proteinase K (Qiagen) in lysis buffer (Qiagen) at 55°C. The digested tissue was centrifuged in high density phase lock gel tubes with phenol chloroform isoamyl alcohol and the resulting aqueous phase was incubated with isopropanol on ice. After further centrifugation the DNA pellet was collected, resuspended in 100% alcohol. and 3 M sodium acetate, and stored at −20°C for 24 h before a final wash in cold 70% alcohol and resuspension in distilled water. The concentration of DNA per sample was calculated using the quanti-iT™ PicoGreen double stranded DNA assay (Molecular Probes). For qRT-PCR, DNA samples were resuspended in sterile H2O at a concentration of 2 ng/μL. Each reaction consisted of 10 ng DNA, 1×Sybergreen Mastermix (Qiagen), 2.5 μL of eGFP or murine GAPDH primer pairs (Qiagen gene globe QT01171611 and QT00309099) to a final volume of 25 μL with sterile H2O. No template controls were included in all experiments. Standard curves were included in all PCR runs which were carried out on the Applied Biosciences StepOnePlus™ instrument (Applied Biosystems). The amount of eGFP DNA present was expressed as a percentage of the GAPDH as read from the standard curve. The sizes of the PCR products were confirmed by electrophoresis of PCR products on a 1.5% agarose gel with 2.5 μL of Sybersafe (Invitrogen).
Statistical analysis
Statistical analysis was carried out using StatsDirect® software. A normal ditribution of data was confirmed using the Shapiro Wilks test. For comparison between groups, a two way analysis of variance (ANOVA) with post hoc Tukey analysis was used. P values of <0.05 were considered significant and results are shown as the mean and standard deviation.
Results
MSC isolation, characterization and transduction
MSCs were isolated from the bone marrow of BALB/c MSCs and were plastic adherent with a fibroblastic morphology. All cells were successfully stimulated to undergo adipogenesis, osteogenesis, and chondrogenesis and this differentiation potential was maintained after transduction with adenoviral constructs (Fig. 1E–G). Cell surface characterization showed them to be strongly positive (>95%) for CD29, CD44, and CD105, weakly positive for Sca1 and CD73 (53% and 17%), and negative for CD45, CD34, and CD31 [18].
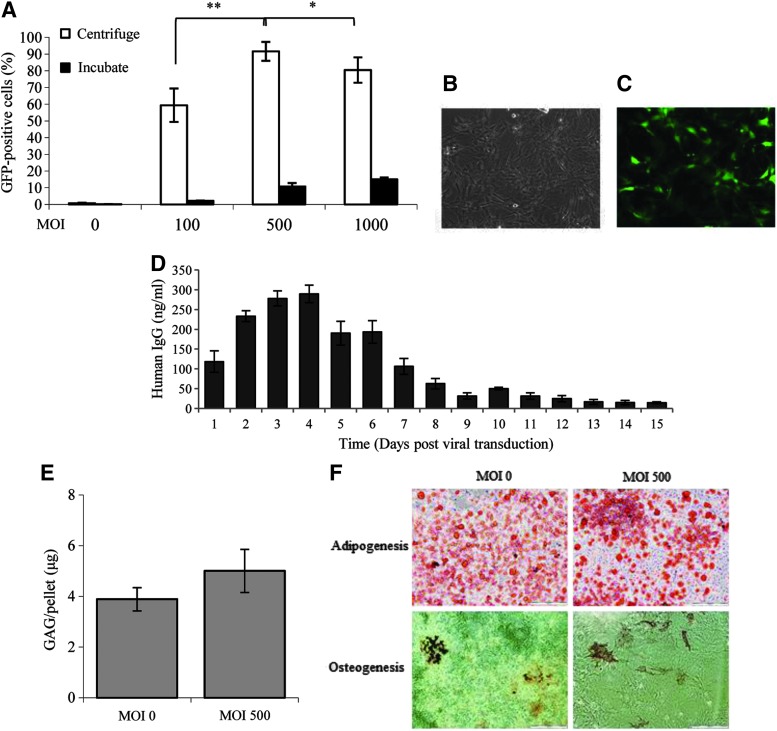
FIG. 1.
Adenoviral transduction of BALB/c MSCs. (A) At all MOIs, the use of high speed centrifugation (white box) enhanced the viral transduction of BALB/c MSCs compared to incubated controls (black box). Transduction at MOI 500 was superior to that at MOI 100 and MOI 1000. Transduced cells retained their fibroblastic morphology under bright field microscopy (B, C), GFP positive cells were also demonstrated by fluorescent microscopy. (D) IgG in the supernatant of MSCs transduced with AdCTLA4Ig was detected at 24 h and peaked at day 4. Levels fell to a plateau at day 9 but remained detectable until day 15. (E) There was no difference in chondrogenesis as measured by GAG per pellet between untransduced MSCs (MOI 0) and MSCAdCTLA4Ig (MOI 500). (F) Both naïve and transduced MSCs underwent adipogenesis and osteogenesis as demonstrated by Oil Red O and von Kossa staining, respectively. All experiments were carried out three times and assayed in triplicate, *P<0.05, **P<0.01 (two-way ANOVA). Data represents mean±SD. ANOVA, analysis of variance; CTLA4Ig, cytotoxic T lymphocyte associated antigen-4 coupled to immunoglobulin-G; GAG, glycosaminoglycan; MOI, multiplicity of infection; MSCs, mesenchymal stem cells; SD, standard deviation.
To optimize MSC transduction with an adenoviral vector, simple incubation was compared to a 90 min centrifugation of MSCs with shallow volume medium containing AdGFP at MOI 100, 500 and 1,000. Viral transduction across all concentrations was superior in cells, which were subjected to centrifugation, P<0.005, confirming results found in rat and goat MSCs [38,39] (Fig. 1A). Within the centrifugation groups, an MOI of 500 was found to be superior with between 80 and 100% of cells expressing GFP and MSC morphology preserved (Fig. 1B). Staining for 7-AAD was minimal (<1%) across all groups (result not shown).
BALB/c MSCs at passage 5 to 7 were transduced with AdCTLA4Ig at an MOI of 500 and 90 min centrifugation. Culture supernatants were collected every 24 h and stored at −20°C. ELISAs for human IgG were carried out on all supernatants after thawing. High levels of IgG were demonstrated after the first 24 h with maximal levels seen at day 4. IgG remained relatively high until day 7 and was detectable until day 14 post-transduction (Fig. 1D).
Homing of MSCs to inflamed joints
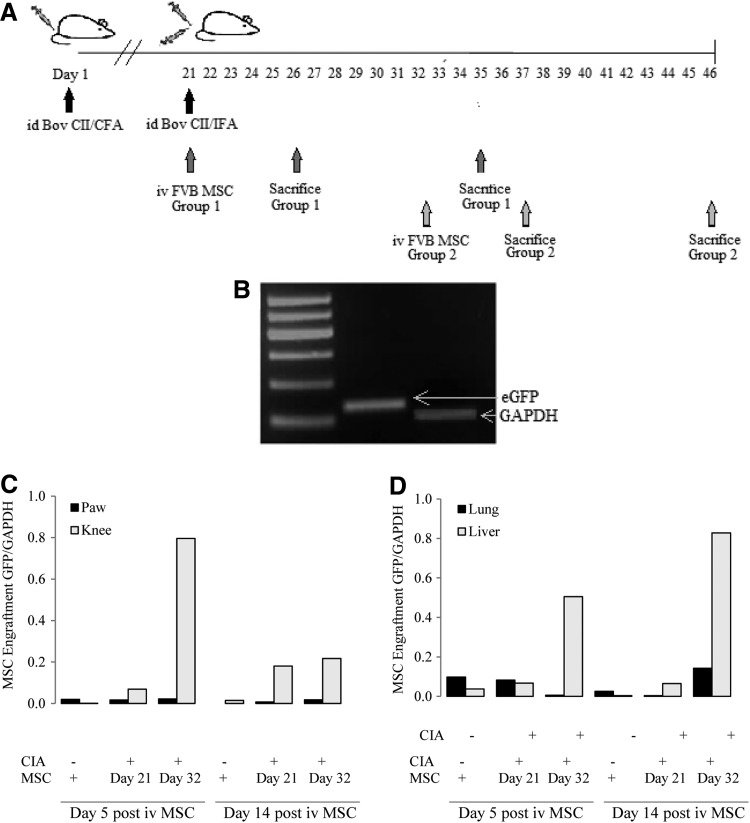
CIA was induced in DBA/1 mice; four mice had FVB-GFP MSCs delivered by iv injection on day 21 (at the time of the booster immunization) and four on day 32 (Fig. 2A). In each group two mice were sacrificed at 5 and 14 days postimmunization. The four control mice had a sham delivery of PBS and were sacrificed on day 26 and 35 (n=2 per group). GAPDH and eGFP PCR products were confirmed by gel electrophoresis as shown in Fig. 2B. When MSCs were delivered to mice without arthritis eGFP was detected in the lung and liver 5 and 14 days after infusion. In arthritic mice, eGFP DNA was detected in both of these organs but to a greater extent in the liver after day 32 delivery of MSCs. GFP DNA was also identified in the knees and paws at both time points after delivery. This was most marked in the knee joints of arthritic mice 5 days after the injection of MSCs during a period of overt clinical disease that is, day 32 (Fig. 2C).
FIG. 2.
Migration of MSCs to joints of mice with CIA. (A) CIA was induced in DBA/1 mice as described. MSCs were delivered intravenously on day 21 or 32. Mice were sacrificed 5 and 14 days after delivery of cells and organs were harvested for DNA isolation and PCR analysis. (B) PCR products after amplification of positive controls were run on agarose gel to confirm the product sizes of eGFP and GAPDH [base pair (bp)]. The detection of eGFP was used to determine the presence of DNA from MSCs isolated from eGFP transgenic mice on an FVB background. (C, D) Only trace amounts of eGFP DNA were detected in the joints of mice with no arthritis. When MSCs were delivered intravenously to symptomatic mice at day 21 or 32 they were detected predominantly in the knee 5 and 14 days later and to a lesser extent in the paw. (D) eGFP was also detected in the lung and the liver 5 and 14 days after injection. The highest levels were found in the liver of mice who had MSCs injected during the period of overt clinical disease (day 32). Results shown are mean values with two mice per treatment group. CIA, collagen induced arthritis; FVB, unbred mouse strain.
Effect of transduced MSCs on T cell cytokine secretion in vitro
DBA/1 CD4+ T cells were isolated and stimulated with anti-CD3 and CD28 beads. T cells were cultured with untransduced BALB/c MSCs, MSCs Adnull or MSCs AdCTLA4Ig at ratios of 1:10 or 1:1 (MSC:T cell ratio). Culture supernatants were collected at 24 and 72 h and assayed by ELISA for pro- and anti-inflammatory cytokines. Neither MSCs alone nor unstimulated T cells produced the assayed cytokines (results not shown).
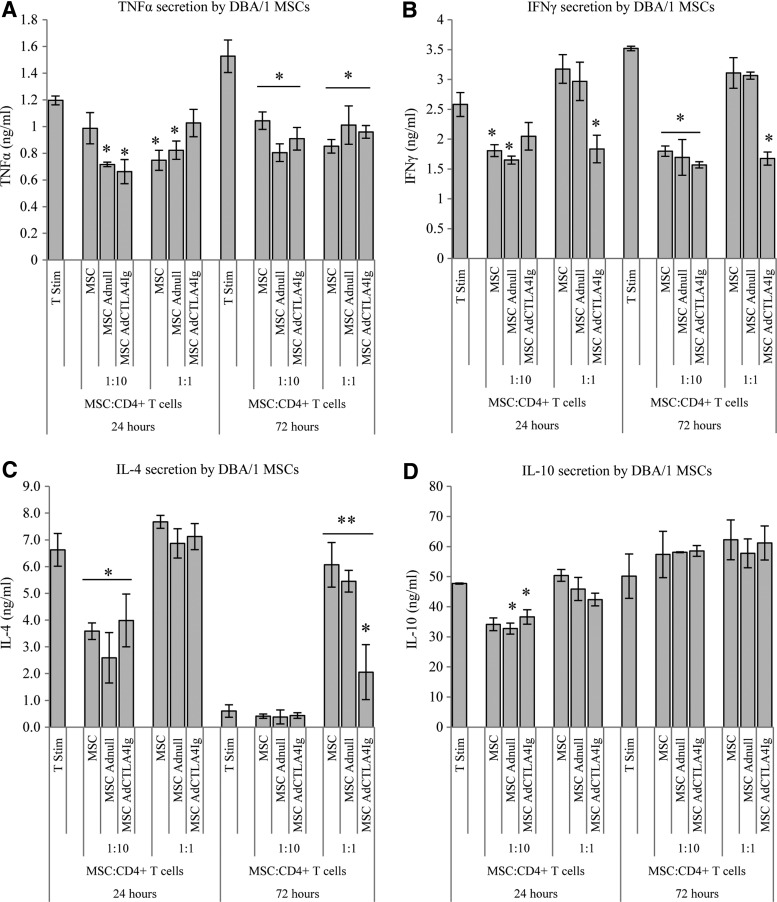
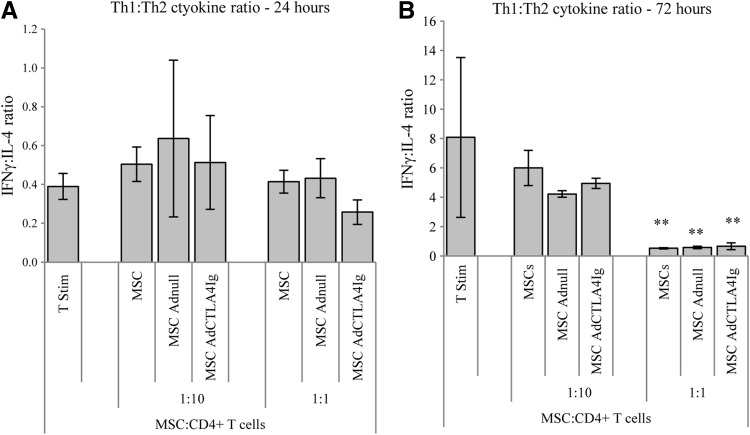
It was demonstrated that coculture with BALB/c MSCs resulted in suppression of TNFα secretion by stimulated DBA/1 T cells at both 24 and 72 h irrespective of viral modification. This effect was seen at both ratios of MSCs to T cells (Fig. 3A). A similar suppression of IFNγ secretion was seen at the ratio of 1:10; however, at the ratio of one MSC to one T-cell, only MSCs expressing CTLA4Ig suppressed IFNγ secretion at both time points (Fig. 3B). At 24 h, there was a suppression of IL-4 production when MSCs were cultured with CD4+ T cells at a ratio of 1:10 with no significant change in IL-4 demonstrated with higher numbers of MSCs. At 72 h this suppression was lost and there was a highly significant augmentation of IL-4 secretion in all groups cultured with MSCs at 1:1 (Fig. 3C). A transient small suppression of IL-10 secretion at 24 h was noted which was not sustained at 72 h (Fig. 3D). The ratio of IFNγ to IL-4 was taken to represent the ratio of Th1 to Th2 cytokines. After 24 h, there was no difference in this ratio between culture groups; however, after 72 h of incubation stimulated T cells cultured with allogeneic MSCs at a ratio of 1:1 had a signficantly reduced Th1:Th2 ratio suggesting a shift towards a more favourable Th2 cytokine profile (Fig. 4).
FIG. 3.
Effect of BALB/c MSCs, unmodified or transduced to express CTLA4Ig, on cytokine secretion by stimulated DBA/1 CD4+ T cells. CD4+ DBA/1 T cells were isolated, stimulated with anti-CD3 and CD28 beads, and cocultured with BALB/c MSCs, MSCs Adnull or MSC AdCTLA4Ig at a ratio of 1:10 or 1:1 (MSC:T cells). Supernatants were collected at 24 and 72 h for cytokine analysis and T cells stimulated in the absence of MSCs served as controls. (A) At both 24 and 72 h, there was significant suppression of TNFα secretion by T cells cultured with MSCs compared to controls; this was independent of viral modification. (B) A decrease in IFNγ production was also noted. This was seen in all groups but was most notable at a ratio of 1:10 after 72 h incubation. At the ratio of 1:1 only MSCs transduced with CTLA4Ig reduced IFNγ production. (C) IL-4 secretion by DBA/1 CD4+ T cells was initially suppressed by MSCs at a ratio of 1:10; however, at 72 h and at a ratio of 1:1 there was a significant augmentation in IL-4 production. All 1:1 MSC groups secreted elevated levels of IL-4 compared to non MSC controls; however, the MSCAdCTLA4Ig group secreted significantly less IL-4 than the other two MSC groups at this timepoint. (D), MSCs had a transient effect on IL-10 production at 24 h, which was not sustained at 72 h. All experiments were carried out three times and assayed in triplicate, *P<0.05, **P<0.01 (two-way ANOVA). Data represents mean±SD.
FIG. 4.
Effect of BALB/c MSCs on the profile of cytokine secretion by stimulate DBA/1 T cells. (A) The ratio of IFNγ to IL-4 was taken to represent the ratio of Th1 to Th2 cytokines. The addition of MSCs to stimulated T cells did not affect this ratio compared to controls at 24 h. (B) After 72 h there was evidence of a significant shift in favour of Th2 cytokine production when MSCs were cultured with stimulated T cells at a ratio of 1:1. All experiments were carried out three times and assayed in triplicate, **P<0.01 (two-way ANOVA). Data represents mean±SD.
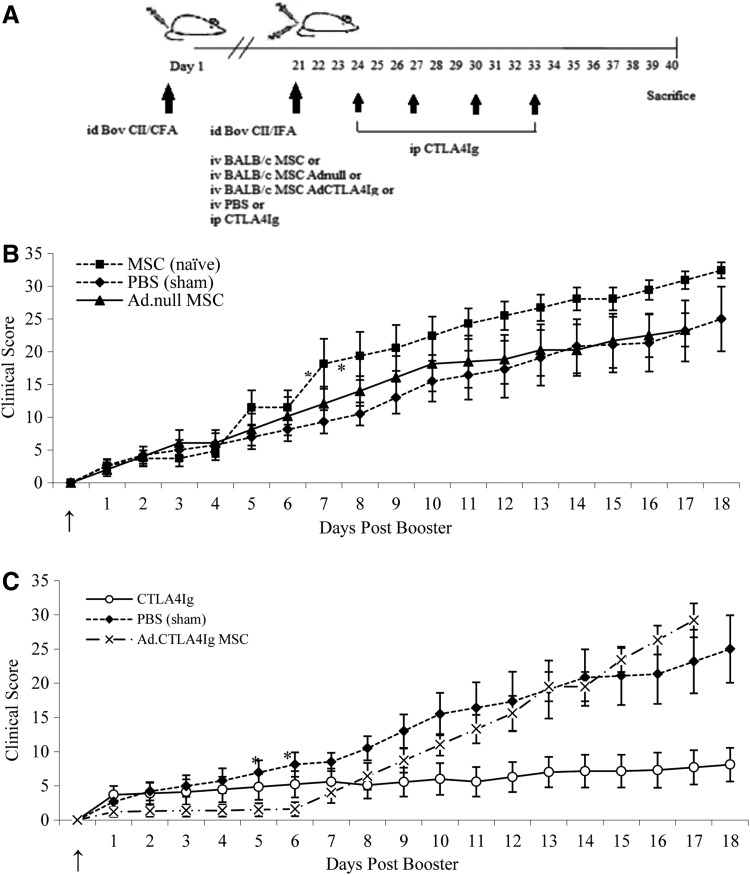
The effect of BALB/c MSCs on the course of CIA
CIA was induced in DBA/1 mice as described. At the time of the booster injection, 1×105 MSCs were delivered iv; cells were either unmodified or transduced with Adnull or AdCTLA4Ig (Fig. 5A). All immunized mice developed clinical signs of arthritis and were scored using the method outlined above. In the group treated with vehicle only, signs of arthritis were evident 24 to 48 h after delivery of the booster immunization and mean disease score at sacrifice was 25. Mice that received a systemic injection of BALB/c MSCs also developed signs of arthritis 24 to 48 h postbooster and by 7 days disease scores in this group were approximately twice that of mice receiving vehicle alone (P<0.05). The MSC treated group continued to have higher scores until the end of the experiment at which stage the mean disease score was 32.5. This did not reach statistical significance compared to the mice treated with vehicle alone. The group which received MSCs Adnull had similar disease scores to the control group (Fig. 5B).
FIG. 5.
The effect of BALB/c MSCs transduced to express CTLA4Ig on the clinical course of CIA in DBA/1 mice. (A) Male DBA/1 mice were immunized with type II collagen and a booster immunization 21 days later. At the time of the booster mice received PBS, MSCs, MSC Adnull, and MSC AdCTLA4Ig by intravenous injection (see arrow). CTLA4Ig was delivered intraperitoneally. Mice receiving untransduced allogeneic MSCs had deterioration in disease scores, which was significant at 1 week and remained elevated until 18 days postinfusion. (B) MSCs expressing CTLA4Ig significantly delayed the onset of active inflammation compared to controls (PBS treatment group). Scores in mice treated with MSCAdCTLA4Ig remained near zero for 6 days indicating no disease activity. (C) Repeated injections of intraperitoneal CTLA4Ig confirmed the efficacy of this treatment in CIA. Results are presented as the mean±SD with n=10 per group, *P<0.05 (two-way ANOVA). PBS, phosphate buffered saline.
The repeated administration of ip CTLA4Ig resulted in a sustained suppression of disease score with a maximum mean score of 8.1. The delivery of MSCs AdCTLA4Ig at day 21 resulted in almost complete disease suppression for 6 days with mean disease scores ranging from 1.3 to 1.6. This was less that than scores measured in mice treated with ip CTLA4Ig during this time period (3.9–5.6) and significantly less than disease scores in the vehicle alone group (4.2–8.1) (P<0.05) and correlated with the duration of gene expression demonstrated in vitro. The scores in this group increased slowly to intercept the scores of the vehicle alone group ∼11 days after cell delivery (Fig. 5C).
Discussion
The role of MSCs in RA remains controversial; conflicting reports on their efficacy in inflammatory arthritis have been difficult to compare due to significant variations in the cell strains used, as well as the number of cells infused, route of administration and clinical assessment. In a rat model of inflammatory arthritis, it was demonstrated that MSCs could only exert a therapeutic benefit when delivered in the preclinical phase of the disease [23], a practice difficult to emulate in the clinical setting. We have previously demonstrated that despite their lack of MHCII, the genetic disparity between the systemically delivered MSC and the host tissue impact on their ability to suppress inflammation in vivo. We showed that allogeneic MSCs resulted in worsening of biochemical, clinical, and histological parameters in mice with CIA, which was not seen after syngeneic MSCs infusion [18]. A recently published study suggested that allogeneic MSCs may in fact contribute to an early increase in systemic inflammation through alterations in T cell profiles and upregulation of Th17 cells by caspase-1 activation, and that the inflammatory environment was not essential to this deleterious effect reinforcing the theory that allogenicity may be a key factor [40]. Despite these results, MSCs have properties, which may still be exploited in the treatment of RA. In particular, their ability to preferentially engraft in inflamed or ischemic tissue warrants further investigation. Endogenous recruitment of MSCs to inflamed joints in CIA has been shown, as well as the mobilization of MSCs from bone marrow to ischaemic tissue [41–43]. Additionally, systemically delivered MSCs may migrate to areas of ischaemia, malignancy, and hyperplasia [44–46], while a recent study demonstrated that the activation of endothelium by TNFα facilitates extravasation of MSCs into inflamed tissue [47].
While there is evidence that infused MSCs home to inflamed tissue, this has not been demonstrated in models of arthritis to date. In a study by Augello et al., MSCs were not demonstrated in joints by immunohistochemistry; however, the use of intraperitoneal injections may have limited the ability of MSCs to enter the systemic circulation [14]. We identified eGFP DNA in the lungs and liver after systemic infusion of FVB-GFP MSCs to mice with and without arthritis. MSCs have been shown to engraft to lung and liver and this is to be expected due to the high circulating blood volume in these organs [48–50]. During active inflammation, hepatocytes become upregulated and increase production of acute phase proteins [51] leading to the hypothesis that the increased hepatocyte activity may generate chemoattractants for MSCs. The migration of systemically delivered MSCs to the joints of mice with arthritis in vivo has been demonstrated for the first time. This effect was most notable in the knees of the CIA mice but the possibility that the large volume of extra-articular material in mouse paw digests may have limited our ability to detect eGFP in these samples cannot be ruled out. MSCs migrated to inflamed joints when injected during both subclinical and overt phases of inflammation and persisted for at least 14 days in the host tissue.
While it has been demonstrated that syngeneic MSCs expressing TGFβ may be of therapeutic benefit in CIA via the down regulation of Th17 [24], the availability of a bank of allogeneic MSCs expressing therapeutic proteins, which could be used when the isolation and delivery of syngeneic MSCs is not practical, remains a potential treatment option in RA refractory to treatment with currently available synthetic and biologic therapies.
As we have previously demonstrated, in vitro T cell testing confirmed the potential for allogeneic BALB/c MSCs to suppress T cell response with significant suppression of TNFα and IFNγ production and an associated augmentation in the secretion of IL-4 [18]. In this study, we see significant suppression of IFNγ when MSCs expressing CTLA4Ig are cultured with T cells at both 24 and 72 h. This reflects the known effects of MSCs on suppressing IFNγ production and the reported reduction in systemic IFNγ post-CTLA4Ig therapy in RA patients [52]. It is also known that MSCs expressing CTLA4Ig may be used to alter the Th1:Th2 cytokine profile to favour Th2 cytokine production and ameliorate experimental autoimmune thyroiditis [34]. It is likely that MSCs and CTLA4Ig act syngertistically to alter suppress T cell activation, reduce production of IFNγ and cause a shift towards a Th2 cytokine response in inflammatory arthritis. A predominant Th2 cytokine response is known to be associated with clinical remission in RA and CIA [53]. Additionally, it has been shown that adenoviral modification of MSCs does not affect the ability of MSCs to suppress T cell responses in vitro nor to affect immune cell population in vivo and therefore, may be considered a suitable gene vector for MSC studies [36]. In view of the possibility of eGFP promoting an immune response in vivo we opted to continue in vivo experiments with BALB/c MSCs. MSCs have broadly similar characteristics across species [54] and it is likely that BALB/c MSCs may migrate to inflamed joints in a similar manner to FVB MSCs. Consistent with data from our group and others, and supported by the hypothesis that MSCs may contribute to disease chronicity, we saw a worsening of clinical disease after treatment with allogeneic BALB/c MSCs. Despite these data, it was demonstrated that MSCs expressing the transgene CTLA4Ig had the ability to significantly suppress disease activity for the duration of maximum transgene expression in vitro. For up to 6 days postdelivery, disease scores in this group remained close to zero, representing complete disease suppression. It may be that expression of CTLA4Ig had a therapeutic benefit on clinical inflammation and suppressed the potentially deleterious effect of allogeneic MSCs or that the very low disease scores seen soon after delivery may reflect homing of the MSCs to sites of inflammation.
Conclusion
We have shown for the first time that systemically infused MSCs reach the inflamed joint in mice with CIA. Also, notwithstanding its limitations, this study demonstrates that despite some initial negative results, allogeneic MSCs may have potential as gene therapy vectors for the treatment of inflammatory arthritis, and that MSCs expressing CTLA4Ig may have a profound effect on T cell responses. Further analysis using a lentiviral vector to prolong transgene expression, with serum measurements of that transgene, would allow further evaluation of this therapeutic strategy.
Acknowledgments
This work has been supported by the Health Research Board of Ireland under grant no. CRT/2007/6, Science Foundation Ireland under grant no. 09/SRC/B1794, the European Union's 7th Framework Programme under grant agreement no. HEALTH-2007-B-223298 (PurStem).
Author Disclosure Statement
The authors have no disclosures.
References
- 1.Choy EH. and Panayi GS. (2001). Cytokine pathways and joint inflammation in rheumatoid arthritis. N Engl J Med 344:907–916 [DOI] [PubMed] [Google Scholar]
- 2.Firestein GS. (2003). Evolving concepts of rheumatoid arthritis. Nature 423:356–361 [DOI] [PubMed] [Google Scholar]
- 3.Furst DE. (2010). Development of TNF inhibitor therapies for the treatment of rheumatoid arthritis. Clin Exp Rheumatol 28(3 Suppl 59):S5–S12 [PubMed] [Google Scholar]
- 4.Finckh A, Ciurea A, Brulhart L, Kyburz D, Moller B, Dehler S, Revaz S, Dudler J. and Gabay C. (2007). B cell depletion may be more effective than switching to an alternative anti-tumor necrosis factor agent in rheumatoid arthritis patients with inadequate response to anti-tumor necrosis factor agents. Arthritis Rheum 56:1417–1423 [DOI] [PubMed] [Google Scholar]
- 5.Genovese MC, McKay JD, Nasonov EL, Mysler EF, da Silva NA, Alecock E, Woodworth T. and Gomez-Reino JJ. (2008). Interleukin-6 receptor inhibition with tocilizumab reduces disease activity in rheumatoid arthritis with inadequate response to disease-modifying antirheumatic drugs: the tocilizumab in combination with traditional disease-modifying antirheumatic drug therapy study. Arthritis Rheum 58:2968–2980 [DOI] [PubMed] [Google Scholar]
- 6.Guyot P, Taylor P, Christensen R, Pericleous L, Poncet C, Lebmeier M, Drost P. and Bergman G. (2011). Abatacept with methotrexate versus other biologic agents in treatment of patients with active rheumatoid arthritis despite methotrexate: a network meta-analysis. Arthritis Res Ther 13:R204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barry FP. and Murphy JM. (2004). Mesenchymal stem cells: clinical applications and biological characterization. Int J Biochem Cell Biol 36:568–584 [DOI] [PubMed] [Google Scholar]
- 8.Duffy MM, Pindjakova J, Hanley SA, McCarthy C, Weidhofer GA, Sweeney EM, English K, Shaw G, Murphy JM, et al. (2011). Mesenchymal stem cell inhibition of T-helper 17 cell- differentiation is triggered by cell-cell contact and mediated by prostaglandin E2 via the EP4 receptor. Eur J Immunol 41:2840–2851 [DOI] [PubMed] [Google Scholar]
- 9.Le Blanc K, Rasmusson I, Gotherstrom C, Seidel C, Sundberg B, Sundin M, Rosendahl K, Tammik C. and Ringden O. (2004). Mesenchymal stem cells inhibit the expression of CD25 (interleukin-2 receptor) and CD38 on phytohaemagglutinin-activated lymphocytes. Scand J Immunol 60:307–315 [DOI] [PubMed] [Google Scholar]
- 10.Potian JA, Aviv H, Ponzio NM, Harrison JS. and Rameshwar P. (2003). Veto-like activity of mesenchymal stem cells: functional discrimination between cellular responses to alloantigens and recall antigens. J Immunol 171:3426–3434 [DOI] [PubMed] [Google Scholar]
- 11.Majumdar MK, Keane-Moore M, Buyaner D, Hardy WB, Moorman MA, McIntosh KR. and Mosca JD. (2003). Characterization and functionality of cell surface molecules on human mesenchymal stem cells. J Biomed Sci 10:228–241 [DOI] [PubMed] [Google Scholar]
- 12.Alm JJ, Koivu HM, Heino TJ, Hentunen TA, Laitinen S. and Aro HT. (2010). Circulating plastic adherent mesenchymal stem cells in aged hip fracture patients. J Orthop Res 28:1634–1642 [DOI] [PubMed] [Google Scholar]
- 13.Loebinger MR, Kyrtatos PG, Turmaine M, Price AN, Pankhurst Q, Lythgoe MF. and Janes SM. (2009). Magnetic resonance imaging of mesenchymal stem cells homing to pulmonary metastases using biocompatible magnetic nanoparticles. Cancer Res 69:8862–8867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Augello A, Tasso R, Negrini SM, Cancedda R. and Pennesi G. (2007). Cell therapy using allogeneic bone marrow mesenchymal stem cells prevents tissue damage in collagen-induced arthritis. Arthritis Rheum 56:1175–1186 [DOI] [PubMed] [Google Scholar]
- 15.González MA, Gonzalez-Rey E, Rico L, Büscher D. and Delgado M. (2009). Treatment of experimental arthritis by inducing immune tolerance with human adipose-derived mesenchymal stem cells. Arthritis Rheum 60:1006–1019 [DOI] [PubMed] [Google Scholar]
- 16.Mao F, Xu WR, Qian H, Zhu W, Yan YM, Shao QX. and Xu HX. (2010). Immunosuppressive effects of mesenchymal stem cells in collagen-induced mouse arthritis. Inflamm Res 59:219–225 [DOI] [PubMed] [Google Scholar]
- 17.Liu Y, Mu R, Wang S, Long L, Liu X, Li R, Sun J, Guo J, Zhang X, et al. (2010). Therapeutic potential of human umbilical cord mesenchymal stem cells in the treatment of rheumatoid arthritis. Arthritis Res Ther 12:R210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sullivan C, Murphy JM, Griffin MD, Porter RM, Evans CH, O'Flatharta C, Shaw G. and Barry F. (2012). Genetic mismatch affects the immunosuppressive properties of mesenchymal stem cells in vitro and their ability to influence the course of collagen-induced arthritis. Arthritis Res Ther 14:R167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Djouad F, Fritz V, Apparailly F, Louis-Plence P, Bony C, Sany J, Jorgensen C. and Noel D. (2005). Reversal of the immunosuppressive properties of mesenchymal stem cells by tumor necrosis factor alpha in collagen-induced arthritis. Arthritis Rheum 52:1595–1603 [DOI] [PubMed] [Google Scholar]
- 20.Schurgers E, Kelchtermans H, Mitera T, Geboes L. and Matthys P. (2010). Discrepancy between the in vitro and in vivo effects of murine mesenchymal stem cells on T-cell proliferation and collagen-induced arthritis. Arthritis Res Ther 12:R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen B, Hu J, Liao L, Sun Z, Han Q, Song Z. and Zhao RC. (2010). Flk-1+mesenchymal stem cells aggravate collagen-induced arthritis by up-regulating interleukin-6. Clin Exp Immunol 159:292–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi JJ, Yoo SA, Park SJ, Kang YJ, Kim WU, Oh IH. and Cho CS. (2008). Mesenchymal stem cells overexpressing interleukin-10 attenuate collagen-induced arthritis in mice. Clin Exp Immunol 153:269–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Papadopoulou A, Yiangou M, Athanasiou E, Zogas N, Kaloyannidis P, Batsis I, Fassas A, Anagnostopoulos A. and Yannaki E. (2012). Mesenchymal stem cells are conditionally therapeutic in preclinical models of rheumatoid arthritis. Ann Rheum Dis 71:1733–1740 [DOI] [PubMed] [Google Scholar]
- 24.Park MJ, Park HS, Cho ML, Oh HJ, Cho YG, Min SY, Chung BH, Lee JW, Kim HY. and Cho SG. (2011). Transforming growth factor beta-transduced mesenchymal stem cells ameliorate experimental autoimmune arthritis through reciprocal regulation of Treg/Th17 cells and osteoclastogenesis. Arthritis Rheum 63:1668–1680 [DOI] [PubMed] [Google Scholar]
- 25.Sharpe AH. and Abbas AK. (2006). T-cell costimulation—biology, therapeutic potential, and challenges. N Engl J Med 355:973–975 [DOI] [PubMed] [Google Scholar]
- 26.Tse WT, Pendleton JD, Beyer WM, Egalka MC. and Guinan EC. (2003). Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation 75:389–397 [DOI] [PubMed] [Google Scholar]
- 27.Zappia E, Casazza S, Pedemonte E, Benvenuto F, Bonanni I, Gerdoni E, Giunti D, Ceravolo A, Cazzanti F, et al. (2005). Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood 106:1755–1761 [DOI] [PubMed] [Google Scholar]
- 28.Aggarwal S. and Pittenger MF. (2005). Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 105:1815–1822 [DOI] [PubMed] [Google Scholar]
- 29.English K, Barry FP. and Mahon BP. (2008). Murine mesenchymal stem cells suppress dendritic cell migration, maturation and antigen presentation. Immunol Lett 115:50–58 [DOI] [PubMed] [Google Scholar]
- 30.Hu W, Wang J, He X, Zhang H, Yu F, Jiang L, Chen D, Chen J. and Dou J. (2011). Human umbilical blood mononuclear cell-derived mesenchymal stem cells serve as interleukin-21 gene delivery vehicles for epithelial ovarian cancer therapy in nude mice. Biotechnol Appl Biochem 58:397–404 [DOI] [PubMed] [Google Scholar]
- 31.Fei S, Qi X, Kedong S, Guangchun J, Jian L. and Wei Q. (2012). The antitumor effect of mesenchymal stem cells transduced with a lentiviral vector expressing cytosine deaminase in a rat glioma model. J Cancer Res Clin Oncol 138:347–357 [DOI] [PubMed] [Google Scholar]
- 32.Cihova M, Altanerova V. and Altaner C. (2011). Stem cell based cancer gene therapy. Mol Pharm 8:1480–1487 [DOI] [PubMed] [Google Scholar]
- 33.Deng Y, Guo X. and Yuan Q. (2003). Efficiency of adenoviral vector mediated CTLA4Ig gene delivery into mesenchymal stem cells. Chin Med J (Engl) 116:1649–1654 [PubMed] [Google Scholar]
- 34.Choi EW, Shin IS, Lee HW, Park SY, Park JH, Nam MH, Kim JS, Woo SK, Yoon EJ, et al. (2011). Transplantation of CTLA4Ig gene-transduced adipose tissue-derived mesenchymal stem cells reduces inflammatory immune response and improves Th1/Th2 balance in experimental autoimmune thyroiditis. J Gene Med 13:3–16 [DOI] [PubMed] [Google Scholar]
- 35.Zhang X, Zhang C, Tang T, Qu Z, Lou J. and Dai K. (2008). Immunomodulatory and osteogenic differentiation effects of mesenchymal stem cells by adenovirus-mediated coexpression of CTLA4Ig and BMP2. J Orthop Res 26:314–321 [DOI] [PubMed] [Google Scholar]
- 36.Treacy O, Ryan AE, Heinzl T, O'Flynn L, Cregg M, Wilk M, Odoardi F, Lohan P, O'Brien T, Nosov M. and Ritter T. (2012). Adenoviral transduction of mesenchymal stem cells: in vitro responses and in vivo immune responses after cell transplantation. PLoS One 7:e42662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gong N, Pleyer U, Yang J, Vogt K, Hill M, Anegon I, Volk HD. and Ritter T. (2006). Influence of local and systemic CTLA4Ig gene transfer on corneal allograft survival. J Gene Med 8:459–467 [DOI] [PubMed] [Google Scholar]
- 38.McMahon JM, Conroy S, Lyons M, Greiser U, O'Shea C, Strappe P, Howard L, Murphy M, Barry F. and O'Brien T. (2006). Gene transfer into rat mesenchymal stem cells: a comparative study of viral and nonviral vectors. Stem Cells Dev 15:87–96 [DOI] [PubMed] [Google Scholar]
- 39.Murphy JM, Fink DJ, Hunziker EB. and Barry FP. (2006). Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum 48:3464–3474 [DOI] [PubMed] [Google Scholar]
- 40.Eljaafari A, Tartelin ML, Aissaoui H, Chevrel G, Osta B, Lavocat F. and Miossec P. (2012). Bone marrow-derived and synovium-derived mesenchymal cells promote Th17 cell expansion and activation through caspase 1 activation: contribution to the chronicity of rheumatoid arthritis. Arthritis Rheum 64:2147–2157 [DOI] [PubMed] [Google Scholar]
- 41.Marinova-Mutafchieva L, Williams RO, Funa K, Maini RN. and Zvaifler NJ. (2002). Inflammation is preceded by tumor necrosis factor-dependent infiltration of mesenchymal cells in experimental arthritis. Arthritis Rheum 46:507–513 [DOI] [PubMed] [Google Scholar]
- 42.Kawada H, Fujita J, Kinjo K, Matsuzaki Y, Tsuma M, Miyatake H, Muguruma Y, Tsuboi K, Itabashi Y, et al. (2004). Nonhematopoietic mesenchymal stem cells can be mobilized and differentiate into cardiomyocytes after myocardial infarction. Blood 104:3581–3587 [DOI] [PubMed] [Google Scholar]
- 43.Li X. and Makarov SS. (2006). An essential role of NF-kappaB in the “tumor-like” phenotype of arthritic synoviocytes. Proc Natl Acad Sci U S A 103:17432–17437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Karp JM. and Leng Teo GS. (2009). Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell 4:206–216 [DOI] [PubMed] [Google Scholar]
- 45.Quante M, Tu SP, Tomita H, Gonda T, Wang SS, Takashi S, Baik GH, Shibata W, Diprete B, et al. (2011). Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell 19:257–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang CH, Cherng WJ, Yang NI, Kuo LT, Hsu CM, Yeh HI, Lan YJ, Yeh CH. and Stanford WL. (2008). Late-outgrowth endothelial cells attenuate intimal hyperplasia contributed by mesenchymal stem cells after vascular injury. Arterioscler Thromb Vasc Biol 28:54–60 [DOI] [PubMed] [Google Scholar]
- 47.Teo GS, Ankrum JA, Martinelli R, Boetto SE, Simms K, Sciuto TE, Dvorak AM, Karp JM. and Carman CV. (2012). Mesenchymal stem cells transmigrate between and directly through tumor necrosis factor-α-activated endothelial cells via both leukocyte-like and novel mechanisms. Stem Cells 30:2472–2486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee RH, Seo MJ, Pulin AA, Gregory CA, Ylostalo J. and Prockop DJ. (2009). The CD34-like protein PODXL and alpha6-integrin (CD49f ) identify early progenitor MSCs with increased clonogenicity and migration to infarcted heart in mice. Blood 113:816–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao W, Li JJ, Cao DY, Li X, Zhang LY, He Y, Yue SQ, Wang DS. and Dou KF. (2012). Intravenous injection of mesenchymal stem cells is effective in treating liver fibrosis. World J Gastroenterol 18:1048–1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baertschiger RM, Serre-Beinier V, Morel P, Bosco D, Peyrou M, Clément S, Sgroi A, Kaelin A, Buhler LH. and Gonelle-Gispert C. (2009). Fibrogenic potential of human multipotent mesenchymal stromal cells in injured liver. PLoS One 4:e6657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Streetz KL, Wüstefeld T, Klein C, Manns MP. and Trautwein C. (2001). Mediators of inflammation and acute phase response in the liver. Cell Mol Biol (Noisy-le-grand) 47:661–673 [PubMed] [Google Scholar]
- 52.Peister A, Mellad JA, Larson BL, Hall BM, Gibson LF. and Prockop DJ. (2004). Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood 103:1662–1668 [DOI] [PubMed] [Google Scholar]
- 53.Billiau A. and Matthys P. (2011). Collagen-induced arthritis and related animal models: how much of their pathogenesis is auto-immune, how much is auto-inflammatory? Cytokine Growth Factor Rev 22:339–344 [DOI] [PubMed] [Google Scholar]
- 54.Marti L, Golmia R, Golmia AP, Paes AT, Guilhen DD, Moreira-Filho CA. and Scheinberg M. (2009). Alterations in cytokine profile and dendritic cells subsets in peripheral blood of rheumatoid arthritis patients before and after biologic therapy. Ann N Y Acad Sci 1173:334–342 [DOI] [PubMed] [Google Scholar]